Combustion Characteristics and Kinetic Analysis of Biomass Pellet Fuel Using Thermogravimetric Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Method
2.2.1. Determination of the Components of Five Kinds of Biomass Pellet Fuel
2.2.2. Thermal Weight Loss Analysis of Five Kinds of Biomass Pellet Fuel
3. Results and Analysis
3.1. Industry Analysis of Five Kinds of Biomass Pellet Fuel
3.2. Analysis of Thermal Weight Loss Characteristics of Five Kinds of Biomass Pellet Fuel
3.3. Ignition Characteristics
3.4. Burnout Characteristics
3.5. Comprehensive Combustion Characteristics
4. Combustion Kinetic Analysis
4.1. Combustion Mechanism
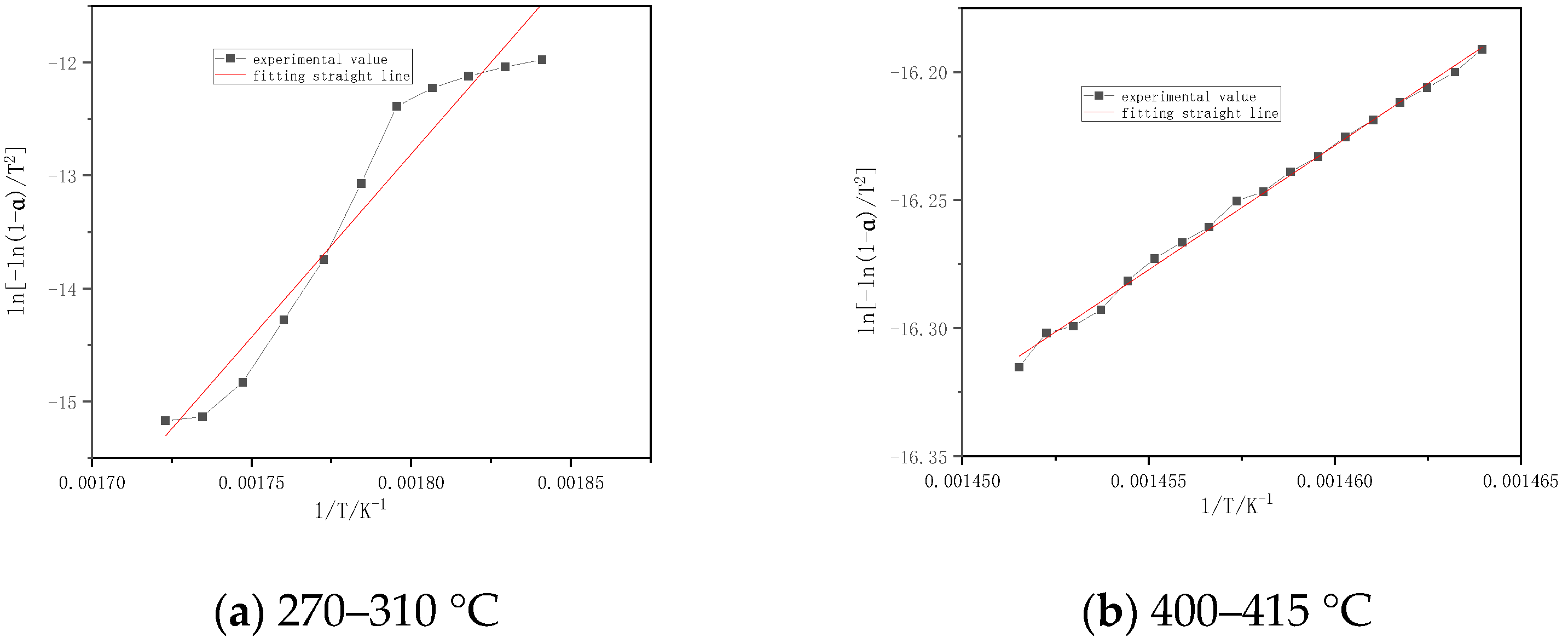
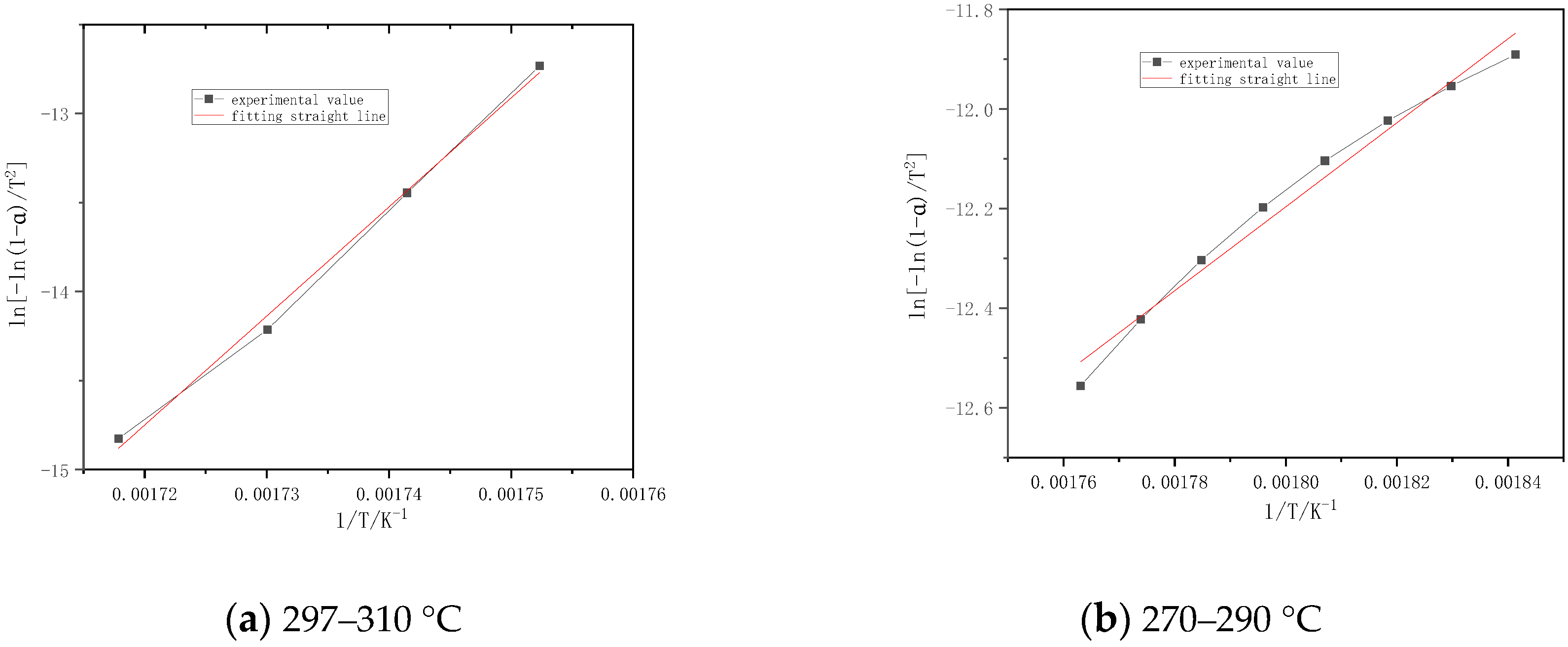
4.2. Kinetic Calculation
5. Conclusions
- (1)
- The pyrolysis of five kinds of biomass pellet fuel mainly includes three stages: (1) water evaporation stage; (2) volatile component combustion stage; (3) fixed carbon oxidation stage.
- (2)
- The TG curves of five kinds of biomass pellet fuel are roughly the same. The peaks of thermal weight loss rate and maximum degradation rate are both in the range of high temperature. The DTG curves of five kinds of biomass pellet fuel have an obvious peak. The range of peak temperature in the DTG curves is 280–310 °C.
- (3)
- The kinetic parameters are obtained by the first-order reaction equation in the combustion stages. The correlation coefficients are all above 0.92, and the fitting results are good. The activation energy of each sample is basically the same in the combustion stage. The activation energy of the volatile matter in combustion stage is 56~542 kJ/mol, and the activation energy of the carbon layer slowly increases rapidly.
- (4)
- The five kinds of biomass pellet fuels have good combustion characteristics and kinetic characteristics, and they can be promoted and applied as biomass pellet fuels in the future.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | the pre-exponential factor(s−1) |
| E | the activation energy(kj/mol) |
| S | the comprehensive combustion characteristic index(min−2·K−3) |
| R | the universal gas constant (8.314J/(K·mol)) |
| T | the absolute temperature(K) |
| m | the mass of the sample |
| Greek letter | |
| α | degree of conversion |
| β | heating rate (K/min) |
| Subscripts and superscripts | |
| 0 | initial value |
| f | burning-out value |
| t | the reaction time(min) |
| i | ignition |
References
- Zuo, H.; Tan, J.; Wei, K.; Huang, Z.; Zhong, D.; Xie, F. Effects of different poses and wind speeds on wind-induced vibration characteristics of a dish solar concentrator system. Renew. Energy 2021, 168, 1308–1326. [Google Scholar] [CrossRef]
- Peng, Q.; Yang, W.; E, J.; Li, S.; Li, Z.; Xu, H.; Fu, G. Effects of propane addition and burner scale on the combustion characteristics and working performance. Appl. Energy 2021, 285, 116484. [Google Scholar] [CrossRef]
- Zuo, H.; Liu, G.; E, J.; Zuo, W.; Wei, K.; Hu, W.; Tan, J.; Zhong, D. Catastrophic analysis on the stability of a large dish solar thermal power generation system with wind-induced vibration. Sol. Energy 2019, 183, 40–49. [Google Scholar] [CrossRef]
- Zhang, B.; Zuo, H.; Huang, Z.; Tan, J.; Zuo, Q. Endpoint forecast of different diesel-biodiesel soot filtration process in diesel particulate filters considering ash deposition. Fuel 2020, 271, 117678. [Google Scholar] [CrossRef]
- Zhong, C.; Tan, J.; Zuo, H.; Wu, X.; Wang, S.; Liu, J. Synergy effects analysis on CDPF regeneration performance enhancement and NOx concentration reduction of NH3–SCR over Cu–ZSM–5. Energy 2021, 230, 120814. [Google Scholar] [CrossRef]
- Mohr, S.; Wang, J.; Ellem, G.; Ward, J.; Giurco, D. Projection of world fossil fuels by country. Fuel 2015, 141, 120–135. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An overview of the organic and inorganic phase composition of biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef]
- Lopez-Velazquez, M.A.; Santes, V.; Balmaseda, J.; Torres-Garcia, E. Pyrolysis of orange waste: A thermo-kinetic study. J. Anal. Appl. Pyrolysis 2013, 99, 170–177. [Google Scholar] [CrossRef]
- Stefanidis, S.D.; Kalogiannis, K.G.; Iliopoulou, E.F.; Michailof, C.M.; Pilavachi, P.A.; Lappas, A.A. A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J. Anal. Appl. Pyrolysis 2014, 105, 143–150. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Wei, Y. Combustion behavior of corncob/bituminous coal and hardwood/bituminous coal. Renew. Energy 2015, 81, 355–365. [Google Scholar] [CrossRef]
- Sharma, P.; Diwan, P.K. Study of thermal decomposition process and the reaction mechanism of the eucalyptus wood. Wood Sci. Technol. 2017, 51, 1081–1094. [Google Scholar] [CrossRef]
- Brebu, M.; Tamminen, T.; Spiridon, I. Thermal degradation of various lignins by TG-MS/FTIR and Py-GC-MS. J. Anal. Appl. Pyrolysis 2013, 104, 531–539. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, Y.; Zhu, X. Determination of effective moisture diffusivity and drying kinetics for poplar sawdust by thermogravimetric analysis under isothermal condition. Bioresour. Technol. 2012, 107, 451–455. [Google Scholar] [CrossRef]
- Chen, D.; Zheng, Y.; Zhu, X. In-depth investigation on the pyrolysis kinetics of raw biomass. Part I: Kinetic analysis for the drying and devolatilization stages. Bioresour. Technol. 2013, 131, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Chakraborty, S. A kinetic study of pyrolysis and combustion of microalgae Chlorella vulgaris using thermo-gravimetric analysis. Bioresour. Technol. 2013, 128, 72–80. [Google Scholar] [CrossRef]
- Gai, C.; Dong, Y.; Zhang, T. The kinetic analysis of the pyrolysis of agricultural residue under non-isothermal conditions. Bioresour. Technol. 2013, 127, 298–305. [Google Scholar] [CrossRef] [PubMed]
- de Jesus, M.S.; Carneiro, A.D.C.O.; Martinez, C.L.M.; Vital, B.R.; Carneiro, A.P.S.; de Assis, M.R. Thermal decomposition fundamentals in large-diameter wooden logs during slow pyrolysis. Wood Sci. Technol. 2019, 53, 1353–1372. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C.; Du, L.; Duan, Y. TG–FTIR and Py–GC/MS analysis on pyrolysis and combustion of pine sawdust. J. Anal. Appl. Pyrolysis 2013, 100, 26–32. [Google Scholar] [CrossRef]
- Garcia-Maraver, A.; Salvachúa, D.; Martínez, M.; Diaz, L.; Zamorano, M. Analysis of the relation between the cellulose, hemicellulose and lignin content and the thermal behavior of residual biomass from olive trees. Waste Manag. 2013, 33, 2245–2249. [Google Scholar] [CrossRef]
- Huang, H.; Liu, J.; Liu, H.; Evrendilek, F.; Buyukada, M. Pyrolysis of water hyacinth biomass parts: Bioenergy, gas emissions, and by-products using TG-FTIR and Py-GC/MS analyses. Energy Convers. Manag. 2020, 207, 112552. [Google Scholar] [CrossRef]
- Liu, X.; Chen, M.; Wei, Y. Kinetics based on two-stage scheme for co-combustion of herbaceous biomass and bituminous coal. Fuel 2015, 143, 577–585. [Google Scholar] [CrossRef]
- Maia, A.A.D.; de Morais, L.C. Kinetic parameters of red pepper waste as biomass to solid biofuel. Bioresour. Technol. 2016, 204, 157–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyakuma, B.B.; Ahmad, A.; Johari, A.; Abdullah, T.T.; Oladokun, O.; Aminu, D.Y. Non-isothermal kinetic analysis of oil palm empty fruit bunch pellets by thermogravimetric analysis. Chem. Eng. Trans. 2015, 45, 1327–1332. [Google Scholar]
- Ceylan, S.; Goldfarb, J.L. Green tide to green fuels: TG–FTIR analysis and kinetic study of Ulva prolifera pyrolysis. Energy Convers. Manag. 2015, 111, 263–270. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Physicochemical characterization and pyrolysis kinetics of wood sawdust. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 38, 2536–2544. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, M.; Zhu, X.; Guo, D.; Liu, S.; Hu, Z.; Xiao, B.; Wang, J.; Laghari, M. Characteristics and kinetic study on pyrolysis of five lignocellulosic biomass via thermogravimetric analysis. Bioresour. Technol. 2015, 192, 441–450. [Google Scholar] [CrossRef]
- Cui, Y.Z.; Qu, Y.X.; Tang, M.T.; Gao, P.; Zhang, G.K. Hot Air Ignition Characteristics of Cotton and Corn Straw Pellet Based on TG-DTG. Appl. Mech. Mater. 2014, 672–674, 164–167. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Dong, A.; Guo, X.; Chen, C.; Xiong, S. Co-pyrolysis of biomass and pingshuo coal. In Proceedings of the International Conference on Materials for Renewable Energy & Environment, Chengdu, China, 19–21 August 2013. [Google Scholar]
- Harun, N.Y.; Afzal, M.T. Chemical and mechanical properties of pellets made from agricultural and woody biomass blends. Trans. ASABE 2015, 58, 921–930. [Google Scholar]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Ceylan, S.; Topçu, Y. Pyrolysis kinetics of hazelnut husk using thermogravimetric analysis. Bioresour. Technol. 2014, 156, 182–188. [Google Scholar] [CrossRef]
- Maryandyshev, P.A.; Popova, E.I.; Chernov, A.A.; Popov, M.S.; Lyubov, V.K.; Trouve, G.; Kehrli, D.; Brillard, A.; Brilhac, J.-F. Thermal decomposition and combustion of biofuels. Solid Fuel Chem. 2017, 51, 370–378. [Google Scholar] [CrossRef]
- Kocabaş-Ataklı, Z.Ö.; Okyay-Öner, F.; Yürüm, Y. Combustion characteristics of Turkish hazelnut shell biomass, lignite coal, and their respective blends via thermogravimetric analysis. J. Therm. Anal. Calorim. 2015, 119, 1723–1729. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Gong, S.; Jia, C.; Wang, Q. TG-FTIR analysis of co-combustion characteristics of oil shale semi-coke and corn straw. J. Therm. Anal. Calorim. 2017, 127, 2531–2544. [Google Scholar] [CrossRef]
- Striūgas, N.; Skvorčinskienė, R.; Paulauskas, R.; Zakarauskas, K.; Vorotinskienė, L. Evaluation of straw with absorbed glycerol thermal degradation during pyrolysis and combustion by TG-FTIR and TG-GC/MS. Fuel 2017, 204, 227–235. [Google Scholar] [CrossRef]
- Sun, C.; Li, C.; Tan, H.; Zhang, Y. Synergistic effects of wood fiber and polylactic acid during co-pyrolysis using TG-FTIR-MS and Py-GC/MS. Energy Convers. Manag. 2019, 202, 112212. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, W.; Liu, H.; Jia, C.; Li, S. Interactions and kinetic analysis of oil shale semi-coke with cornstalk during co-combustion. Appl. Energy 2011, 88, 2080–2087. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, T.; Wu, J.; Wu, J. Multi-Gaussian-DAEM-reaction model for thermal decompositions of cellulose, hemicellulose and lignin: Comparison of N-2 and CO2 atmosphere. Bioresour. Technol. 2014, 166C, 87–95. [Google Scholar] [CrossRef]


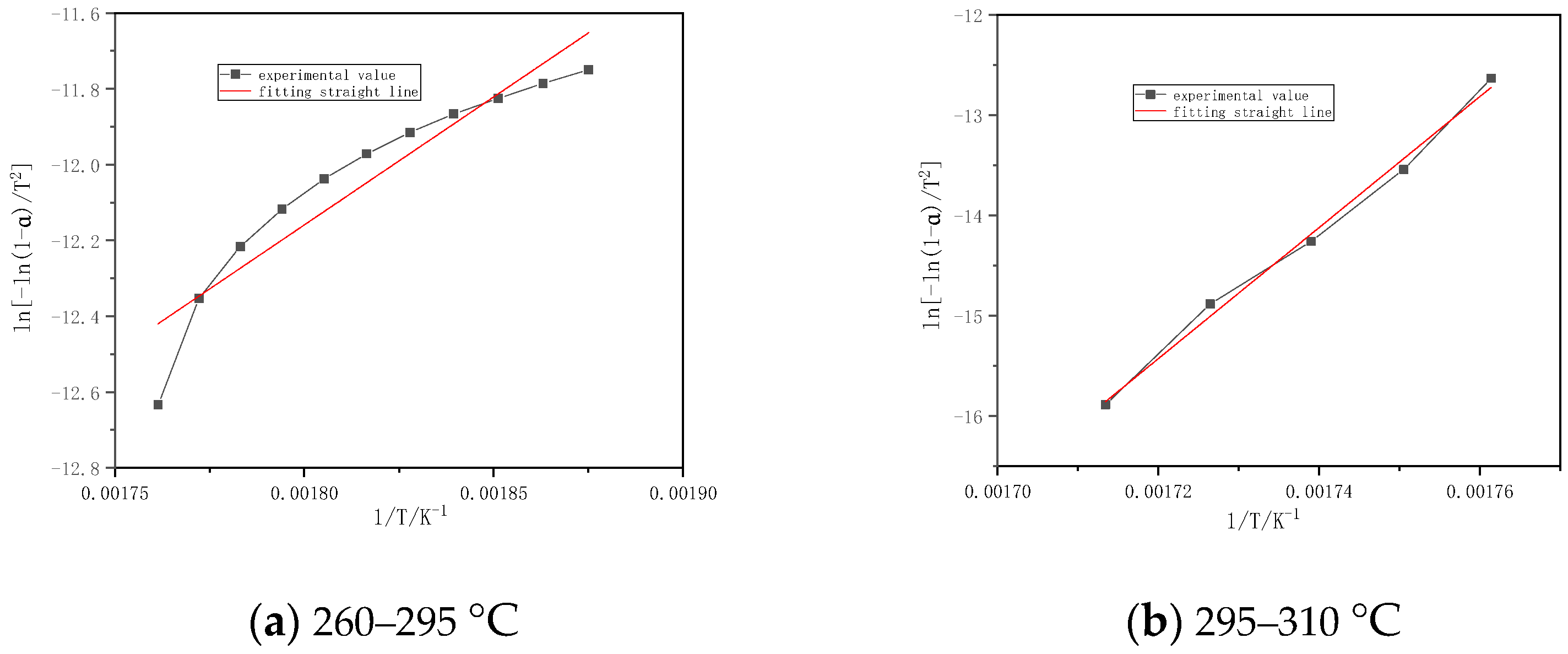


| Sample | Moisture | Volatile | Ash |
|---|---|---|---|
| Masson pine | 12.09 | 62.65 | 1.36 |
| Chinese fir | 11.05 | 78.86 | 0.52 |
| Willow | 10.21 | 69.58 | 1.95 |
| Slash pine | 15.46 | 77.75 | 1.34 |
| Poplar | 10.57 | 75.14 | 1.59 |
| Combustion Characteristics | Masson Pine | Chinese Fir | Willow | Slash Pine | Poplar |
|---|---|---|---|---|---|
| Ignition temperature/°C | 241 | 267 | 266 | 256 | 271 |
| Burnout temperature/°C | 318 | 311 | 331 | 419 | 316 |
| Combustion Characteristics | Masson Pine | Chinese Fir | Willow | Slash Pine | Poplar |
|---|---|---|---|---|---|
| Ignition temperature Ti/°C | 241 | 267 | 266 | 256 | 271 |
| Burnout temperature Tf/°C | 318 | 311 | 331 | 419 | 316 |
| Maximum burning rate (%/min) | 103.35 | 134.1 | 68.11 | 158.25 | 117.45 |
| Average burning rate (%/min) | 22.1 | 37.71 | 25.23 | 10.3 | 36.08 |
| Comprehensive combustion characteristic index S (min−2·K−3) | 1.24 × 10−8 | 2.28 × 10−8 | 7.34 × 10−9 | 5.94 × 10−9 | 1.83 × 10−8 |
| Sample | Temperature Interval (°C) | Fitting Equation | Activation Energy E (kj/mol) | Pre-Reference factor A (min−1) | Correlation Coefficient r |
|---|---|---|---|---|---|
| Chinese fir | 260–295 | y = −6753.69x + 24.316 | 56.15 | 4.91 × 1015 | 0.9401 |
| 295–310 | y = −65,266.75x + 27.685 | 542.63 | 1.38 × 1018 | 0.9966 | |
| Masson pine | 260–310 | y = −20,807.17x + 20.274 | 172.99 | 2.65 × 1014 | 0.9241 |
| willow | 270–320 | y = −15,697.41x + 20.456 | 130.51 | 2.40 × 1014 | 0.9252 |
| Slash pine | 270–310 | y = −32,414.51x + 21.157 | 269.49 | 1.00 × 1015 | 0.9704 |
| 400–415 | y = −9706.38x + 20.400 | 80.70 | 1.41 × 1014 | 0.9984 | |
| Poplar | 270–297 | y = −8432.88x + 27.376 | 70.11 | 8.97 × 1016 | 0.9904 |
| 297–310 | y = −61,236.51x + 20.076 | 509.12 | 6.41 × 1014 | 0.9978 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, G. Combustion Characteristics and Kinetic Analysis of Biomass Pellet Fuel Using Thermogravimetric Analysis. Processes 2021, 9, 868. https://doi.org/10.3390/pr9050868
Jia G. Combustion Characteristics and Kinetic Analysis of Biomass Pellet Fuel Using Thermogravimetric Analysis. Processes. 2021; 9(5):868. https://doi.org/10.3390/pr9050868
Chicago/Turabian StyleJia, Guohai. 2021. "Combustion Characteristics and Kinetic Analysis of Biomass Pellet Fuel Using Thermogravimetric Analysis" Processes 9, no. 5: 868. https://doi.org/10.3390/pr9050868
APA StyleJia, G. (2021). Combustion Characteristics and Kinetic Analysis of Biomass Pellet Fuel Using Thermogravimetric Analysis. Processes, 9(5), 868. https://doi.org/10.3390/pr9050868





