Long-Term Stability of Nitrifying Granules in a Membrane Bioreactor without Hydraulic Selection Pressure
Abstract
1. Introduction
2. Materials and Methods
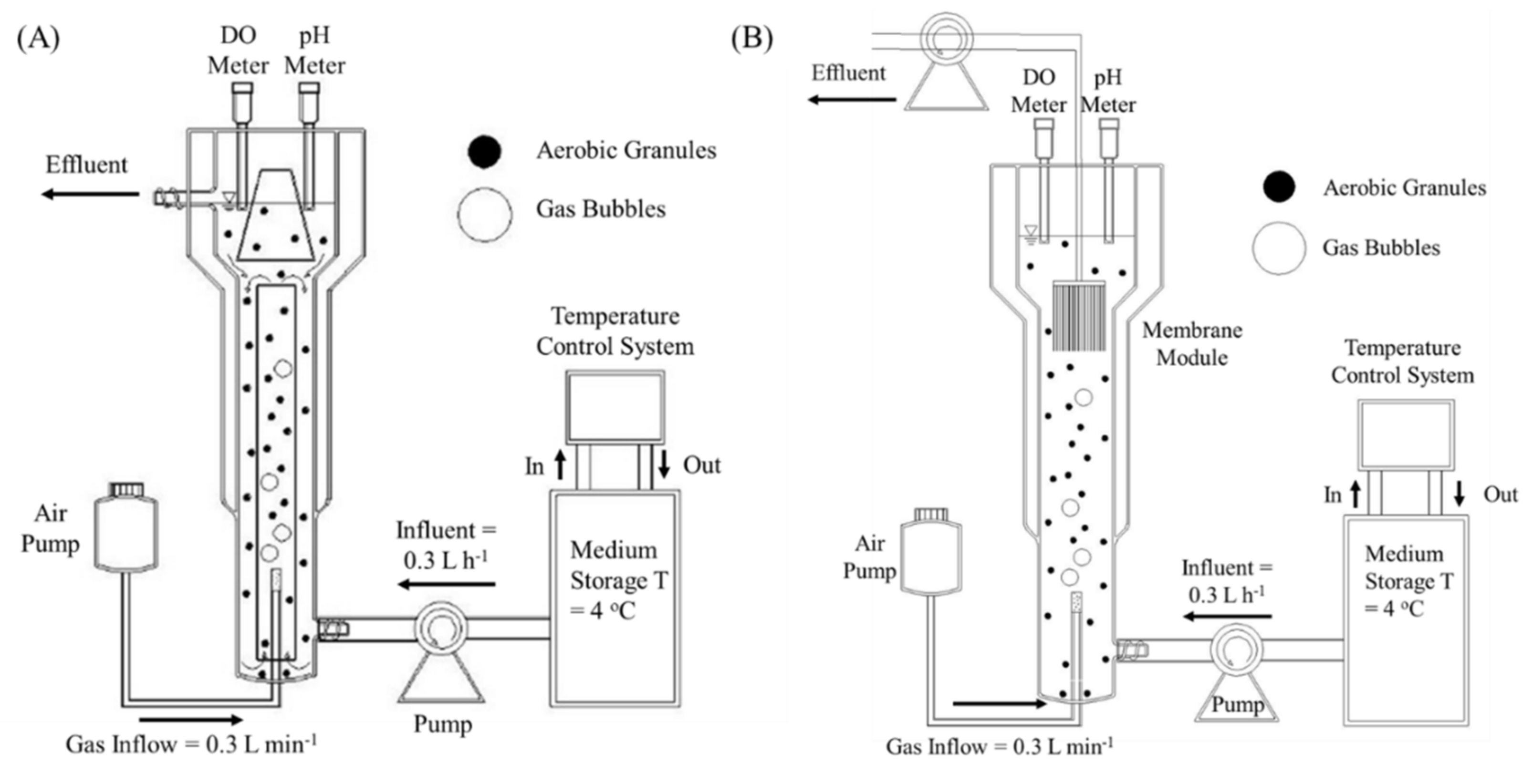
2.1. Reactor Setup
2.2. Analytical Methods
2.3. Model Simulation
3. Results
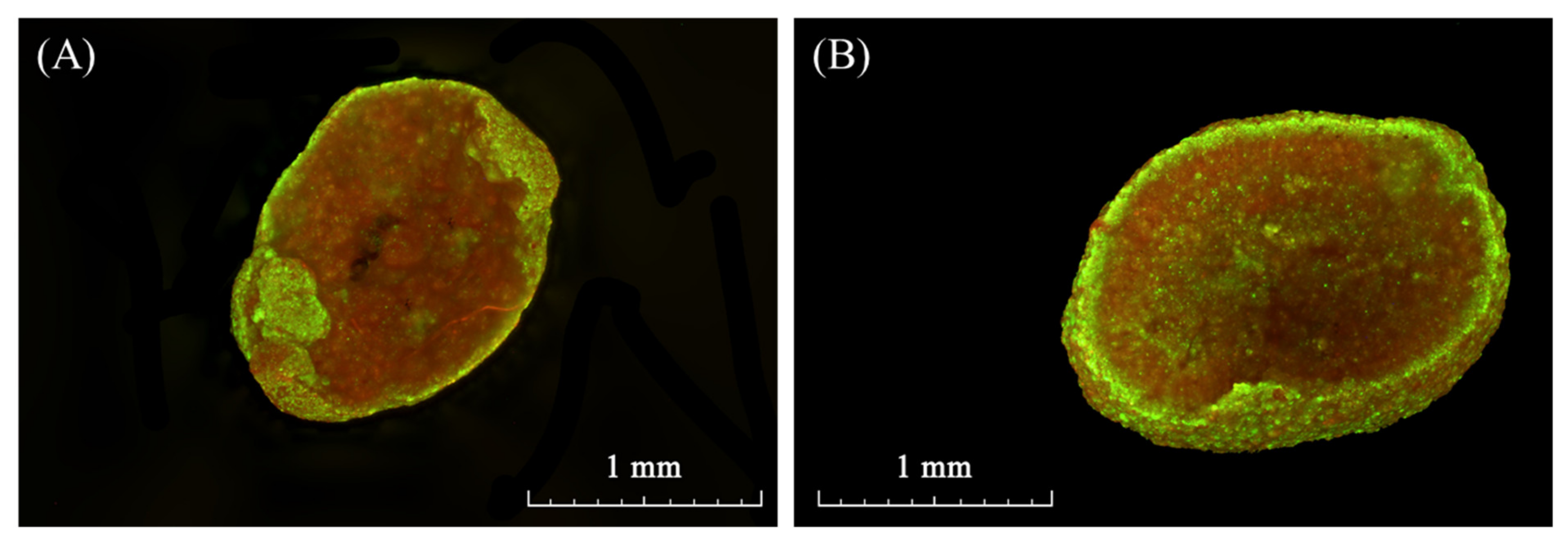
3.1. Characteristics of Seed Granules
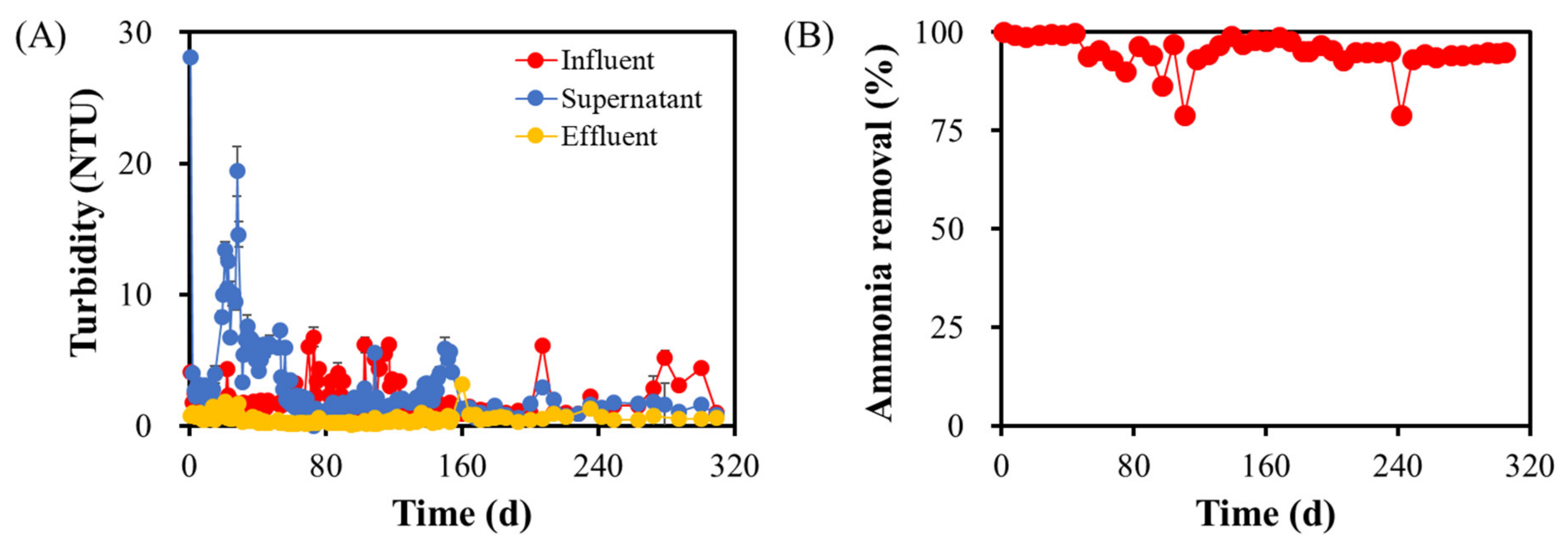
3.2. Response of Seed Granules to Membrane Insertion
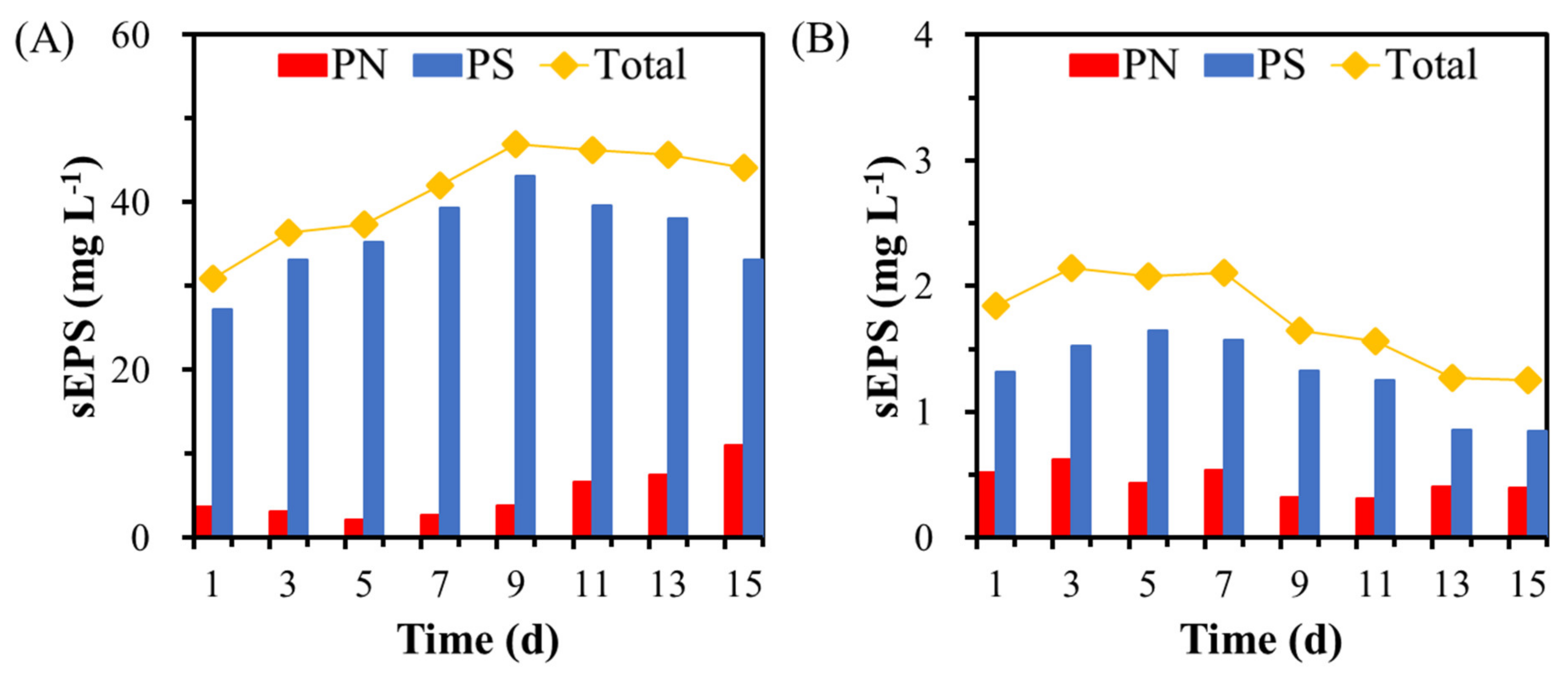
3.3. Characteristics of the Nitrifying Granules Stabilized in the GMBR after 305 Days
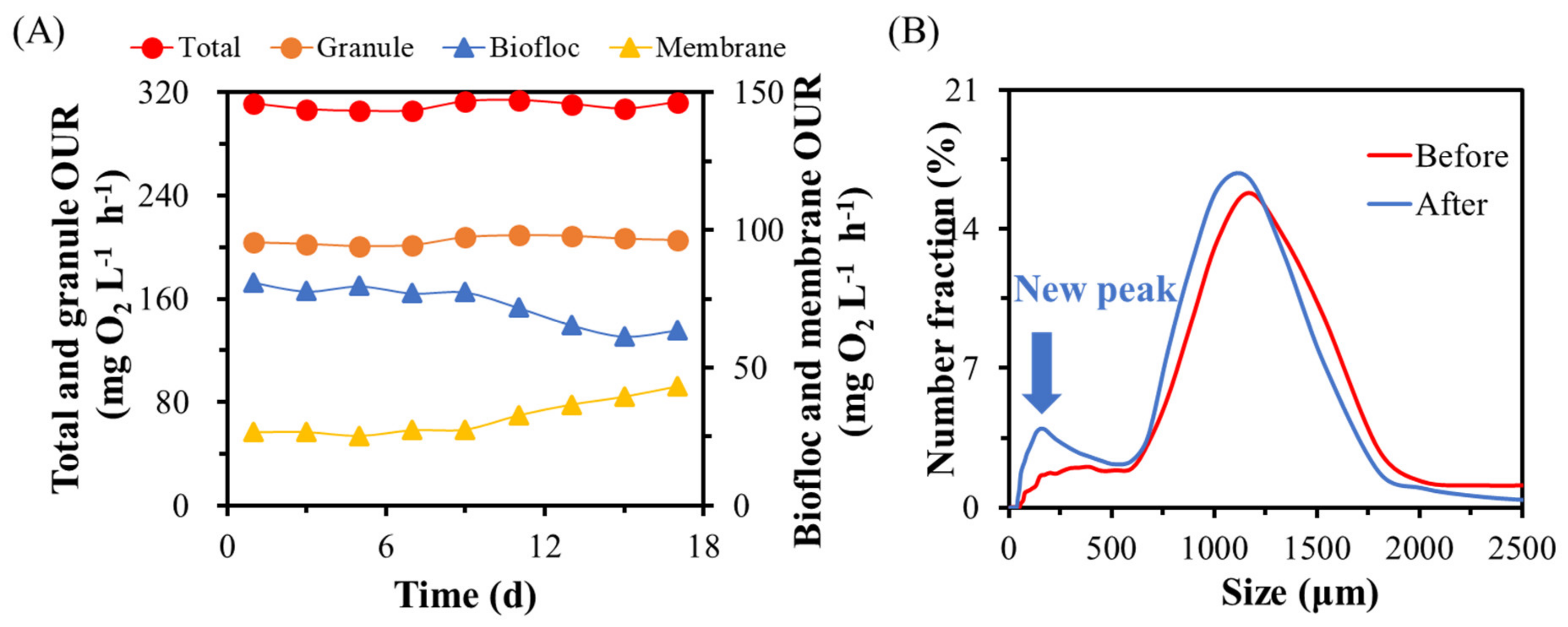
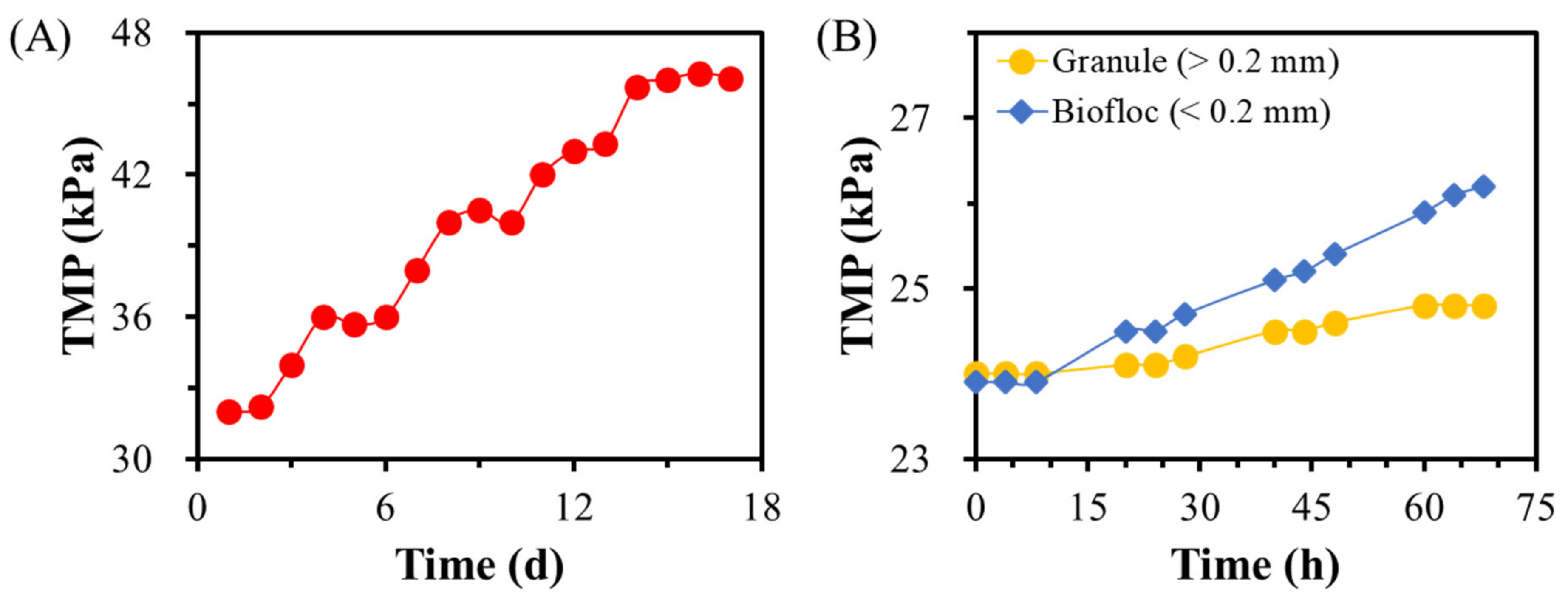
3.4. Dynamic Equilibrium within a Backwash Cycle of GMBR at the Steady State
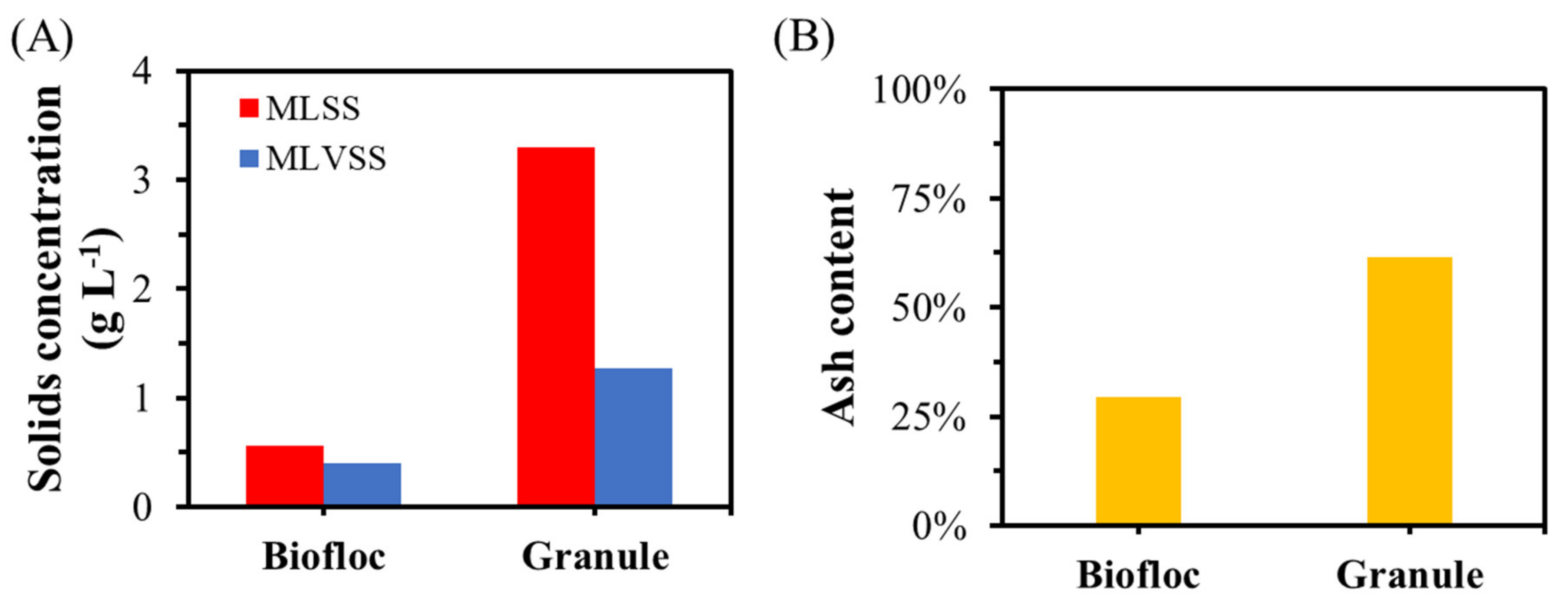
3.5. Contributions of Granules and Bioflocs to Membrane Fouling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kent, T.R.; Bott, C.B.; Wang, Z.-W. State of the art of aerobic granulation in continuous flow bioreactors. Biotechnol. Adv. 2018, 36, 1139–1166. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Lan, G.-H.; Zeng, P. Excessive precipitation of CaCO3 as aragonite in a continuous aerobic granular sludge reactor. Appl. Microbiol. Biotechnol. 2015, 99, 8225–8234. [Google Scholar] [CrossRef]
- Corsino, S.; Campo, R.; Di Bella, G.; Torregrossa, M.; Viviani, G. Study of aerobic granular sludge stability in a continuous-flow membrane bioreactor. Bioresour. Technol. 2016, 200, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Kim, I.S.; Kim, K.S. Development of a novel process to mitigate membrane fouling in a continuous sludge system by seeding aerobic granules at pilot plant. J. Membr. Sci. 2016, 497, 90–98. [Google Scholar] [CrossRef]
- Chen, C.; Bin, L.; Tang, B.; Huang, S.; Fu, F.; Chen, Q.; Wu, L.; Wu, C. Cultivating granular sludge directly in a continuous-flow membrane bioreactor with internal circulation. Chem. Eng. J. 2017, 309, 108–117. [Google Scholar] [CrossRef]
- An, Z.; Kent, T.R.; Sun, Y.; Bott, C.B.; Wang, Z.-W. Free ammonia resistance of nitrite-oxidizing bacteria developed in aerobic granular sludge cultivated in continuous upflow airlift reactors performing partial nitritation. Water Environ. Res. 2021, 93, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Martin, K.; Shaw, A.; de Clippeleir, H.; Sturm, B. “Accidental granular sludge?”: Understanding process design and operational conditions that lead to low SVI-30 values through a survey of full scale facilities in North America. Proc. Water Environ. Fed. 2016, 2016, 3385–3394. [Google Scholar] [CrossRef]
- Baird, R.; Bridgewater, L. Standard Methods for Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Liu, H.; Fang, H.H. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [CrossRef]
- Kent, T.R.; Sun, Y.; An, Z.; Bott, C.B.; Wang, Z.-W. Mechanistic understanding of the NOB suppression by free ammonia inhibition in continuous flow aerobic granulation bioreactors. Environ. Int. 2019, 131, 105005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, Z.; Qiu, Z.; Jin, M.; Chen, Z.; Chen, Z.; Li, J.; Wang, X.; Wang, J. Dynamic and distribution of ammonia-oxidizing bacteria communities during sludge granulation in an anaerobic–aerobic sequencing batch reactor. Water Res. 2011, 45, 6207–6216. [Google Scholar]
- Jin, R.-C.; Zheng, P.; Mahmood, Q.; Zhang, L. Performance of a nitrifying airlift reactor using granular sludge. Sep. Purif. Technol. 2008, 63, 670–675. [Google Scholar] [CrossRef]
- Shi, X.-Y.; Yu, H.-Q.; Sun, Y.-J.; Huang, X. Characteristics of aerobic granules rich in autotrophic ammonium-oxidizing bacteria in a sequencing batch reactor. Chem. Eng. J. 2009, 147, 102–109. [Google Scholar] [CrossRef]
- Zheng, Y.-M.; Yu, H.-Q.; Sheng, G.-P. Physical and chemical characteristics of granular activated sludge from a sequencing batch airlift reactor. Process Biochem. 2005, 40, 645–650. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.-W.; Liu, Y.Q.; Qin, L.; Tay, J.H. A generalized model for settling velocity of aerobic granular sludge. Biotechnol. Progr. 2005, 21, 621–626. [Google Scholar] [CrossRef]
- Sun, Y.; Angelotti, B.; Wang, Z.-W. Continuous-flow aerobic granulation in plug-flow bioreactors fed with real domestic wastewater. Sci. Total Environ. 2019, 688, 762–770. [Google Scholar] [CrossRef]
- Tsuneda, S.; Nagano, T.; Hoshino, T.; Ejiri, Y.; Noda, N.; Hirata, A. Characterization of nitrifying granules produced in an aerobic upflow fluidized bed reactor. Water Res. 2003, 37, 4965–4973. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, X.; Zhao, Z.; Li, J. Organics and nitrogen removal and sludge stability in aerobic granular sludge membrane bioreactor. Appl. Microbiol. Biotechnol. 2008, 79, 679–685. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Liu, H.; Hua, Z.; Du, G.; Chen, J. Characteristics of aerobic biogranules from membrane bioreactor system. J. Membr. Sci. 2007, 287, 294–299. [Google Scholar] [CrossRef]
- Geng, Z.; Hall, E.R. A comparative study of fouling-related properties of sludge from conventional and membrane enhanced biological phosphorus removal processes. Water Res. 2007, 41, 4329–4338. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Yao, Y.; Xu, D. Mechanism of Membrane Fouling Control by HMBR: Effect of Microbial Community on EPS. Int. J. Environ. Res. Public Health 2020, 17, 1681. [Google Scholar] [CrossRef]
- Zuthi, M.; Ngo, H.; Guo, W. Modelling bioprocesses and membrane fouling in membrane bioreactor (MBR): A review towards finding an integrated model framework. Bioresour. Technol. 2012, 122, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiu, Z.; Chen, Z.; Li, J.; Zhang, Y.; Wang, X.; Zhang, B. Comparison and analysis of membrane fouling between flocculent sludge membrane bioreactor and granular sludge membrane bioreactor. PLoS ONE 2012, 7, e40819. [Google Scholar]
- Wang, X.; Zhang, B.; Shen, Z.; Qiu, Z.; Chen, Z.; Jin, M.; Li, J.; Wang, J. The EPS characteristics of sludge in an aerobic granule membrane bioreactor. Bioresour. Technol. 2010, 101, 8046–8050. [Google Scholar]
- Liu, Y.-Q.; Lan, G.-H.; Zeng, P. Size-dependent calcium carbonate precipitation induced microbiologically in aerobic granules. Chem. Eng. J. 2016, 285, 341–348. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, H.; Yang, F.; Wang, Y.; Gao, M. Long-term storage and subsequent reactivation of aerobic granules. Bioresour. Technol. 2008, 99, 8304–8309. [Google Scholar] [CrossRef]
- De la Torre, T.; Rodriguez, C.; Gomez, M.; Alonso, E.; Malfeito, J. The IFAS-MBR process: A compact combination of biofilm and MBR tecnnology as RO pretreatment. Desalin. Water Treat. 2013, 51, 1063–1069. [Google Scholar] [CrossRef]
- Iorhemen, O.T.; Hamza, R.A.; Tay, J.H. Membrane bioreactor (MBR) technology for wastewater treatment and reclamation: Membrane fouling. Membranes 2016, 6, 33. [Google Scholar] [CrossRef]
- Thanh, B.X.; Visvanathan, C.; Aim, R.B. Fouling characterization and nitrogen removal in a batch granulation membrane bioreactor. Int. Biodeterior. Biodegrad. 2013, 85, 491–498. [Google Scholar] [CrossRef]
- Li, X.; Du, R.; Peng, Y.; Zhang, Q.; Wang, J. Characteristics of sludge granulation and EPS production in development of stable partial nitrification. Bioresour. Technol. 2020, 303, 122937. [Google Scholar] [CrossRef]


| Parameters | Unit | Influent | In Reactor | Effluent |
|---|---|---|---|---|
| DO | mg L−1 | - | 4.63 ± 0.84 | - |
| pH | - | 8.22 ± 0.14 | 7.23 ± 0.23 | 7.24 ± 0.22 |
| NH4+-N | mg L−1 | 49.70 ± 2.50 | 3.26 ± 4.17 | 2.66 ± 5.83 |
| NO2−-N | mg L−1 | 0.28 ± 0.21 | 3.42 ± 4.53 | 3.85 ± 5.83 |
| NO3−-N | mg L−1 | 0.79 ± 0.53 | 45.56 ± 6.73 | 45.93 ± 5.97 |
| Parameters | Unit | Granules | Influent | Effluent |
|---|---|---|---|---|
| Particle size | mm | 1.8 ± 0.6 | - | - |
| Settling velocity | m h−1 | 54.2 ± 4.4 | - | - |
| DO | mg L−1 | - | 4.11 ± 0.72 | - |
| pH | - | - | 8.34 ± 0.16 | 7.26 ± 0.19 |
| Turbidity | NTU | - | 2.12 ± 1.12 | 0.77 ± 0.31 |
| NH4+-N | mg L−1 | - | 50.74 ± 5.36 | 1.70 ± 1.62 |
| NO2--N | mg L−1 | - | 0.80 ± 0.28 | 0.55 ± 0.32 |
| NO3--N | mg L−1 | - | 0.65 ± 0.19 | 49.83 ± 5.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Z.; Zhang, X.; Bott, C.B.; Wang, Z.-W. Long-Term Stability of Nitrifying Granules in a Membrane Bioreactor without Hydraulic Selection Pressure. Processes 2021, 9, 1024. https://doi.org/10.3390/pr9061024
An Z, Zhang X, Bott CB, Wang Z-W. Long-Term Stability of Nitrifying Granules in a Membrane Bioreactor without Hydraulic Selection Pressure. Processes. 2021; 9(6):1024. https://doi.org/10.3390/pr9061024
Chicago/Turabian StyleAn, Zhaohui, Xueyao Zhang, Charles B. Bott, and Zhi-Wu Wang. 2021. "Long-Term Stability of Nitrifying Granules in a Membrane Bioreactor without Hydraulic Selection Pressure" Processes 9, no. 6: 1024. https://doi.org/10.3390/pr9061024
APA StyleAn, Z., Zhang, X., Bott, C. B., & Wang, Z.-W. (2021). Long-Term Stability of Nitrifying Granules in a Membrane Bioreactor without Hydraulic Selection Pressure. Processes, 9(6), 1024. https://doi.org/10.3390/pr9061024







