Mulberry Leaf and Radix Astragali Regulates Differentially Expressed Genes and Proteins in the Streptozotocin-Induced Diabetic Mice Liver
Abstract
:1. Introduction
2. Materials and Methods
2.1. Intragastric Administration of Mulberry Leaf and Radix Astragali in Diabetic Mice
2.2. Transcriptome Sequencing of Mice Liver
2.3. Proteomic Analysis of Mice Liver
2.4. Gene Ontology (GO) and KEGG Enrichment Analysis of Targets
2.5. Protein–Protein Interaction Network Construction
2.6. Test and Verification DEGs by qRT-PCR
2.7. Antibody Preparation and Western Blot Analysis
3. Results
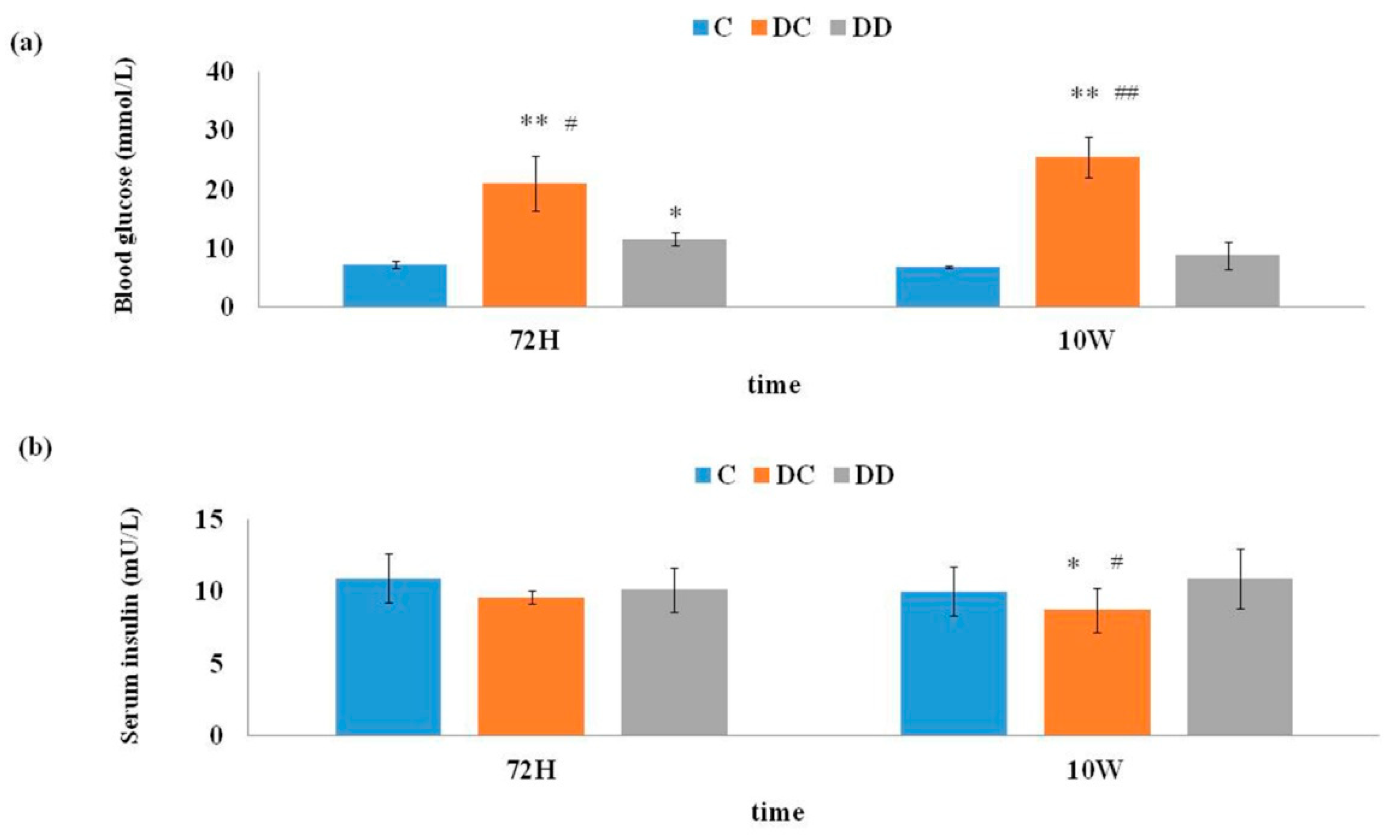
3.1. Hypoglycemic Effect of Mulberry Leaf Combined with Astragali on Diabetic Mice
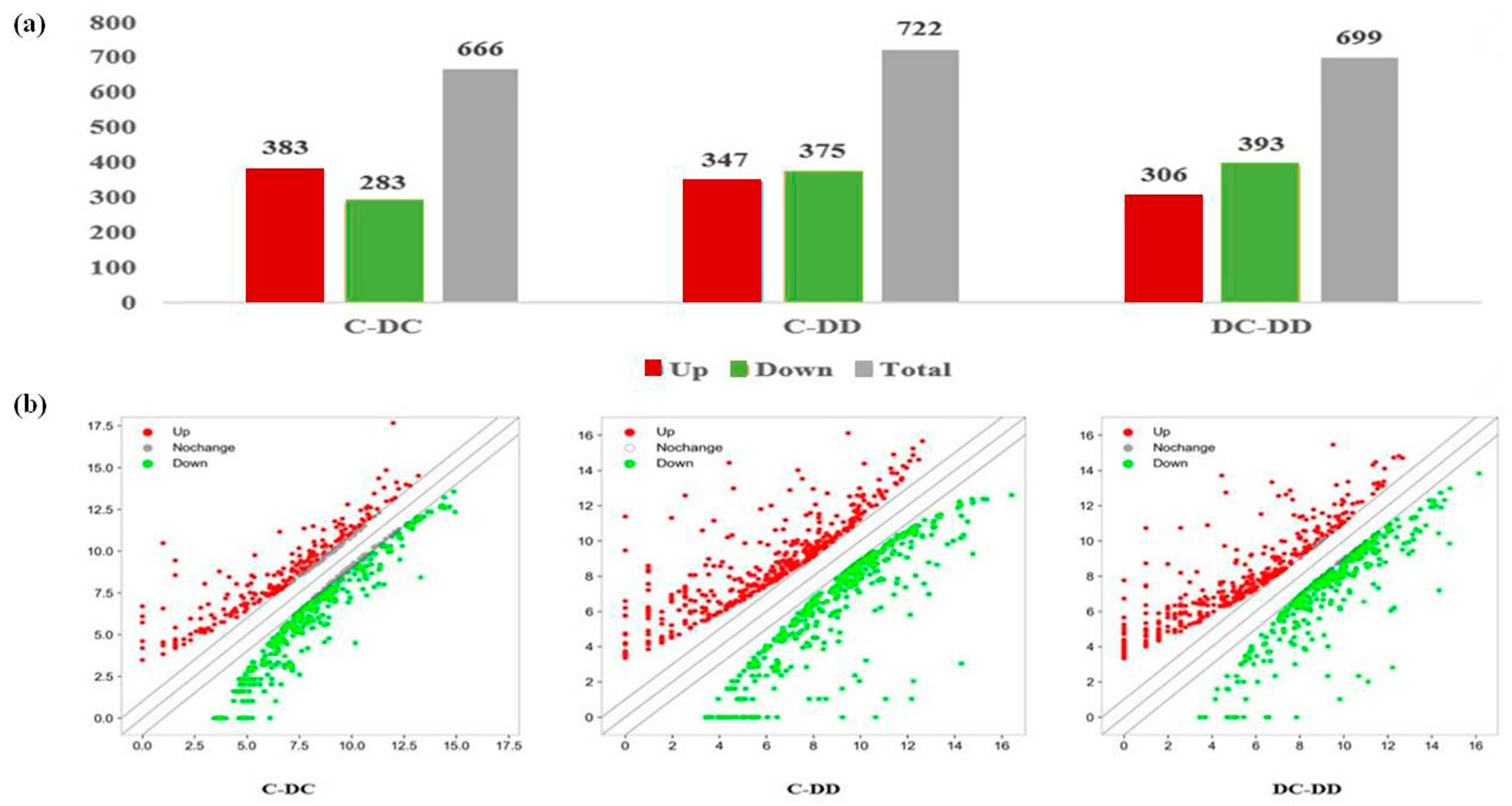
3.2. Analysis of Differential Genes and Protein Expression in Three Mouse Liver Tissues
3.2.1. Differential Gene Expression Changes
3.2.2. Differential Protein Expression Changes
3.2.3. Differential Gene and Protein Expression by Venn Analysis
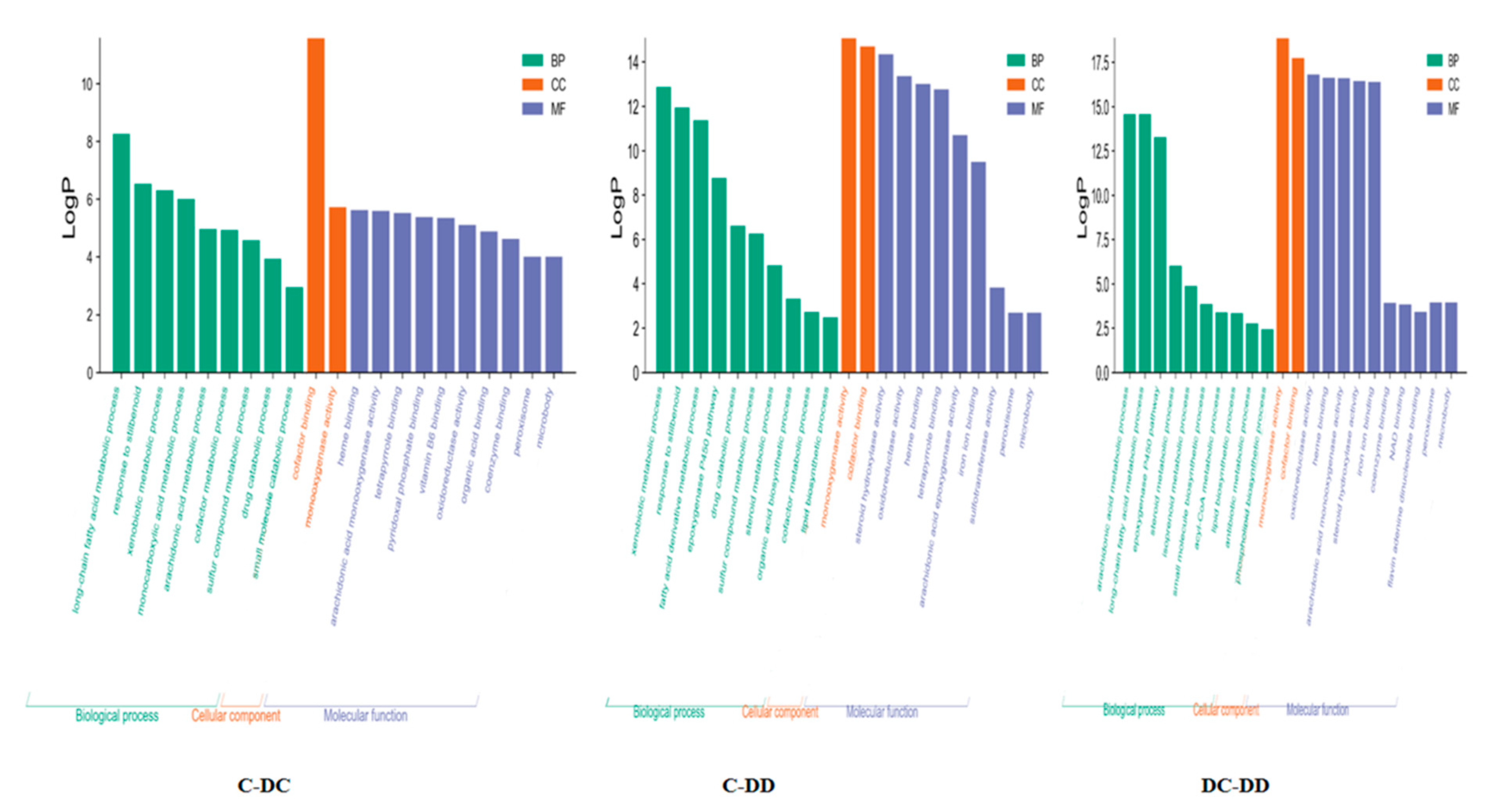
3.3. Go Enrichment Analysis of Common Differential Genes in Transcriptome and Proteome of Three Sample Groups
3.4. KEGG Annotation and Enrichment Analysis of Common Differential Genes in Transcriptome and Proteome of Three Sample Groups
3.5. Analysis of Protein–Protein Interaction
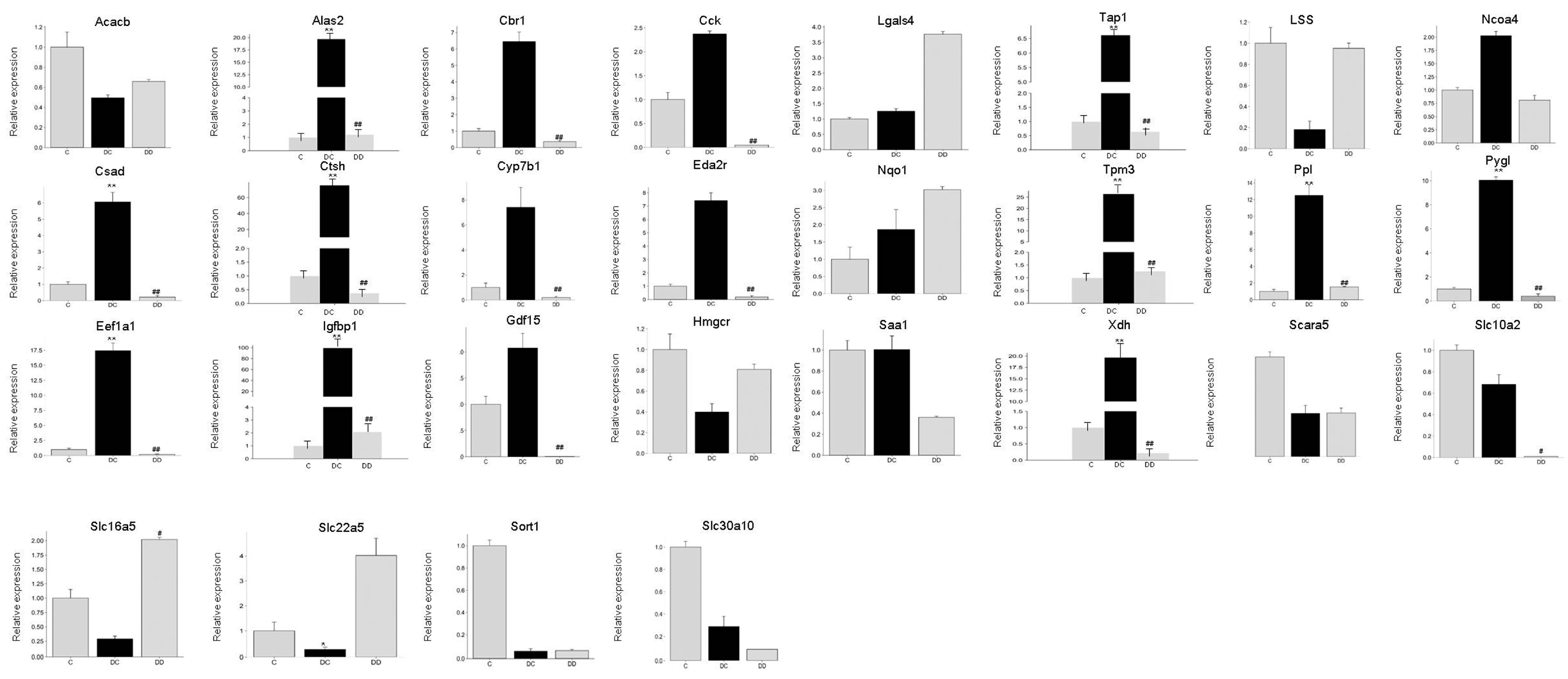
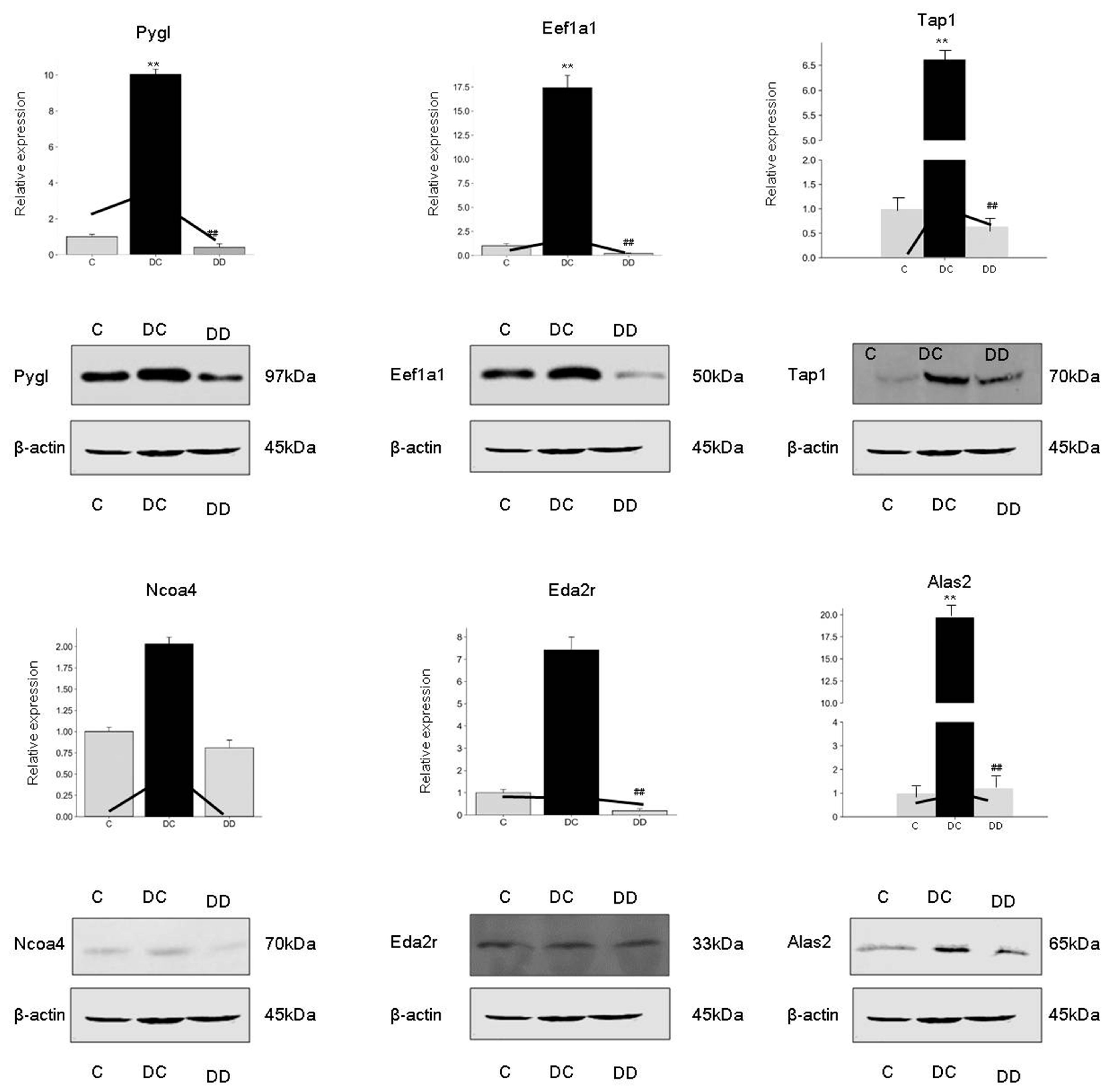
3.6. Identification of DEGs by qRT-PCR
3.7. Western Blot Validates Differentially Expressed Proteins
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Teng, D.; Shi, X.; Qin, G.; Qin, Y.; Quan, H.; Shi, B.; Sun, H.; Ba, J.; Chen, B.; et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: National cross sectional study. BMJ 2020, 369, m997. [Google Scholar] [CrossRef]
- Zhou, L.; Liao, Y.; Yin, P.; Zeng, Z.; Li, J.; Lu, X.; Zheng, L.; Xu, G. Metabolic profiling study of early and late recurrence of hepatocellular carcinoma based on liquid chromatography-mass spectrometry. J. Chromatogr. B 2014, 966, 163–170. [Google Scholar] [CrossRef]
- Shen, X.; Vaidya, A.; Wu, S.; Gao, X. The diabetes epidemic in china: An integrated review of national surveys. Endocr. Pract. 2016, 22, 1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Li, X.; Xie, C.; Luo, X.; Bao, Y.; Wu, B.; Hu, Y.; Zhong, Z.; Liu, C.; Li, M.J. Prevention effects and possible molecular mechanism of Mulberry Leaf extract and its formulation on rats with Insulin-Insensitivity. PLoS ONE 2016, 11, e152728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ty, C.; Preston, T.R.; Ly, J. The use of ensiled cassava leaves in diets for growing pigs. 2. The influence of type of palm oil and cassava leaf maturity on digestibility and N balance for growing pigs. Livest. Res. Rural Dev. 2003, 15, 25–37. [Google Scholar]
- Wang, G.Q.; Zhu, L.; Ma, M.L.; Chen, X.C.; Gao, Y.; Yu, T.Y.; Yang, G.S.; Pang, W.J. Mulberry 1-Deoxynojirimycin inhibits adipogenesis by repression of the ERK/PPARγ signaling pathway in porcine intramuscular adipocytes. J. Agric. Food Chem. 2015, 63, 6212. [Google Scholar] [CrossRef]
- Li, Q.; Dai, Y.; Zou, Y.; Liao, S.; Shen, W.; Hu, T.; Liu, F. Mulberry (Morus atropurpurea Roxb.) leaf polyphenols inhibits adipogenesis and lipogenesis-related gene expression in 3T3-L1 adipocytes. J. Food Biochem. 2018, 42, e12599. [Google Scholar] [CrossRef]
- Wei, F.M.; Chen, C.; Chao, L.I.; Xiong, F.U. Antioxidant and hypoglycemic activities of mulberry leaves extract, tea polyphenols and their compounds. Sci. Technol. Food Ind. 2018, 39, 299–305. [Google Scholar]
- Hong, H.D.; Wen, J.M.; Chen, Z.J. The Research Progress on Pharmacological Effects of Astragalus Membranaceus. World Latest Med. Inf. 2016, 14, 49–50, 69. [Google Scholar]
- Ma, X.Q.; Shi, Q.; Duan, J.A.; Dong, T.T.X.; Tsim, K.W.K. Chemical analysis of Radix Astragali (Huangqi) in China: A comparison with its adulterants and seasonal variations. J. Agric. Food Chem. 2002, 50, 4861–4866. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, Y.; Song, F.; Liu, Z.; Liu, S. Studies on principal components and antioxidant activity of different Radix Astragali samples using high-performance liquid chromatography/electrospray ionization multiple-stage tandem mass spectrometry. Talanta 2009, 78, 1090–1101. [Google Scholar] [CrossRef]
- Qi, L.W.; Cao, J.; Ping, L.; Yu, Q.T.; Wen, X.D.; Wang, Y.X.; Li, C.Y.; Bao, K.D.; Ge, X.X.; Cheng, X.L. Qualitative and quantitative analysis of Radix Astragali products by fast high-performance liquid chromatography-diode array detection coupled with time-of-flight mass spectrometry through dynamic adjustment of fragmentor voltage. J. Chromatogr. A 2008, 1203, 27–35. [Google Scholar] [CrossRef]
- Lin, Y.W.; Chiang, B.H. Anti-tumor activity of the fermentation broth of Cordyceps militaris cultured in the medium of Radix Astragali. Process. Biochem. 2008, 43, 244–250. [Google Scholar] [CrossRef]
- Hoo, R.L.; Wong, J.Y.; Qiao, C.F.; Xu, A.; Xu, H.X.; Lam, K.S. The effective fraction isolated from Radix Astragali alleviates glucose intolerance, insulin resistance and hypertriglyceridemia in db/db diabetic mice through its anti-inflammatory activity. Nutr. Metab. 2010, 7, 67. [Google Scholar] [CrossRef] [Green Version]
- Jia, R.; Cao, L.; Xu, P.; Jeney, G.; Yin, G. In vitro and in vivo hepatoprotective and antioxidant effects of Astragalus polysaccharides against carbon tetrachloride-induced hepatocyte damage in common carp (Cyprinus carpio). Fish Physiol. Biochem. 2012, 38, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.P.; Hingorani, A.D.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Ference, B.A.; Robinson, J.G.; Brook, R.D.; Catapano, A.L.; Sabatine, M.S. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N. Engl. J. Med. 2016, 375, 2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kathiresan, S. Developing medicines that mimic the natural successes of the human genome: Lessons from NPC1L1, HMGCR, PCSK9, APOC3, and CETP. J. Am. Coll. Cardiol. 2015, 65, 1562–1566. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.L.; Lan, T.; Tan, M.S.; Wang, H.F.; Yu, J.T. Association of HMGCR polymorphism with late-onset Alzheimer’s disease in Han Chinese. Oncotarget 2016, 7, 22746–22751. [Google Scholar] [CrossRef] [Green Version]
- Agellon, L.B.; Drover, V.; Cheema, S.K.; Gbaguidi, G.F.; Walsh, A. Dietary cholesterol fails to stimulate the human cholesterol 7α-Hydroxylase gene (CYP7A1) in transgenic mice. J. Biol. Chem. 2002, 277, 20131–20134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, D.J.; Campos, J.A.; Gil, G.; Osborne, T.F. PGC-1α activates CYP7A1 and bile acid biosynthesis. J. Biol. Chem. 2003, 278, 50047. [Google Scholar] [CrossRef] [Green Version]
- Pullinger, C.R.; Eng, C.; Salen, G.; Shefer, S.; Batta, A.K.; Erickson, S.K.; Verhagen, A.; Rivera, C.R.; Mulvihill, S.J.; Malloy, M.J. Human cholesterol 7α-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J. Clin. Investig. 2002, 110, 109–117. [Google Scholar] [CrossRef]
- Koukouritaki, S.B.; Simpson, P.; Yeung, C.K.; Rettie, A.E.; Hines, R.N. Human hepatic flavin-containing monooxygenases 1 (FMO1) and 3 (FMO3) developmental expression. Pediatr. Res. 2002, 51, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Klapper, M.; Böhme, M.; Nitz, I.; Döring, F. The human intestinal fatty acid binding protein (hFABP2) gene is regulated by HNF-4α. Biochem. Biophys. Res. Commun. 2007, 356, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Takakura, Y.; Yoshioka, K.; Umekawa, T.; Kogure, A.; Toda, H.; Yoshikawa, T.; Yoshida, T. Thr54 allele of the FABP2 gene affects resting metabolic rate and visceral obesity. Diabetes Res. Clin. Pract. 2005, 67, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Formanack, M.L.; Baier, L.J. Variation in the FABP2 promoter affects gene expression: Implications for prior association studies. Diabetologia 2004, 47, 349–351. [Google Scholar]
- Venkatachalam, A.B.; Sawler, D.L.; Wright, J.M. Tissue-specific transcriptional modulation of fatty acid-binding protein genes, fabp2, fabp3 and fabp6, by fatty acids and the peroxisome proliferator, clofibrate, in zebrafish (Danio rerio). Gene 2013, 520, 14–21. [Google Scholar] [CrossRef]
- Weiss, E.P.; Brandauer, J.; Kulaputana, O.; Ghiu, I.A.; Wohn, C.R.; Phares, D.A.; Shuldiner, A.R.; Hagberg, J.M. FABP2 Ala54Thr genotype is associated with glucoregulatory function and lipid oxidation after a high-fat meal in sedentary nondiabetic men and women. Am. J. Clin. Nutr. 2007, 85, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Gaudet, R.; Wiley, D.C. Structure of the ABC ATPase domain of human TAP1, the transporter associated with antigen processing. EMBO J. 2014, 20, 4964–4972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallfelz, M.; Jung, D.; Hilmes, C.; Knuth, A.; Seliger, B. Induction of immunogenicity of a human renal-cell carcinoma cell line by TAP1-gene transfer. Int. J. Cancer 2015, 81, 125–133. [Google Scholar] [CrossRef]
- Shen, L.; Chiang, A.; Liu, W.; Li, G.; Liang, R.; Srivastava, G. Expression of HLA class I, beta(2)-microglobulin, TAP1 and IL-10 in Epstein-Barr virus-associated nasal NK/T-cell lymphoma: Implications for tumor immune escape mechanism. Int. J. Cancer 2015, 92, 692–696. [Google Scholar] [CrossRef]
- Wagener, F.; Toonen, E.; Wigman, L.; Fransen, J.; Russel, F. HMOX1 promoter polymorphism modulates the relationship between disease activity and joint damage in rheumatoid arthritis. Arthritis Rheum. 2010, 58, 3388–3393. [Google Scholar] [CrossRef]
- Walther, M.; Caul, A.D.; Aka, P.; Njie, M.; Amambua-Ngwa, A.; Walther, B.; Predazzi, I.M.; Cunnington, A.; Deininger, S.; Takem, E.N.; et al. HMOX1 gene promoter alleles and high HO-1 levels are associated with severe malaria in gambian children. PLoS Pathog. 2012, 8, e1002579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keenan, B.T.; Chibnik, L.B.; Cui, J.; Ding, B.; Padyukov, L.; Kallberg, H.; Bengtsson, C.; Klareskog, L.; Alfredsson, L.; Karlson, E.W. Effect of interactions of glutathione S-transferase T1, M1, and P1 and HMOX1 gene promoter polymorphisms with heavy smoking on the risk of rheumatoid arthritis. Arthritis Rheum. 2010, 62, 3196–3210. [Google Scholar] [CrossRef]
- Curjuric, I.; Imboden, M.; Schindler, C.; Downs, S.H.; Probst-Hensch, N.M. HMOX1 and GST variants modify attenuation of FEF25-75% decline due to PM10 reduction. Eur. Respir. J. 2010, 35, 505–514. [Google Scholar] [CrossRef]
- Leaf, D.E.; Rajapurkar, M.; Lele, S.S.; Mukhopadhyay, B.; Boerger, E.A.; Mc Causland, F.R.; Eisenga, M.F.; Singh, K.; Babitt, J.L.; Kellum, J.A.; et al. Iron, hepcidin, and death in human AKI. J. Am. Soc. Nephrol. 2019, 30, 493–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, A.; Worwood, M. Iron and sudden infant death syndrome. BMJ 2019, 298, 1248, (Clinical research ed.). [Google Scholar] [CrossRef] [Green Version]
- Nakmura, T.; Naguro, I.; Ichijo, H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta (BBA) Gen. Subj. 2019, 1863, 1398–1409. [Google Scholar] [CrossRef] [PubMed]
- Martin, V.; Distefano, A.; Dixon, S.; Bellido, A.; Soto, D.; Cordoba, J.P.; D’Ippolito, S.; Colman, S.; Bartoli, C.; Fiol, D. An iron dependent death pathway involved in the response of plants to biotic and abiotic stresses. Biocell 2014, 38, 80. [Google Scholar]
- Tang, H.M.; Cheung, P. Gallic acid triggers Iron-Dependent cell death with apoptotic, ferroptotic, and necroptotic features. Toxins 2019, 11, 492. [Google Scholar] [CrossRef] [Green Version]
- Perring, J.; Crawshay-Williams, F.; Huang, C.; Townley, H.E. Bio-inspired melanin nanoparticles induce cancer cell death by iron adsorption. J. Mater. Sci. Mater. Med. 2018, 29, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robb, S.J.; Robb-Gaspers, L.D.; Scaduto, J.; Thomas, A.P.; Connor, J.R. Influence of calcium and iron on cell death and mitochondrial function in oxidatively stressed astrocytes. J. Neurosci. Res. 2015, 55, 674–686. [Google Scholar] [CrossRef]
- Gil, L.D.; Kyoung, K.M.; Min, K.K.; Seop, K.H.; Oh-Shin, K.; Hyun-Shik, L.; Dong-Seok, L. Peroxiredoxin 5 prevents iron overload-induced neuronal death by inhibiting mitochondrial fragmentation and endoplasmic reticulum stress in mouse hippocampal HT-22 cells. Int. J. Biochem. Cell Biol. 2018, 102, 10–19. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Ge, Q.; Chen, L.; Chen, K. Mulberry Leaf and Radix Astragali Regulates Differentially Expressed Genes and Proteins in the Streptozotocin-Induced Diabetic Mice Liver. Processes 2021, 9, 1898. https://doi.org/10.3390/pr9111898
Zhang S, Ge Q, Chen L, Chen K. Mulberry Leaf and Radix Astragali Regulates Differentially Expressed Genes and Proteins in the Streptozotocin-Induced Diabetic Mice Liver. Processes. 2021; 9(11):1898. https://doi.org/10.3390/pr9111898
Chicago/Turabian StyleZhang, Shu, Qi Ge, Liang Chen, and Keping Chen. 2021. "Mulberry Leaf and Radix Astragali Regulates Differentially Expressed Genes and Proteins in the Streptozotocin-Induced Diabetic Mice Liver" Processes 9, no. 11: 1898. https://doi.org/10.3390/pr9111898





