Novel Hemocompatible Imine Compounds as Alternatives for Antimicrobial Therapy in Pharmaceutical Application
Abstract
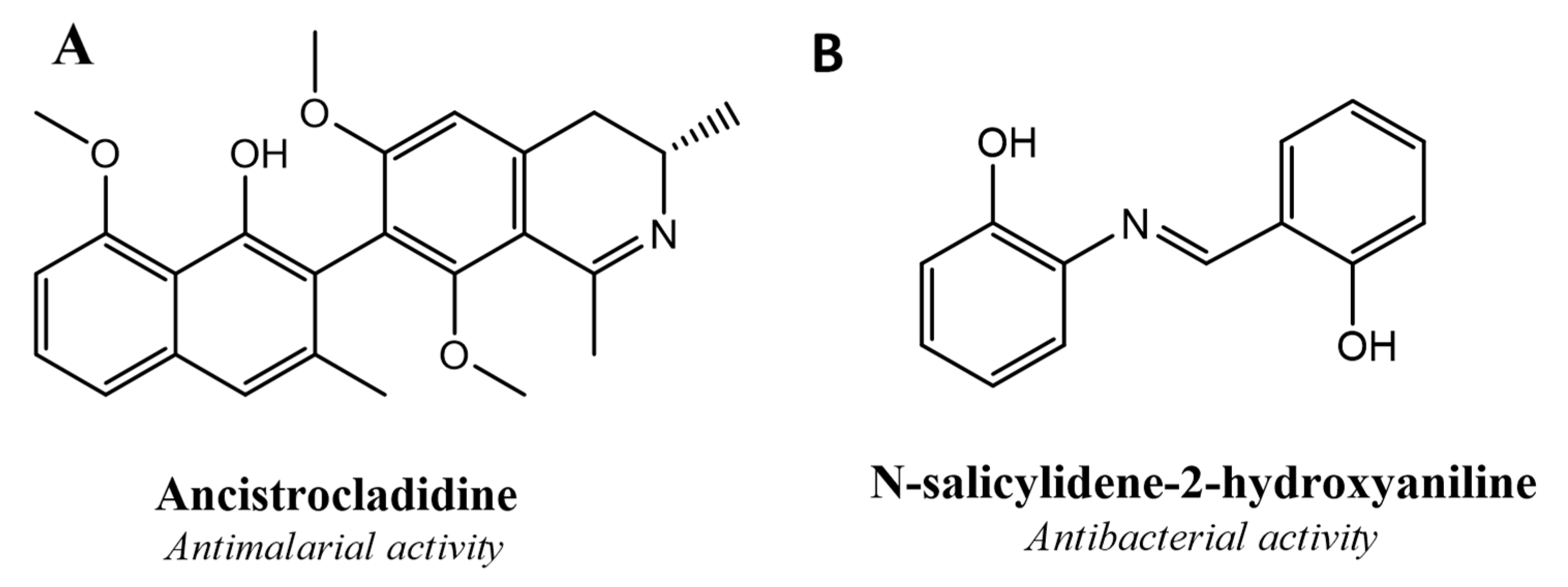
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
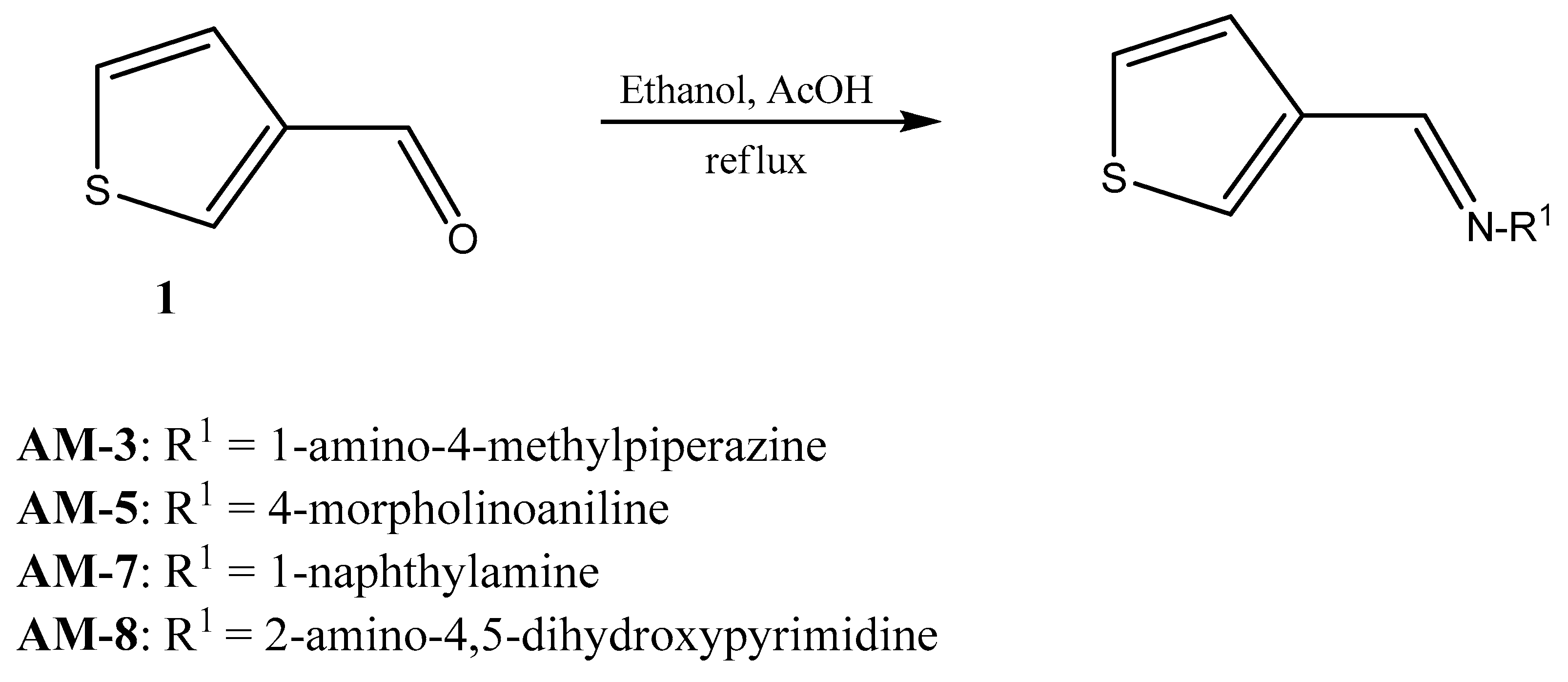
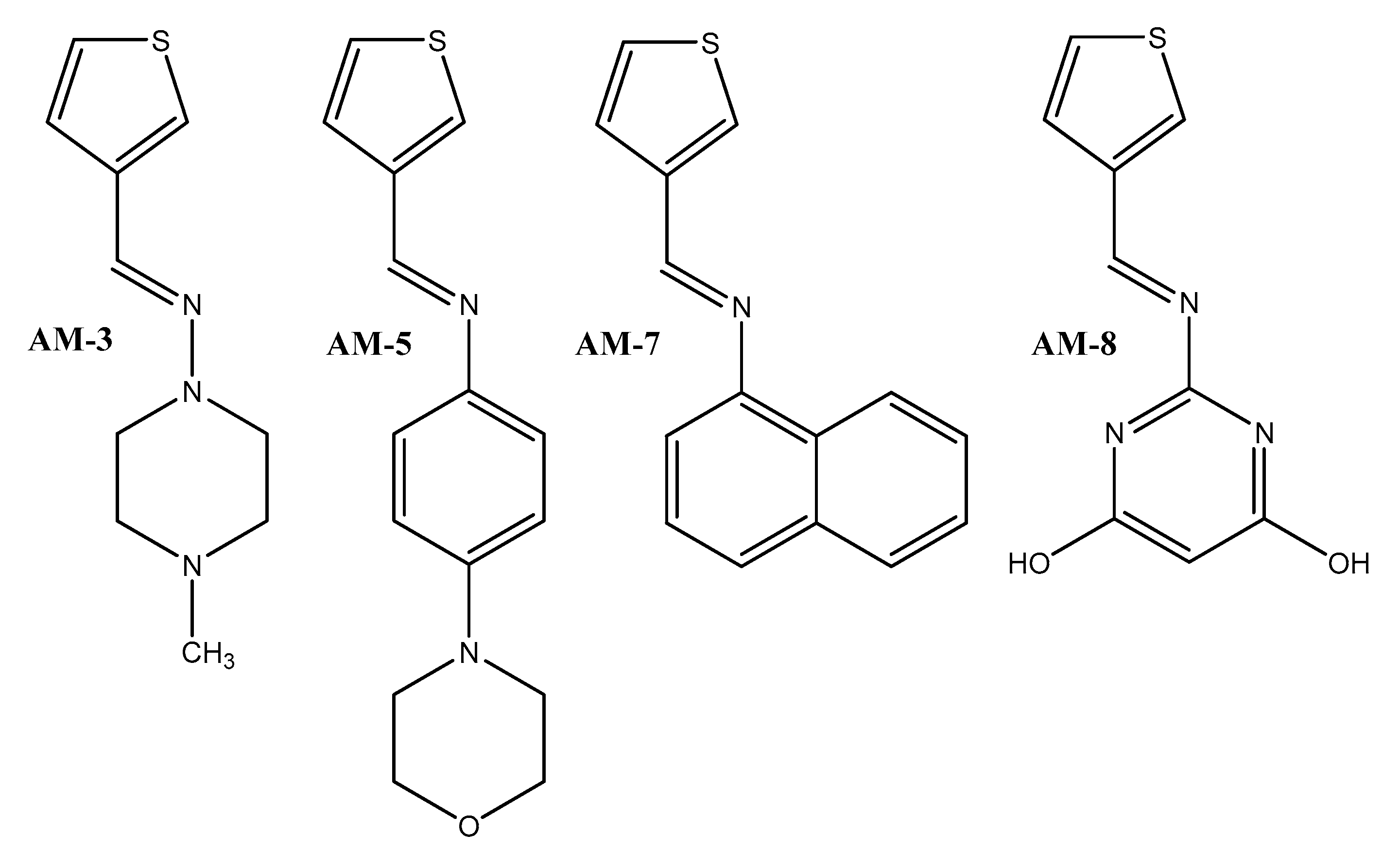
2.2.1. Chemical Synthesis
2.2.2. Nuclear Magnetic Resonance Spectroscopy
2.2.3. Fourier Transform Infrared Spectroscopy
2.2.4. Differential Scanning Calorimetry
2.2.5. Powder X-ray Diffractometry
2.2.6. Morphological Analysis Using Scanning Electron Microscopy
2.2.7. Solubility Analysis
2.2.8. In Vitro Antimicrobial Activities
2.2.9. Measurement of Hemolysis
3. Results and Discussion
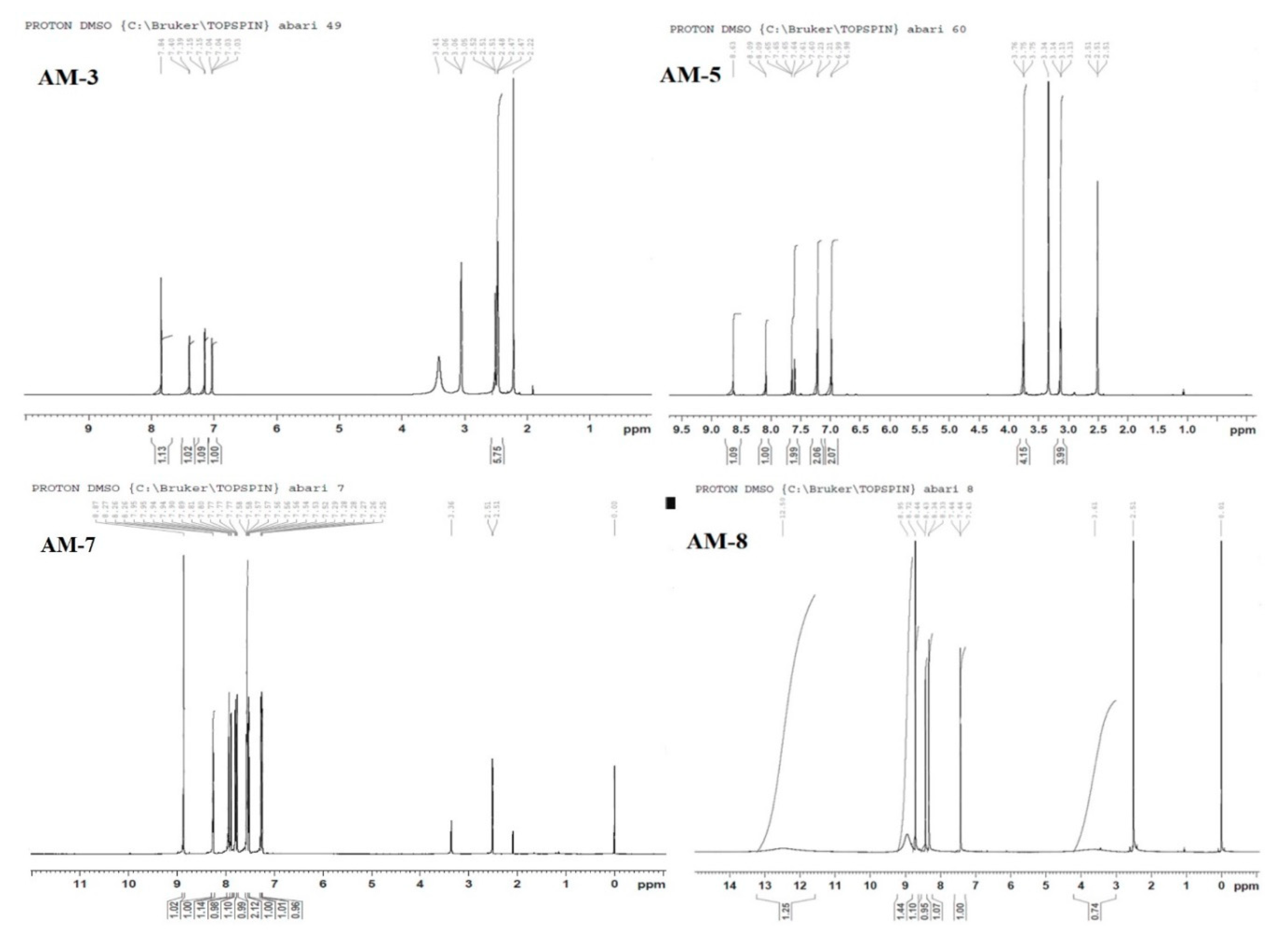
3.1. Nuclear Magnetic Resonance Spectroscopy
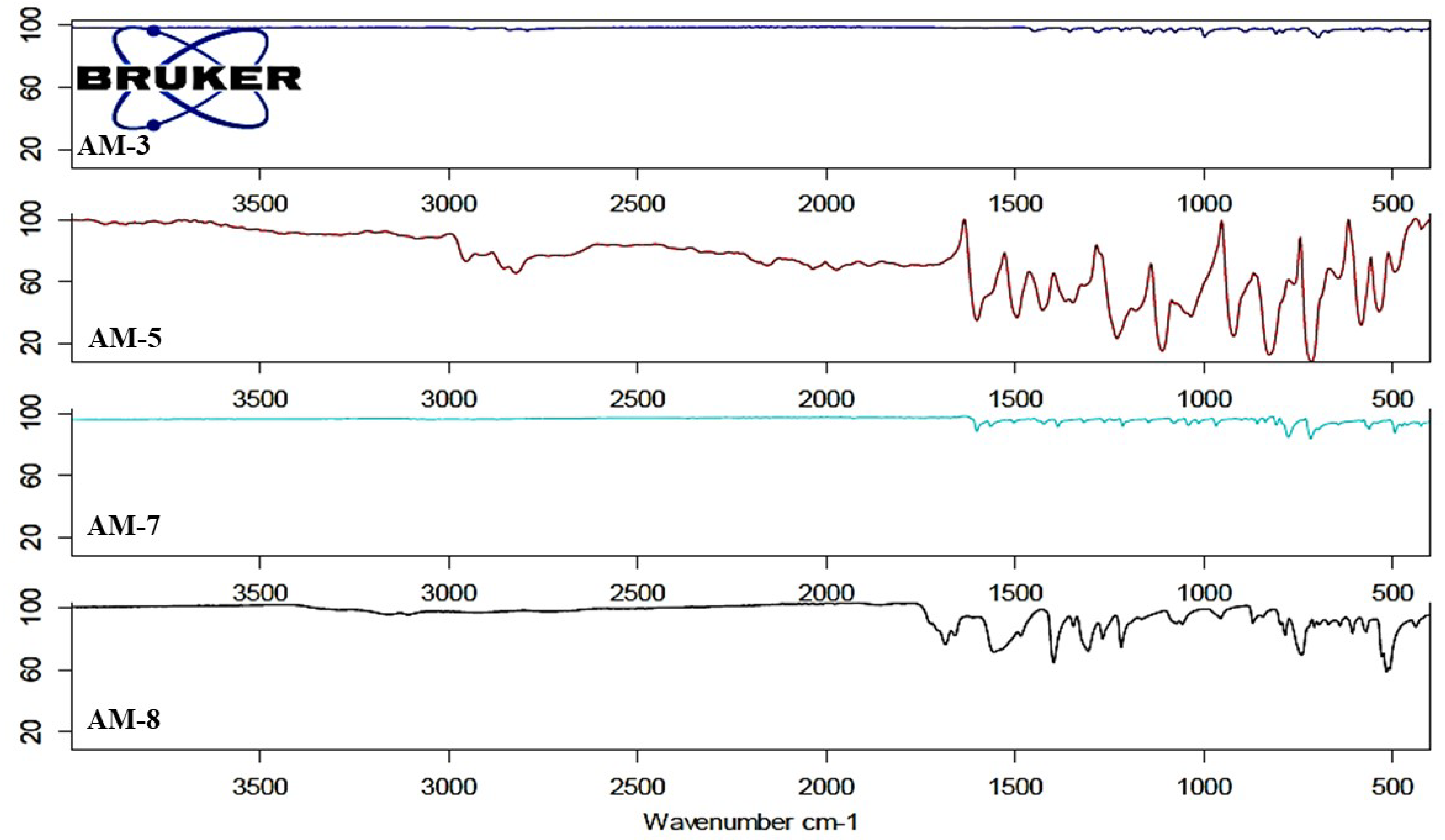
3.2. Fourier Transform Infrared Spectroscopy
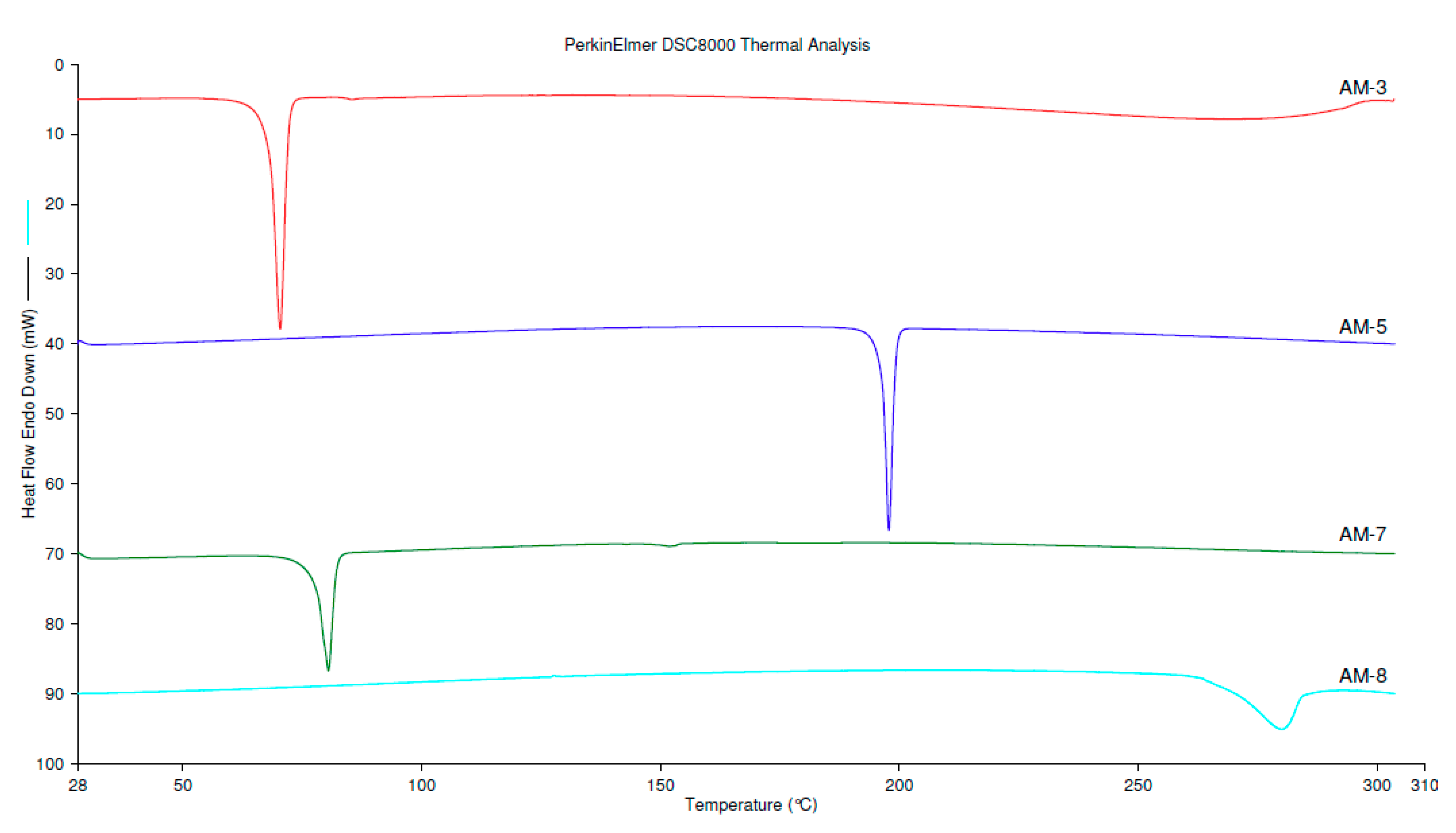
3.3. Differential Scanning Calorimetry
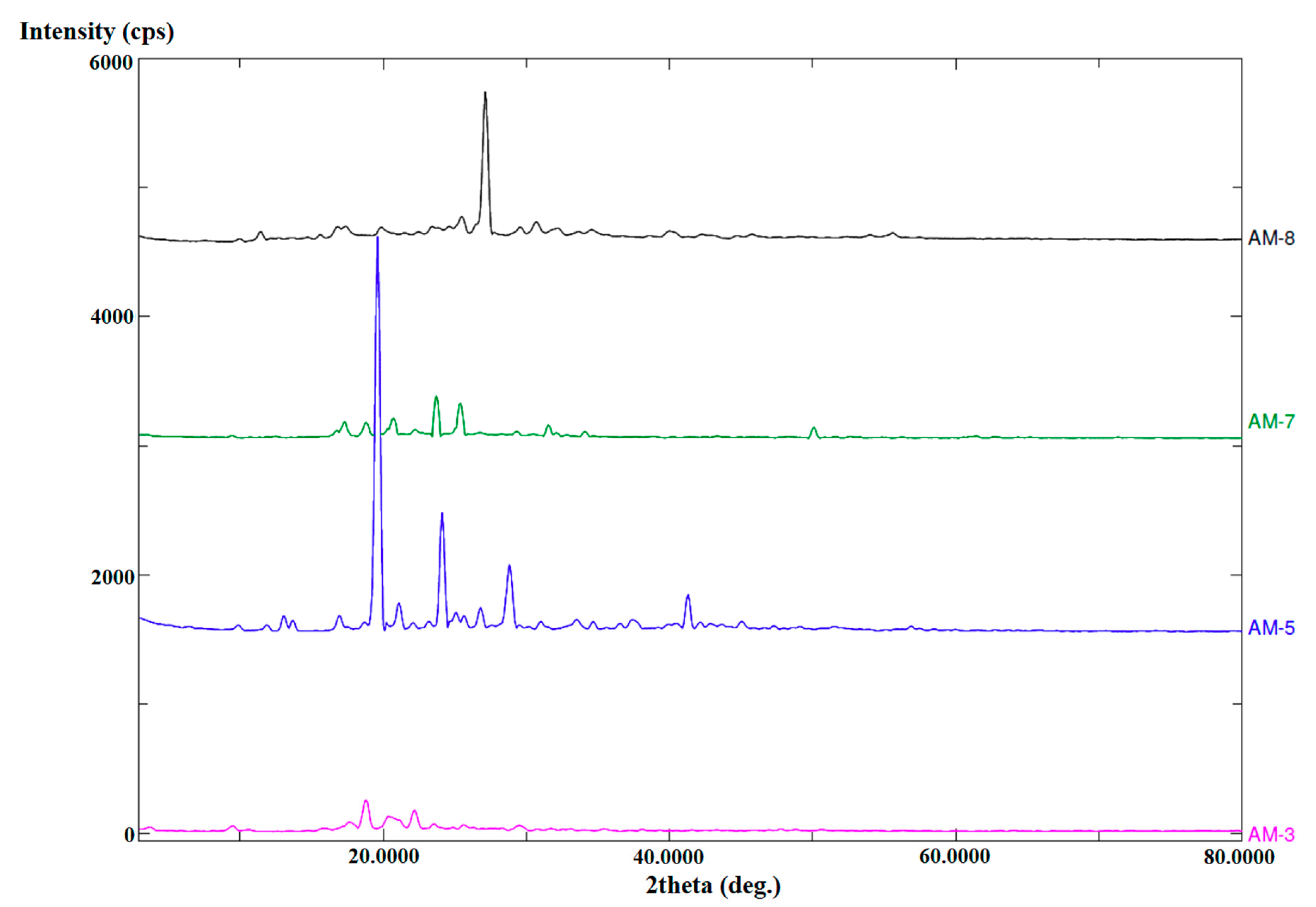
3.4. Powder X-ray Diffraction
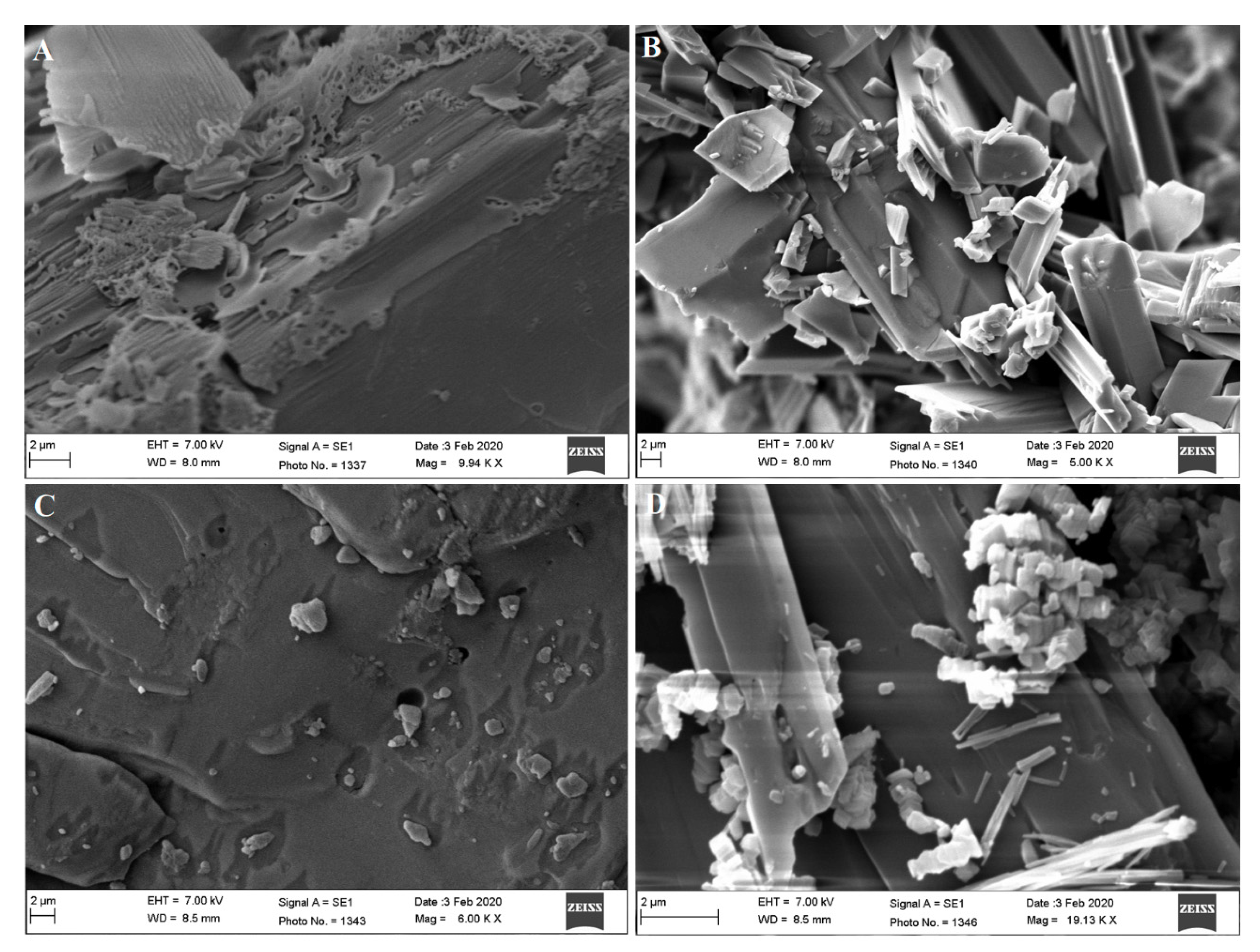
3.5. Assessment of Morphology and Composition using EDX-SEM
3.6. Solubility Study in Various Solvents and Surfactants
3.7. In Vitro Antimicrobial Activities
3.7.1. Zone of Inhibition (ZOI)
3.7.2. Minimum Inhibitory Concentration
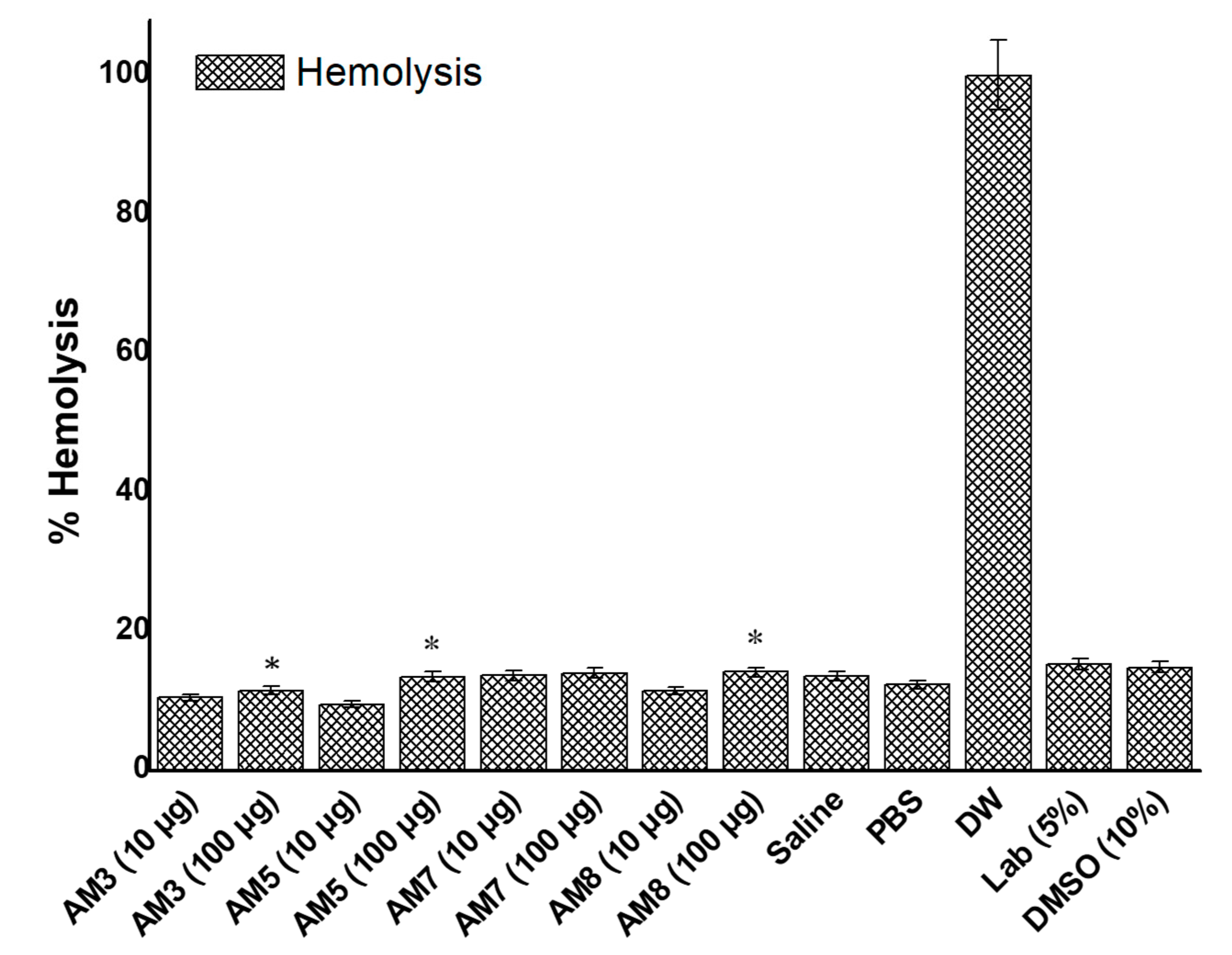
3.8. In Vitro Hemolysis Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution. Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar]
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.B.; de Fatima, A. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Al Zoubi, W. Biological activities of Schiff bases and their complexes: A review of recent works. Int. J. Org. Chem. 2013, 3, 73–95. [Google Scholar] [CrossRef] [Green Version]
- Souzam, A.O.; Galetti, F.; Silva, C.L.; Bicalho, B.; Parma, M.M.; Fonseca, S.F.; Marsaioli, A.J.; Trindade, A.C.; Gil, R.P.F.; Bezerra, F.S. Antimycobacterial and cytotoxicity activity of synthetic and natural compounds. Química Nova 2007, 30, 1563–1566. [Google Scholar]
- Tobriya, S.K. Biological applications of schiff base and its metal complexes-a review. Int. J. Sci. Res. 2014, 3, 1254–1256. [Google Scholar]
- Shi, L.; Ge, H.-M.; Tan, S.-H.; Li, H.-Q.; Song, Y.-C.; Zhu, H.-L.; Tan, R.-X. Synthesis and antimicrobial activities of Schiff bases derived from 5-chloro-salicylaldehyde. Eur. J. Med. Chem. 2014, 42, 558–564. [Google Scholar] [CrossRef]
- Hearn, M.J.; Cynamon, M.H. Design and synthesis of antituberculars: Preparation and evaluation against Mycobacterium tuberculosis of an isoniazid Schiff base. J. Antimicrob. Chemother. 2004, 53, 185–191. [Google Scholar] [CrossRef]
- Rajappa, S. Thiophenes and their benzo derivatives: (ii) reactivity. Compr. Heterocycl. Chem. 1984, 1984, 741–861. [Google Scholar]
- Niedzielski, E.L.; Nord, F. On the Mechanism of the Gattermann Aldehyde Synthesis. I. J. Am. Chem. Soc. 1941, 63, 1462–1463. [Google Scholar] [CrossRef]
- Layer, R.W. The Chemistry of Imines. Chem. Rev. 1963, 63, 489–510. [Google Scholar] [CrossRef]
- Cui, W.; Zhu, H.; Jia, M.; Ao, W.; Zhang, Y.; Zhaorigetu, B. One-pot synthesis of imines from benzyl alcohol and amines on Au/ZrO2 catalyst. React. Kinet. Mech. Catal. 2013, 109, 551–562. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Al-Hamdani, A.A.S.; Ahmed, S.D.; Ko, Y.G. Synthesis, characterization, and biological activity of Schiff bases metal complexes. J. Phys. Org. Chem. 2017, 31, e3752. [Google Scholar] [CrossRef]
- Amézquita-Valencia, M.; Suárez-Ortiz, G.A.; Cabrera, A. Synthesis of Prochiral Imines from Aromatic Ketones Using Magnesium Perchlorate as Catalytic Promotor. Synth. Commun. 2013, 43, 1947–1954. [Google Scholar] [CrossRef]
- Radulović, N.S.; Miltojević, A.B.; Vukićević, R.D. Simple and efficient one-pot solvent-free synthesis of N-methyl imines of aromatic aldehydes. Comptes Rendus Chim. 2013, 16, 257–270. [Google Scholar] [CrossRef]
- Hitge, R.; Smit, S.; Petzer, A.; Petzer, J.P. Evaluation of nitrocatechol chalcone and pyrazoline derivatives as inhibitors of catechol-O-methyltransferase and monoamine oxidase. Bioorganic Med. Chem. Lett. 2020, 30, 127188. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cai, X.; Shi, M.; Xue, L.; Kuang, S.; Xu, R.; Qi, W.; Li, Y.; Ma, X.; Zhang, R. Identification and optimization of piperine analogues as neuroprotective agents for the treatment of Parkinson’s disease via the activation of Nrf2/keap1 pathway. Eur. J. Med. Chem. 2020, 199, 112385. [Google Scholar] [CrossRef]
- Billman, J.H.; Tai, K.M. Reduction of schiff bases. II. Benzhydrylamines and structurally related compounds. J. Org. Chem. 1958, 23, 535–539. [Google Scholar] [CrossRef]
- Pandeya, S.; Sriram, D.; Nath, G.; De Clercq, E. Synthesis and antimicrobial activity of Schiff and Mannich bases of isatin and its derivatives with pyrimidine. Il Farm. 1999, 54, 624–628. [Google Scholar] [CrossRef]
- Love, B.E.; Ren, J. Synthesis of sterically hindered imines. J. Org. Chem. 1993, 58, 5556–5557. [Google Scholar] [CrossRef]
- Rao, V.K.; Reddy, S.S.; Krishna, B.S.; Naidu, K.R.M.; Raju, C.N.; Ghosh, S.K. Synthesis of Schiff’s bases in aqueous medium: A green alternative approach with effective mass yield and high reaction rates, Green Chemistry. Green Chem. Lett. Rev. 2010, 3, 217–223. [Google Scholar] [CrossRef] [Green Version]
- Le-Thanh, H.; Vocelle, D. 1H NMR studies of proton transfer in Schiff base and carboxylic acid systems. Can. J. Chem. 1990, 68, 1909–1916. [Google Scholar] [CrossRef]
- Pesek, J.J.; Frost, J.H. Nuclear magnetic resonance spectroscopy of schiff bases. I. Imines in Aqueous Solution. J. Magn. Reson. 1974, 15, 520–528. [Google Scholar] [CrossRef]
- Chittur, K.K. FTIR/ATR for protein adsorption to biomaterial surfaces. Biomaterials 1998, 19, 357–369. [Google Scholar] [CrossRef]
- Belfer, S.; Fainchtain, R.; Purinson, Y.; Kedem, O. Surface characterization by FTIR-ATR spectroscopy of polyethersulfone membranes-unmodified, modified and protein fouled. J. Membr. Sci. 2000, 172, 113–124. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Neau, S.H. Use of the Flory-Huggins theory to predict the solubility of nifedipine and sulfamethoxazole in the triblock, graft copolymer Soluplus. Drug Dev. Ind. Pharm. 2016, 42, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Marsac, P.J.; Shamblin, S.L.; Taylor, L.S. Theoretical and practical approaches for prediction of drug–polymer miscibility and solubility. Pharm. Res. 2006, 23, 2417–2426. [Google Scholar] [CrossRef]
- Tantishaiyakul, V.; Kaewnopparat, N.; Ingkatawornwong, S. Properties of solid dispersions of piroxicam in polyvinylpyrrolidone. Int. J. Pharm. 1999, 181, 143–151. [Google Scholar] [CrossRef]
- Yamashita, K.; Nakate, T.; Okimoto, K.; Ohike, A.; Tokunaga, Y.; Ibuki, R.; Higaki, K.; Kimura, T. Establishment of new preparation method for solid dispersion formulation of tacrolimus. Int. J. Pharm. 2003, 267, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Zidan, A.S.; Rahman, Z.; Sayeed, V.; Raw, A.; Yu, L.; Khan, M.A. Crystallinity evaluation of tacrolimus solid dispersions by chemometric analysis. Int. J. Pharm. 2012, 423, 341–350. [Google Scholar] [CrossRef]
- Nagaveni, V.; Mahadevan, K.; Vijayakumar, G.; Nagabhushana, H.; Naveen, S.; Lokanath, N. Synthesis, crystal structure and excellent photoluminescence properties of copper (II) and cobalt (II) complexes with Bis (1 [(4-butylphenyl) imino] methyl naphthalen-2-ol) Schiff base. J. Sci. Adv. Mater. Devices 2018, 3, 51–58. [Google Scholar] [CrossRef]
- Hussain, A.; Singh, V.K.; Singh, O.P.; Shafaat, K.; Kumar, S.; Ahmad, F.J. Formulation and optimization of nanoemulsion using antifungal lipid and surfactant for accentuated topical delivery of Amphotericin B. Drug Deliv. 2016, 23, 3101–3110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altamimi, M.A.; Elzayat, E.M.; Alhowyan, A.A.; Alshehri, S.; Shakeel, F. Effect of β-cyclodextrin and different surfactants on solubility, stability, and permeability of hydrochlorothiazide. J. Mol. Liq. 2018, 250, 323–328. [Google Scholar] [CrossRef]
- Hussain, A.; Samad, A.; Singh, S.; Ahsan, M.; Haque, M.; Faruk, A.; Ahmed, F. Nanoemulsion gel-based topical delivery of an antifungal drug: In vitro activity and in vivo evaluation. Drug. Deliv. 2016, 23, 642–657. [Google Scholar] [CrossRef]
- Wikler, M.A. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard; CLSI (NCCLS): Wayne, PA, USA, 2006; Volume 26, p. M7-A7. [Google Scholar]
- Hussain, A.; Altamimi, M.A.; Alshehri, S.; Imam, S.S.; Shakeel, F.; Singh, S.K. Novel Approach for Transdermal Delivery of Rifampicin to Induce Synergistic Antimycobacterial Effects Against Cutaneous and Systemic Tuberculosis Using a Cationic Nanoemulsion Gel. Int. J. Nanomed. 2020, 15, 1073–1094. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, V.; Gaivoronskaya, L.; Prostakov, N. 1 H and 13 C NMR spectra, isomers, and imine-enamine tautomers of N-(1, 2, 5-trimethyl-4-piperidylidene) aniline. Chem. Heterocycl. Compd. 1989, 25, 1176–1179. [Google Scholar]
- Liu, R.; Liu, Z. Polythiophene: Synthesis in aqueous medium and controllable morphology. Chin. Sci. Bull. 2009, 54, 2028–2032. [Google Scholar] [CrossRef] [Green Version]
- Mahalakshmi, G.; Balachandran, V. NBO, HOMO, LUMO analysis and vibrational spectra (FTIR and FT Raman) of 1-Amino 4-methylpiperazine using ab initio HF and DFT methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 135, 321–334. [Google Scholar] [CrossRef]
- Shaikh, M.A.; Farooqui, M.; Abed, S. Morpholinium glycolate as an efficient and reusable catalyst for the synthesis of bis (pyrazol-5-ol) derivatives under solvent-free conditions. Iran. J. Cat 2018, 8, 73–80. [Google Scholar]
- Singh, K.; Ingole, P.G.; Bhrambhatt, H.; Bhattachayra, A.; Bajaj, H.C. Preparation, characterization and performance evaluation of chiral selective composite membranes. Sep. Purif. Technol. 2011, 78, 138–146. [Google Scholar] [CrossRef]
- Alanazi, A.; Alshehri, S.; Altamimi, M.; Shakeel, F. Solubility determination and three dimensional Hansen solubility parameters of gefitinib in different organic solvents: Experimental and computational approaches. J. Mol. Liq. 2020, 299, 112211. [Google Scholar] [CrossRef]
- Paengsri, W.; Lee, V.; Chong, W.; Wahab, H.; Baramee, A. Synthesis, antituberculosis activity and molecular docking studies for novel naphthoquinone derivatives. Int. J. Biol. Chem. 2012, 6, 69–88. [Google Scholar] [CrossRef] [Green Version]
- Yempalla, K.R.; Munagala, G.; Singh, S.; Magotra, A.; Kumar, S.; Rajput, V.S.; Bharate, S.S.; Tikoo, M.; Singh, G.; Khan, I.A. Nitrofuranyl methyl piperazines as new anti-TB agents: Identification, validation, medicinal chemistry, and PK studies. ACS Med. Chem. Lett. 2015, 6, 1041–1046. [Google Scholar] [CrossRef] [Green Version]
- Panneerselvam, P.; Nair, R.R.; Vijayalakshmi, G.; Subramanian, E.H.; Sridhar, S.K. Synthesis of Schiff bases of 4-(4-aminophenyl)-morpholine as potential antimicrobial agents. Eur. J. Med. Chem. 2005, 40, 225–229. [Google Scholar] [CrossRef]
- Sharma, V.; Chitranshi, N.; Agarwal, A.K. Significance and biological importance of pyrimidine in the microbial world. Int. J. Med. Chem. 2014, 2014, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Bemer-Melchior, P.; Bryskier, A.; Drugeon, H.B. Comparison of the in vitro activities of rifapentin and rifampicin against mycobacterium tuberculosis complex. J. Antimicrob. Chemother. 2000, 46, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Thornsberry, C.; Hill, B.C.; Swenson, J.M.; McDougal, L.K. Rifampin: Spectrum of Antibacterial Activity. Rev. Infect. Dis. 1983, 5, S412–S417. [Google Scholar] [CrossRef]
- Bo-Young, H.; Wei-Hsun, C.; Yi-Jing, X.; Jyh-Mirn, L. A Ketoconazole Susceptibility Test for Malassezia pachydermatis Using Modified Leeming–Notman Agar. J. Fungi 2018, 4, 1–6. [Google Scholar]
- Gouveia, M.; Figueira, J.; Jardim, M.G.; Castro, R.; Tomás, H.; Rissanen, K.; Rodrigues, J. Poly(alkylidenimine) Dendrimers Functionalized with the OrganometallicMoiety [Ru(5-C5H5)(PPh3)2]+ as Promising Drugs Against Cisplatin-Resistant Cancer Cells and Human Mesenchymal Stem Cells. Molecules 2018, 23, 1471. [Google Scholar] [CrossRef] [Green Version]
- Abdulrahman, H.S.; Mohammed, M.H.; Al-Ani, L.A.; Ahmad, M.H.; Hashim, N.M.; Yehye, W.A. Synthesis of Phthalimide Imine Derivatives as a Potential Anticancer Agent. J. Chem. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
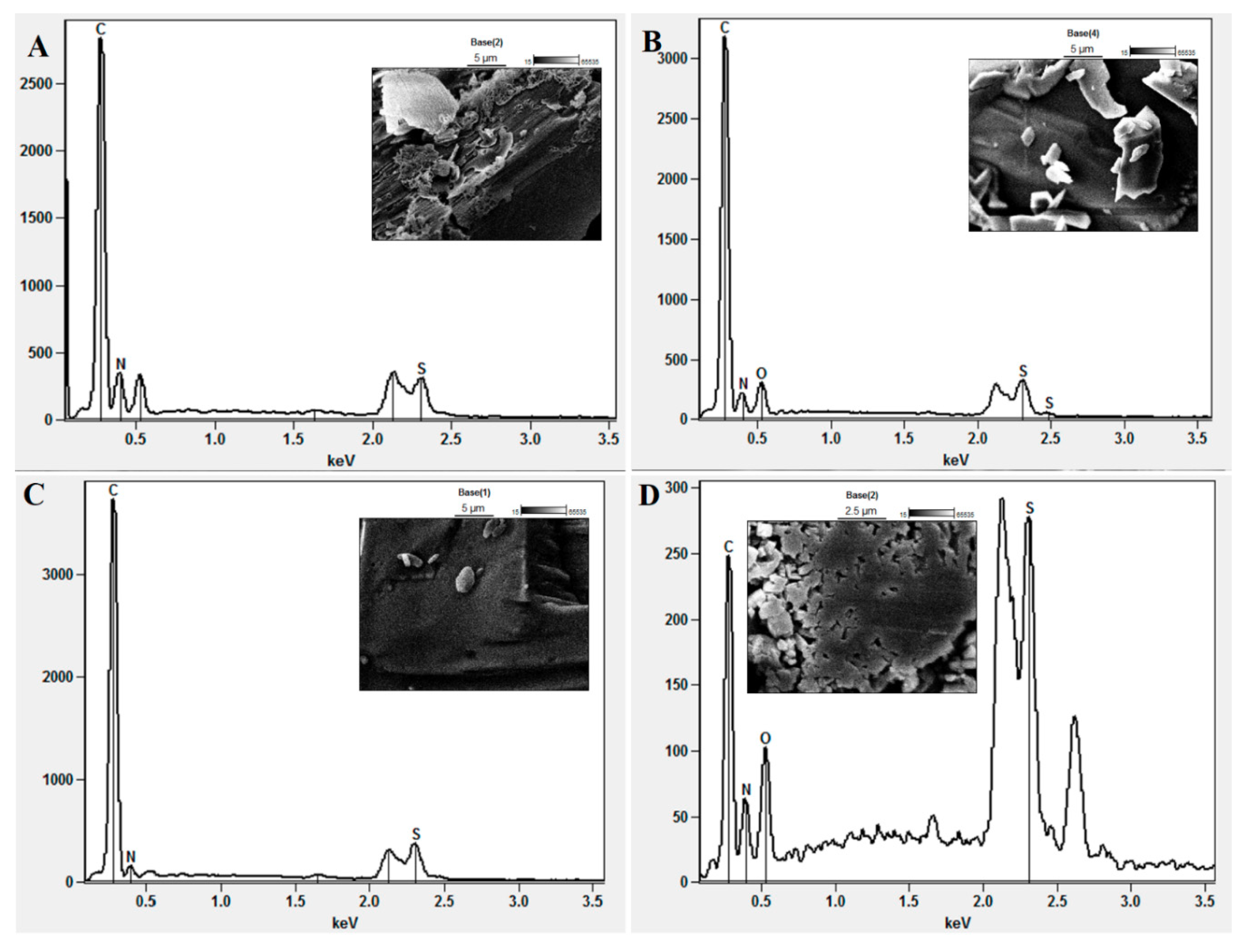

| Scanning specification | EDX summary report on scanned image | |||
| AM-3 | AM-5 | AM-7 | AM-8 | |
| Image Resolution | 512 × 384 | 512 × 384 | 512 × 384 | 512 × 384 |
| Image Pixel Size (µm) | 0.06 | 0.06 | 0.06 | 0.06 |
| Map Resolution | 256 × 192 | 256 × 192 | 256 × 192 | 256 × 192 |
| Map Pixel Size (µm) | 0.11 | 0.11 | 0.11 | 0.11 |
| Acc. Voltage (kV) | 7 | 7 | 7 | 7 |
| Magnification | 10,000 | 10,000 | 10,000 | 10,000 |
| Take-off angle | 33.4° | 35.0° | 35.0° | 35.0° |
| Element scanned | Composition analysis report (%) | |||
| AM-3 | AM-5 | AM-7 | AM-8 | |
| Carbon | 53.77 | 68.23 | 78.70 | 42.26 |
| Nitrogen | 40.82 | 21.39 | 16.44 | 18.71 |
| Oxygen | – | 6.16 | – | 9.28 |
| Sulphur | 5.51 | 4.22 | 4.87 | 29.75 |
| Compounds | Zone of Inhibition (mm) * | |||||||
|---|---|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | A. baumannii | M. smegmatis | E. faecalis | C. albicans | A. niger | |
| AM-3 | – | 16.0 (0.81) | 15.6 (0.78) | 18.0 (0.91) | 16.0 (0.82) | 14.6 (0.73) | 15.3 (0.76) | 17.3 (0.86) |
| AM-5 | – | 9.3 (0.46) | 7.3 (0.36) | 9.7 (0.48) | – | – | 9.7 (0.48) | 9.3 (0.47) |
| AM-7 | – | 12.0 (0.65) | 16.3 (0.81) | 16.0 (0.83) | 14.3 (0.71) | – | 14.7 (0.73) | 15.7 (0.79) |
| AM-8 | – | 5.3 (0.26) | 8.6 (0.43) | 7.3 (0.36) | – | – | 7.3 (0.35) | 7.7 (0.39) |
| DMSO (5%) | – | – | – | – | – | – | – | – |
| LAB (5%) | – | – | – | – | – | – | – | – |
| Compound | Minimum Inhibitory Concentration (µg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|
| S. aureus | B. subtilis | E. coli | A. baumannii | M. smegmatis | E. faecalis | C. albicans | A. niger | |
| AM-3 | – | 62.5 | 31.25 | 125.0 | 31.25 | 125.0 | 62.5 | 31.25 |
| AM-5 | – | 100.0 | 25.0 | 200.0 | – | – | 25.0 | 100.0 |
| AM-7 | – | 31.25 | 31.25 | 125.0 | 15.62 | – | 62.5 | 15.62 |
| AM-8 | – | 100.0 | 50.0 | 12.5 | – | – | 50.0 | 50.0 |
| * Ketoconazole | – | – | – | – | – | – | 0.5 | 0.25 |
| * Isoniazid | NS | NS | NS | NS | 0.125 | NS | NS | NS |
| * Rifampicin | 0.25 | 0.15 | 16 | 32 | 0.64 | 8.0 | NS | NS |
| DMSO (5%) | – | – | – | – | – | – | – | – |
| LAB (5%) | – | – | – | – | – | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altamimi, M.A.; Hussain, A.; Alshehri, S.; Imam, S.S.; Alnami, A.; Bari, A. Novel Hemocompatible Imine Compounds as Alternatives for Antimicrobial Therapy in Pharmaceutical Application. Processes 2020, 8, 1476. https://doi.org/10.3390/pr8111476
Altamimi MA, Hussain A, Alshehri S, Imam SS, Alnami A, Bari A. Novel Hemocompatible Imine Compounds as Alternatives for Antimicrobial Therapy in Pharmaceutical Application. Processes. 2020; 8(11):1476. https://doi.org/10.3390/pr8111476
Chicago/Turabian StyleAltamimi, Mohammad A., Afzal Hussain, Sultan Alshehri, Syed Sarim Imam, Abdulmalik Alnami, and Ahmed Bari. 2020. "Novel Hemocompatible Imine Compounds as Alternatives for Antimicrobial Therapy in Pharmaceutical Application" Processes 8, no. 11: 1476. https://doi.org/10.3390/pr8111476
APA StyleAltamimi, M. A., Hussain, A., Alshehri, S., Imam, S. S., Alnami, A., & Bari, A. (2020). Novel Hemocompatible Imine Compounds as Alternatives for Antimicrobial Therapy in Pharmaceutical Application. Processes, 8(11), 1476. https://doi.org/10.3390/pr8111476








