Low-Cost Synthesis of Alumina Nanoparticles and Their Usage for Bisphenol-A Removal from Aqueous Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis and Modification of Gamma-Alumina Nanoparticles (γ-ANPs)
2.2.2. Preparation of Gamma-Alumina Nanoparticles (γANPs) Modified by CTAB (γANPs-CTAB)
2.2.3. Characterization Methods
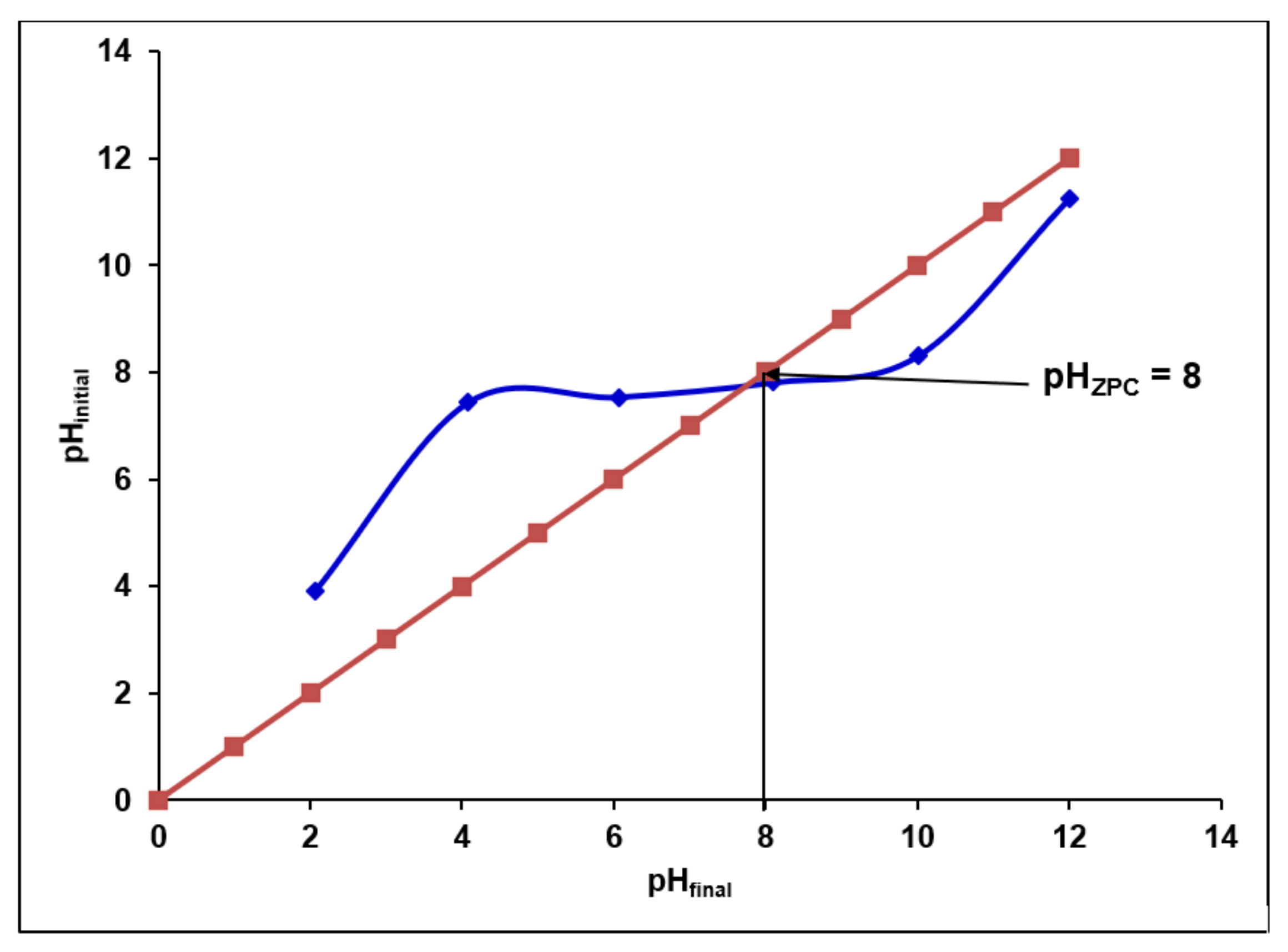
2.2.4. Determination of pHZPC
2.3. Batch Bisphenol-A (BPA) Adsorption Investigations
2.4. Statistics and Data Analysis
3. Results and Discussion
3.1. Raw Bauxite Characterization
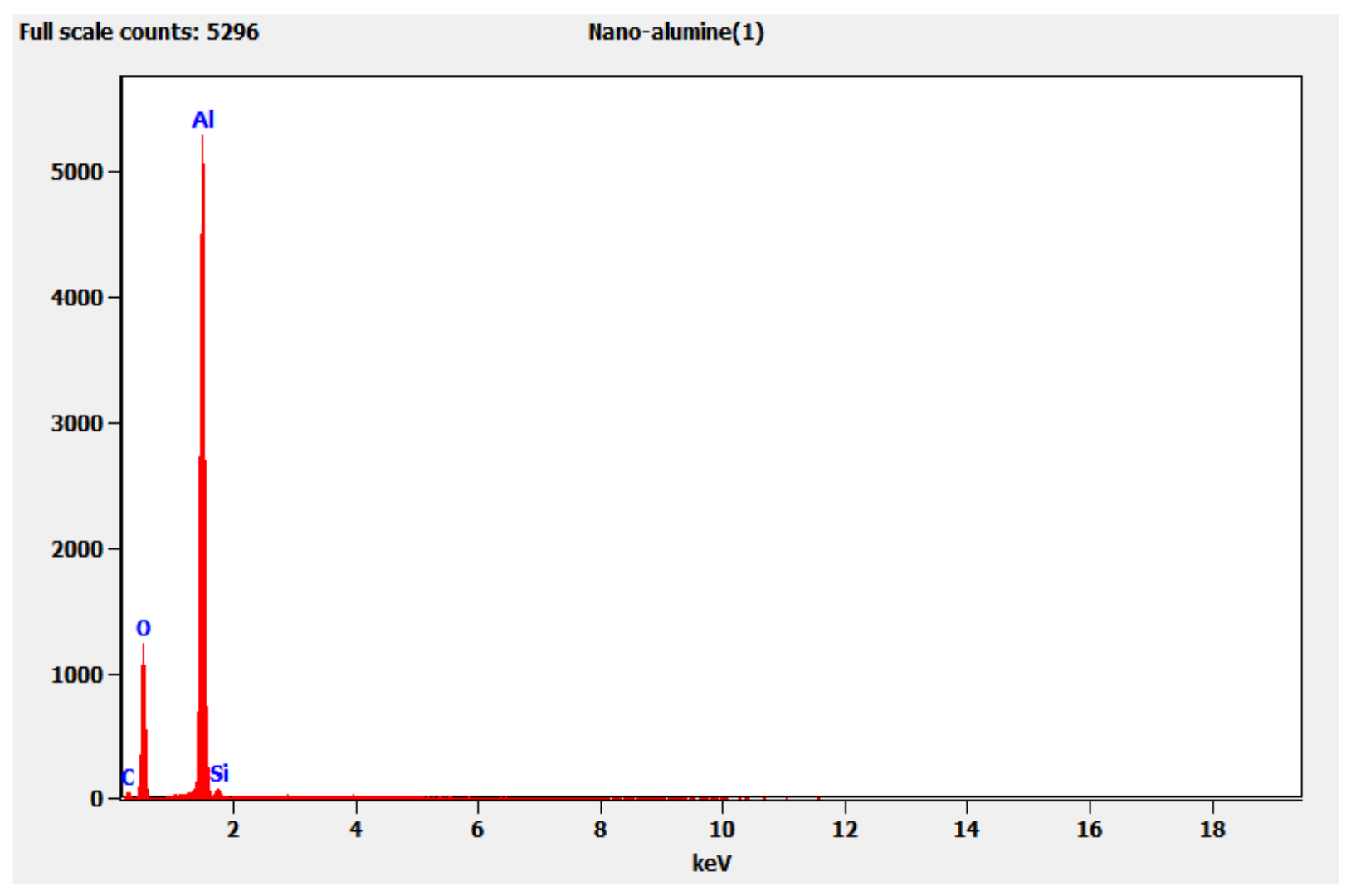
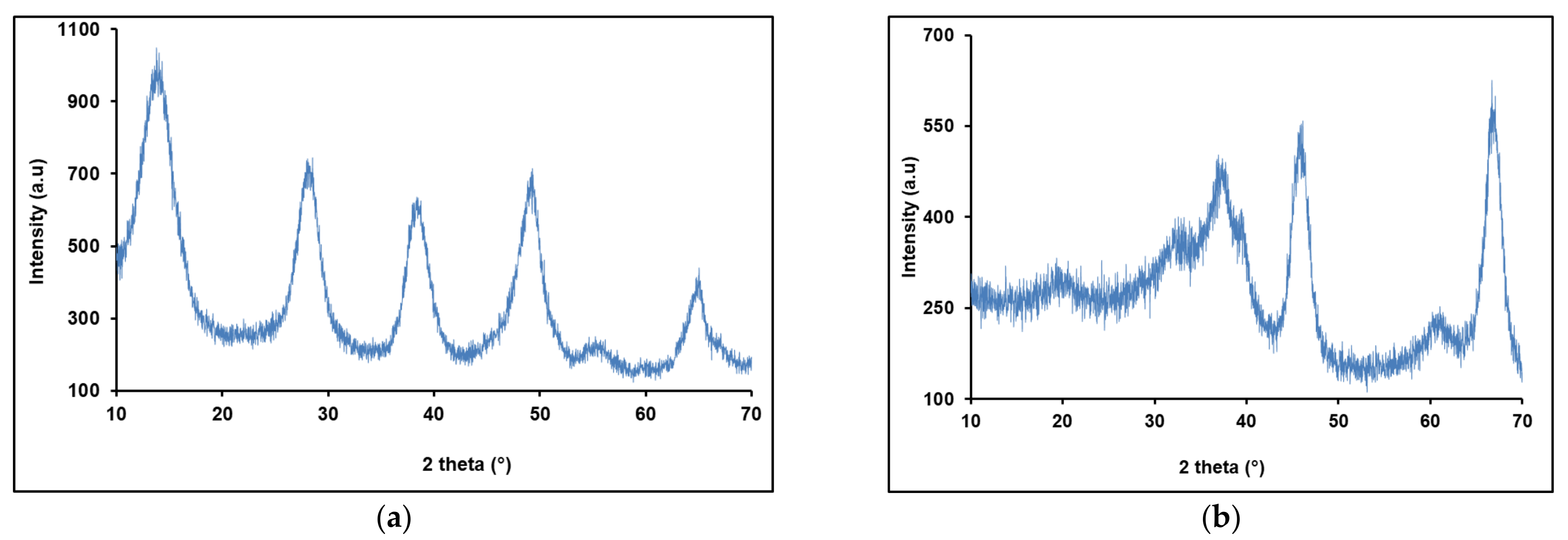
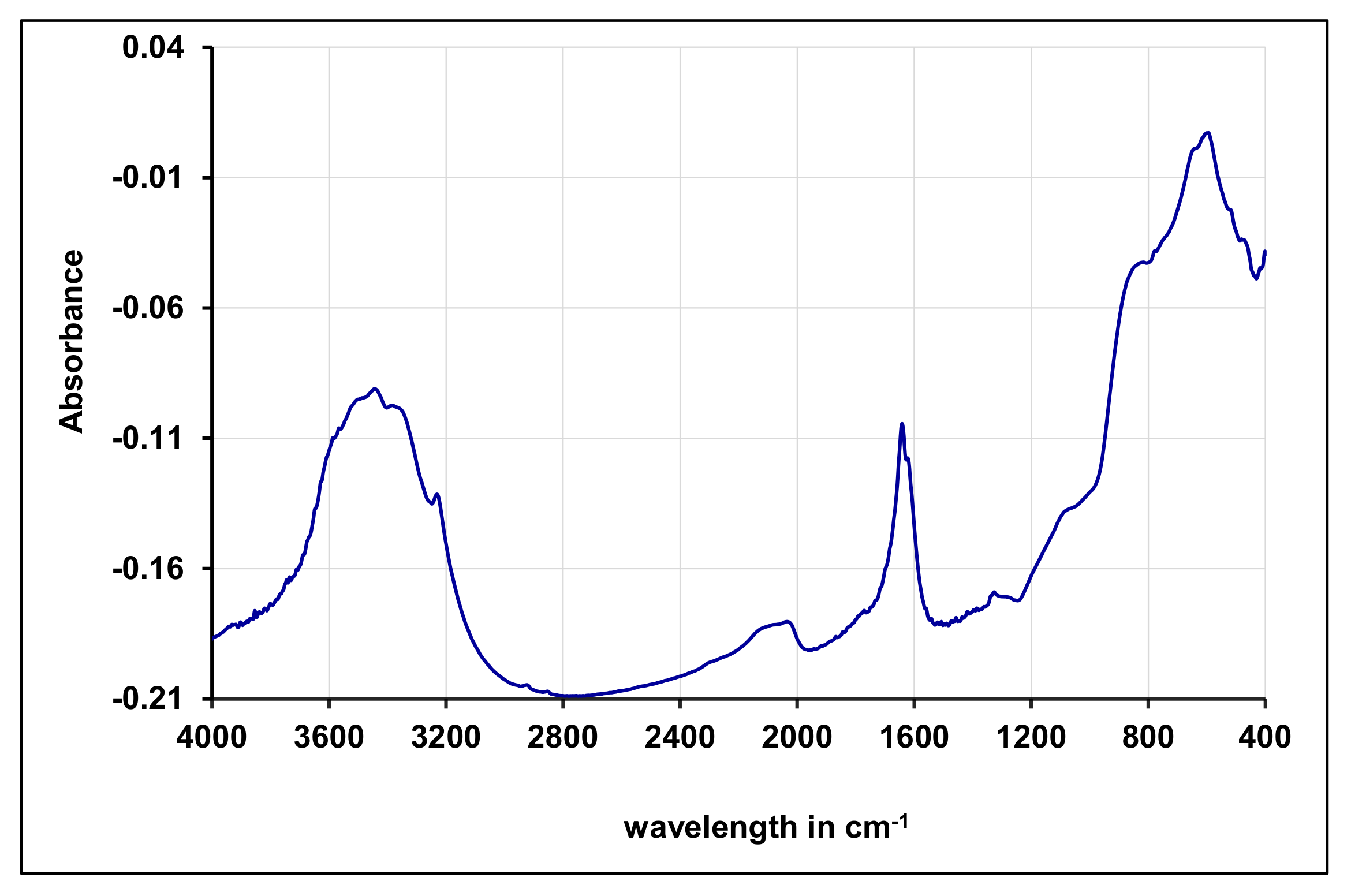
3.2. Characterizations of Synthesized Gamma-Alumina Nanoparticles (γANPs)
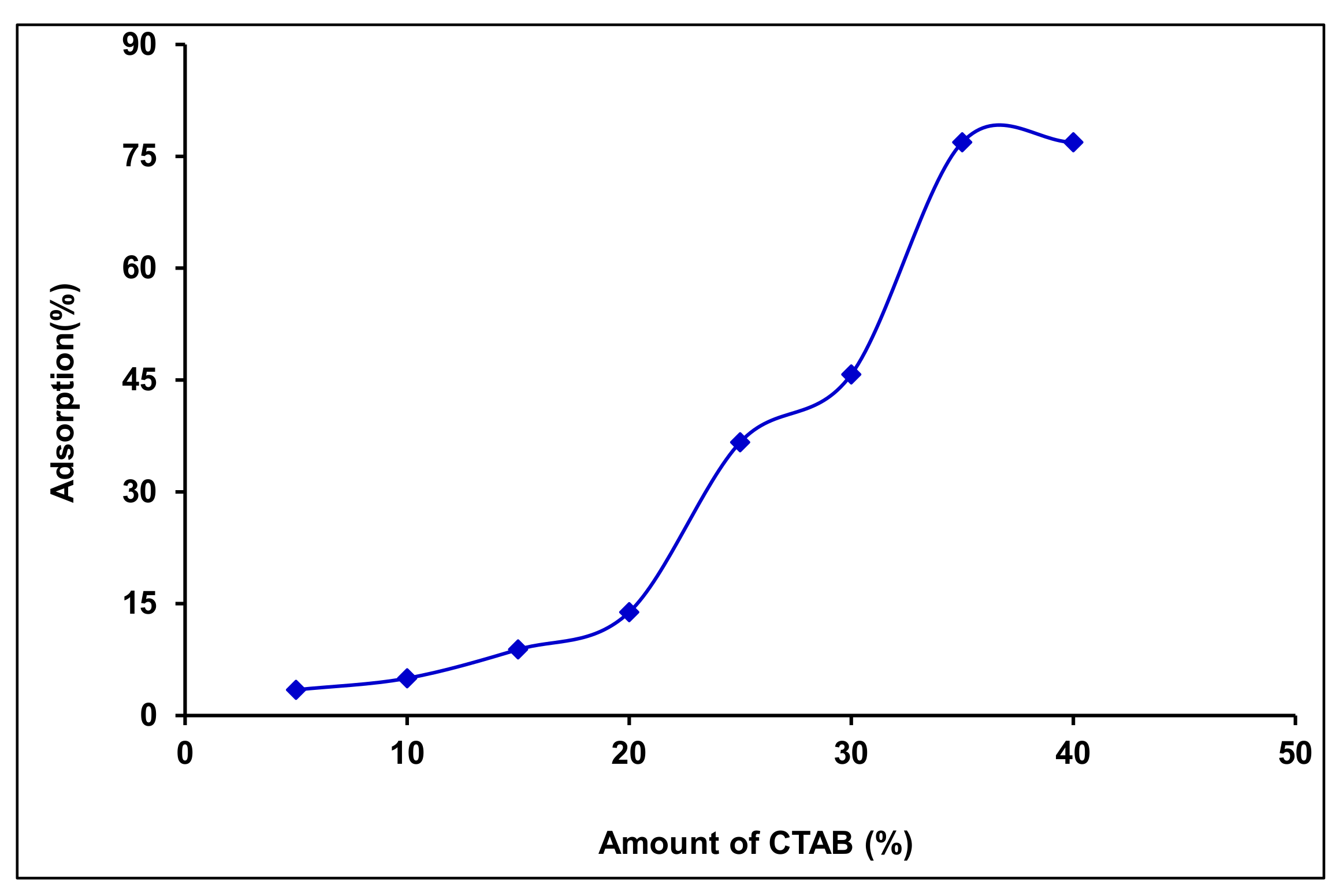
3.3. Effect of Surfactant (CTAB) Loading Levels
3.4. Batch Investigations of BPA Adsorption onto γANPs-CTAB
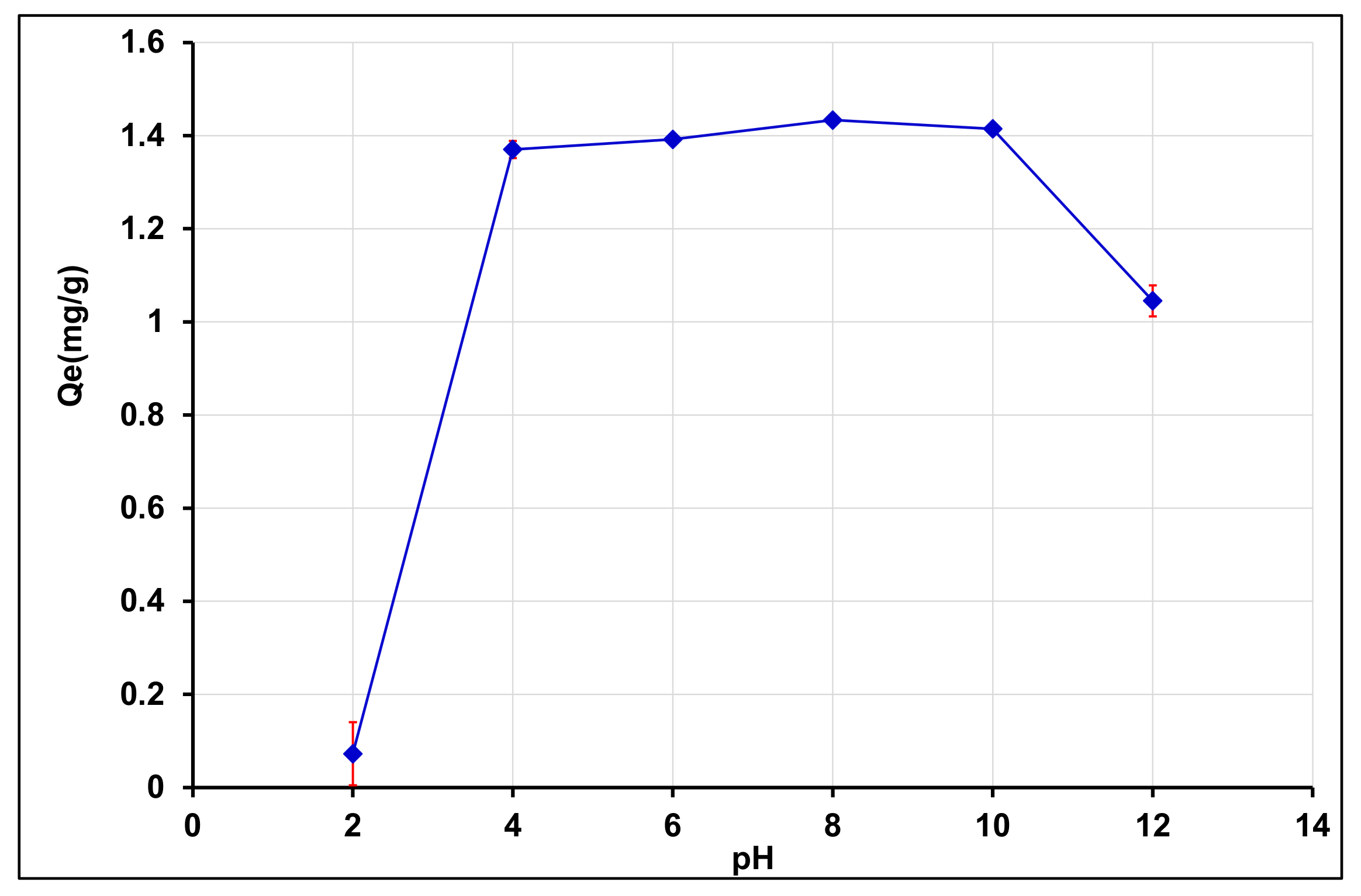
3.4.1. Effect of Initial pH
3.4.2. Effect of Adsorbent Dose
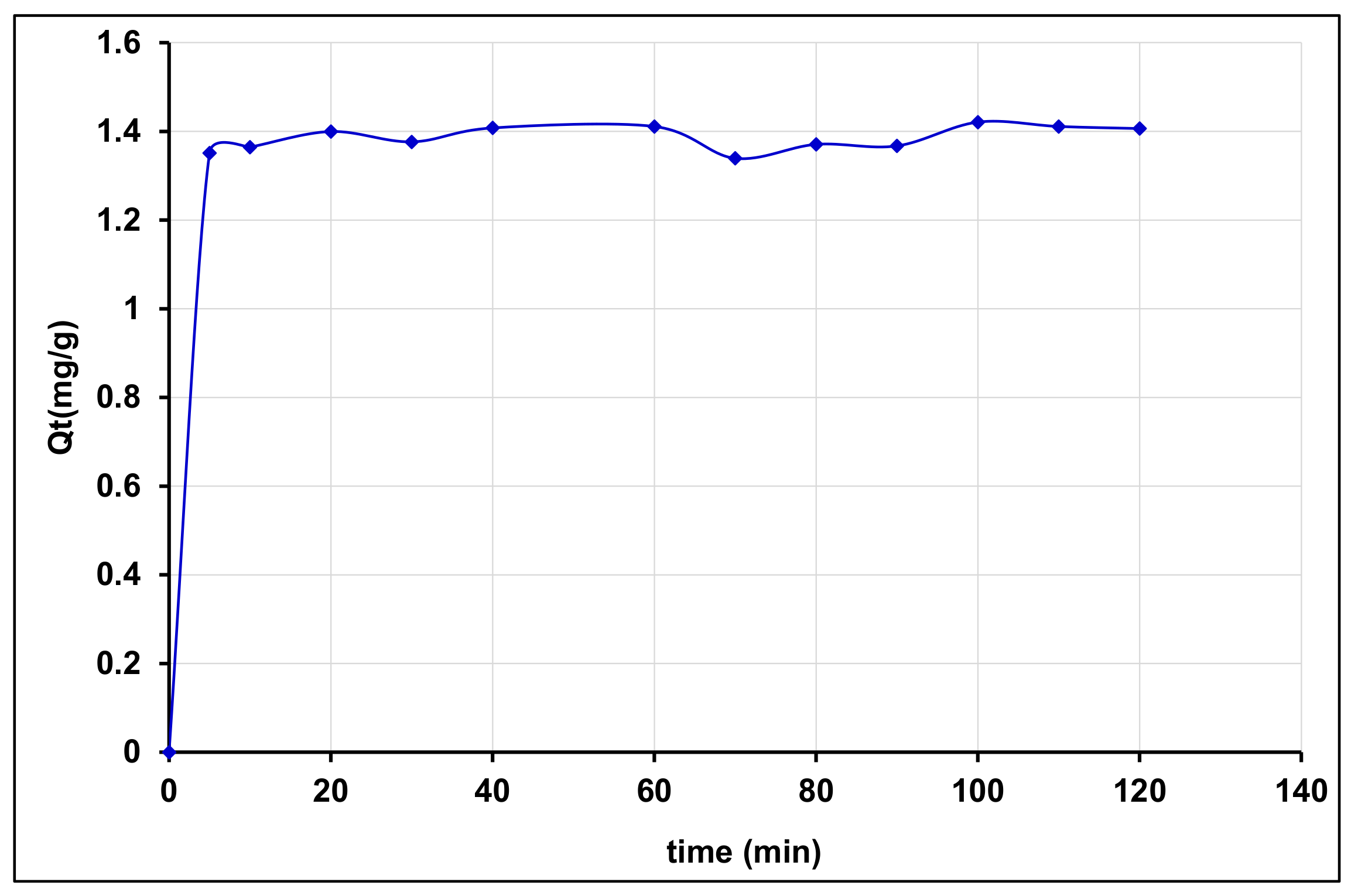
3.4.3. Effect of the Agitation Time on BPA Adsorption and Kinetics
Effect of the Agitation Time on BPA Adsorption
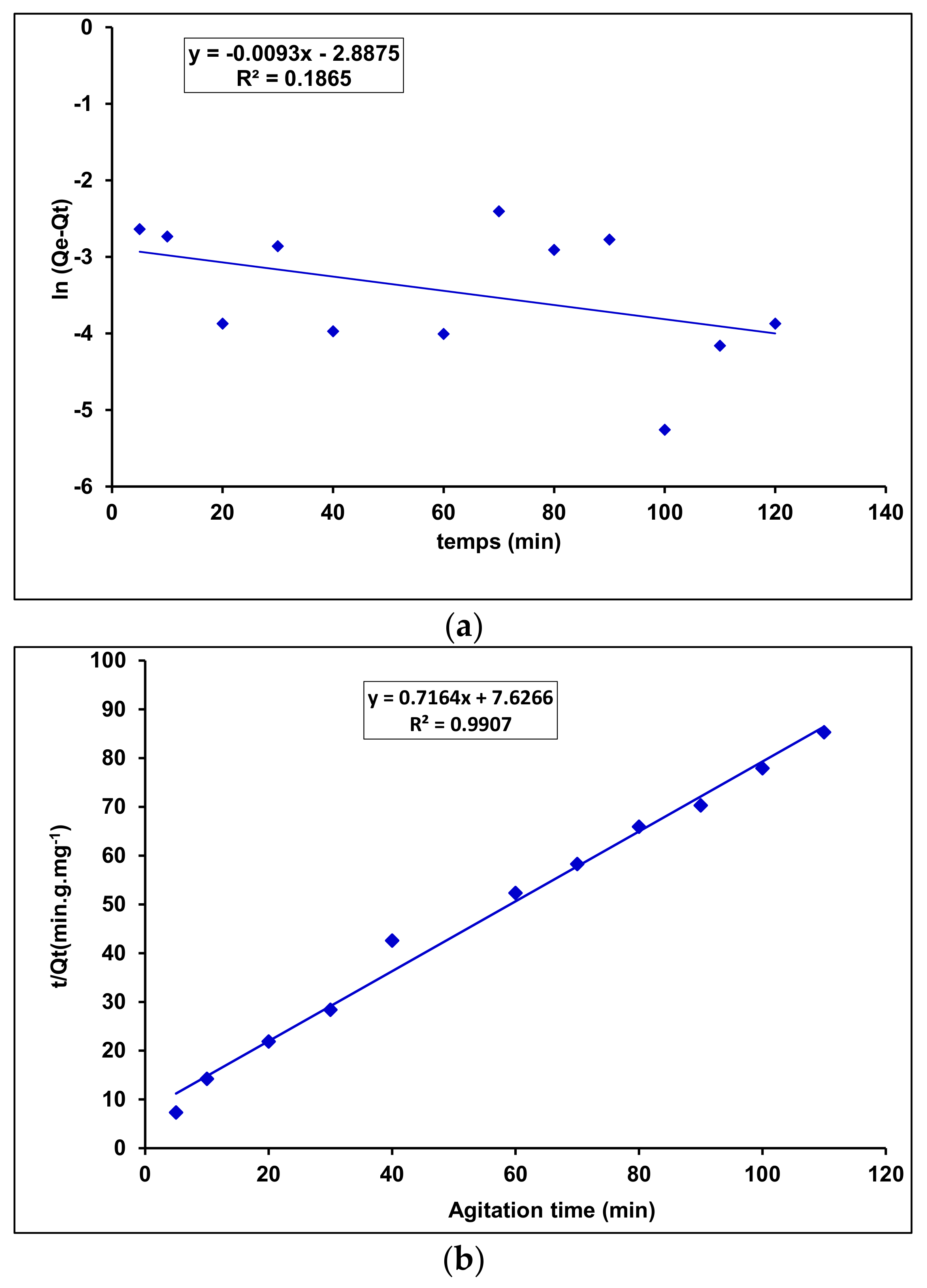
Adsorption Kinetics
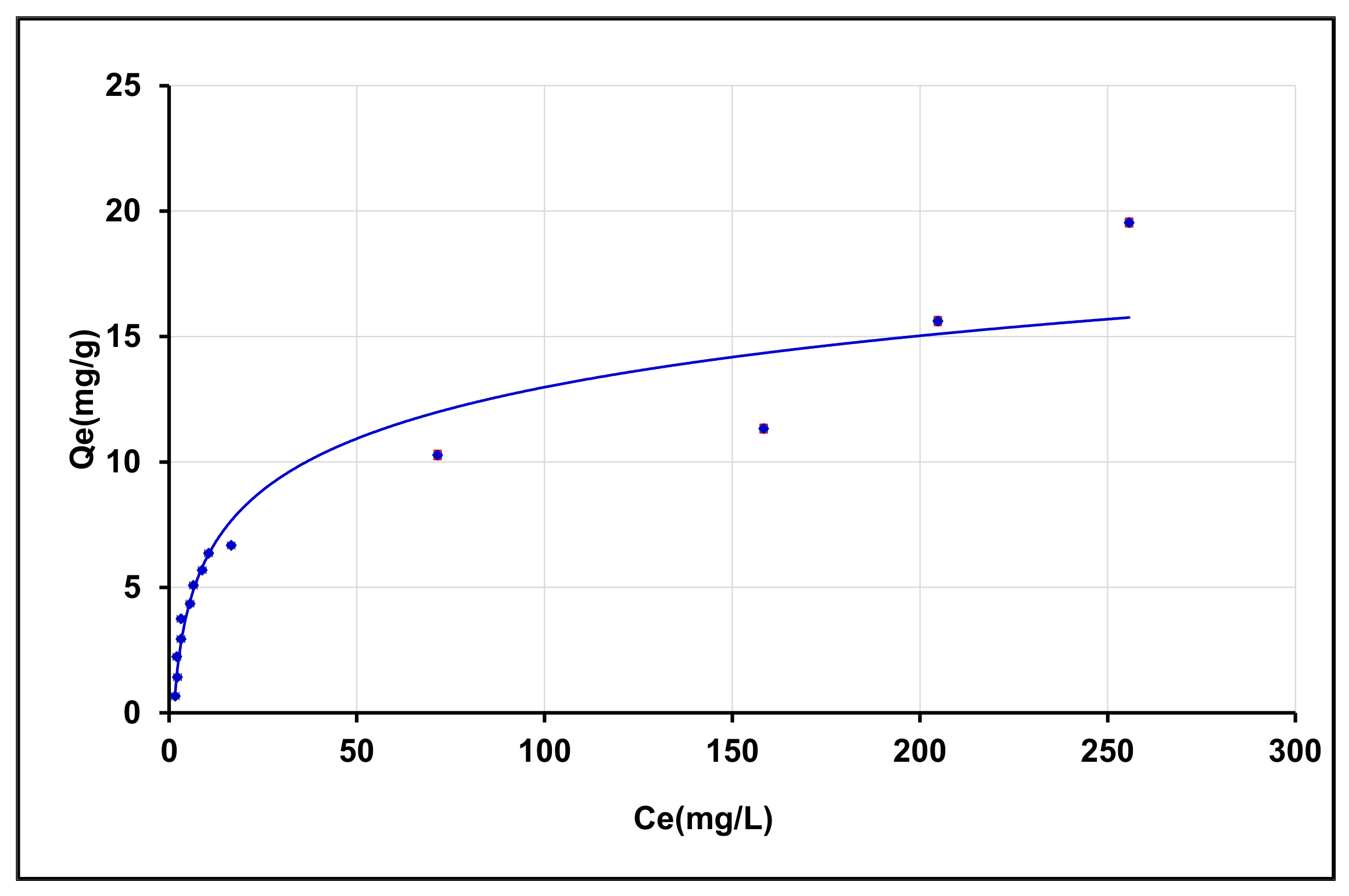
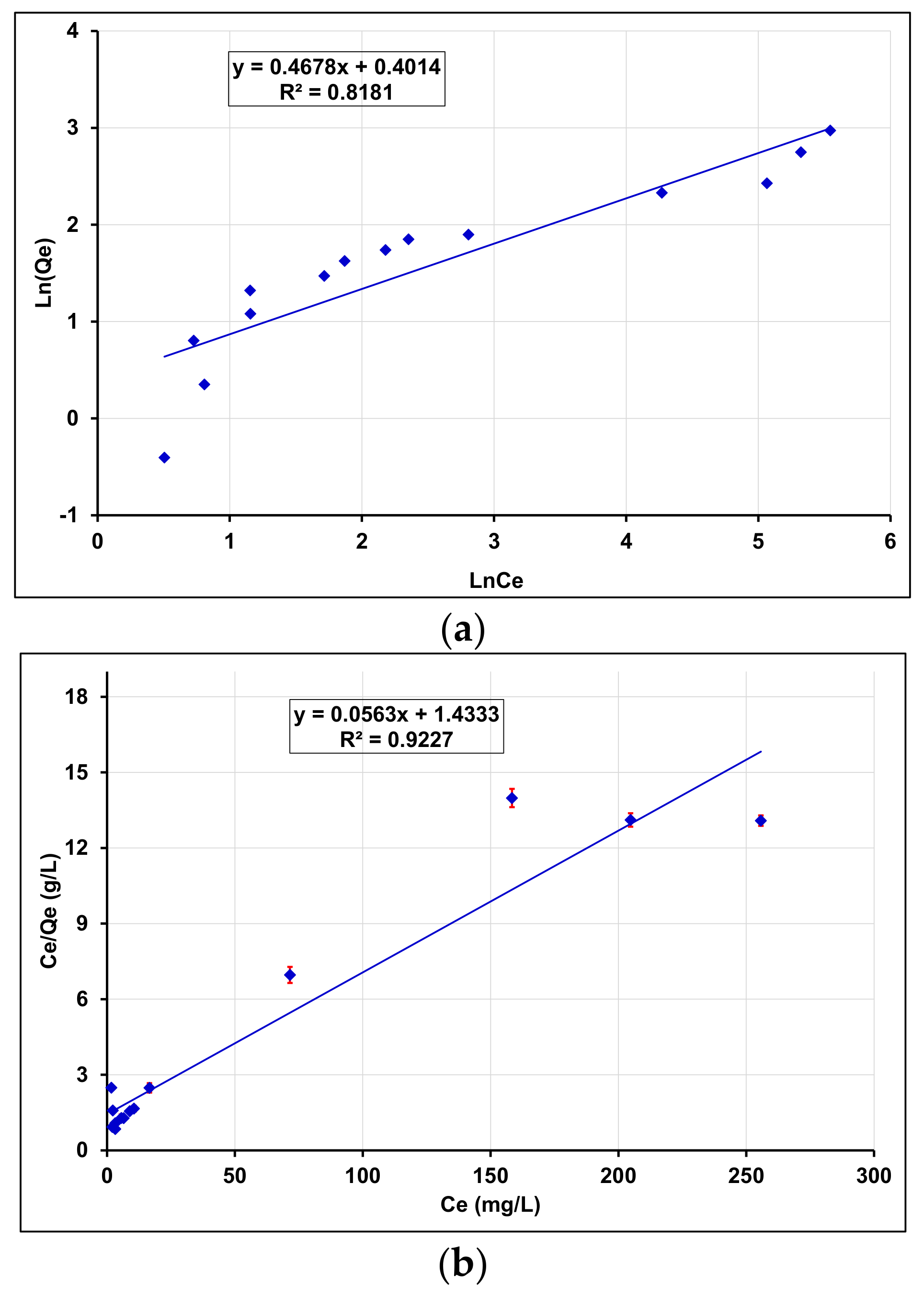
3.4.4. Effect of Initial Concentration of BPA Solution and Isotherms
Effect of Initial Concentration of BPA Solution
Adsorption Isotherms
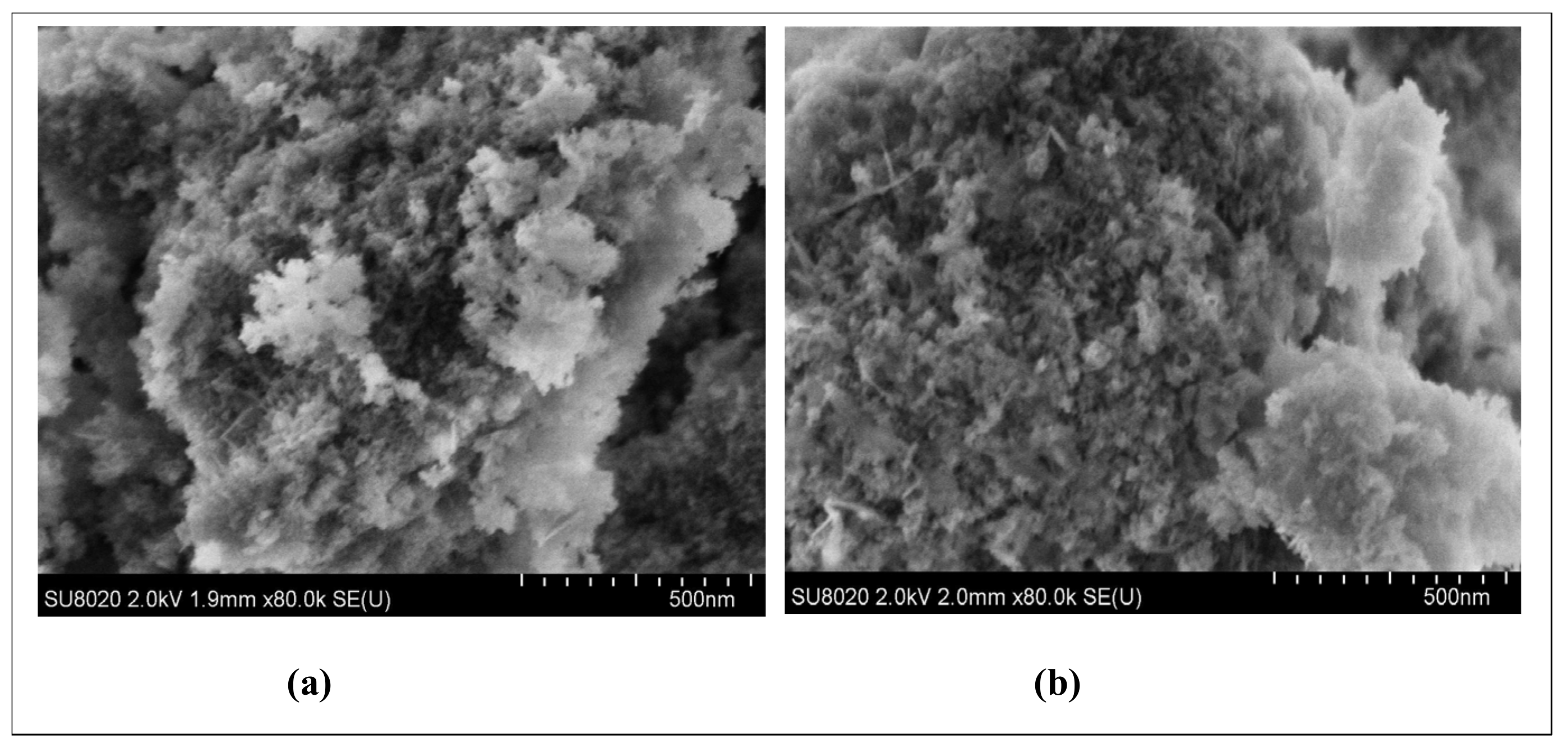
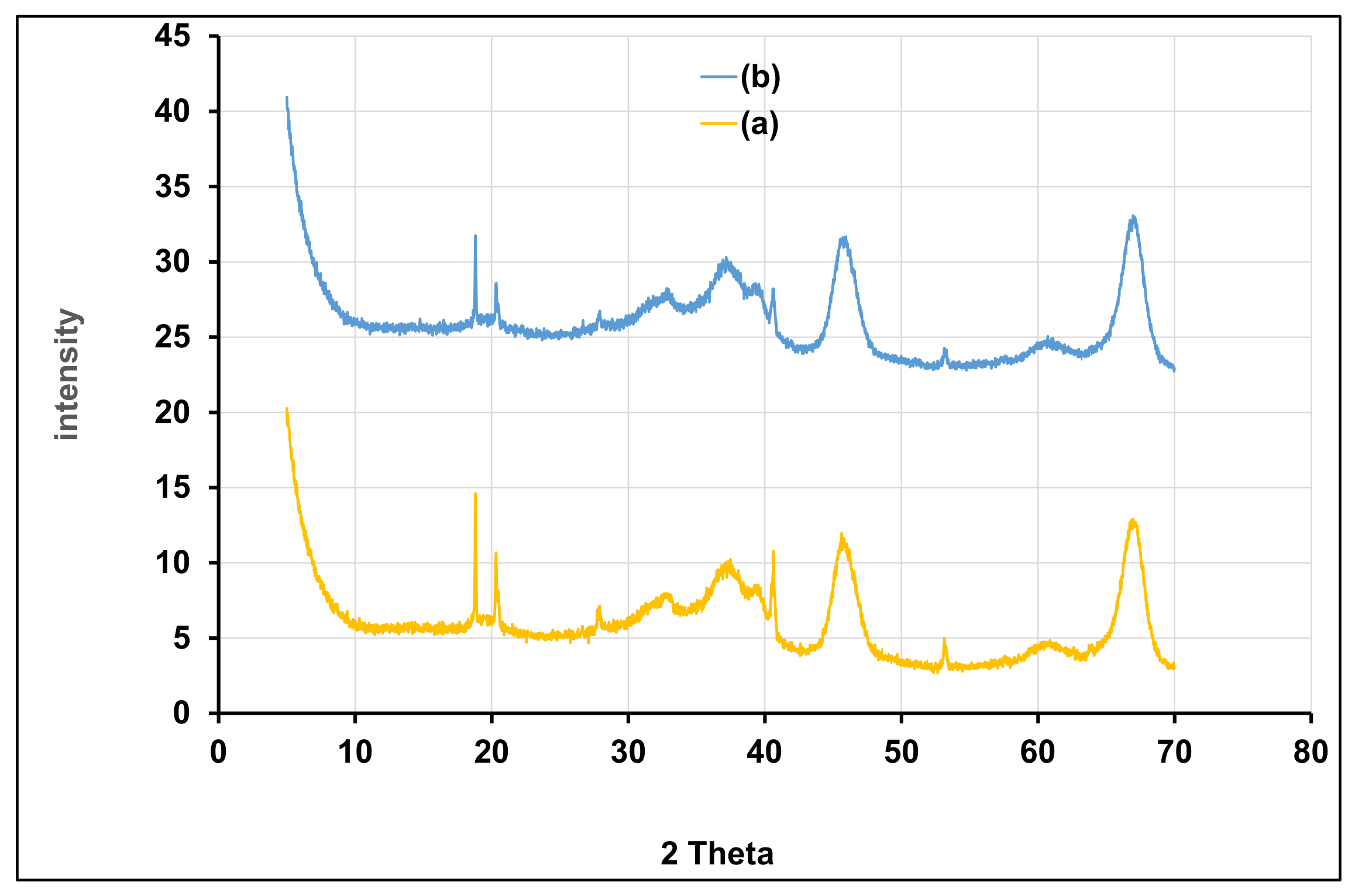
3.4.5. Investigations on γANPs-CTAB before and after BPA Adsorption
FTIR Investigations
SEM Investigations
XRPD Investigations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, S.M.; Clark, K.E.; Staples, C.A.; Klecka, G.M.; Dimond, S.S.; Caspers, N.; Hentges, S.G. Relevance of drinking water as a source of human exposure to bisphenol A. J. Expo. Sci. Environ. Epidemiol. 2012, 23, 137–144. [Google Scholar] [CrossRef]
- Saal, F.S.V.; Welshons, W.V. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environ. Res. 2006, 100, 50–76. [Google Scholar] [CrossRef]
- Staples, C.A.; Dome, P.B.; Klecka, G.M.; Oblock, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
- Chevalier, N. Les perturbateurs endocriniens: De nouveaux acteurs dans l’épidémie d’obésité et de diabète de type 2? Corresp. Métab. Horm. Diabètes Nutr. 2014, 100, 248–252. [Google Scholar]
- ANSES. Evaluation des Risques du Bisphénol A (BPA) pour la Santé Humaine; Tome 1; Agence Nationale de Sécurité Sanitaire de l’Alimentation, de l’Environnement et du Travail (ANSES): Paris, France, 2013.
- Pop, C.-E.; Draga, S.; Măciucă, R.; Niță, R.; Crăciun, N.; Wolff, R. Bisphenol A Effects in Aqueous Environment on Lemna minor. Processes 2021, 9, 1512. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Ooga, H.; Hirotsu, T.; Wang, W.; Wu, Q.Y.; Hu, H.-Y. Matrix-enhanced adsorption removal of trace BPA by controlling the interlayer hydrophobic environment of montmorillonite. Appl. Clay Sci. 2015, 104, 81–87. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Special Report on Environmental Endocrine Disruption: An Effects Assessment and Analysis; USEPA: Washington, DC, USA, 1997.
- Bautista-Toledo, I.; Ferro-Garcia, M.; Rivera-Utrilla, J.; Moreno-Castilla, C.; Vegas Fernández, F. Bisphenol A removal from water by activated carbon. Effects of carbon characteristics and solution chemistry. Environ. Sci. Technol. 2005, 39, 6246–6250. [Google Scholar] [CrossRef]
- Park, Y.; Sun, Z.; Ayoko, G.A.; Frost, R.L. Bisphenol A sorption by organo-montmorillonite: Implications for the removal of organic contaminants from water. Chemosphere 2014, 107, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Rivas, F.J.; Encinas, Á.; Acedo, B.; Beltran, F.J. Mineralization of bisphenol A by advanced oxidation processes. J. Chem. Technol. Biotechnol. 2009, 84, 589–594. [Google Scholar] [CrossRef]
- Yüksel, S.; Kabay, N.; Yüksel, M. Removal of bisphenol A (BPA) from water by various nanofiltration (NF) and reverse osmosis (RO) membranes. J. Hazard. Mater. 2013, 263, 307–310. [Google Scholar] [CrossRef]
- Cui, Y.-H.; Li, X.-Y.; Chen, G. Electrochemical degradation of bisphenol A on different anodes. Water Res. 2009, 43, 1968–1976. [Google Scholar] [CrossRef]
- Murugananthan, M.; Yoshihara, S.; Rakuma, T.; Shirakashi, T. Mineralization of bisphenol A (BPA) by anodic oxidation with boron-doped diamond (BDD) electrode. J. Hazard. Mater. 2008, 154, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Roddick, F.; Fan, L.; Aziz, H.A. Application of ozone for the removal of bisphenol A from water and wastewater—A review. Chemosphere 2013, 90, 2197–2207. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tian, P.-F.; Zhang, C.; Deng, Y.-Q.; Xu, J.; Gong, J.; Han, Y.-F. Au/carbon as Fenton-like catalysts for the oxidative degradation of bisphenol A. Appl. Catal. B Environ. 2013, 134–135, 145–152. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Li, F.; Zhu, L. Degradation and mineralization of bisphenol A by mesoporous Bi2WO6 under simulated solar light irradiation. Environ. Sci. Technol. 2010, 44, 6843–6848. [Google Scholar] [CrossRef]
- Torres, R.A.; Pétrier, C.; Combet, E.; Moulet, F.; Pulgarin, C. Bisphenol A mineralization by integrated ultrasound-UV-iron (II) treatment. Environ. Sci. Technol. 2007, 41, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Cajthaml, T.; Krӗsinová, Z.; Svobodová, K.; Möder, M. Biodegradation of endocrine-disrupting compounds and suppression of estrogenic activity by ligninolytic fungi. Chemosphere 2009, 75, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T.; Lai, C.-W.; Su, T.-Y. Adsorption of bisphenol-A from aqueous solution onto minerals and carbon adsorbents. J. Hazard. Mater. 2006, 134, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.T.; Hsu, H.C.; Su, T.Y.; Lin, K.Y.; Lin, C.M. Adsorption characteristics of bisphenol-A in aqueous solutions onto hydrophobic zeolitea. J. Colloid Interface Sci. 2006, 299, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhan, Y.; Lin, J.; Jiang, A.; Xi, W. Removal of bisphenol A from aqueous solution using cetylpyridinium bromide (CPB)-modified natural zeolites as adsorbents. Environ. Earth Sci. 2014, 72, 3969–3980. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, D.; Chen, X.; Lin, Y. Adsorption of bisphenol A from water by surfactant-modified zeolite. J. Colloid Interface Sci. 2010, 348, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Sun, Z.; Park, Y.; Ayoko, G.A.; Frost, R.L. Removal of bisphenol A from wastewater by Ca-montmorillonite modified with selected surfactants. Chem. Eng. J. 2013, 234, 416–422. [Google Scholar] [CrossRef] [Green Version]
- Rathnayake, S.I.; Xi, Y.; Frost, R.L.; Ayoko, G.A. Environmental applications of inorganic–organic clays for recalcitrant organic pollutants removal: Bisphenol A. J. Colloid Interface Sci. 2016, 470, 183–195. [Google Scholar] [CrossRef] [Green Version]
- Garikoé, I.; Sorgho, B.; Yaméogo, A.; Guel, B.; Andala, D. Removal of bisphenol A by adsorption on organically modified clays from Burkina Faso. Bioremediat. J. 2021, 25, 22–47. [Google Scholar] [CrossRef]
- Kimura, Y.; Yamamoto, M.; Shimazaki, R.; Kashiwada, A.; Matsuda, K.; Yamada, K. Use of Chitosan for Removal of Bisphenol A from Aqueous Solutions Through Quinone Oxidation by Polyphenol Oxidase. J. Appl. Polym. Sci. 2012, 124, 796–804. [Google Scholar] [CrossRef]
- Dehghani, M.H.; Ghadermazi, M.; Bhatnagar, A.; Sadighara, P.; Jahed-Khaniki, G.; Heibati, B.; McKay, G. Adsorptive removal of endocrine disrupting bisphenol A from aqueous solution using chitosan. J. Environ. Chem. Eng. 2016, 4, 2647–2655. [Google Scholar] [CrossRef]
- Lazim, Z.M.; Salmiati; Hadibarata, T.; Yusop, Z.; Nazifa, T.H.; Abdullah, N.H.; Nuid, M.; Salim, N.A.A.; Zainuddin, N.A.; Ahmad, N. Bisphenol A Removal by Adsorption Using Waste Biomass: Isotherm and Kinetic Studies. Biointerface Res. Appl. Chem. 2021, 11, 8467–8481. [Google Scholar]
- Balarak, D. Kinetics, Isotherm and Thermodynamics Studies on Bisphenol A Adsorption using Barley husk. Inter. J. ChemTech Res. 2016, 9, 681–690. [Google Scholar]
- Olawale Orimolade, B.; Amoo Adekola, F.; Aminat Mohammed, A.; Olayiwola Idris, A.; Damilare Saliu, O.; Yusuf, T. Removal of Bisphenol-A from Aqueous Solution Using Rice Husk Nanosilica: Adsorption Kinetics, Equilibrium and Thermodynamic Studies. J. Appl. Chem. Res. 2018, 12, 8–21. [Google Scholar]
- Şimşek, S.; Ulusoy, H.I. Synthesis of a Useful and Economic Polymeric Material for Effective Removal of Bisphenol A. J. Polym. Environ. 2017, 26, 1605–1612. [Google Scholar] [CrossRef]
- Xiang, Y.; Yan, H.; Zheng, B.; Faheem, A.; Chen, W.; Hu, Y. E. coli@UiO-67 composites as a recyclable adsorbent for bisphenol A removal. Chemosphere 2021, 270, 128672. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Pan, F.; Li, W.; Li, D.; Xu, H.; Xia, D.; Li, A. Enhanced Adsorption of Bisphenol A from Aqueous Solution with 2-Vinylpyridine Functionalized Magnetic Nanoparticles. Polymers 2018, 10, 1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balarak, D.; Ansari, H.; Dashtizadeh, M. Maryam Bazi Synthesis of Graphene Oxide as an Adsorbent for the Removal of Bisphenol A. J. Hum. Environ. Health Promot. 2019, 5, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Adak, A.; Bandyopadhyay, M.; Pal, A. Removal of crystal violet dye from wastewater by surfactant-modified alumina. Sep. Purif. Technol. 2005, 44, 139–144. [Google Scholar] [CrossRef]
- Adak, A.; Pal, A.; Bandyopadhyay, M. Removal of phenol from water environment by surfactant-modified alumina through adsolubilization. Colloids Surf. A Physicochem. Eng. Asp. 2006, 277, 63–68. [Google Scholar] [CrossRef]
- Khobragade, M.; Pal, A. Adsorptive removal of Mn(II) from water and wastewater by surfactant-modified alumina. Desalination Water Treat. 2014, 57, 2775–2786. [Google Scholar] [CrossRef]
- Khobragade, M.U.; Pal, A. Fixed-bed column study on removal of Mn(II), Ni(II) and Cu(II) from aqueous solution by surfactant bilayer supported alumina. Sep. Sci. Technol. 2016, 51, 1287–1298. [Google Scholar] [CrossRef]
- Khobragade, M.U.; Nayak, A.K.; Pal, A. Solid-Phase Extraction of Cu(II) from Aqueous Solution Using Surfactant-Modified Alumina. J. Hazard. Toxic Radioact. Waste 2017, 21, 04016017. [Google Scholar] [CrossRef]
- Dhawale, V.P.; Khobragade, V.; Kulkarni, S.D. Synthesis and Characterization of Aluminium Oxide (Al2O3) Nanoparticles and its Application in Azodye Decolourisation. Int. J. Environ. Chem. 2018, 2, 10. [Google Scholar] [CrossRef] [Green Version]
- Chu, T.P.M.; Nguyen, N.T.; Vu, T.L.; Dao, T.H.; Dinh, L.C.; Nguyen, H.L.; Hoang, T.H.; Le, T.S.; Pham, T.D. Synthesis, Characterization, and Modification of Alumina Nanoparticles for Cationic Dye Removal. Materials 2019, 12, 450. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Dubey, S.; Gautam, R.K.; Chattopadhyaya, M.C.; Sharma, Y.C. Adsorption characteristics of alumina nanoparticles for the removal of hazardous dye, Orange G from aqueous solutions. Arabian J. Chem. 2019, 12, 5339–5354. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Luo, L.; Shi, Y.; Chen, Y.; Wang, S.; Bian, L.; Jiang, F. One-step hydrothermal synthesis of CTAB-modified SiO2 for removal of bisphenol A. Water Sci. Technol. 2017, 76, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Rovani, S.; Santos, J.J.; Guilhen, S.N.; Corio, P.; Fungaro, D.A. Fast, efficient and clean adsorption of bisphenol-A using renewable mesoporous silica nanoparticles from sugarcane waste ash. RSC Adv. 2020, 10, 27706–27712. [Google Scholar] [CrossRef]
- Syarif, D.G.; Prajitno, D.H.; Umar, E. Synthesis of A12O3 Nanoparticles from Local Bauxite for Water-A12O3 Nanofluids egy. J. Phys. Conf. Ser. 2017, 799, 12014. [Google Scholar] [CrossRef]
- Meteab, H.S.; Karem, H.A.; Salih, W.K. Synthesis and characterization of nano gamma aluminium oxide from iraqi bauxite using extraction method. ARPN J. Eng. Appl. Sci. 2018, 13, 814–818. [Google Scholar]
- Isfahani, T.D.; Javadpour, J.; Khavandi, A.; Dinnebier, R.; Goodarzia, M.; Rezaie, H.R. Mechanochemical synthesis of alumina nanoparticles: Formation mechanism and phase transformation. Powder Technol. 2012, 229, 17–23. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Garces, J.M. Hydrothermal synthesis of novel alpha alumina nano-materials with controlled morphologies and high thermal stabilities. CrystEngComm 2010, 12, 2996–3002. [Google Scholar] [CrossRef]
- Behera, P.S.; Sarkar, R.; Bhattacharyya, S. Nano Alumina: A Review of the Powder Synthesis Method. Interceram—Int. Ceram. Rev. 2016, 65, 10–16. [Google Scholar] [CrossRef]
- Zaki, T.; Kabel, K.; Hassan, H. Preparation of high pure α-Al2O3 nanoparticles at low temperatures using Pechini method. Ceram. Int. 2012, 38, 2021–2026. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Manivasakan, P.; Rajendran, V.; Rauta, P.R.; Sahu, B.B.; Panda, B.K. Direct synthesis of nano alumina from natural bauxite. Adv. Mater. Res. 2009, 67, 143–148. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, E.; Kim, T.; Wang, J.; Hableel, G.; Reardon, P.J.T.; Ananthakrishna, S.J.; Wang, T.; Arconada-Alvarez, S.; Knowles, J.C.; et al. Organosilica Nanoparticles with an Intrinsic Secondary Amine: An Efficient and Reusable Adsorbent for Dyes. ACS Appl. Mater. Interfaces 2017, 9, 15566–15576. [Google Scholar] [CrossRef] [PubMed]
- Nayab, S.; Farrukh, A.; Oluz, Z.; Tuncel, E.; Tariq, S.R.; Rahman, H.U.; Kirchhoff, K.; Duran, H.; Yameen, B. Design and Fabrication of Branched Polyamine Functionalized Mesoporous Silica: An Efficient Absorbent for Water Remediation. ACS Appl. Mater. Interfaces 2014, 6, 4408–4417. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.F.; Xiao, B.; Thomas, K.M. Adsorption of Metal Ions on Nitrogen Surface Functional Groups in Activated Carbons. Langmuir 2002, 18, 470–478. [Google Scholar] [CrossRef]
- Vijayakumar, G.; Tamilarasan, R.; Dharmendirakumar, M. Adsorption, Kinetic, Equilibrium and Thermodynamic studies on the removal of basic dye Rhodamine-B from aqueous solution by the use of natural adsorbent perlite. J. Mater. Environ. Sci. 2012, 3, 157–170. [Google Scholar]
- Geyikçi, F. Adsorption of Acid Blue 161 (AB 161) Dye from Water by Multi-walled Carbon Nanotubes. Full-Nanotub. Carbon Nanostructures 2013, 21, 579–593. [Google Scholar] [CrossRef]
- Bawa, S.G.; Ahmed, A.S.; Okonkwo, P.C. The study of Thermal effect on the surface properties of Gamma-Alumina synthesed from Kankara Kaolin. Niger. J. Technol. 2016, 35, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Paranjpe, K.Y. Alpha, Beta and Gamma Alumina as a catalyst—A Review. Pharma Innov. J. 2017, 6, 236–238. [Google Scholar]
- Rodríguez, J.A.; García, M.F. Synthesis, Properties, and Applications of Oxide Nanomaterials; John Wiley & Sons: Hobeken, NJ, USA, 2007. [Google Scholar]
- Rahmanpour, O.; Shariati, A.; Nikou, M.R.K. New Method for Synthesis Nano Size γ-Al2O3 Catalyst for Dehydration of Methanol to Dimethyl Ether. Int. J. Chem. Eng. Appl. 2012, 3, 125–128. [Google Scholar] [CrossRef] [Green Version]
- Yimin, L.; Dianqing, L.; Pinggui, T.; Yongjun, F. A simple and promoter free way to synthesize spherical γ-alumina with high hydrothermal stability. Mater. Lett. 2015, 155, 75–77. [Google Scholar]
- Bazyari, A.; Mortazavi, Y.; Khodadadi, A.A.; Thompson, L.T.; Tafreshi, R.; Zaker, A.; Ajenifujah, O.T. Effects of alumina phases as nickel supports on deep reactive adsorption of (4,6-dimethyl) dibenzothiophene: Comparison between γ, δ, and θ-alumina. Appl. Catal. B Environ. 2016, 180, 312–323. [Google Scholar] [CrossRef]
- Ysla, F.A.; Martins, A.R.; Rodrigo Estevam, C.; Cesário, F.d.V.; Adriana, D.B.; Carvalho, L.S. A Simple Way to Produce γ-Alumina From Aluminum Cans by Precipitation Reactions. Mater. Res. 2016, 19, 977–982. [Google Scholar]
- Khosla, E.; Kaur, S.; Dave, P.N. Mechanistic study of adsorption of acid orange-7 over aluminum oxide nanoparticles. J. Eng. 2013, 2013, 593534. [Google Scholar] [CrossRef] [Green Version]
- Sharma, Y.C.; Srivastava, V.; Upadhyay, S.N.; Weng, C.H. Alumina nanoparticles for the removal of Ni(II) from aqueous solutions. Ind. Eng. Chem. Res. 2008, 47, 8095–8100. [Google Scholar] [CrossRef]
- Afkhami, A.; Saber-Tehrani, M.; Bagheri, H. Simultaneous removal of heavy-metal ions in wastewater samples using nano-alumina modified with 2,4-dinitrophenylhydrazine. J. Hazard. Mater. 2010, 181, 836–844. [Google Scholar] [CrossRef]
- Al-Rubayee, W.T.; Abdul-Rasheed, O.F.; Ali, N.M. Preparation of a Modified Nanoalumina Sorbent for the Removal of Alizarin Yellow R and Methylene Blue Dyes from Aqueous Solutions. J. Chem. 2016, 2016, 4683859. [Google Scholar] [CrossRef]
- Li, W.; Cao, C.Y.; Wu, L.Y.; Ge, M.F.; Song, W.G. Superb fluoride and arsenic removal performance of highly ordere mesoporous aluminas. J. Hazard. Mater. 2011, 198, 143–150. [Google Scholar] [CrossRef]
- Srivastava, V.; Weng, C.H.; Singh, V.K.; Sharma, Y.C. Adsorption of nickel ions from aqueous solutions by nano alumina: Kinetic, mass transfer, and equilibrium studies. J. Chem. Eng. Data 2011, 56, 1414–1422. [Google Scholar] [CrossRef]
- Pham, T.D.; Kobayashi, M.; Adachi, Y. Interfacial characterization of α-alumina with small surface area by streaming potential and chromatography. Colloids Surf. A 2013, 436, 148–157. [Google Scholar] [CrossRef]
- Dovi, E.; Kani, A.N.; Aryee, A.A.; Jie, M.; Li, J.; Li, Z.; Li, Q.; Han, R. Decontamination of bisph en ol A and Congo red dye from solution by using CTAB functionalised walnut shell. Environ. Sci. Pollut. Res. 2021, 28, 28732–28749. [Google Scholar] [CrossRef]
- Royer, B.; Cardoso, N.F.; Lima, E.C.; Vaghetti, J.C.P.; Simon, N.M.; Calvete, T.; Veses, R.C. Applications of Brazilian pine-fruit shell in natural and carbonized forms as adsorbents to removal of methylene blue from aqueous solutions—kinetic and equilibrium study. J. Hazard. Mater. 2009, 164, 1213–1222. [Google Scholar] [CrossRef]
- Makhlouf, M.; Hamacha, R.; Villièras, F.; Bengueddach, A. Kinetics and thermodynamics adsorption of phenolic compounds on organic-inorganic hybrid mesoporous material. Int. J. Innov. Appl. Stud. 2013, 3, 1116–1124. [Google Scholar]
- Tavlieva, M.P.; Genieva, S.D.; Georgieva, V.G.; Vlaev, L.T. Kinetic study of brilliant green adsorption from aqueous solution onto white rice husk ash. J. Colloid Interface Sci. 2013, 409, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, L.; Zhu, Y. Decontamination of Bisphenol A from Aqueous Solution by Graphene Adsorption. Langmuir 2012, 28, 8418–8425. [Google Scholar] [CrossRef]
- Zhao, Z.; Fu, D.; Ma, Q. Adsorption Characteristics of Bisphenol A from Aqueous Solution onto HDTMAB-Modified Palygorskite. Sep. Sci. Technol. 2014, 49, 81–89. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Jiang, J.-Q.; Ma, X. Adsorption of bisphenol A onto cationic-modified zeolite. Desalination Water Treat. 2016, 57, 26299–26306. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Gao, M.; Luo, Z.; Yang, S. Enhanced removal of bisphenol A from aqueous solution by organo-montmorillonites modified with novel Gemini pyridinium surfactants containing long alkyl chain. Chem. Eng. J. 2016, 285, 27–38. [Google Scholar] [CrossRef]
- Li, Y.; Jin, F.; Wang, C.; Chen, Y.; Wang, Q.; Zhang, W.; Wang, D. Modification of bentonite with cationic surfactant for the enhanced retention of bisphenol A from landfill leachate. Environ. Sci. Pollut. Res. 2015, 22, 8618–8628. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Anastopoulos, I. Adsorptive removal of bisphenol A (BPA) from aqueous solution: A review. Chemosphere 2017, 168, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, P.; Zhu, Y.; Tran, L. Simultaneous adsorption of Cd2+ and BPA on amphoteric surfactant activated montmorillonite. Chemosphere 2016, 144, 1026–1032. [Google Scholar] [CrossRef]
- Vithanage, M.; Mayakaduwa, S.; Herath, I.; Ok, Y.S.; Mohan, D. Kinetics, thermodynamics and mechanistic studies of carbofuran removal using biochars from tea waste and rice husks. Chemosphere 2016, 150, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, A.A.; Adeoye, I.O.; Bello, O.S. Adsorption of dyes using different types of clay: A review. Appl. Water Sci. 2017, 7, 543–568. [Google Scholar] [CrossRef] [Green Version]
- Gorzin, F.; Abadi, M.B.R. Adsorption of Cr(VI) from aqueous solution by adsorbent prepared from paper mill sludge: Kinetics and thermodynamics studies. Adsorpt. Sci. Technol. 2018, 36, 149–169. [Google Scholar] [CrossRef]
- Liu, S.; Wu, P.; Chen, M.; Yu, L.; Kang, C.; Zhu, N.; Dang, Z. Amphoteric modified vermiculites as adsorbents for enhancing removal of organic pollutants: Bisphenol A and Tetrabromobisphenol A. Environ. Pollut. 2017, 228, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.H.; Tan, I.A.W.; Ahmad, A.L. Adsorption isotherm, kinetic modeling and mechanism of 2,4,6-trichlorophenol on coconut husk-based activated carbon. Chem. Eng. J. 2008, 144, 235–244. [Google Scholar] [CrossRef]
- Dayane, J.A.; Hélen, C.R.; Érica, L.O.; Ione, L.S.A.; Nívia, M.M.C.; Túlio, N.M.; Cleide, S.T.A. Characterization of Pequi (Caryocar brasiliense) Shells and Evaluation of Their Potential for the Adsorption of Pb(II) Ions in Aqueous Systems. J. Braz. Chem. Soc. 2016, 27, 616–623. [Google Scholar]
- Hind, A.R.; Bhargava, S.K.; McKinnon, A. At the solid/liquid interface: FTIR/ATR—The tool of choice. Adv. Colloid Interface Sci. 2001, 93, 91–114. [Google Scholar] [CrossRef]




| Oxides | Al2O3 | SiO2 | Fe2O3 | TiO2 | P2O5 | CaO | V2O5 | Na2O | Cr2O3 | MgO | MnO | K2O | ZnO | L.O.I: | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 61.66 | 25.44 | 5.71 | 4.00 | 0.15 | 0.06 | 0.06 | 0.05 | 0.04 | 0.04 | 0.03 | 0.01 | 0.006 | 2.74 | 99.94 |
| Oxides | Al2O3 | SiO2 | Fe2O3 | P2O5 | V2O5 | Na2O | MgO | K2O | ZnO |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | 88.00 | 5.101 | 0.100 | 0.170 | 0.020 | 0.090 | 0.020 | 0.010 | 0.005 |
| Sorbent | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| K1 (min−1) | Qe1 (mg/g) | R2 | K2 (min−1) | Qe2 (g/mg · min) | R2 | |
| γANPs-CTAB | 0.0093 | 0.0557 | 0.1865 | 0.0673 | 1.3959 | 0.9907 |
| Sorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| KL (L/mg) | Qm (mg/g) | R2 | KF (L/g) | n | R2 | |
| γANPs-CTAB | 0.0403 | 17.4825 | 0.9201 | 1.5324 | 2.1786 | 0.8077 |
| [BPA] mg/L | 10 | 20 | 30 | 40 | 60 | 70 | 80 | 90 | 100 | 200 | 300 | 400 | 500 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RL | 0.712 | 0.553 | 0.452 | 0.382 | 0.292 | 0.261 | 0.236 | 0.216 | 0.198 | 0.110 | 0.076 | 0.058 | 0.047 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kam, O.R.; Garikoe, I.; Bakouan, C.; Guel, B. Low-Cost Synthesis of Alumina Nanoparticles and Their Usage for Bisphenol-A Removal from Aqueous Solutions. Processes 2021, 9, 1709. https://doi.org/10.3390/pr9101709
Kam OR, Garikoe I, Bakouan C, Guel B. Low-Cost Synthesis of Alumina Nanoparticles and Their Usage for Bisphenol-A Removal from Aqueous Solutions. Processes. 2021; 9(10):1709. https://doi.org/10.3390/pr9101709
Chicago/Turabian StyleKam, Ollé Rodrigue, Issaka Garikoe, Corneille Bakouan, and Boubié Guel. 2021. "Low-Cost Synthesis of Alumina Nanoparticles and Their Usage for Bisphenol-A Removal from Aqueous Solutions" Processes 9, no. 10: 1709. https://doi.org/10.3390/pr9101709









