Optimized Organosolv Pretreatment of Biomass Residues Using 2-Methyltetrahydrofuran and n-Butanol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomasses
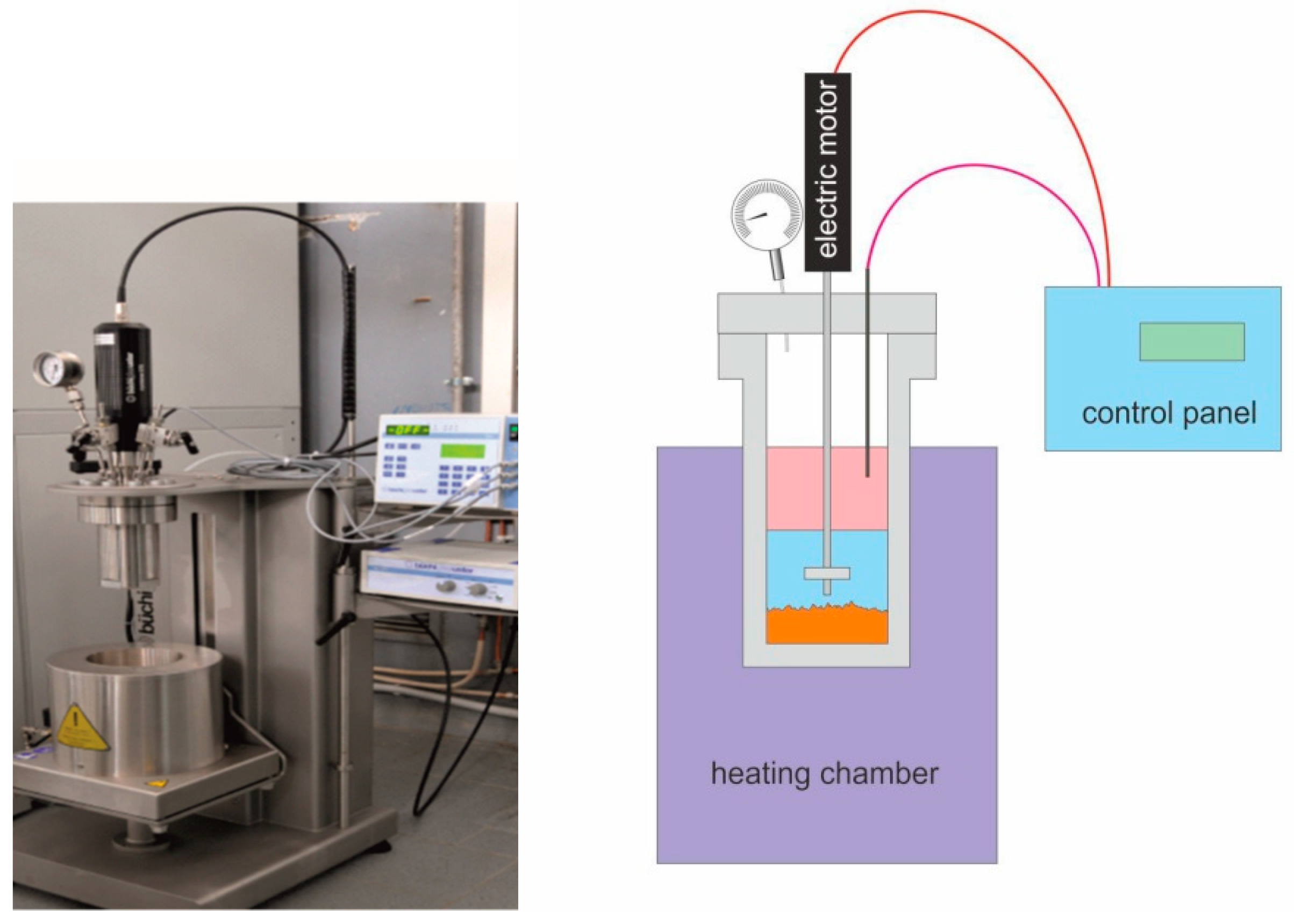
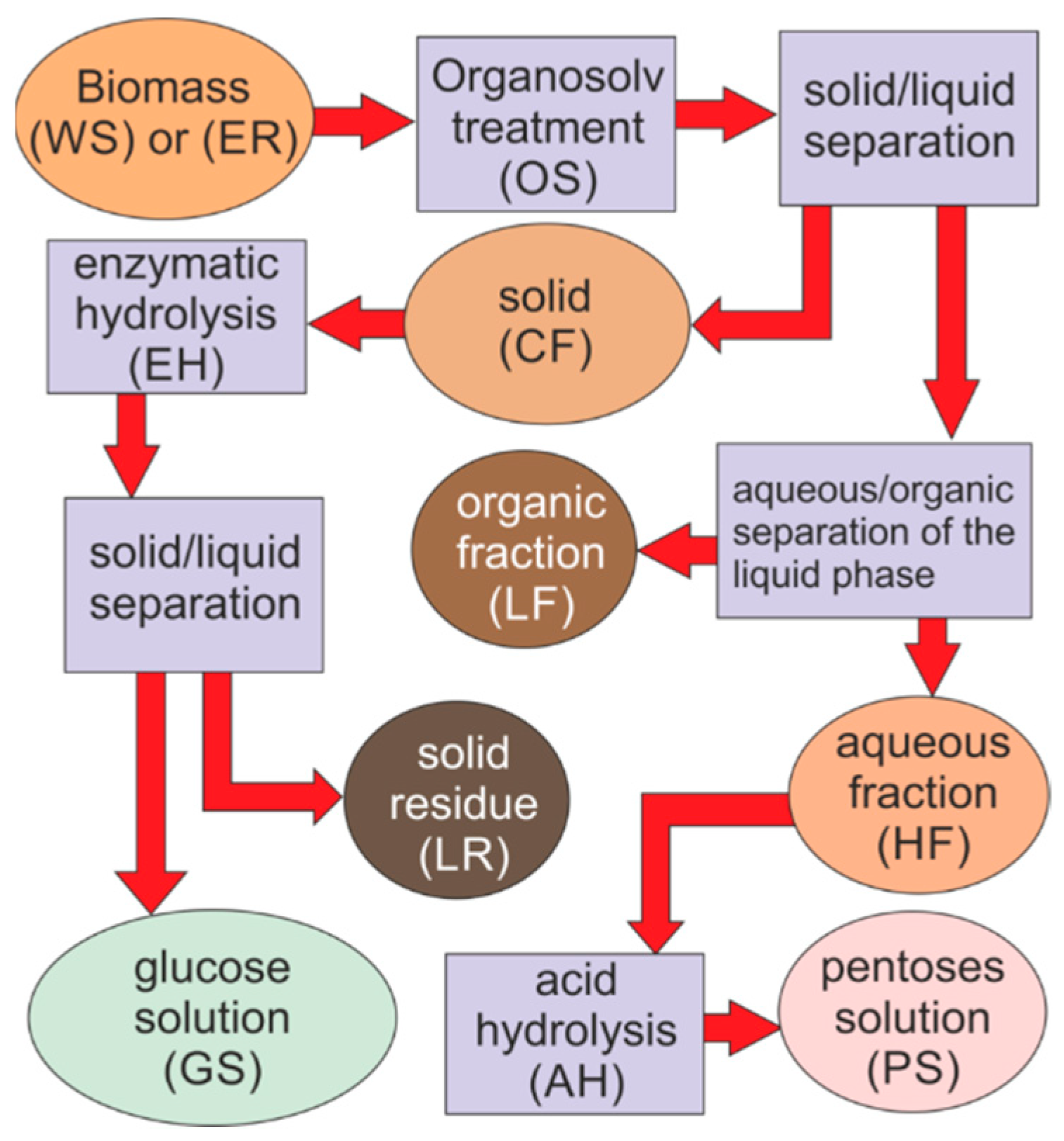
2.2. OrganoSolv (OS) Pretreatment and Fractionating
2.3. Design of Experiments (DOE)
- 1.
- Temperature, in the range 140–180 °C;
- 2.
- Time, in the range 30–90 min;
- 3.
- Catalyst (oxalic acid), in the range of 0–10 as wt.% respect the dry biomass.
| Study Type: | Response Surface; |
| Subtype: | Randomized; |
| Design Type: | Box-Behnken; |
| Runs: | 15 (3 center points); |
| Design Model: | Quadratic. |
| Study Type: | Response Surface; |
| Subtype: | Randomized; |
| Design Type: | Central Composite; |
| Runs: | 10 (2 center points); |
| Design Model: | Quadratic. |
- CF purity (i.e., the mass percentage of glucan in the CF)
- HF purity (i.e., the mass percentage of carbohydrates in HF)
- Glucose yield (the produced glucose compared to the glucan content in raw feedstock, as a percentage on the theoretical quantitative hydrolysis).
2.4. Enzymatic Hydrolysis
2.5. Analytical Methods
3. Results and Discussion
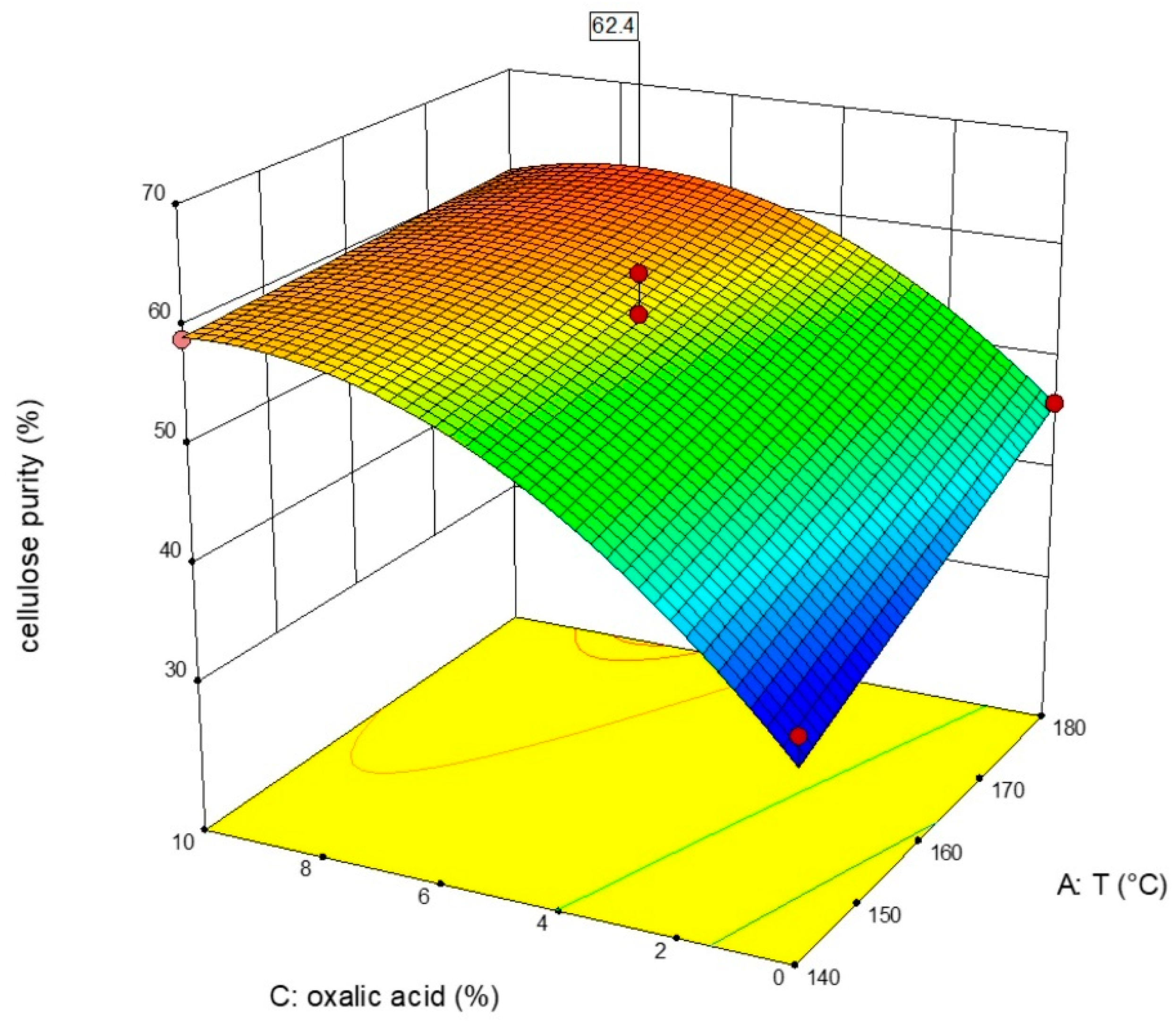
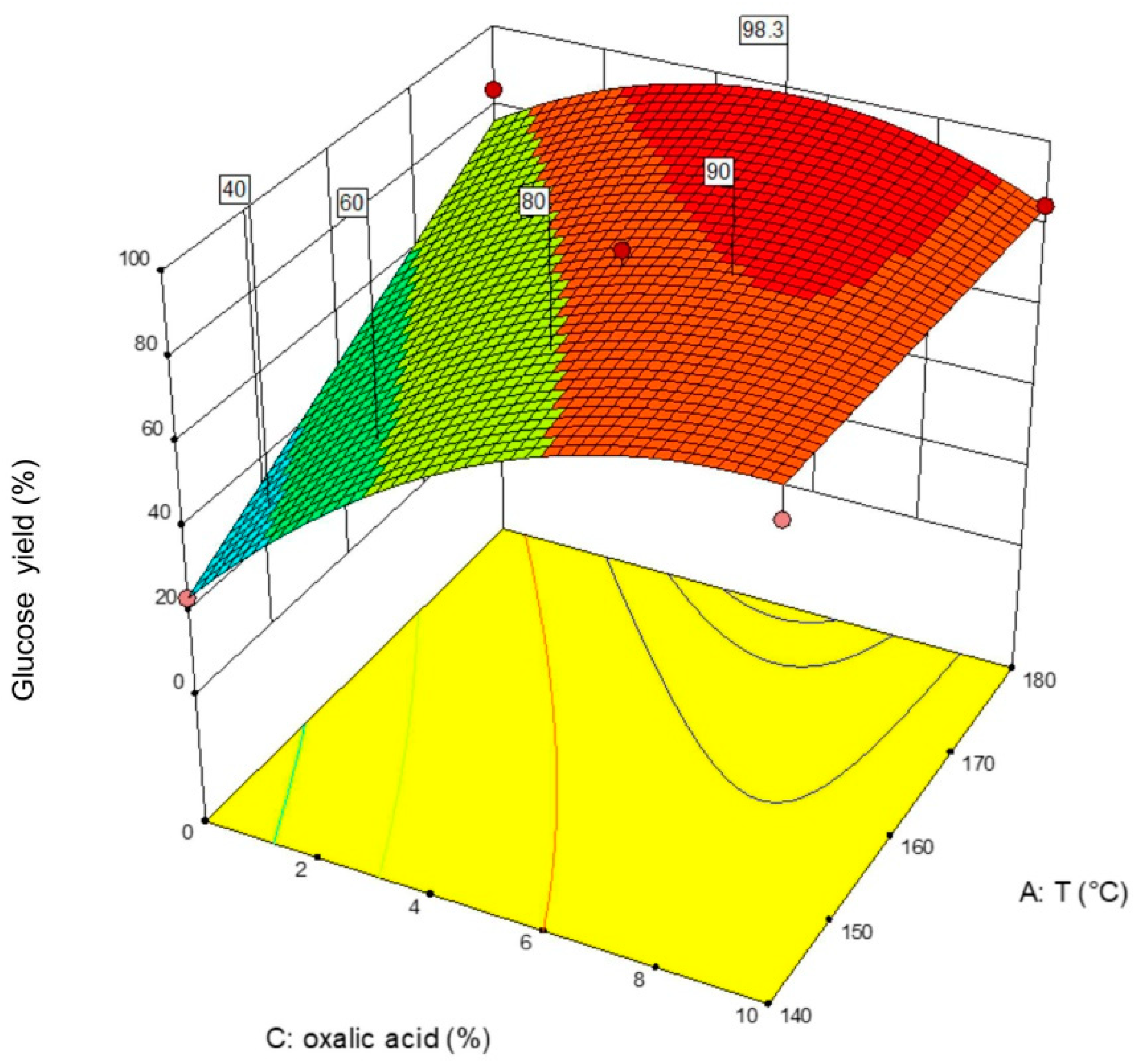
3.1. DOE Analysis of WS Pretreated with Butanol
3.2. DOE Analysis for ER Pretreated with Butanol
3.3. DOE Analysis of WS Pretreated with 2M-THF OS
3.4. Optimization
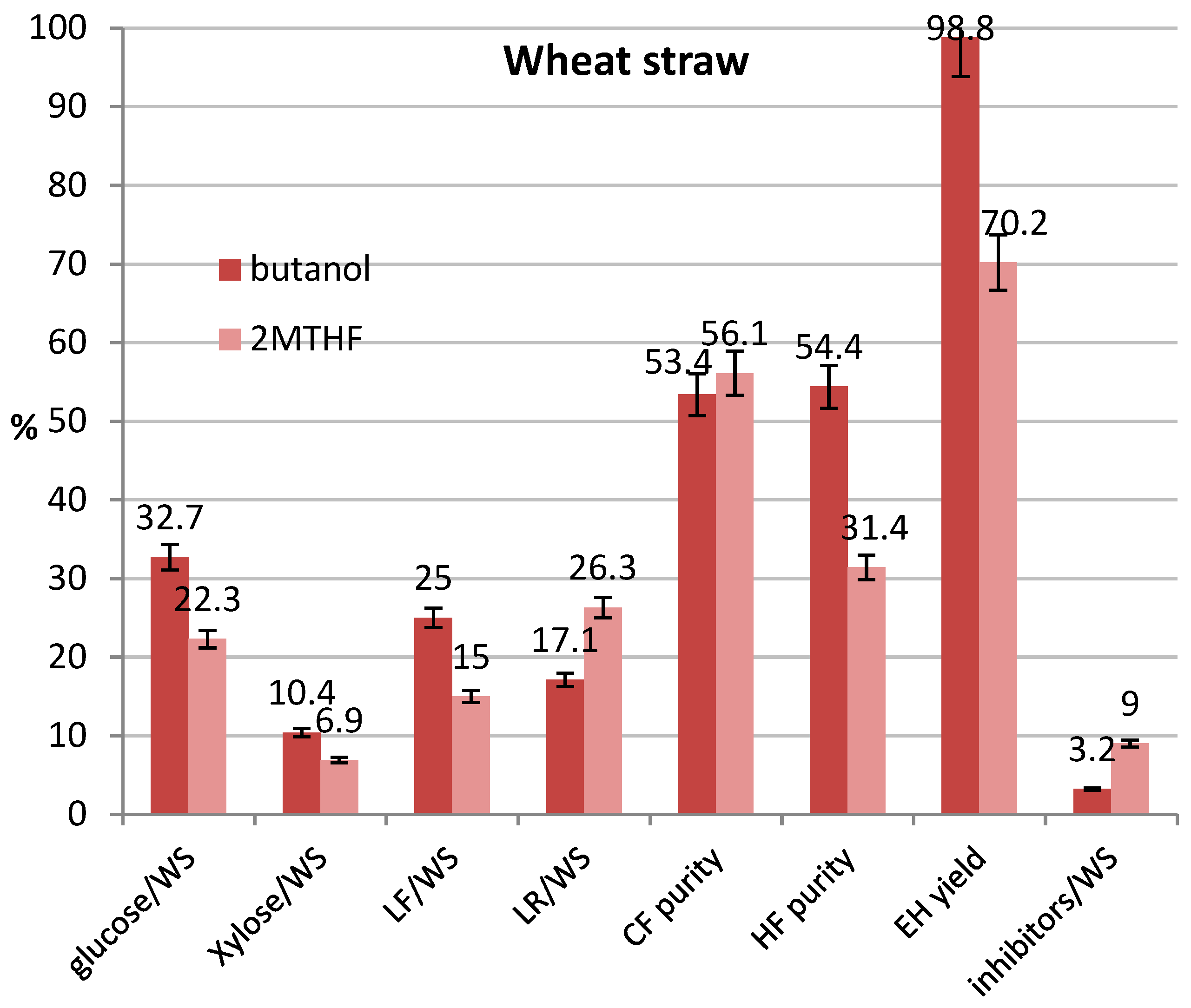
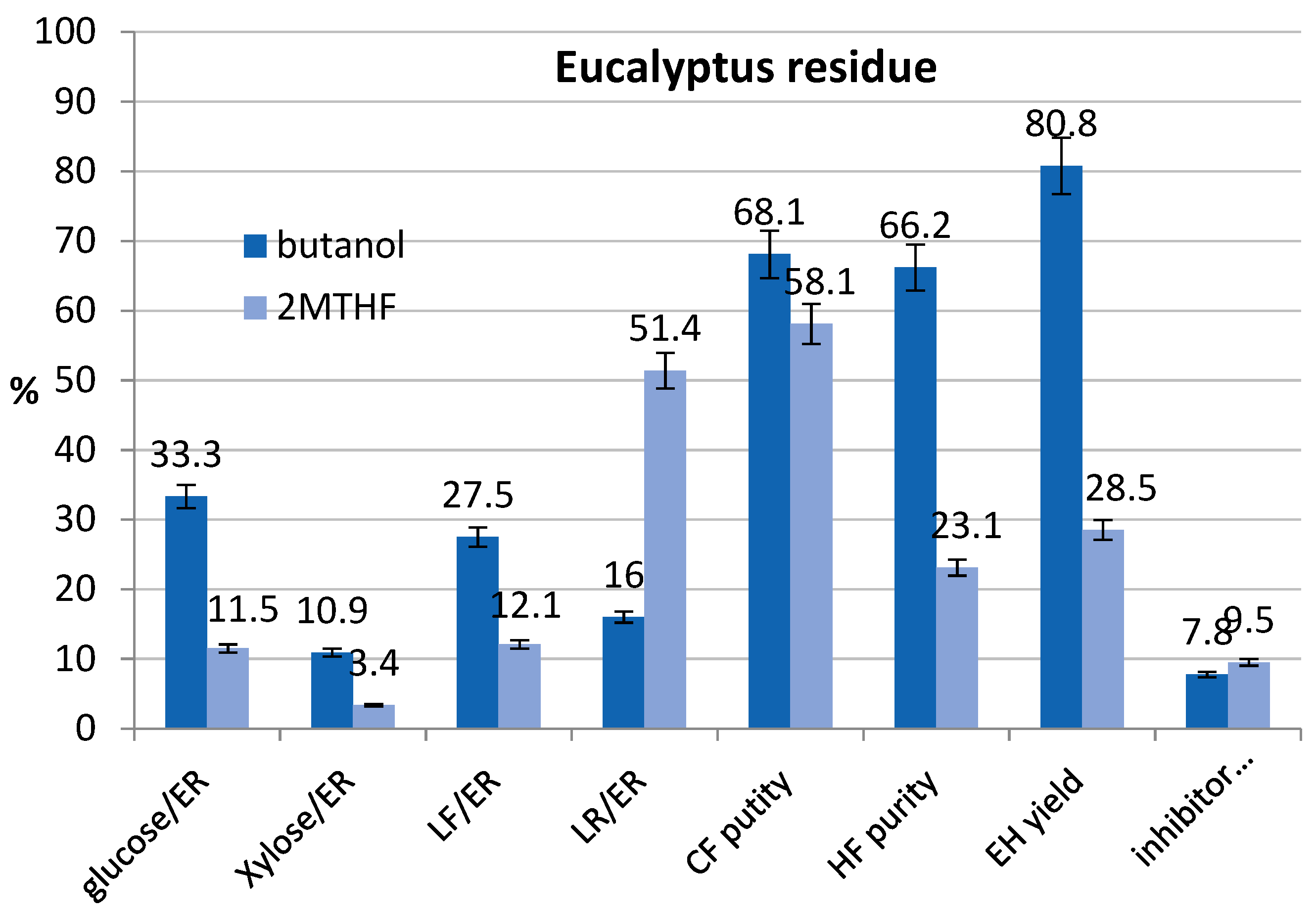
3.5. Tests at Optimized Conditions and Mass Balances
3.6. Trials at Reduced Temperature and Enzyme Loading
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2M-THF | 2-methyltetrahydrofuran |
| AH | Acid Hydrolysis |
| CF | Cellulosic Fraction |
| DOE | Design of Experiments |
| EH | Enzymatic Hydrolysis |
| ER | Eucalyptus Residues |
| GS | Glucose Solution |
| HF | Hemicellulose Fraction |
| LF | Lignin Fraction |
| LR | Lignin Residue |
| OS | Organic Solvent |
| PS | Pentose Solution |
| WS | Wheat Straw |
References
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Enzyme Res. 2011, 2011, 1–17. [Google Scholar] [CrossRef]
- Olsson, L.; Hägerdal, B.H. Fermentation of lignocellulosic hydrolysates for ethanol production. Enzyme Microb. Technol. 1996, 18, 312–331. [Google Scholar] [CrossRef]
- Kim, H.; Block, D.E.; Mills, D.A. Simultaneous consumption of pentose and hexose sugars: An optimal microbial phenotype for efficient fermentation of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2010, 88, 1077–1085. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Wei Kit Chin, D.; Lim, S.; Pang, Y.L.; Lam, M.K. Fundamental review of organosolv pretreatment and its challenges in emerging consolidated bioprocessing. Biofuel Bioprod. Bioref. 2020, 14, 808–829. [Google Scholar] [CrossRef]
- Pan, X.; Arato, C.; Gilkes, N.; Gregg, D.; Mabee, W.; Pye, K.; Xiao, Z.; Zhang, X.; Saddler, J. Biorefining of softwoods using ethanol organosolv pulping: Preliminary evaluation of process streams for manufacture of fuel-grade ethanol and co-products. Biotechnol. Bioeng. 2005, 90, 473–481. [Google Scholar] [CrossRef]
- Jiménez, L.; Maestre, F.; Pérez, I. Use of butanol-water mixtures for making wheat straw pulp. Wood Sci. Technol. 1999, 33, 97–109. [Google Scholar] [CrossRef]
- Stein, T.; Grande, P.M.; Kayser, H.; Sibilla, F.; Leitner, W.; María, P.D. From biomass to feedstock: One-step fractionation of lignocellulose components by the selective organic acid-catalyzed depolymerization of hemicellulose in a biphasic system. Green Chem. 2011, 13, 1772–1777. [Google Scholar] [CrossRef]
- Moity, L.; Durand, M.; Benazzouz, A.; Molinier, V.; Aubry, J.M. In Silico Search for Alternative Green Solvents. In Alternative Solvents for Natural Products Extraction; Farid, C., Vian, M.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 381, p. 21. [Google Scholar]
- Pace, V.; Hoyos, P.; Castoldi, L.; Maria, D.d.P.; Alcántara, A.R. 2-Methyltetrahydrofuran (2-MeTHF): A biomass-derived solvent with broad application in organic chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef]
- Li, S.X.; Li, M.F.; Bian, J.; Sun, S.N.; Peng, F.; Xue, Z.M. Biphasic 2-methyltetrahydrofuran/oxalic acid/water pretreatment to enhance cellulose enzymatic hydrolysis and lignin valorization. Bioresour. Technol. 2017, 243, 1105–1111. [Google Scholar] [CrossRef]
- Xue, B.; Yang, Y.; Zhu, M.; Sun, Y.; Li, X. Lewis acid-catalyzed biphasic 2-methyltetrahydrofuran/H2O pretreatment of lignocelluloses to enhance cellulose enzymatic hydrolysis and lignin valorization. Bioresour. Technol. 2018, 270, 55–61. [Google Scholar] [CrossRef]
- Grande, P.M.; Viell, J.; Theyssen, N.; Marquardt, W.; de María, P.D.; Leitner, W. Fractionation of lignocellulosic biomass using the OrganoCat process. Green Chem. 2015, 17, 3533–3539. [Google Scholar] [CrossRef] [Green Version]
- Weidener, D.; Dama, M.; Dietrich, S.K.; Ohrem, B.; Pauly, M.; Leitner, W.; María, P.d.D.; Grande, P.M.; Klose, H. Multiscale analysis of lignocellulose recalcitrance towards OrganoCat pretreatment and fractionation. Biotechnol. Biofuels 2020, 13, 1–13. [Google Scholar] [CrossRef]
- Zhang, Z.; Harrison, M.D.; Rackemann, D.W.; Doherty, W.O.; O’Hara, I.M. Organosolv pretreatment of plant biomass for enhanced enzymatic saccharification. Green Chem. 2016, 18, 360–381. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sust. Energ. Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Agricultural Residues as Feedstocks for Biofuels Production. Available online: https://www.etipbioenergy.eu/ (accessed on 15 November 2021).
- Deswarte, F.E.; Clark, J.H.; Wilson, A.J.; Hardy, J.J.; Marriott, R.; Chahal, S.P.; Jackson, C.; Heslop, G.; Birkett, M.; Bruce, T.J.; et al. Toward an integrated straw-based biorefinery. Biofuel. Bioprod. Bioref. Innov. Sustain. Econ. 2007, 1, 245–254. [Google Scholar] [CrossRef]
- Ghaffar, S.H. Wheat straw biorefinery for agricultural waste valorisation. Green Mater. 2019, 8, 60–67. [Google Scholar] [CrossRef]
- Zhang, H.; Lopez, P.C.; Holland, C.; Lunde, A.; Ambye-Jensen, M.; Felby, C.; Thomsen, S.T. The multi-feedstock biorefinery–Assessing the compatibility of alternative feedstocks in a 2G wheat straw biorefinery process. GCB Bioenergy 2018, 10, 946–959. [Google Scholar] [CrossRef]
- Clarke, C.R.E.; Palmer, B.; Gounden, D. Understanding and adding value to Eucalyptus fibre. Sout. For. 2008, 70, 169–174. [Google Scholar] [CrossRef]
- Reina, L.; Botto, E.; Mantero, C.; Moyna, P.; Menéndez, P. Production of second generation ethanol using Eucalyptus dunnii bark residues and ionic liquid pretreatment. Biomass Bioenergy 2016, 93, 116–121. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Patinha, D.J.S.; Sousa, G.D.A.; Villaverde, J.J.; Silva, C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Pascoal Neto, C. Eucalyptus biomass residues from agro-forest and pulping industries as sources of high-value triterpenic compounds. Cellulose Chem. Technol. 2011, 45, 475–481. [Google Scholar]
- Nogueira, G.P.; McManus, M.C.; Leak, D.J.; Franco, T.T.; de Souza Dias, M.O.; Cavaliero, C.K.N. Are eucalyptus harvest residues a truly burden-free biomass source for bioenergy? A deeper look into biorefinery process design and Life Cycle Assessment. J. Clean. Prod. 2021, 299, 126956. [Google Scholar] [CrossRef]
- Dantas, E.R.S.; Bonhivers, J.C.; Maciel Filho, R.; Mariano, A.P. Biochemical conversion of sugarcane bagasse into the alcohol fuel mixture of isopropanol-butanol-ethanol (IBE): Is it economically competitive with cellulosic ethanol? Bioresour. Technol. 2020, 314, 123712. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass, Laboratory Analytical Procedure (LAP) 2012. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 5 October 2021).
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]




| WS, wt.% | ER, wt.% | |
|---|---|---|
| Extractives in water | 5.6 | 2.2 |
| Extractives in ethanol | 2.2 | 1.0 |
| Glucan | 29.9 | 40.4 |
| Xylan | 18.9 | 14.3 |
| Arabinan | 2.4 | 0.7 |
| Galactan | 0.9 | 1.1 |
| Mannan | 0.0 | 1.0 |
| Acetyl groups | 2.6 | 3.9 |
| Lignin (acid insoluble) | 13.5 | 22.5 |
| Lignin (acid soluble) | 1.8 | 3.7 |
| Ash | 13.8 | 1.0 |
| nd | 8.4 | 8.1 |
| Moisture | 13.0 | 10.5 |
| Run | A: T | B: t | C: Oxalic Acid | R1 CF Purity | R2 HF Purity | R3 Glucose Recovery |
|---|---|---|---|---|---|---|
| °C | min | % | % | % | % | |
| 1 | 180 | 90 | 5 | 62.1 | 43.2 | 84.1 |
| 2 | 160 | 90 | 10 | 64.5 | 61.4 | 83.7 |
| 3 | 160 | 30 | 0 | 38.0 | 26 | 33.2 |
| 4 | 160 | 60 | 5 | 57.5 | 53.4 | 89.3 |
| 5 | 160 | 90 | 0 | 38.5 | 57.4 | 45.9 |
| 6 | 140 | 60 | 10 | 59.0 | 60.4 | 80.3 |
| 7 | 180 | 60 | 0 | 46.0 | 89.9 | 84.1 |
| 8 | 140 | 30 | 5 | 49.1 | 58.8 | 64.5 |
| 9 | 180 | 60 | 10 | 58.0 | 39.6 | 84.8 |
| 10 | 160 | 60 | 5 | 52.9 | 69.8 | 83.9 |
| 11 | 160 | 30 | 10 | 58.3 | 80.9 | 82.3 |
| 12 | 180 | 30 | 5 | 61.3 | 65.1 | 90.5 |
| 13 | 140 | 60 | 0 | 35.6 | 28.1 | 23.7 |
| 14 | 140 | 90 | 5 | 55.0 | 62.1 | 76.2 |
| 15 | 160 | 60 | 5 | 61.0 | 60.5 | 84.3 |
| Run | A: T | B: Oxalic Acid | R1 CF Purity | R2 HF Purity | R3 Glucose Recovery Yield |
|---|---|---|---|---|---|
| °C | % | % | % | % | |
| 1 | 140 | 9 | 59.4 | 50.7 | 53 |
| 2 | 180 | 9 | 72 | 50.3 | 78.3 |
| 3 | 180 | 3 | 70.1 | 58.7 | 70.4 |
| 4 | 140 | 3 | 56.6 | 42 | 41.4 |
| 5 | 140 | 6 | 63.7 | 40.9 | 50.5 |
| 6 | 160 | 3 | 74.4 | 51.5 | 65 |
| 7 | 180 | 6 | 77.4 | 55.5 | 86.9 |
| 8 | 160 | 9 | 79.4 | 53 | 80.7 |
| 9 | 160 | 6 | 74.3 | 55.7 | 80.2 |
| 10 | 160 | 6 | 73 | 54.2 | 81.6 |
| Run | A: T | B: Oxalic Acid | R1 CF Purity | R2 HF Purity | R3 Glucose Recovery Yield |
|---|---|---|---|---|---|
| °C | % | % | % | % | |
| 1 | 160 | 4 | 52.4 | 45.6 | 57.4 |
| 2 | 140 | 4 | 53.4 | 49.4 | 48.3 |
| 3 | 180 | 4 | 55.7 | 30.9 | 64.8 |
| 4 | 160 | 1 | 47.0 | 37.6 | 44.6 |
| 5 | 180 | 1 | 56.6 | 36.8 | 56.6 |
| 6 | 160 | 4 | 53.3 | 42.5 | 55.8 |
| 7 | 140 | 1 | 35.1 | 28.6 | 27.8 |
| 8 | 140 | 7 | 52.9 | 27.8 | 51.4 |
| 9 | 160 | 7 | 54.3 | 30.9 | 59.1 |
| 10 | 180 | 7 | 53.4 | 24.1 | 62.1 |
| T, °C | Oxalic Acid % | t, min | CF Purity, % | HF Purity, % | Glucose Yield, % | Inhibitors * % | |
|---|---|---|---|---|---|---|---|
| WS, butanol | 175 | 4.6 | 55 | 59.0 | 58.1 | 94.0 | 3.3 |
| WS, 2M-THF | 180 | 3.2 | 60 | 55.9 | 42.2 | 64.8 | 8.3 |
| ER, butanol | 170 | 4.2 | 60 | 75.9 | 55.1 | 79.5 | 9.4 |
| ER, 2M-THF ** | 170 | 4.2 | 60 |
| WS/Butanol | WS/2M-THF | ER/Butanol | ER/2M-THF | |
|---|---|---|---|---|
| acetic acid, % | 0.24 | 1.5 | 1.02 | 3.08 |
| formic acid, % | 2.4 | 1.2 | 6.25 | 2.62 |
| furfural, % | 0.51 | 5.8 | 0.47 | 3.28 |
| 5HMF, % | 0.06 | 0.5 | 0.06 | 0.51 |
| Total, % | 3.21 | 9.0 | 7.8 | 9.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viola, E.; Zimbardi, F.; Morgana, M.; Cerone, N.; Valerio, V.; Romanelli, A. Optimized Organosolv Pretreatment of Biomass Residues Using 2-Methyltetrahydrofuran and n-Butanol. Processes 2021, 9, 2051. https://doi.org/10.3390/pr9112051
Viola E, Zimbardi F, Morgana M, Cerone N, Valerio V, Romanelli A. Optimized Organosolv Pretreatment of Biomass Residues Using 2-Methyltetrahydrofuran and n-Butanol. Processes. 2021; 9(11):2051. https://doi.org/10.3390/pr9112051
Chicago/Turabian StyleViola, Egidio, Francesco Zimbardi, Massimo Morgana, Nadia Cerone, Vito Valerio, and Assunta Romanelli. 2021. "Optimized Organosolv Pretreatment of Biomass Residues Using 2-Methyltetrahydrofuran and n-Butanol" Processes 9, no. 11: 2051. https://doi.org/10.3390/pr9112051
APA StyleViola, E., Zimbardi, F., Morgana, M., Cerone, N., Valerio, V., & Romanelli, A. (2021). Optimized Organosolv Pretreatment of Biomass Residues Using 2-Methyltetrahydrofuran and n-Butanol. Processes, 9(11), 2051. https://doi.org/10.3390/pr9112051







