Insights on Monosaccharides and Bioethanol Production from Sweet Sorghum Stalks Using Dilute Acid Pretreatment
Abstract
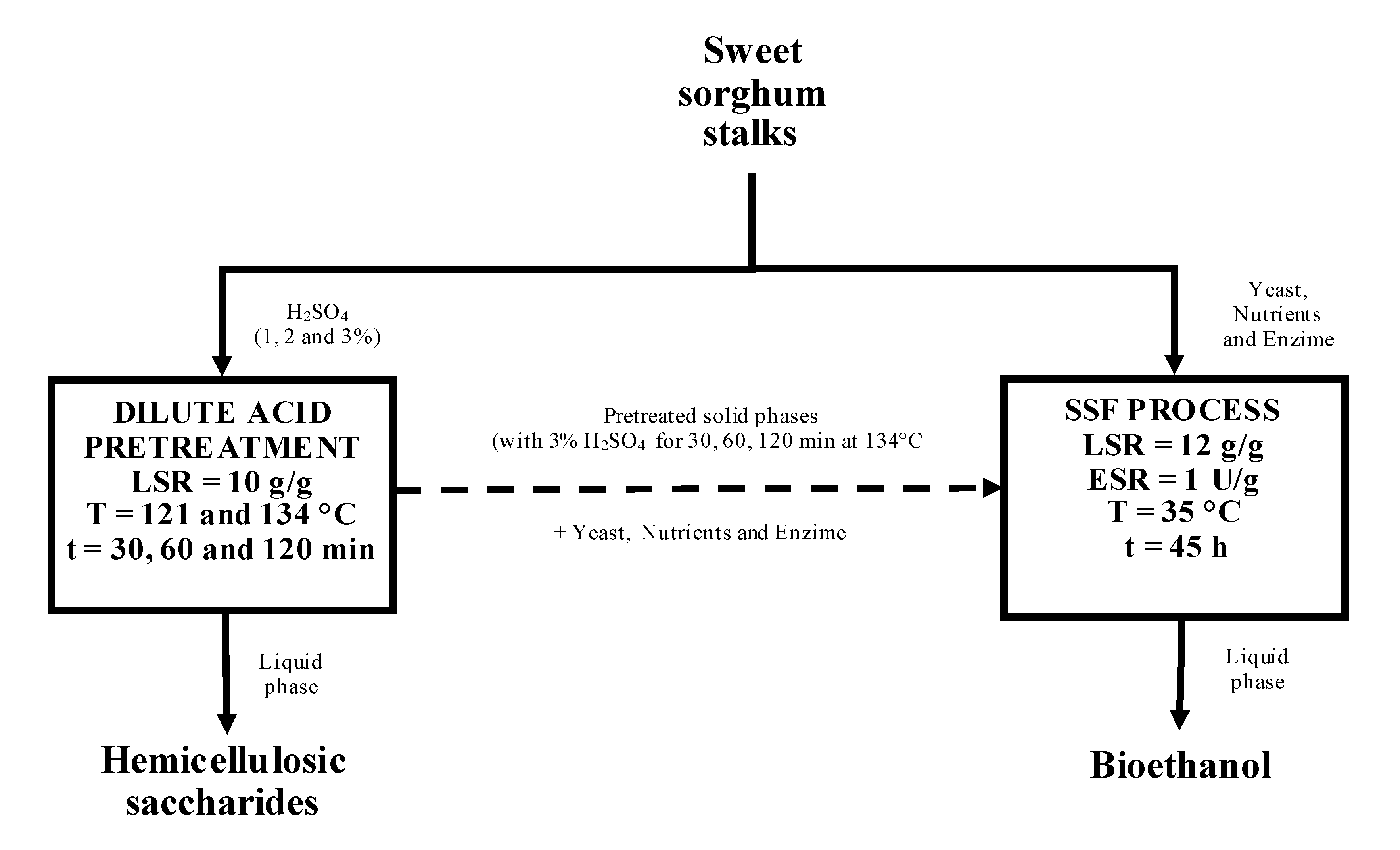
:1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Dilute Acid Pretreatment of SSS
2.3. Yeast Cultivation and Inoculum Preparation
2.4. Simultaneous Saccharification and Fermentation (SSF)
2.5. Analytical Methods
3. Results and Discussion
3.1. SSS Fractionation and Saccharides Production
3.2. SSF of Untreated and Pretreated SSS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Girard, P.; Fallot, A. Review of existing and emerging technologies for the production of biofuels in developing countries. Energy Sustain. Dev. 2006, 10, 92–108. [Google Scholar] [CrossRef]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef] [Green Version]
- Union, E. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources and amending and subsequently repealing Directives 2001/77/EC and 2003/30/EC. Off. J. Eur. Union 2009, L 140, 16–62. [Google Scholar] [CrossRef]
- Union, E. Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the promotion of the use of energy from renewable sources. Off. J. Eur. Union 2018, L 328, 82–209. [Google Scholar]
- Dar, R.A.; Dar, E.A.; Kaur, A.; Phutela, U.G. Sweet sorghum-a promising alternative feedstock for biofuel production. Renew. Sustain. Energy Rev. 2018, 82, 4070–4090. [Google Scholar] [CrossRef]
- Cifuentes, R.; Bressani, R.; Rolz, C. The potential of sweet sorghum as a source of ethanol and protein. Energy Sustain. Dev. 2014, 21, 13–19. [Google Scholar] [CrossRef]
- Appiah-Nkansah, N.B.; Li, J.; Rooney, W.; Wang, D. A review of sweet sorghum as a viable renewable bioenergy crop and its techno-economic analysis. Renew. Energy 2019, 143, 1121–1132. [Google Scholar] [CrossRef]
- Perazzo, A.F.; Carvalho, G.G.P.; Santos, E.M.; Bezerra, H.F.C.; Silva, T.C.; Pereira, G.A.; Ramos, R.C.S.; Rodrigues, J.A.S. Agronomic Evaluation of Sorghum Hybrids for Silage Production Cultivated in Semiarid Conditions. Front. Plant Sci. 2017, 8, 1088. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Monti, A. Are we ready to cultivate sweet sorghum as a bioenergy feedstock? A review on field management practices. Biomass Bioenergy 2012, 40, 1–12. [Google Scholar] [CrossRef]
- Aguado-Santacruz, G.A.; Moreno-Gómez, B.; Gómez-Torres, L.M.; Anaya-López, J.L. Biotechnology of sorghum: Prospects for improvement of nutritional and biofuel traits. In Sorghum: Food and Energy Source; Vázquez, M., de Ramírez León, J.A., Eds.; Nova Science Publishers, Inc.: Hauppauge, New York, NY, USA, 2012; pp. 113–151. [Google Scholar]
- Mathur, S.; Umakanth, A.V.; Tonapi, V.A.; Sharma, R.; Sharma, M.K. Sweet sorghum as biofuel feedstock: Recent advances and available resources. Biotechnol. Biofuels 2017, 10, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Mekala, N.K.; Potumarthi, R.; Baadhe, R.R.; Gupta, V.K. Current Bioenergy Researches: Strengths and Future Challenges. In Bioenergy Research: Advances and Applications; Gupta, V.K., Tuohy, M.G., Kubicek, C.P., Saddler, J., Xu, F., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2014; pp. 1–21. [Google Scholar] [CrossRef]
- Peral, C. Biomass Pretreatment Strategies (Technologies, Environmental Performance, Economic Considerations, Industrial Implementation). In Biotransformation of Agricultural Waste and By-Products: The Food, Feed, Fibre, Fuel (4F) Economy; Poltronieri, P., D’Urso, O.F., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 125–160. [Google Scholar] [CrossRef]
- López-Linares, J.C.; Gómez-Cruz, I.; Ruiz, E.; Romero, I.; Castro, E. Production of Ethanol from Hemicellulosic Sugars of Exhausted Olive Pomace by Escherichia coli. Processes 2020, 8, 533. [Google Scholar] [CrossRef]
- Deshavath, N.N.; Mohan, M.; Venkatadasu, V.; Goud, V.; Pinnamaneni, S.R.; Benarjee, T. Dilute acid pretreatment of sorghum biomass to maximize the hemicellulose hydrolysis with minimized levels of fermentative inhibitors for bioethanol production. 3 Biotech 2017, 7, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, Y.H.; Kim, K.H. Acidic Pretreatment. In Pretreatment of Biomass: Processes and Technologies; Pandey, A., Negi, S., Binod, P., Larroche, C., Eds.; Elsevier, B.V.: Amsterdam, The Netherlands, 2015; pp. 27–50. [Google Scholar] [CrossRef]
- Weiß, K.; Alt, M. Determination of Single Sugars, Including Inulin, in Plants and Feed Materials by High-Performance Liquid Chromatography and Refraction Index Detection. Fermentation 2017, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Shintani, T. Food Industrial Production of Monosaccharides Using Microbial, Enzymatic, and Chemical Methods. Fermentation 2019, 5, 47. [Google Scholar] [CrossRef] [Green Version]
- Regassa, T.H.; Wortmann, C.S. Sweet sorghum as a bioenergy crop: Literature review. Biomass Bioenergy 2014, 64, 348–355. [Google Scholar] [CrossRef]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic agriculture wastes as biomass feedstocks for second-generation bioethanol production: Concepts and recent developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef] [Green Version]
- Idris, A.S.O.; Pandey, A.; Rao, S.; Sukumaran, R.K. Cellulase production through solid-state tray fermentation, and its use for bioethanol from sorghum stover. Bioresour. Technol. 2017, 242, 265–271. [Google Scholar] [CrossRef]
- Tesfaw, A.; Assefa, F. Current Trends in Bioethanol Production by Saccharomyces cerevisiae: Substrate, Inhibitor Reduction, Growth Variables, Coculture, and Immobilization. Int. Sch. Res. Not. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Vancov, T.; McIntosh, S. Mild acid pretreatment and enzyme saccharification of Sorghum bicolor straw. Appl. Energy 2012, 92, 421–428. [Google Scholar] [CrossRef]
- Ostovareh, S.; Karimi, K.; Zamani, A. Efficient conversion of sweet sorghum stalks to biogas and ethanol using organosolv pretreatment. Ind. Crop. Prod. 2015, 66, 170–177. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Romero, I.; Ruiz, E.; Cara, C.; Romero-García, J.M.; Castro, E. Design and Optimization of Sulfuric Acid Pretreatment of Extracted Olive Tree Biomass Using Response Surface Methodology. BioResources 2017, 12, 1779–1797. [Google Scholar] [CrossRef] [Green Version]
- Choudhary, S.J.; Mehmood, S.; Naz, H.; Jaafar, H.Z.E.; Zia-Ul-Haq, M. Effect of Sulfuric Acid on Pretreatment of YSS-10R Variety of Sorghum and Analysis of Its Interaction with Temperature and Time. BioResources 2015, 10, 2103–2112. [Google Scholar] [CrossRef] [Green Version]
- Deshavath, N.N.; Venkatadasu, V.; Goud, V.; Rao, P.S. Development of dilute sulfuric acid pretreatment method for the enhancement of xylose fermentability. Biocatal. Agric. Biotechnol. 2017, 11, 224–230. [Google Scholar] [CrossRef]
- Romaní, A.; Garrote, G.; Alonso, J.L.; Parajó, J.C. Bioethanol production from hydrothermally pretreated Eucalyptus globulus wood. Bioresour. Technol. 2010, 101, 8706–8712. [Google Scholar] [CrossRef] [PubMed]
- Nozari, B.; Mirmohamadsadeghi, S.; Karimi, K. Bioenergy production from sweet sorghum stalks via a biorefinery perspective. Appl. Microbiol. Biotechnol. 2018, 102, 3425–3438. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, K.; Bertilsson, M.; Lidén, G. A short review on SSF—An interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| H2SO4 conc. (%) | Untreated | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
|---|---|---|---|---|---|---|---|---|---|---|
| Reaction Time (min.) | SSS | 30 | 30 | 30 | 60 | 60 | 60 | 120 | 120 | 120 |
| Material balance data (g PSS/100 g raw material, oven-dry basis) | ||||||||||
| Solid recovered | - | 53.49 | 49.04 | 46.31 | 51.69 | 46.20 | 45.82 | 50.73 | 47.99 | 42.21 |
| Non-volatile compounds | - | 38.11 | 44.29 | 53.57 | 36.05 | 44.51 | 53.22 | 41.39 | 46.64 | 57.26 |
| Solid phase composition (g/100 g PSS, oven-dry basis) | ||||||||||
| Glucan | 44.07 ± 0.56 | 55.92 ± 0.48 | 58.79 ± 0.62 | 62.92 ± 0.35 | 58.25 ± 0.45 | 60.80 ± 0.32 | 64.42 ± 0.32 | 61.06 ± 0.68 | 64.81 ± 0.12 | 66.00 ± 0.50 |
| Xylan | 14.31 ± 0.15 | 14.94 ± 0.11 | 8.39 ± 0.12 | 6.64 ± 0.13 | 12.41 ± 0.02 | 7.10 ± 0.08 | 5.05 ± 0.12 | 9.35 ± 0.12 | 5.21 ± 0.16 | 3.38 ± 0.10 |
| Klason lignin | 19.46 ± 0.19 | 24.21 ± 0.23 | 27.99 ± 0.13 | 30.77 ± 0.15 | 28.18 ± 0.35 | 32.34 ± 0.12 | 33.27 ± 0.12 | 29.45 ± 0.21 | 32.58 ± 0.25 | 34.39 ± 0.32 |
| Extractives | 14.67 ± 0.03 | |||||||||
| Ash | 4.00 ± 0.02 | |||||||||
| Proteins | 1.33 ± 0.02 | |||||||||
| Liquid phase composition (g/L or g monomer equivalent/L) | ||||||||||
| Glucose | - | 14.62 ± 0.06 | 13.84 ± 0.06 | 14.68 ± 0.12 | 13.97 ± 0.02 | 14.06 ± 0.09 | 13.89 ± 0.13 | 14.11 ± 0.14 | 13.81 ± 0.21 | 14.89 ± 0.05 |
| Xylose | - | 12.34 ± 0.05 | 15.81 ± 0.09 | 16.27 ± 0.14 | 13.31 ± 0.17 | 16.22 ± 0.05 | 15.27 ± 0.17 | 15.73 ± 0.09 | 15.39 ± 0.15 | 14.45 ± 0.02 |
| Arabinose | - | 2.26 ± 0.01 | 2.20 ± 0.05 | 2.50 ± 0.04 | 2.13 ± 0.04 | 2.27 ± 0.08 | 2.42 ± 0.07 | 2.07 ± 0.05 | 2.31 ± 0.06 | 2.58 ± 0.01 |
| Acetic acid | - | 1.45 ± 0.01 | 2.69 ± 0.08 | 3.02 ± 0.06 | 2.08 ± 0.02 | 3.13 ± 0.04 | 3.14 ± 0.08 | 2.73 ± 0.05 | 2.94 ± 0.02 | 3.61 ± 0.01 |
| Gluco-oligomers | - | 1.79 ± 0.02 | 1.84 ± 0.09 | 1.13 ± 0.03 | 2.33 ± 0.03 | 2.06 ± 0.04 | 1.27 ± 0.08 | 1.39 ± 0.05 | 1.16 ± 0.01 | 0.48 ± 0.01 |
| Arabino-oligomers | - | 0.28 ± 0.01 | 0.42 ± 0.08 | 0.16 ± 0.08 | 0.41 ± 0.07 | 0.44 ± 0.01 | 0.28 ± 0.05 | 0.37 ± 0.08 | 0.21 ± 0.06 | 0.02 ± 0.08 |
| Acetyl groups-oligomers | - | 0.16 ± 0.01 | 0.18 ± 0.01 | 0.02 ± 0.00 | 0.27 ± 0.02 | 0.05 ± 0.00 | 0.34 ± 0.02 | 0.02 ± 0.00 | 0.51 ± 0.01 | 0.00 ± 0.00 |
| H2SO4 conc. (%) | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
|---|---|---|---|---|---|---|---|---|---|
| Reaction Time (min.) | 30 | 30 | 30 | 60 | 60 | 60 | 120 | 120 | 120 |
| Material balance data (g PSS/100 g raw material, oven-dry basis) | |||||||||
| Solid recovered | 46.26 | 45.63 | 42.40 | 45.59 | 42.40 | 42.16 | 44.54 | 42.53 | 41.85 |
| Non-volatile compounds | 46.05 | 47.15 | 54.66 | 45.27 | 49.08 | 57.05 | 45.84 | 47.43 | 55.63 |
| Solid phase composition (g/100 g PSS, oven-dry basis) | |||||||||
| Glucan | 60.56 ± 0.23 | 66.29 ± 0.56 | 67.23 ± 0.52 | 65.60 ± 0.41 | 66.75 ± 0.63 | 66.85 ± 0.29 | 66.21 ± 0.35 | 66.61 ± 0.31 | 66.69 ± 0.29 |
| Xylan | 7.50 ± 0.09 | 3.40 ± 0.12 | 1.88 ± 0.23 | 6.33 ± 0.14 | 2.16 ± 0.05 | 0.96 ± 0.01 | 3.78 ± 0.06 | 1.10 ± 0.01 | 0.50 ± 0.01 |
| Klason lignin | 30.54 ± 0.14 | 34.25 ± 0.03 | 36.01 ± 0.08 | 30.59 ± 0.18 | 35.08 ± 0.12 | 36.79 ± 0.05 | 32.69 ± 0.06 | 38.06 ± 0.06 | 38.80 ± 0.11 |
| Liquid phase composition (g/L or g monomer equivalent/L) | |||||||||
| Glucose | 15.97 ± 0.13 | 14.88 ± 0.08 | 17.22 ± 0.09 | 14.82 ± 0.18 | 15.73 ± 0.17 | 15.31 ± 0.07 | 16.24 ± 0.18 | 16.01 ± 0.04 | 15.27 ± 0.05 |
| Xylose | 16.64 ± 0.04 | 14.10 ± 0.19 | 12.44 ± 0.02 | 15.37 ± 0.14 | 14.24 ± 0.01 | 11.74 ± 0.09 | 15.23 ± 0.09 | 11.76 ± 0.04 | 9.97 ± 0.12 |
| Arabinose | 2.06 ± 0.02 | 2.29 ± 0.01 | 2.59 ± 0.08 | 1.99 ± 0.06 | 2.34 ± 0.04 | 2.47 ± 0.07 | 1.92 ± 0.09 | 2.24 ± 0.02 | 2.22 ± 0.02 |
| Acetic acid | 2.81 ± 0.05 | 3.28 ± 0.06 | 3.37 ± 0.07 | 2.90 ± 0.01 | 3.66 ± 0.06 | 3.44 ± 0.09 | 3.43 ± 0.09 | 2.70 ± 0.07 | 3.30 ± 0.08 |
| Gluco-oligomers | 2.10 ± 0.05 | 1.30 ± 0.05 | 0.00 ± 0.00 | 1.44 ± 0.04 | 1.05 ± 0.01 | 1.47 ± 0.01 | 1.06 ± 0.02 | 1.08 ± 0.01 | 0.85 ± 0.01 |
| Arabino-oligomers | 0.51 ± 0.01 | 0.27 ± 0.01 | 0.16 ± 0.01 | 0.35 ± 0.02 | 0.23 ± 0.01 | 0.21 ± 0.01 | 0.19 ± 0.01 | 0.10 ± 0.01 | 0.13 ± 0.01 |
| Acetyl groups-oligomers | 0.20 ± 0.01 | 0.16 ± 0.01 | 0.09 ± 0.01 | 0.13 ± 0.01 | 0.00 ± 0.00 | 0.39 ± 0.02 | 0.15 ± 0.01 | 0.75 ± 0.03 | 0.22 ± 0.02 |
| Experiment | Maximum Ethanol Concentration (g/L) | Maximum Ethanol Conversion (%) |
|---|---|---|
| SSF1 | 9.78 ± 0.19 | 49.80 ± 0.31 |
| SSF2 | 1.23 ± 0.12 | 4.06 ± 0.08 |
| SSF3 | 1.39 ± 0.13 | 4.62 ± 0.06 |
| SSF4 | 1.24 ± 0.12 | 4.10 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buruiană, C.-T.; Georgescu, L.; Isticioaia, S.-F.; Constantin, O.E.; Vizireanu, C.; Dinică, R.M.; Furdui, B. Insights on Monosaccharides and Bioethanol Production from Sweet Sorghum Stalks Using Dilute Acid Pretreatment. Processes 2020, 8, 1486. https://doi.org/10.3390/pr8111486
Buruiană C-T, Georgescu L, Isticioaia S-F, Constantin OE, Vizireanu C, Dinică RM, Furdui B. Insights on Monosaccharides and Bioethanol Production from Sweet Sorghum Stalks Using Dilute Acid Pretreatment. Processes. 2020; 8(11):1486. https://doi.org/10.3390/pr8111486
Chicago/Turabian StyleBuruiană, Cristian-Teodor, Luminița Georgescu, Simona-Florina Isticioaia, Oana Emilia Constantin, Camelia Vizireanu, Rodica Mihaela Dinică, and Bianca Furdui. 2020. "Insights on Monosaccharides and Bioethanol Production from Sweet Sorghum Stalks Using Dilute Acid Pretreatment" Processes 8, no. 11: 1486. https://doi.org/10.3390/pr8111486







