Techno-Economic Analysis of a Kilo-Watt Scale Hydrogen-Bromine Flow Battery System for Sustainable Energy Storage
Abstract
1. Introduction
2. Principle of Hydrogen-Bromine Flow Battery (HBFB) Technology
3. Methodology and Assumptions
3.1. HBFB Performance Parameters and System Model
3.1.1. Size
3.1.2. System Efficiency Model
3.1.3. HBFB Model System
3.2. HBFB Performance Parameters and Stack Model
3.2.1. Stack Efficiencies Model
3.2.2. Stack Polarization and Cycling Behavior Model
3.3. System Component Parameters
3.3.1. Electrolyte System
3.3.2. Hydrogen System
3.3.3. Bi-Directional Inverters and System Controls
3.3.4. Housing
3.4. Stack Component Parameters
3.4.1. Liquid Diffusion Electrode
3.4.2. Gas Diffusion Electrode
3.4.3. Membrane
3.4.4. Graphite Plate and Gasket
3.4.5. End Plate
3.5. Financial Assumptions
- Prices are reported in 2020 US dollars accounting for a 3.0% inflation rate (discount rate for capital, with no taxes assumed).
- The costs of electricity from wind and solar parks are assumed to be constant at $0.03/kWh [4].
- The stack assembly costs are $180/m2 for low volume production (base case) according to [17]. For the future case, this is likely to reduce to $100/m2 through automation and large volume production.
- Once a year, there is minor maintenance that requires two field technicians working one full day. Maintenance consists of inspection and electrolyte balancing. Associated costs are $1600 per year.
- After 10 years of operation, major maintenance is performed to replace the stack membranes and laminated GDEs.
- No dedicated operator is required to manage the HBFB system because the targeted scale of 500 kW is relatively small. The system is monitored off-site by a service team that monitors multiple systems simultaneously.
- One-third of the renewable energy generated will be stored in an HBFB storage system.
3.6. Summary of Component Costs
3.7. Economic Model and Calculation
4. Results and Discussions
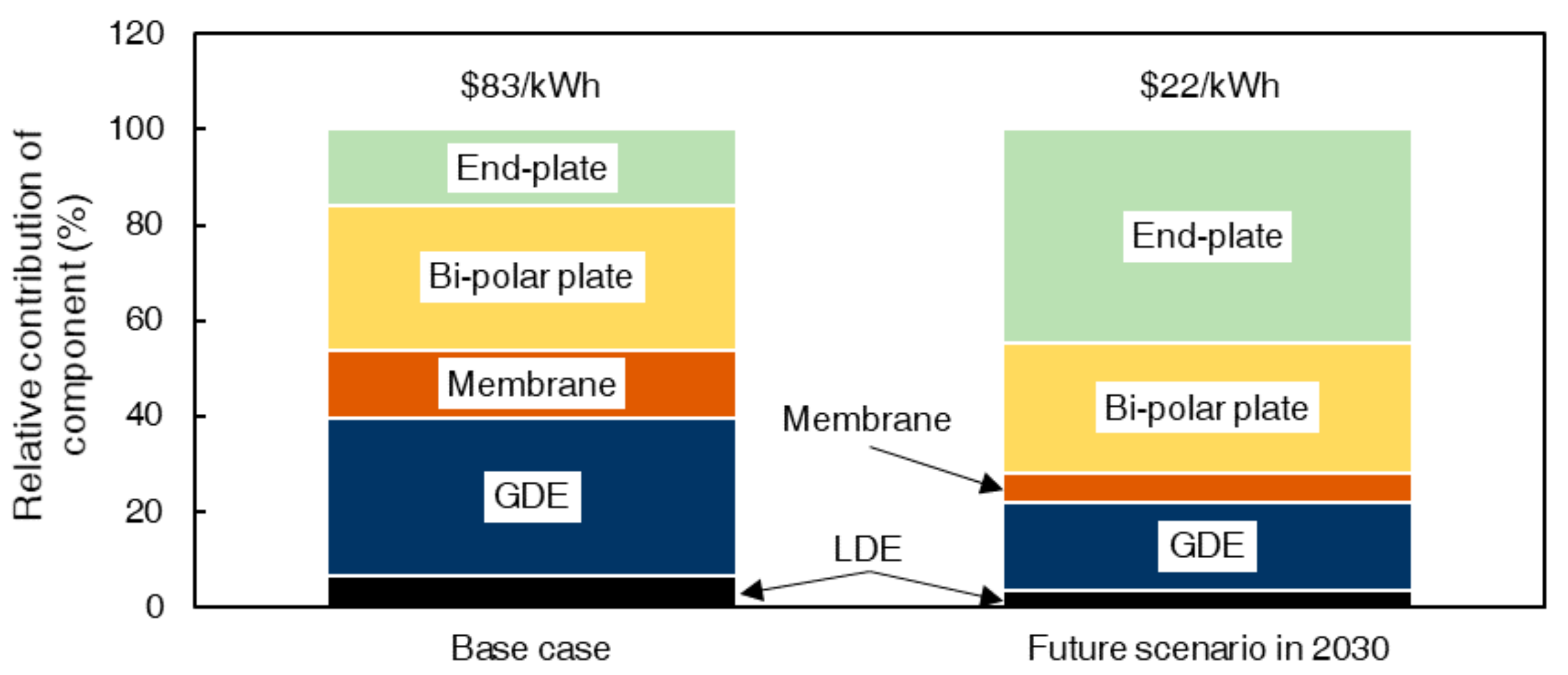
4.1. HBFB Stack Component Costs
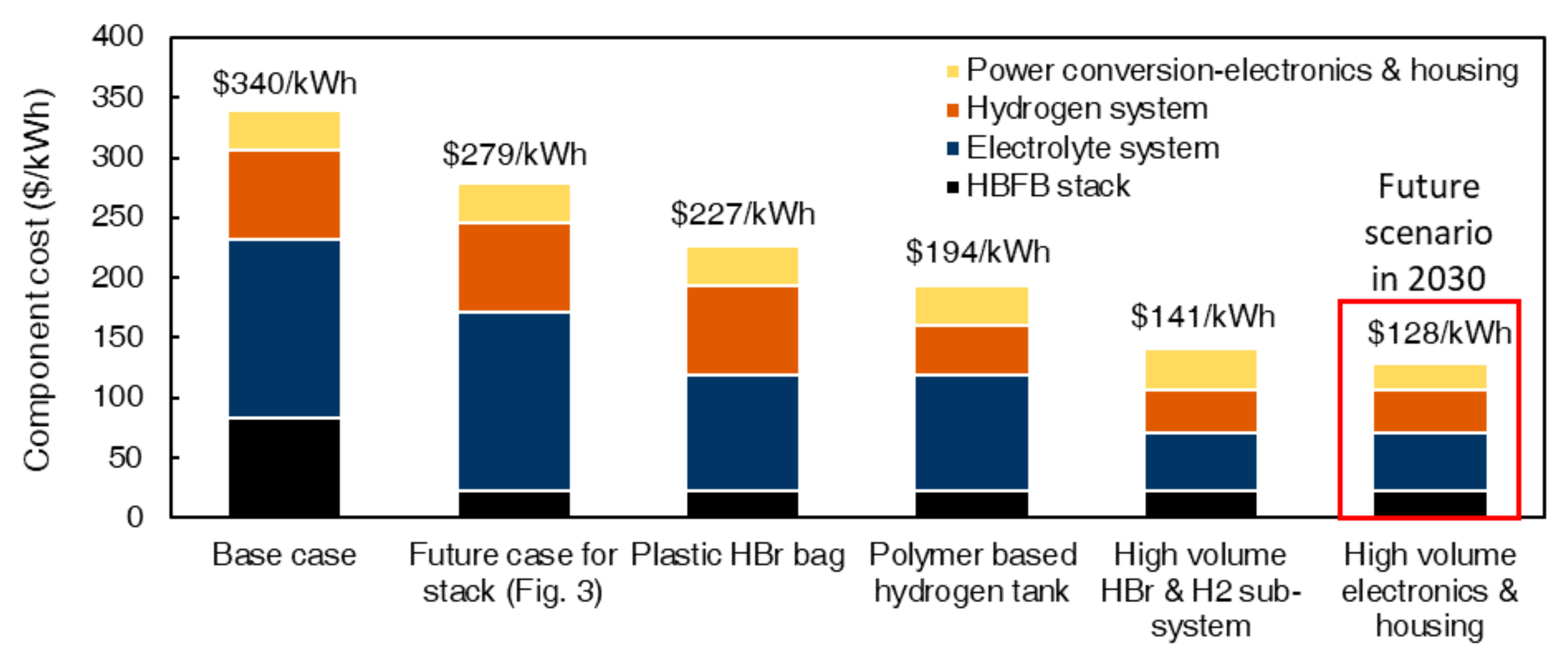
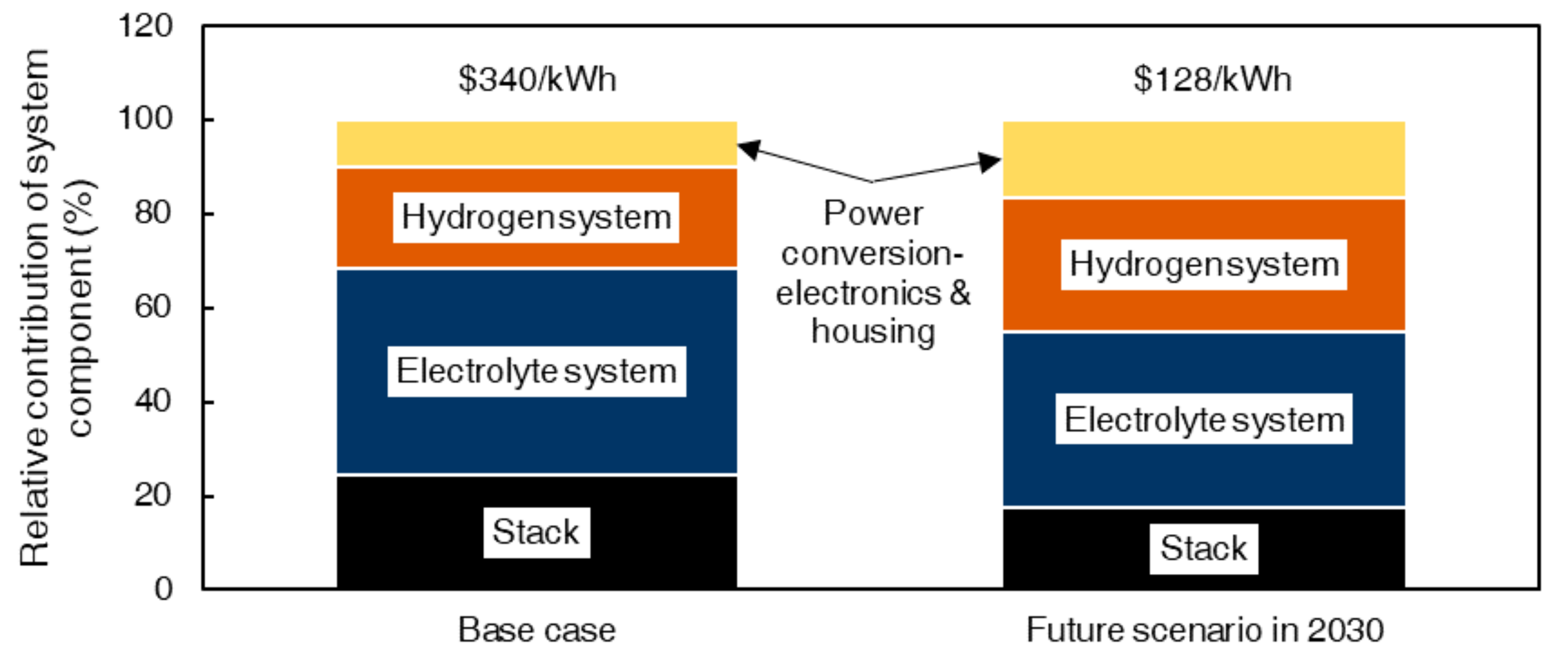
4.2. HBFB Total System Costs
4.3. Cost of Power ($/kW) and Cost of Energy ($/kWh)
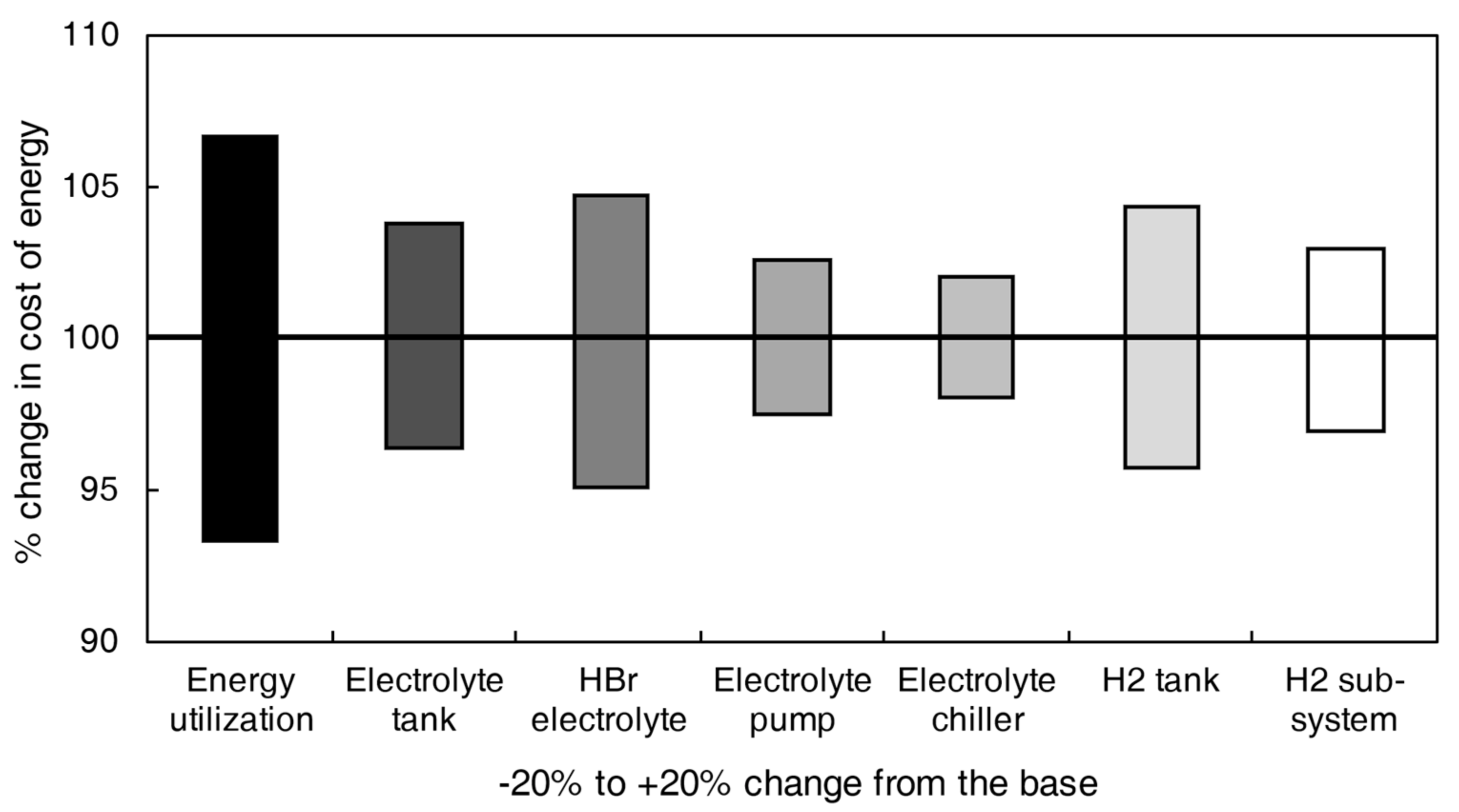
4.4. Cash Flow and Levelized Cost of Storage
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations Climate Change Paris Agreement 2016. Available online: https://unfccc.int/files/essential_background/convention/application/pdf/english_paris_agreement.pdf (accessed on 22 July 2020).
- Communication and roadmap on the European Green Deal; European Commission: Brussels, Belgium, 2019; Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:b828d165-1c22-11ea-8c1f-01aa75ed71a1.0002.02/DOC_1&format=PDF (accessed on 31 October 2020).
- The Netherlands Dutch Reflection on the European Green Deal 2019. Available online: https://www.permanentrepresentations.nl/binaries/nlatio/documents/publications/2019/11/25/dutch-reflection-on-the-green-deal/Dutch+Reflection+on+the+Green+Deal+%28nov+2019%29.pdf (accessed on 31 October 2020).
- IRENA, Renewable Power Generation Costs in 2018; International Renewable Energy Agency: Abu Dhabi, UAE, 2019; Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2019/May/IRENA_Renewable-Power-Generations-Costs-in-2018.pdf (accessed on 18 September 2020).
- IRENA. Global Energy Transformation: A Roadmap to 2050; International Renewable Energy Agency: Abu Dhabi, UAE, 2018. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2018/Apr/IRENA_Report_GET_2018.pdf (accessed on 22 July 2020).
- Teplin, C.; Dyson, M.; Engel, A.; Glazer, G. The Growing Market for Clean Energy Portfolios: Economic Opportunities for a Shift from New Gas-Fired Generation to Clean Energy across the United States Electricity Industry; Rocky Mountain Institute: Baslot, CO, USA, 2019; Available online: https://rmi.org/cep-reports (accessed on 29 September 2020).
- Fu, R.; Remo, T.; Margolis, R. 2018 U.S. Utility-Scale Photovoltaics-Plus-Energy Storage System Costs Benchmark; National Renewable Energy Laboratory: Golden, CO, USA, 2018. Available online: www.nrel.gov/docs/fy19osti/71714.pdf (accessed on 29 September 2020).
- IRENA. Electricity Storage and Renewables: Costs and Markets to 2030; International Renewable Energy Agency: Abu Dhabi, UAE, 2017. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2017/Oct/IRENA_Electricity_Storage_Costs_2017_Summary.pdf?la=en&hash=2FDC44939920F8D2BA29CB762C607BC9E882D4E9 (accessed on 21 September 2020).
- Zakeri, B.; Syri, S. Electrical energy storage systems: A comparative life cycle cost analysis. Renew. Sustain. Energy Rev. 2015, 42, 569–596. [Google Scholar] [CrossRef]
- Chen, H.; Cong, T.N.; Yang, W.; Tan, C.; Li, Y.; Ding, Y. Progress in electrical energy storage system: A critical review. Prog. Nat. Sci. 2009, 19, 291–312. [Google Scholar] [CrossRef]
- Cho, K.T.; Albertus, P.; Battaglia, V.; Kojic, A.; Srinivasan, V.; Weber, A.Z. Optimization and Analysis of High-Power Hydrogen/Bromine-Flow Batteries for Grid-Scale Energy Storage. Energy Technol. 2013, 1, 596–608. [Google Scholar] [CrossRef]
- Livshits, V.; Ulus, A.; Peled, E. High-power H2/Br2 fuel cell. Electrochem. Commun. 2006, 8, 1358–1362. [Google Scholar] [CrossRef]
- Kreutzer, H.; Yarlagadda, V.; van Nguyen, T. Performance evaluation of a regenerative hydrogen-bromine fuel cell. J. Electrochem. Soc. 2012, 159, F331–F337. [Google Scholar] [CrossRef]
- Saadi, K.; Nanikashvili, P.; Tatus-Portnoy, Z.; Hardisty, S.; Shokhen, V.; Zysler, M.; Zitoun, D. Crossover-tolerant coated platinum catalysts in hydrogen/bromine redox flow battery. J. Power Sources 2019, 422, 84–91. [Google Scholar] [CrossRef]
- Noack, J.; Wietschel, L.; Roznyatovskaya, N.; Pinkwart, K.; Tübke, J. Techno-economic modeling and analysis of redox flow battery systems. Energies 2016, 9, 627. [Google Scholar] [CrossRef]
- Dmello, R.; Milshtein, J.D.; Brushett, F.R.; Smith, K.C. Cost-driven materials selection criteria for redox flow battery electrolytes. J. Power Sources 2016, 330, 261–272. [Google Scholar] [CrossRef]
- Singh, N.; McFarland, E.W. Levelized cost of energy and sensitivity analysis for the hydrogen–bromine flow battery. J. Power Sources 2015, 288, 187–198. [Google Scholar] [CrossRef]
- Yeo, R.S.; Chin, D.T. A Hydrogen-Bromine Cell for Energy Storage Applications. J. Electrochem. Soc. 1980, 127, 549–555. [Google Scholar] [CrossRef]
- Trumpf Huttinger TruConvert AC 3020—Power Conversion System. Available online: https://www.trumpf.cn/filestorage/TRUMPF_Master/Products/Power_Electronics/Energy_storage/TRUMPF_Power_conversion_system_TruConvert_AC_3020-EN.pdf.pdf (accessed on 24 January 2020).
- Faraday, M., VI. Experimental researches in electricity.-Seventh Series. Philos. Trans. R. Soc. Lond. 1834, 124, 77–122. [Google Scholar] [CrossRef]
- Trovò, A.; Marini, G.; Sutto, A.; Alotto, P.; Giomo, M.; Moro, F.; Guarnieri, M. Standby thermal model of a vanadium redox flow battery stack with crossover and shunt-current effects. Appl. Energy 2019, 240, 893–906. [Google Scholar] [CrossRef]
- Lin, G.; Chong, P.Y.; Yarlagadda, V.; Nguyen, T.V.; Wycisk, R.J.; Pintauro, P.N.; Bates, M.; Mukerjee, S.; Tucker, M.C.; Weber, A.Z. Advanced Hydrogen-Bromine Flow Batteries with Improved Efficiency, Durability and Cost. J. Electrochem. Soc. 2016, 163, A5049–A5056. [Google Scholar] [CrossRef]
- Forner-Cuenca, A.; Penn, E.E.; Oliveira, A.M.; Brushett, F.R. Exploring the Role of Electrode Microstructure on the Performance of Non-Aqueous Redox Flow Batteries. J. Electrochem. Soc. 2019, 166, A2230. [Google Scholar] [CrossRef]
- Tariq, F.; Rubio-Garcia, J.; Yufit, V.; Bertei, A.K.; Chakrabarti, B.; Kucernak, A.; Brandon, N. Uncovering the mechanisms of electrolyte permeation in porous electrodes for redox flow batteries through real time in situ 3D imaging. Sustain. Energy Fuels 2018, 2, 2068–2080. [Google Scholar] [CrossRef]
- Schweiss, R.; Meiser, C.; Damjanovic, T.; Galbiati, I.; Haak, N. SIGRACET® Gas Diffusion Layers for PEM Fuel Cells, Electrolyzers and Batteries (White Paper); SGL Carbon GmbH: Bonn, Germany, 2016. [Google Scholar]
- Nunna, S.; Blanchard, P.; Buckmaster, D.; Davis, S.; Naebe, M. Development of a cost model for the production of carbon fibres. Heliyon 2019, 5, e02698. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.; Kleen, G.; Papageorgopoulos, D. Fuel Cell System Cost—2017; DOE Hydrogen and Fuel Cells Program Record; DOE: Washington, DC, USA, 2017. [Google Scholar]
- Hugo, Y.A.; Kout, W.; Sikkema, F.; Borneman, Z.; Nijmeijer, K. In situ long-term membrane performance evaluation of hydrogen-bromine flow batteries. J. Energy Storage 2019, 27, 101068. [Google Scholar] [CrossRef]
- Cho, K.T.; Tucker, M.C.; Ding, M.; Ridgway, P.; Battaglia, V.S.; Srinivasan, V.; Weber, A.Z. Cyclic Performance Analysis of Hydrogen/Bromine Flow Batteries for Grid-Scale Energy Storage. ChemPlusChem 2014. [Google Scholar] [CrossRef]
- Uchida, M.; Fukuoka, Y.; Sugawara, Y.; Ohara, H.; Ohta, A. Improved Preparation Process of Very-Low-Platinum-Loading Electrodes for Polymer Electrolyte Fuel Cells. J. Electrochem. Soc. 1998, 145, 3708–3713. [Google Scholar] [CrossRef]
- Hugo, Y.A.; Kout, W.; Sikkema, F.; Borneman, Z.; Nijmeijer, K. Performance mapping of cation exchange membranes for hydrogen-bromine flow batteries for energy storage. J. Membr. Sci. 2018, 566, 406–414. [Google Scholar] [CrossRef]
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Tucker, M.C.; Cho, K.T.; Spingler, F.B.; Weber, A.Z.; Lin, G. Impact of membrane characteristics on the performance and cycling of the Br2–H2 redox flow cell. J. Power Sources 2015, 284, 212–221. [Google Scholar] [CrossRef]
- Park, J.W.; Wycisk, R.; Pintauro, P.N. Nafion/PVDF nanofiber composite membranes for regenerative hydrogen/bromine fuel cells. J. Membr. Sci. 2015, 490, 103–112. [Google Scholar] [CrossRef]
- Park, J.W.; Wycisk, R.; Lin, G.; Chong, P.Y.; Powers, D.; van Nguyen, T.; Dowd, R.P., Jr.; Pintauro, P.N. Electrospun Nafion/PVDF single-fiber blended membranes for regenerative H2/Br2 fuel cells. J. Membr. Sci. 2017, 541, 85–92. [Google Scholar] [CrossRef]
- Cho, K.T.; Ridgway, P.; Weber, A.Z.; Haussener, S.; Battaglia, V.; Srinivasan, V. High Performance Hydrogen/Bromine Redox Flow Battery for Grid-Scale Energy Storage. J. Electrochem. Soc. 2012, 159, A1806–A1815. [Google Scholar] [CrossRef]
- Minke, C.; Hickmann, T.; dos Santos, A.R.; Kunz, U.; Turek, T. Cost and performance prospects for composite bipolar plates in fuel cells and redox flow batteries. J. Power Sources 2016, 305, 182–190. [Google Scholar] [CrossRef]
- James, B.D.; Kalinoski, J.A.; Baum, K.N. Mass Production Cost Estimation For Direct H2 PEM Fuel Cell Systesm for Automotive Applications. 2010 Update; Directed Technologies Inc.: Arlington, VA, USA, 2010. [Google Scholar]
- The International Flow Battery Forum: Mercure Manchester Piccadilly Hotel, Manchester, UK, 27–29 June 2017; Conference papers; (trading as the International Flow Battery Forum); IFBF Administration Office, Swanbarton Limited: Manchester, UK, 2017.
- Li, Y.; Nguyen, T.V. Core-shell rhodium sulfide catalyst for hydrogen evolution reaction/hydrogen oxidation reaction in hydrogen-bromine reversible fuel cell. J. Power Sources 2018, 382, 152–159. [Google Scholar] [CrossRef]
- FlowBox. Available online: https://www.innoenergy.com/for-innovators/innoenergy-thematic-fields/energy-storage/flowbox/ (accessed on 22 November 2019).
- Wagner, R.; Preschitschek, N.; Passerini, S.; Leker, J.; Winter, M. Current research trends and prospects among the various materials and designs used in lithium-based batteries. J. Appl. Electrochem. 2013, 43, 481–496. [Google Scholar] [CrossRef]
- Braff, W.A.; Bazant, M.Z.; Buie, C.R. Membrane-less hydrogen bromine flow battery. Nat. Commun. 2013, 4, 2346. [Google Scholar] [CrossRef]






| Operating Condition | Parameter |
|---|---|
| Temperature | ≤60 °C |
| CHBr dis. | 6 M |
| CHBr ch. | 2 M |
| CBr2 dis. | 0.3 M |
| CBr2 ch. | 2.3 M |
| Br2 stoichiometry ratio | ≥3 |
| H2 dis. | 1 bar |
| H2 ch. | 8 bar |
| Parameter | Base Case | Future Case | Unit |
|---|---|---|---|
| Nominal (dis.) power | 500 | 500 | kW |
| Stack resistance | 0.30 | 0.22 | Ω·cm2 |
| Current density | 0.33 | 0.33 | A/cm2 |
| Power density | 0.266 | 0.274 | W/cm2 |
| Voltaic efficiency | 80 | 85 | % |
| Coulombic efficiency | 97 | 99 | % |
| Energy efficiency | 78 | 84 | % |
| Pump loss | 2 | 2 | % |
| Cooling loss | 3 | 3 | % |
| Bi-directional inverter efficiency | 96 | 96 | % |
| System efficiency | 70 | 77 | % |
| Req. active area | ~210 | ~200 | m2 |
| Req. electrolyte tank volume | 75 | 65 | m3 |
| Component | Base Case | Future Case | Cost Unit |
|---|---|---|---|
| HBFB system | |||
| Electrolyte tank | 56 | 4 | $/kWh |
| HBr electrolyte | 50 | 8 | $/kWh |
| Electrolyte pump | 25 | 21 | $/kWh |
| Electrolyte chiller | 18 | 15 | $/kWh |
| H2 tank | 44 | 11 | $/kWh |
| H2 sub-system | 30 | 26 | $/kWh |
| Bi-directional inverter | 180 | 80 | $/kW |
| Power electronics | 45,000 | 38,250 | $/unit |
| Housing | 33,000 | 28,050 | $/unit |
| System labor | 150 | 100 | $/kW |
| HBFB stack | |||
| Liquid diffusion electrode (LDE) | 135 | 20 | $/m2 |
| Gas diffusion electrode (GDL) | 650 | 100 | $/m2 |
| Replacement LDE | 250 | ||
| Membrane | 285 | 35 | $/m2 |
| Replacement membrane | 120 | ||
| Graphite plate | 550 | 125 | $/m2 |
| Gasket | 50 | 25 | $/m2 |
| End plate | 132 | 100 | $/kW |
| Stack labor | 180 | 100 | $/m2 |
| Cost Breakdown | Year | ||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | |
| Cumulative stored energy (MWh) | 0 | 10,000 | 20,000 | 30,000 | 40,000 |
| Capital costs | $1,811,090 | $115,039 | |||
| CRF cost | $152,238 | $113,956 | $113,188 | $101,394 | |
| Maintenance | $8,000 | $8,000 | $8,000 | $8,000 | |
| Electricity | $130,974 | $130,974 | $130,974 | $130,974 | |
| Cumulative costs | $1,811,090 | $2,102,302 | $2,470,270 | $2,722,432 | $2,962,800 |
| LCoS (/kWh) | $0.210 | $0.124 | $0.091 | $0.074 | |
| Cost Breakdown | Year | ||||
|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | |
| Cumulative stored energy (MWh) | 0 | 10,000 | 20,000 | 30,000 | 40,000 |
| Capital costs | $712,761 | $47,703 | |||
| CRF cost | $59,914 | $44,848 | $44,750 | $40,057 | |
| Maintenance | $8,000 | $8,000 | $8,000 | $8,000 | |
| Electricity | $90,437 | $90,437 | $90,437 | $90,437 | |
| Cumulative costs | $712,761 | $871,112 | $1,062,100 | $1,205,287 | $1,343,781 |
| LCoS (/kWh) | $0.087 | $0.053 | $0.040 | $0.034 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hugo, Y.A.; Kout, W.; Dalessi, G.; Forner-Cuenca, A.; Borneman, Z.; Nijmeijer, K. Techno-Economic Analysis of a Kilo-Watt Scale Hydrogen-Bromine Flow Battery System for Sustainable Energy Storage. Processes 2020, 8, 1492. https://doi.org/10.3390/pr8111492
Hugo YA, Kout W, Dalessi G, Forner-Cuenca A, Borneman Z, Nijmeijer K. Techno-Economic Analysis of a Kilo-Watt Scale Hydrogen-Bromine Flow Battery System for Sustainable Energy Storage. Processes. 2020; 8(11):1492. https://doi.org/10.3390/pr8111492
Chicago/Turabian StyleHugo, Yohanes Antonius, Wiebrand Kout, Guido Dalessi, Antoni Forner-Cuenca, Zandrie Borneman, and Kitty Nijmeijer. 2020. "Techno-Economic Analysis of a Kilo-Watt Scale Hydrogen-Bromine Flow Battery System for Sustainable Energy Storage" Processes 8, no. 11: 1492. https://doi.org/10.3390/pr8111492
APA StyleHugo, Y. A., Kout, W., Dalessi, G., Forner-Cuenca, A., Borneman, Z., & Nijmeijer, K. (2020). Techno-Economic Analysis of a Kilo-Watt Scale Hydrogen-Bromine Flow Battery System for Sustainable Energy Storage. Processes, 8(11), 1492. https://doi.org/10.3390/pr8111492






