Bioactive and Topographically-Modified Electrospun Membranes for the Creation of New Bone Regeneration Models
Abstract
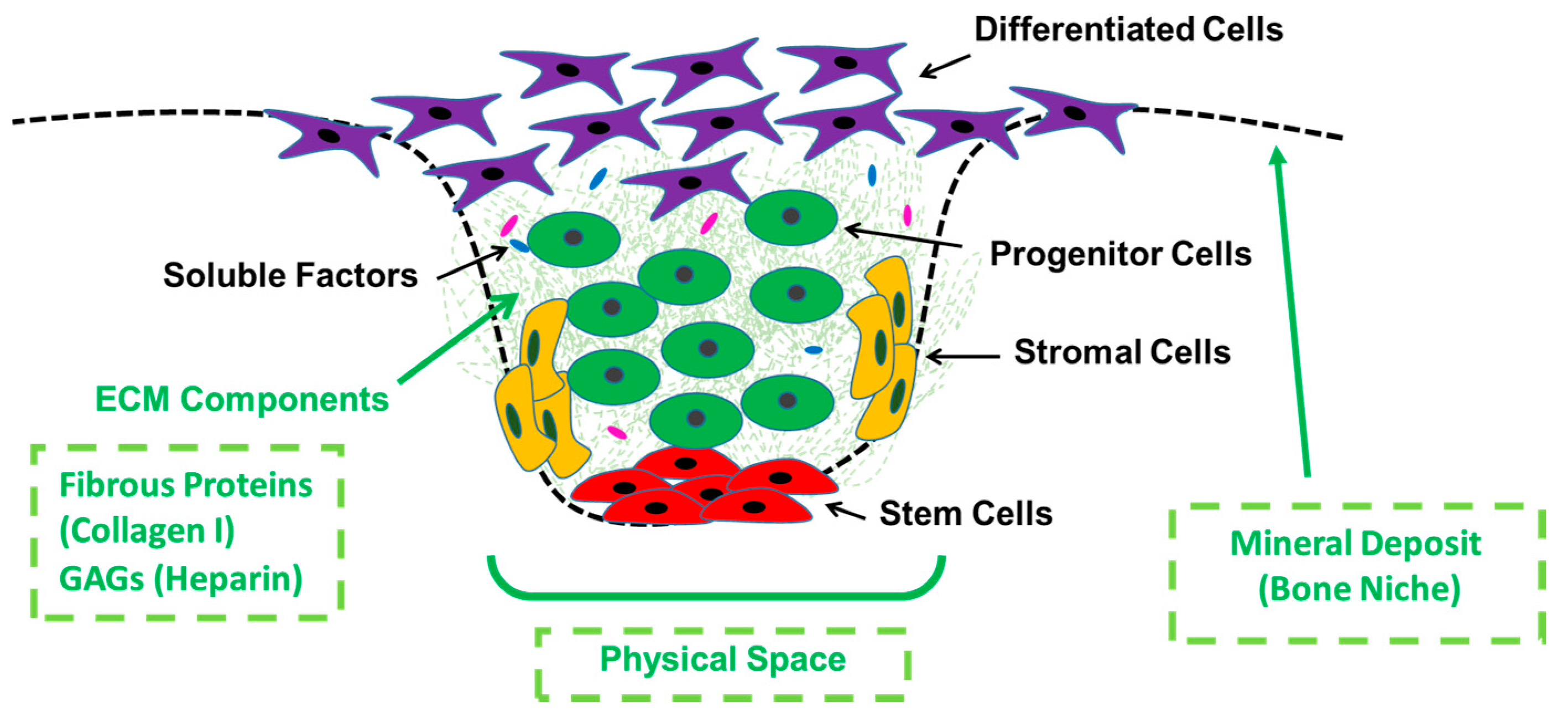
1. Introduction
2. Materials and Methods
2.1. Manufacture of Electrospun Membranes
2.2. Characterization of PCL Electrospun Scaffolds Prior to Biofunctionalization
2.3. Biofunctionalisation of PCL Electrospun Scaffolds
2.3.1. Absorption of Collagen and Heparin
2.3.2. Incorporation of Inorganic Component (Bioactive Glass)
2.4. Materials Characterization after Incorporation of Biofunctional Agents
2.5. Biological Assessment
2.5.1. Cell Culture
2.5.2. Cell Viability and Cell Morphology
2.5.3. Alkaline Phosphatase Assay (ALP)
2.6. Statistical Analysis
3. Results
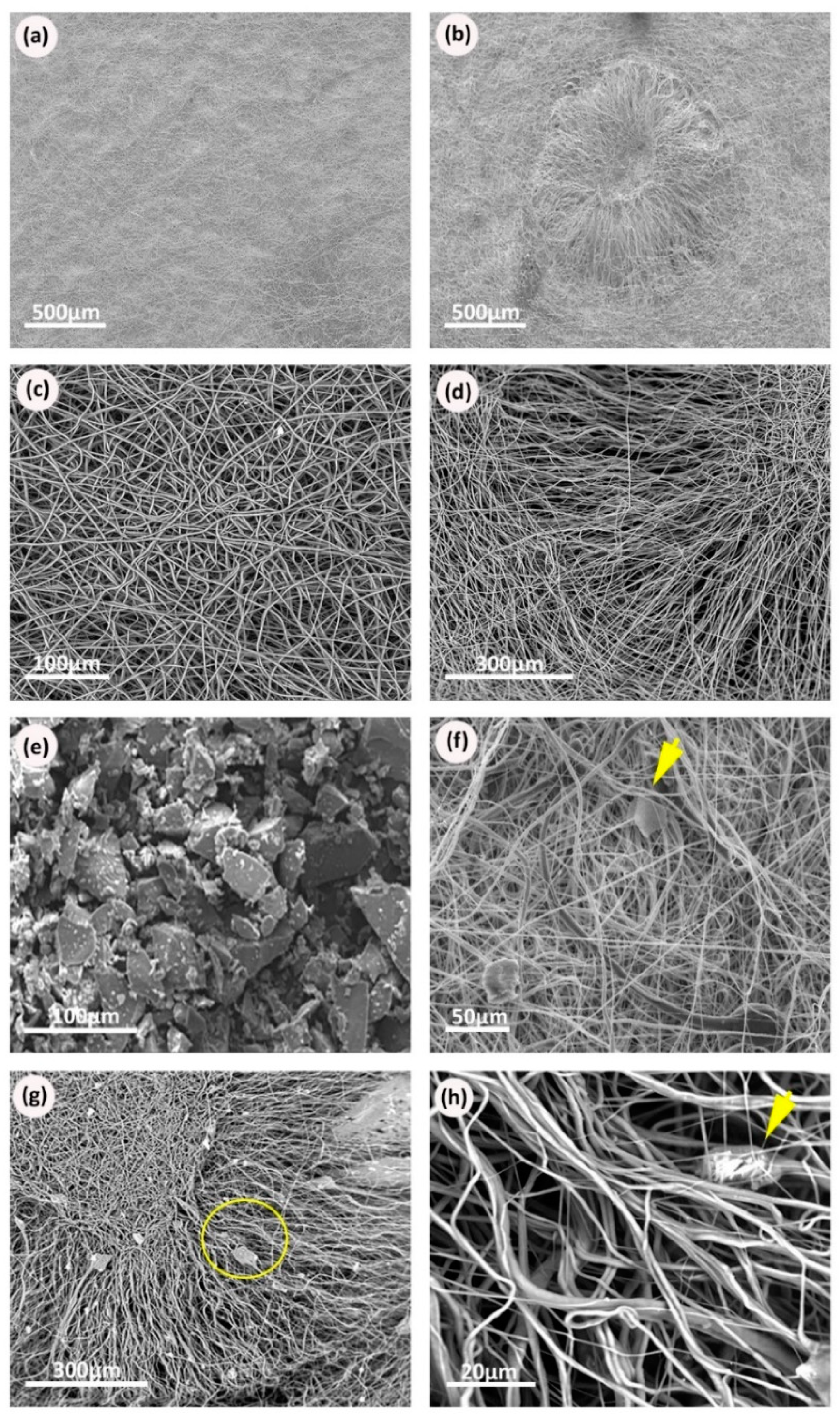
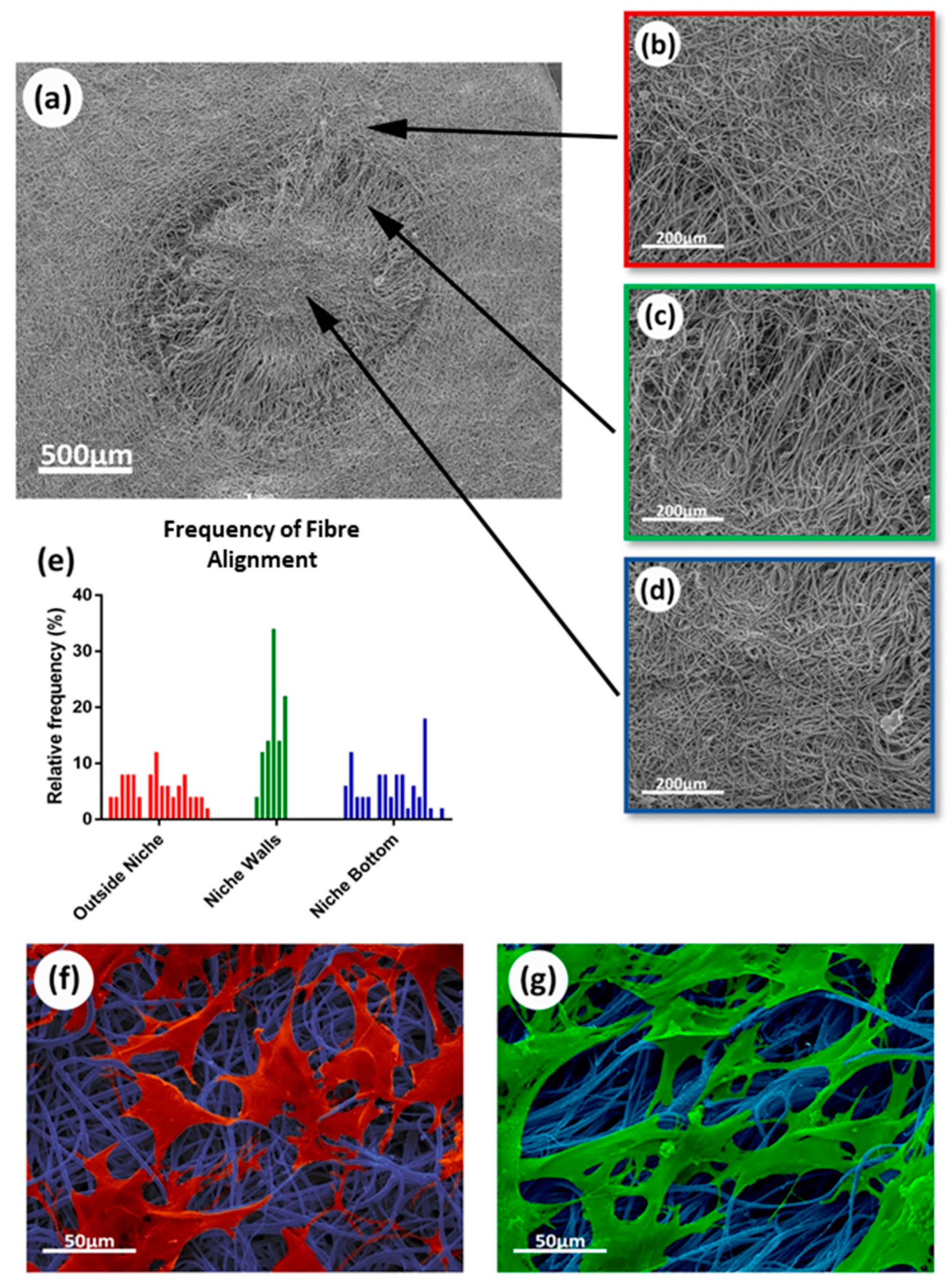
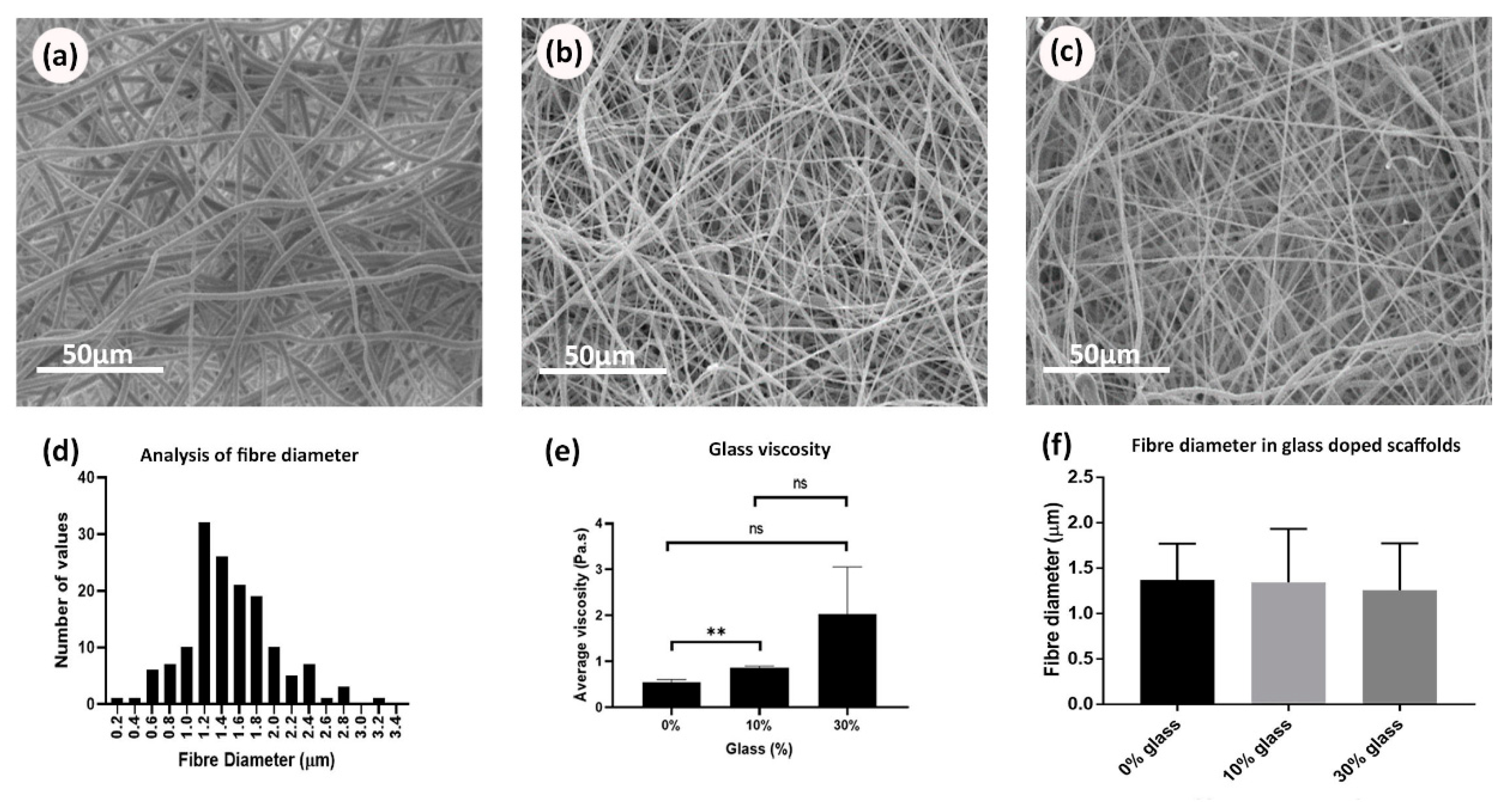
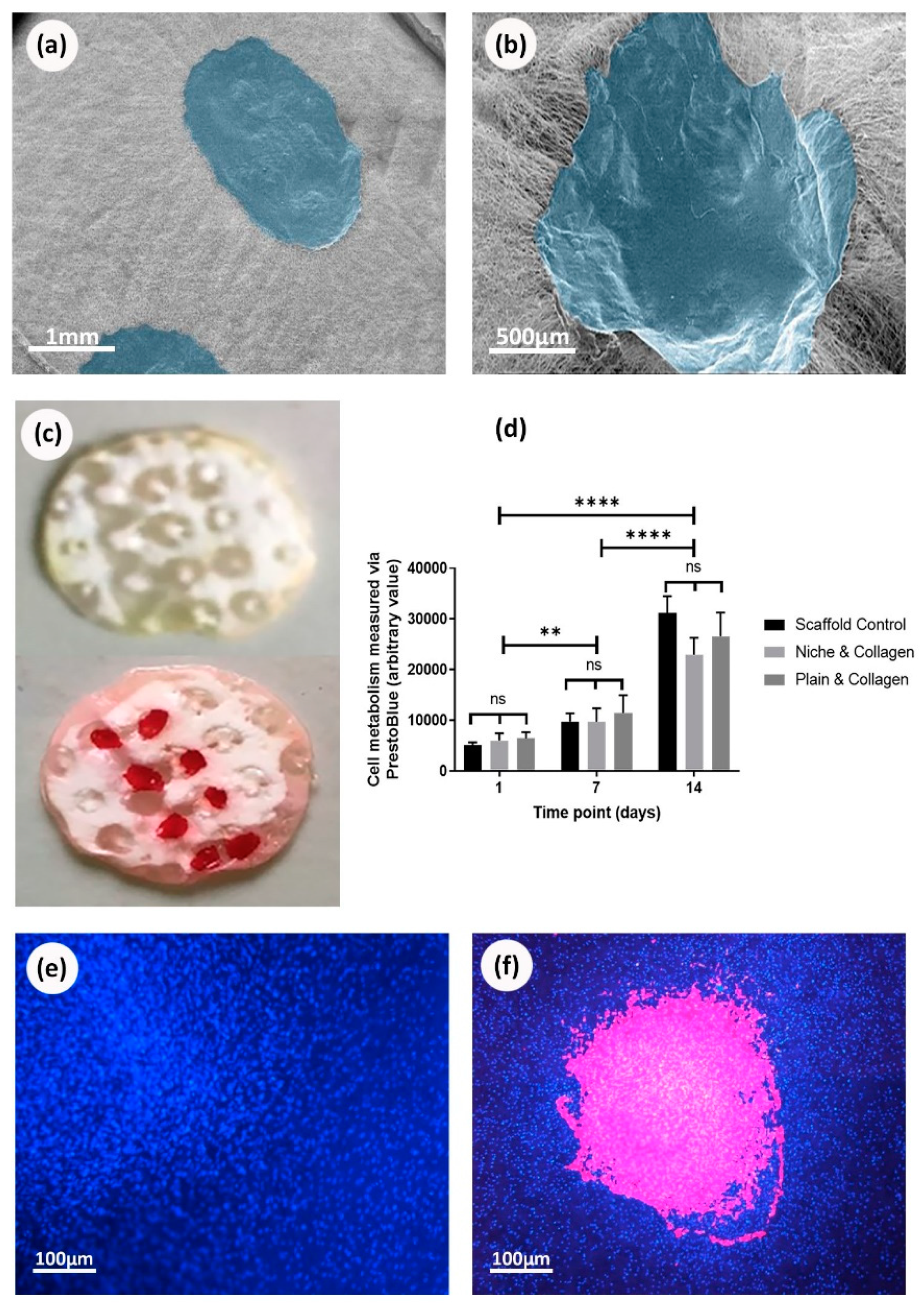
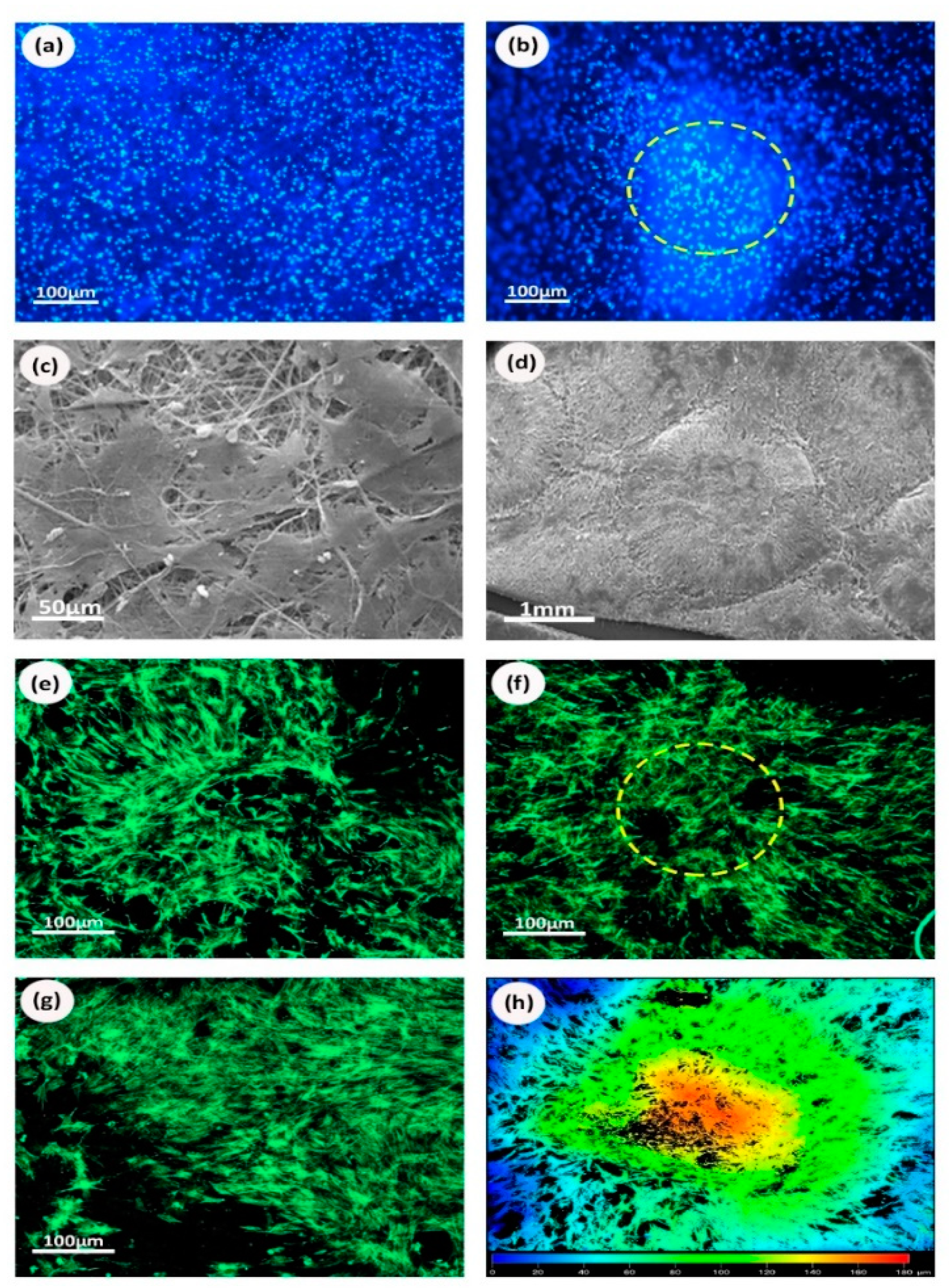
3.1. Electrospun Scaffold’s Shape, Thickness, and Fiber Morphology
3.2. Rheological Properties for Bioglass-Doped Electrospinning Solutions
3.3. Incorporation of Biomolecules on the PCL Scaffolds
3.4. Cells Viability on Biofunctionalized PCL Scaffolds
3.5. Cells Viability on Bioglass Doped Scaffolds
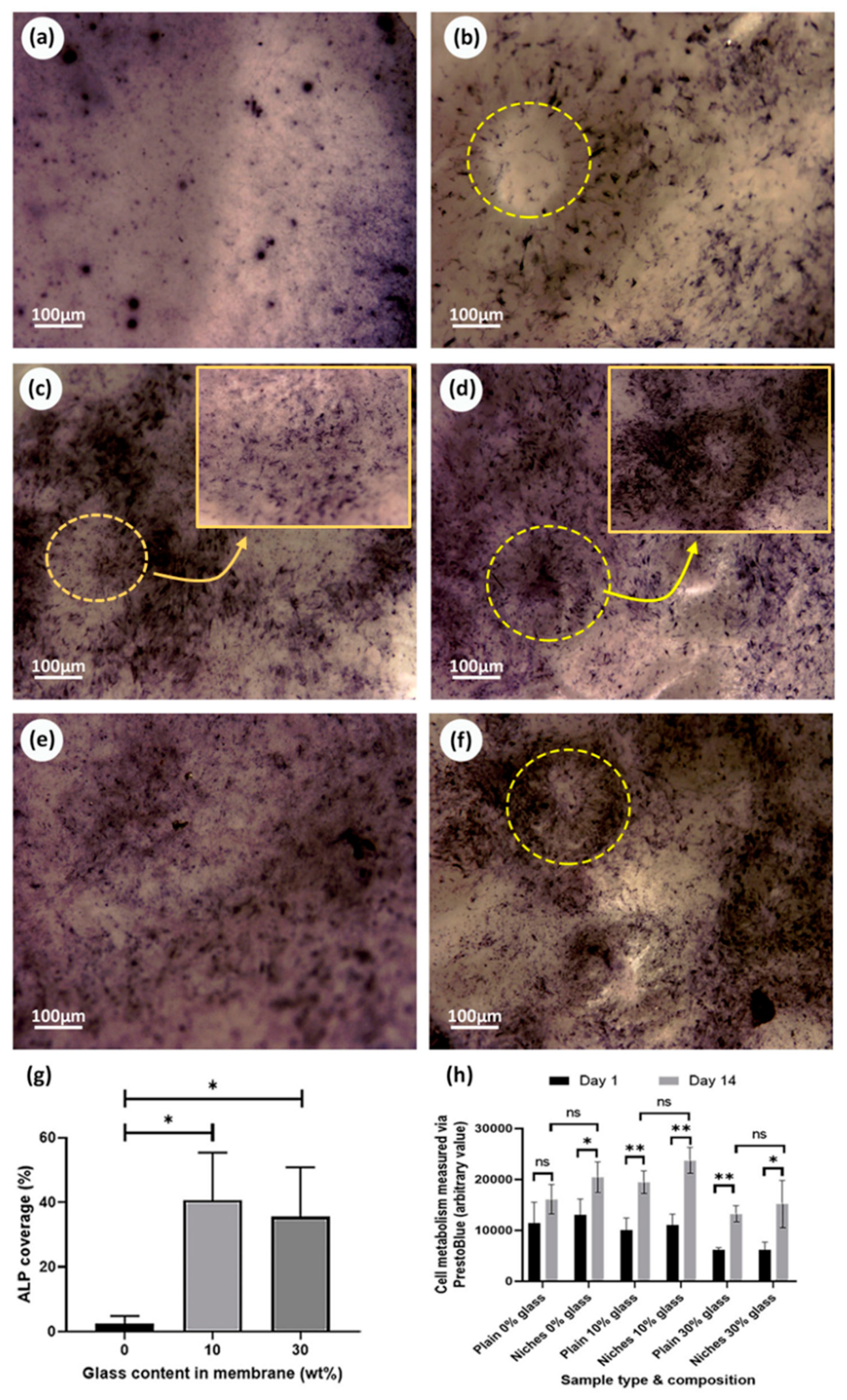
3.6. Osteogenic Properties of Bioactive Glass
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Storheim, K.; Zwart, J.A. Musculoskeletal disorders and the Global Burden of Disease study. Ann. Rheum Dis. 2014, 73, 949–950. [Google Scholar] [CrossRef] [PubMed]
- Hernlund, E.; Svedbom, A.; Ivergard, M.; Compston, J.; Cooper, C.; Stenmark, J.; McCloskey, E.V.; Jonsson, B.; Kanis, J.A. Osteoporosis in the European Union: Medical management, epidemiology and economic burden. A report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch. Osteoporos. 2013, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Gautschi, O.P.; Frey, S.P.; Zellweger, R. Bone morphogenetic proteins in clinical applications. ANZ J. Surg. 2007, 77, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Polo-Corrales, L.; Latorre-Esteves, M.; Ramirez-Vick, J.E. Scaffold Design for Bone Regeneration. J. Nanosci. Nanotech. 2014, 14, 15–56. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef]
- Schofield, R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 1978, 4, 7–25. [Google Scholar]
- Cotsarelis, G.; Sun, T.T.; Lavker, R.M. Label-retaining cells reside in the bulge area of pilosebaceous unit: Implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 1990, 61, 1329–1337. [Google Scholar] [CrossRef]
- Potten, C.S.; Owen, G.; Booth, D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 2002, 115, 2381–2388. [Google Scholar]
- Cotsarelis, G.; Cheng, S.Z.; Dong, G.; Sun, T.T.; Lavker, R.M. Existence of Slow-Cycling Limbal Epithelial Basal Cells That Can Be Preferentially Stimulated to Proliferate - Implications on Epithelial Stem-Cells. Cell 1989, 57, 201–209. [Google Scholar] [CrossRef]
- Schermer, A.; Galvin, S.; Sun, T.T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J. Cell Biol. 1986, 103, 49–62. [Google Scholar] [CrossRef]
- Gage, F.H.; Kempermann, G.; Palmer, T.D.; Peterson, D.A.; Ray, J. Multipotent progenitor cells in the adult dentate gyrus. J. Neurobiol. 1998, 36, 249–266. [Google Scholar] [CrossRef]
- Loeffler, M.; Roeder, I. Tissue stem cells: Definition, plasticity, heterogeneity, self-organization and models—A conceptual approach. Cells Tissues Organs. 2002, 171, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Blau, H.M. Artificial Stem Cell Niches. Adv. Mater. 2009, 21, 3255–3268. [Google Scholar] [CrossRef] [PubMed]
- Roeder, I.; Loeffler, M.; Glauche, I.; Participants, O. Towards a quantitative understanding of stem cell-niche interaction: Experiments, models, and technologies. Blood Cell Mol. Dis. 2011, 46, 308–317. [Google Scholar] [CrossRef]
- Paterson, T.E.; Beal, S.N.; Santocildes-Romero, M.E.; Sidambe, A.T.; Hatton, P.V.; Asencio, I.O. Selective laser melting-enabled electrospinning: Introducing complexity within electrospun membranes. Proc. Inst. Mech. Eng. Part H 2017, 231, 565–574. [Google Scholar] [CrossRef]
- Raimondi, M.T.; Eaton, S.M.; Lagana, M.; Aprile, V.; Nava, M.M.; Cerullo, G.; Osellame, R. Three-dimensional structural niches engineered via two-photon laser polymerization promote stem cell homing. Acta Biomater. 2013, 9, 4579–4584. [Google Scholar] [CrossRef]
- Kaukua, N.; Shahidi, M.K.; Konstantinidou, C.; Dyachuk, V.; Kaucka, M.; Furlan, A.; An, Z.W.; Wang, L.L.; Hultman, I.; Ahrlund-Richter, L.; et al. Glial origin of mesenchymal stem cells in a tooth model system. Nature 2014, 513. [Google Scholar] [CrossRef]
- Gill, A.A.; Ortega, I.; Kelly, S.; Claeyssens, F. Towards the fabrication of artificial 3D microdevices for neural cell networks. Biomed. Microdevices 2015, 17. [Google Scholar] [CrossRef]
- Ortega, I.; Deshpande, P.; Gill, A.A.; MacNeil, S.; Claeyssens, F. Development of a microfabricated artificial limbus with micropockets for cell delivery to the cornea. Biofabrication 2013, 5. [Google Scholar] [CrossRef]
- Ortega, I.; McKean, R.; Ryan, A.J.; MacNeil, S.; Claeyssens, F. Characterisation and evaluation of the impact of microfabricated pockets on the performance of limbal epithelial stem cells in biodegradable PLGA membranes for corneal regeneration. Biomater. Sci. UK 2014, 2, 723–734. [Google Scholar] [CrossRef]
- Ortega, I.; Ryan, A.J.; Deshpande, P.; MacNeil, S.; Claeyssens, F. Combined microfabrication and electrospinning to produce 3-D architectures for corneal repair. Acta Biomater. 2013, 9, 5511–5520. [Google Scholar] [CrossRef] [PubMed]
- Ortega, I.; Sefat, F.; Deshpande, P.; Paterson, T.; Ramachandran, C.; Ryan, A.J.; MacNeil, S.; Claeyssens, F. Combination of Microstereolithography and Electrospinning to Produce Membranes Equipped with Niches for Corneal Regeneration. JOVE J. Vis. Exp. 2014. [Google Scholar] [CrossRef]
- Fisher, O.Z.; Khademhosseini, A.; Langer, R.; Peppas, N.A. Bioinspired Materials for Controlling Stem Cell Fate. Acc. Chem. Res. 2010, 43, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Moeller, H.C.; Mian, M.K.; Shrivastava, S.; Chung, B.G.; Khademhosseini, A. A microwell array system for stem cell culture. Biomaterials 2008, 29, 752–763. [Google Scholar] [CrossRef]
- Muller, E.; Grinenko, T.; Pompe, T.; Waskow, C.; Werner, C. Space constraints govern fate of hematopoietic stem and progenitor cells in vitro. Biomaterials 2015, 53, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Li, L.H. The stem cell niches in bone. J. Clin. Investig. 2006, 116, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Minardi, S.; Corradetti, B.; Taraballi, F.; Sandri, M.; Van Eps, J.; Cabrera, F.J.; Weiner, B.K.; Tampieri, A.; Tasciotti, E. Evaluation of the osteoinductive potential of a bio-inspired scaffold mimicking the osteogenic niche for bone augmentation. Biomaterials 2015, 62, 128–137. [Google Scholar] [CrossRef]
- Danti, S.; Serino, L.P.; D′Alessandro, D.; Moscato, S.; Danti, S.; Trombi, L.; Dinucci, D.; Chiellini, F.; Pietrabissa, A.; Lisanti, M.; et al. Growing bone tissue-engineered niches with graded osteogenicity: An in vitro method for biomimetic construct assembly. Tissue Eng. Part C 2013, 19, 911–924. [Google Scholar] [CrossRef]
- Muerza-Cascante, M.L.; Shokoohmand, A.; Khosrotehrani, K.; Haylock, D.; Dalton, P.D.; Hutmacher, D.W.; Loessner, D. Endosteal-like extracellular matrix expression on melt electrospun written scaffolds. Acta Biomater. 2017, 52, 145–158. [Google Scholar] [CrossRef]
- Tang, W.; Lin, D.; Yu, Y.; Niu, H.; Guo, H.; Yuan, Y.; Liu, C. Bioinspired trimodal macro/micro/nano-porous scaffolds loading rhBMP-2 for complete regeneration of critical size bone defect. Acta Biomater. 2016, 32, 309–323. [Google Scholar] [CrossRef]
- Di Maggio, N.; Piccinini, E.; Jaworski, M.; Trumpp, A.; Wendt, D.J.; Martin, I. Toward modeling the bone marrow niche using scaffold-based 3D culture systems. Biomaterials 2011, 32, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Kular, J.K.; Basu, S.; Sharma, R.I. The extracellular matrix: Structure, composition, age-related differences, tools for analysis and applications for tissue engineering. J. Tissue Eng. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Capila, I.; Linhardt, R.J. Heparin-protein interactions. Angew. Chem. Int. Ed. Engl. 2002, 41, 391–412. [Google Scholar] [CrossRef]
- Farbod, K.; Nejadnik, M.R.; Jansen, J.A.; Leeuwenburgh, S.C.G. Interactions Between Inorganic and Organic Phases in Bone Tissue as a Source of Inspiration for Design of Novel Nanocomposites. Tissue Eng. Part B 2014, 20, 173–188. [Google Scholar] [CrossRef]
- Chuenjitkuntaworn, B.; Osathanon, T.; Nowwarote, N.; Supaphol, P.; Pavasant, P. The efficacy of polycaprolactone/hydroxyapatite scaffold in combination with mesenchymal stem cells for bone tissue engineering. J. Biomed. Mater. Res. A 2016, 104, 264–271. [Google Scholar] [CrossRef]
- Ning, L.; Malmstrom, H.; Ren, Y.F. Porous collagen-hydroxyapatite scaffolds with mesenchymal stem cells for bone regeneration. J. Oral. Implantol. 2015, 41, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Sanaei-Rad, P.; Jafarzadeh Kashi, T.S.; Seyedjafari, E.; Soleimani, M. Enhancement of stem cell differentiation to osteogenic lineage on hydroxyapatite-coated hybrid PLGA/gelatin nanofiber scaffolds. Biologicals 2016, 44, 511–516. [Google Scholar] [CrossRef]
- Fiume, E.; Barberi, J.; Verne, E.; Baino, F. Bioactive Glasses: From Parent 45S5 Composition to Scaffold-Assisted Tissue-Healing Therapies. J. Funct. Biomater. 2018, 9, 24. [Google Scholar] [CrossRef]
- Fu, Q.; Saiz, E.; Rahaman, M.N.; Tomsia, A.P. Bioactive glass scaffolds for bone tissue engineering: State of the art and future perspectives. Mat. Sci. Eng. C Mater. 2011, 31, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Mezger, T. The Rheology Handbook; Vincentz Network: Hnaover, Germany, 2011. [Google Scholar]
- Santocildes-Romero, M.E.; Crawford, A.; Hatton, P.V.; Goodchild, R.L.; Reaney, I.M.; Miller, C.A. The osteogenic response of mesenchymal stromal cells to strontium-substituted bioactive glasses. J. Tissue Eng. Regen. M 2015, 9, 619–631. [Google Scholar] [CrossRef]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef]
- Li, X.M.; Wang, L.; Fan, Y.B.; Feng, Q.L.; Cui, F.Z.; Watari, F. Nanostructured scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 2424–2435. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.F.; Ding, X.L.; Zhou, G.; Li, P.; Wei, X.; Fan, Y.B. Electrospinning of Nanofibers for Tissue Engineering Applications. J. Nanomater. 2013. [Google Scholar] [CrossRef]
- Schutte, J.; Yuan, X.W.; Dirven, S.; Potgieter, J. The opportunity of Electrospinning as a form of Additive Manufacturing in Biotechnology. I C Mech. Mach. Vis. Pr. 2017, 131–136. [Google Scholar] [CrossRef]
- Ortega, I.; Dew, L.; Kelly, A.G.; Chong, C.K.; MacNeil, S.; Claeyssens, F. Fabrication of biodegradable synthetic perfusable vascular networks via a combination of electrospinning and robocasting. Biomater. Sci. UK 2015, 3, 592–596. [Google Scholar] [CrossRef]
- Discher, D.E.; Mooney, D.J.; Zandstra, P.W. Growth factors, matrices, and forces combine and control stem cells. Science 2009, 324, 1673–1677. [Google Scholar] [CrossRef]
- Huang, Z.; Ren, P.G.; Ma, T.; Smith, R.L.; Goodman, S.B. Modulating osteogenesis of mesenchymal stem cells by modifying growth factor availability. Cytokine 2010, 51, 305–310. [Google Scholar] [CrossRef]
- Murali, S.; Rai, B.; Dombrowski, C.; Lee, J.L.; Lim, Z.X.; Bramono, D.S.; Ling, L.; Bell, T.; Hinkley, S.; Nathan, S.S.; et al. Affinity-selected heparan sulfate for bone repair. Biomaterials 2013, 34, 5594–5605. [Google Scholar] [CrossRef]
- Mathews, S.; Mathew, S.A.; Gupta, P.K.; Bhonde, R.; Totey, S. Glycosaminoglycans enhance osteoblast differentiation of bone marrow derived human mesenchymal stem cells. J. Tissue Eng. Regen. Med. 2014, 8, 143–152. [Google Scholar] [CrossRef]
- Gong, T.; Xie, J.; Liao, J.; Zhang, T.; Lin, S.; Lin, Y. Nanomaterials and bone regeneration. Bone Res. 2015, 3, 15029. [Google Scholar] [CrossRef]
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Lobel, K.D.; Hench, L.L. In vitro adsorption and activity of enzymes on reaction layers of bioactive glass substrates. J. Biomed. Mater. Res. 1998, 39, 575–579. [Google Scholar] [CrossRef]
- Keshaw, H.; Forbes, A.; Day, R.M. Release of angiogenic growth factors from cells encapsulated in alginate beads with bioactive glass. Biomaterials 2005, 26, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.H.; Tang, A.; Oh, S.C.; Spalazzi, J.P.; Dionisio, K. Compositional effects on the formation of a calcium phosphate layer and the response of osteoblast-like cells on polymer-bioactive glass composites. Biomaterials 2005, 26, 6323–6334. [Google Scholar] [CrossRef]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Ionic products of bioactive glass dissolution increase proliferation of human osteoblasts and induce insulin-like growth factor II mRNA expression and protein synthesis. Biochem. Res. 2000, 276, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, K.; Kotaki, M.; Ramakrishna, S. Guided bone regeneration membrane made of polycaprolactone/calcium carbonate composite nano-fibers. Biomaterials 2005, 26, 4139–4147. [Google Scholar] [CrossRef] [PubMed]
- Kouhi, M.; Morshed, M.; Varshosaz, J.; Fathi, M.H. Poly (epsilon-caprolactone) incorporated bioactive glass nanoparticles and simvastatin nanocomposite nanofibers: Preparation, characterization and in vitro drug release for bone regeneration applications. Chem. Eng. J. 2013, 228, 1057–1065. [Google Scholar] [CrossRef]
- Lin, H.M.; Lin, Y.H.; Hsu, F.Y. Preparation and characterization of mesoporous bioactive glass/polycaprolactone nanofibrous matrix for bone tissues engineering. J. Mater. Sci. Mater. M 2012, 23, 2619–2630. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Nelson, E.R.; Smith, R.L.; Goodman, S.B. The sequential expression profiles of growth factors from osteoprogenitors [correction of osteroprogenitors] to osteoblasts in vitro. Tissue Eng. 2007, 13, 2311–2320. [Google Scholar] [CrossRef]
- Shi, J.; Wang, L.; Zhang, F.; Li, H.; Lei, L.; Liu, L.; Chen, Y. Incorporating Protein Gradient into Electrospun Nanofibers As Scaffolds for Tissue Engineering. ACS Appl. Mater. Inter. 2010, 2, 1025–1030. [Google Scholar] [CrossRef]
- Wallace, K.E.; Hill, R.G.; Pembroke, J.T.; Brown, C.J.; Hatton, P.V. Influence of sodium oxide content on bioactive glass properties. J. Mater. Sci. Mater. Med. 1999, 10, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, P.; Cannillo, V.; Sola, A.; Dorigato, A.; Chiellini, F. Highly porous polycaprolactone-45S5 Bioglass (R) scaffolds for bone tissue engineering. Compos. Sci. Technol. 2010, 70, 1869–1878. [Google Scholar] [CrossRef]
- Kaufmann, E.A.B.E.; Ducheyne, P.; Shapiro, I.M. Evaluation of osteoblast response to porous bioactive glass (45S5) substrates by RT-PCR analysis. Tissue Eng. 2000, 6, 19–28. [Google Scholar] [CrossRef]
- Yoshida, M.; Langer, R.; Lendlein, A.; Lahann, J. From advanced biomedical coatings to multi-functionalized biomaterials. Polym. Rev. 2006, 46, 347–375. [Google Scholar] [CrossRef]
- Yoo, H.S.; Kim, T.G.; Park, T.G. Surface-functionalized electrospun nanofibers for tissue engineering and drug delivery. Adv. Drug Deliv. Rev. 2009, 61, 1033–1042. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelmoneim, D.; Alhamdani, G.M.; Paterson, T.E.; Santocildes Romero, M.E.; Monteiro, B.J.C.; Hatton, P.V.; Ortega Asencio, I. Bioactive and Topographically-Modified Electrospun Membranes for the Creation of New Bone Regeneration Models. Processes 2020, 8, 1341. https://doi.org/10.3390/pr8111341
Abdelmoneim D, Alhamdani GM, Paterson TE, Santocildes Romero ME, Monteiro BJC, Hatton PV, Ortega Asencio I. Bioactive and Topographically-Modified Electrospun Membranes for the Creation of New Bone Regeneration Models. Processes. 2020; 8(11):1341. https://doi.org/10.3390/pr8111341
Chicago/Turabian StyleAbdelmoneim, Dina, Ghsaq M. Alhamdani, Thomas E. Paterson, Martin E. Santocildes Romero, Beatriz J. C. Monteiro, Paul V. Hatton, and Ilida Ortega Asencio. 2020. "Bioactive and Topographically-Modified Electrospun Membranes for the Creation of New Bone Regeneration Models" Processes 8, no. 11: 1341. https://doi.org/10.3390/pr8111341
APA StyleAbdelmoneim, D., Alhamdani, G. M., Paterson, T. E., Santocildes Romero, M. E., Monteiro, B. J. C., Hatton, P. V., & Ortega Asencio, I. (2020). Bioactive and Topographically-Modified Electrospun Membranes for the Creation of New Bone Regeneration Models. Processes, 8(11), 1341. https://doi.org/10.3390/pr8111341





