Utility of Low-Cost Multichannel Data Acquisition System for Photoacoustic Computed Tomography
Abstract
1. Introduction
2. Materials and Methods
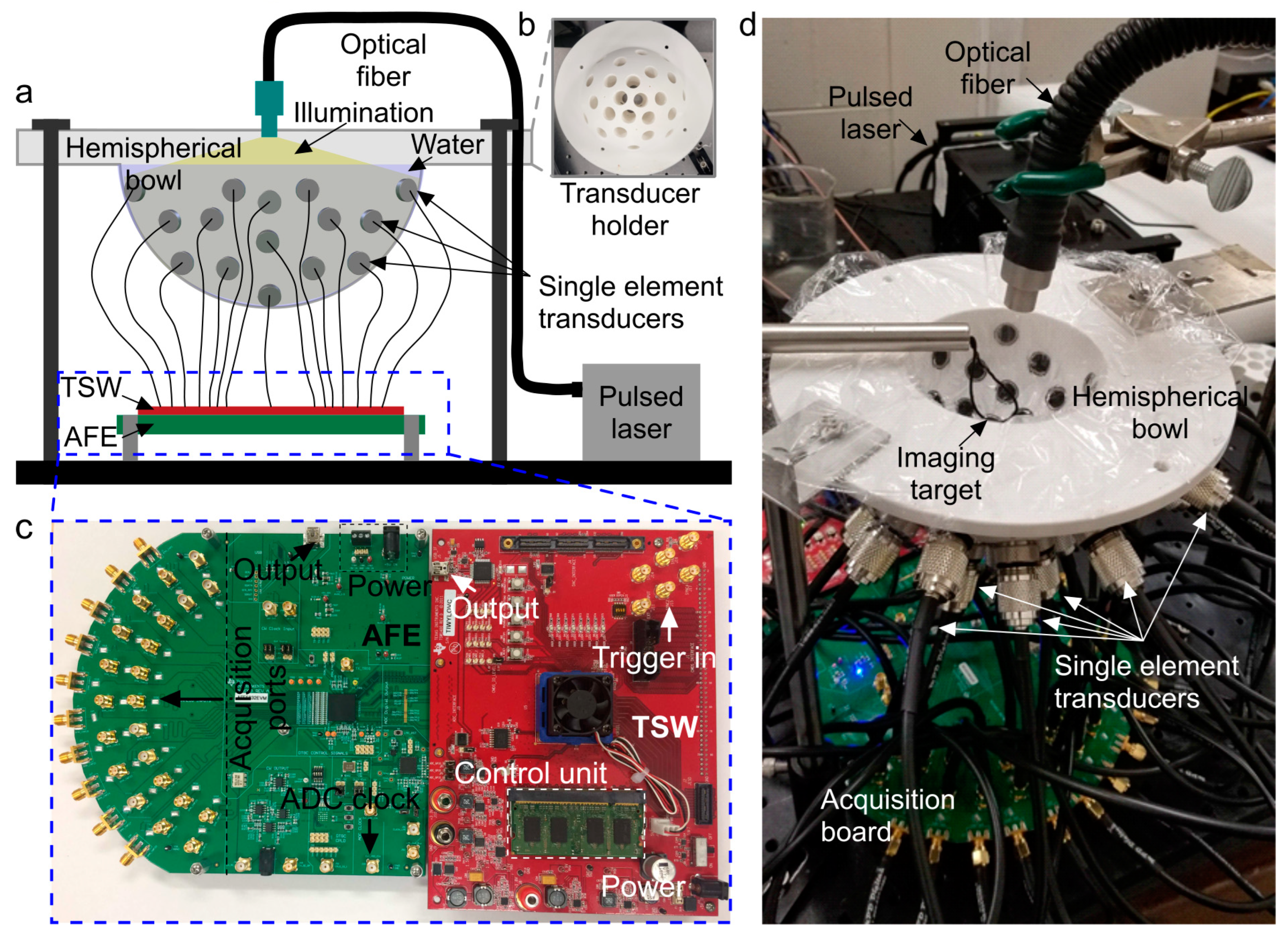
2.1. PACT System Setup
2.2. Low-Cost Data Acquisition Framework:
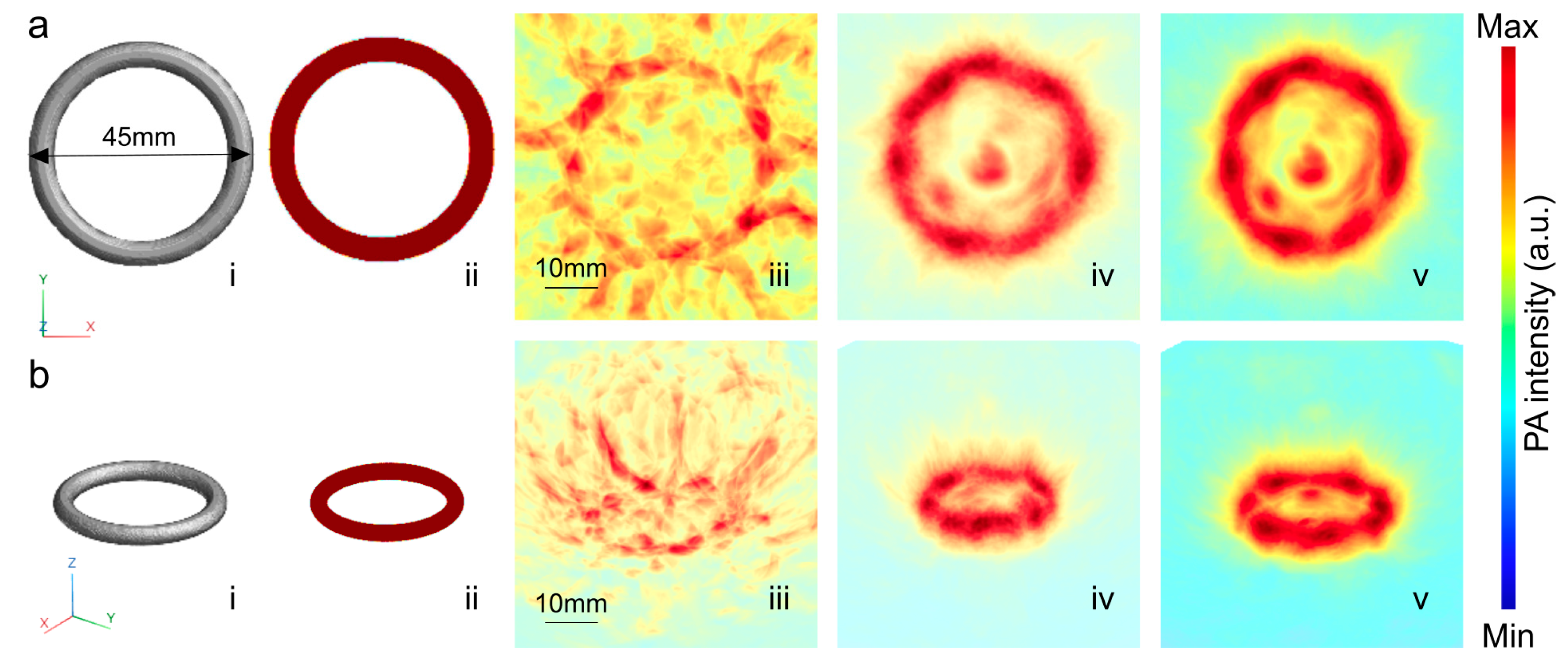
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, E.Z.; Povazay, B.; Laufer, J.; Alex, A.; Hofer, B.; Pedley, B.; Glittenberg, C.; Treeby, B.; Cox, B.; Beard, P.; et al. Multimodal photoacoustic and optical coherence tomography scanner using an all optical detection scheme for 3D morphological skin imaging. Biomed. Opt. Express 2011, 2, 2202–2215. [Google Scholar] [CrossRef]
- Chen, Z.; Rank, E.; Meiburger, K.M.; Sinz, C.; Hodul, A.; Zhang, E.; Hoover, E.; Minneman, M.; Ensher, J.; Beard, P.C.; et al. Non-invasive multimodal optical coherence and photoacoustic tomography for human skin imaging. Sci. Rep. 2017, 7, 17975. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Yang, S.; Wang, Y.; Gu, Y.; Xing, D. Noninvasive and high-resolving photoacoustic dermoscopy of human skin. Biomed. Opt. Express 2016, 7, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Kratkiewicz, K.; Manwar, R.; Rajabi-Estarabadi, A.; Fakhoury, J.; Meiliute, J.; Daveluy, S.; Mehregan, D.; Avanaki, K. Photoacoustic/ultrasound/optical coherence tomography evaluation of melanoma lesion and healthy skin in a Swine model. Sensors 2019, 19, 2815. [Google Scholar] [CrossRef] [PubMed]
- Olds, H.; Mehregan, D.; Kratiewicz, K.; Manwar, R.; Xu, Q.; Reddy, S.; Mahmoodkayayeh, S.; Avanaki, K. Is photoacoustic imaging clinically safe: Evaluation of possible thermal damage due to laser-tissue interaction. In Medical Student Research Symposium; Wayne State University: Detroit, MI, USA, 2020; Available online: https://digitalcommons.wayne.edu/som_srs/56 (accessed on 8 April 2025).
- Ermilov, S.A.; Khamapirad, T.; Conjusteau, A.; Leonard, M.H.; Lacewell, R.; Mehta, K.; Miller, T.; Oraevsky, A. Laser optoacoustic imaging system for detection of breast cancer. J. Biomed. Opt. 2009, 14, 024007. [Google Scholar] [CrossRef]
- Copland, J.A.; Eghtedari, M.; Popov, V.L.; Kotov, N.; Mamedova, N.; Motamedi, M.; Oraevsky, A.A. Bioconjugated gold nanoparticles as a molecular based contrast agent: Implications for imaging of deep tumors using optoacoustic tomography. Mol. Imaging Biol. 2004, 6, 341–349. [Google Scholar] [CrossRef]
- Manohar, S.; Vaartjes, S.E.; van Hespen, J.C.G.; Klaase, J.M.; Engh, F.M.v.D.; Steenbergen, W.; van Leeuwen, T.G. Initial results of in vivo non-invasive cancer imaging in the human breast using near-infrared photoacoustics. Opt. Express 2007, 15, 12277–12285. [Google Scholar] [CrossRef]
- Mallidi, S.; Luke, G.P.; Emelianov, S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends Biotechnol. 2011, 29, 213–221. [Google Scholar] [CrossRef]
- Oraevsky, A.A.; Karabutov, A.A.; Solomatin, S.V.; Savateeva, E.V.; Andreev, V.A.; Gatalica, Z.; Singh, H.; Fleming, R.D. Laser optoacoustic imaging of breast cancer in vivo. In Proceedings of the BiOS 2001 the International Symposium on Biomedical Optics, San Jose, CA, USA, 15 June 2001; International Society for Optics and Photonics: Bellingham, WA, USA, 2001. [Google Scholar]
- Li, M.-L.; Oh, J.-T.; Xie, X.; Ku, G.; Wang, W.; Li, C.; Lungu, G.; Stoica, G.; Wang, L.V. Simultaneous Molecular and Hypoxia Imaging of Brain Tumors In Vivo Using Spectroscopic Photoacoustic Tomography. Proc. IEEE 2008, 96, 481–489. [Google Scholar] [CrossRef]
- Nie, L.; Cai, X.; Maslov, K.; Garcia-Uribe, A.; Anastasio, M.A.; Wang, L.V. Photoacoustic tomography through a whole adult human skull with a photon recycler. J. Biomed. Opt. 2012, 17, 110506. [Google Scholar] [CrossRef]
- Ranjbaran, S.M.; Zafar, M.; Saint-Martin, L.; Islam, T.; Avanaki, K. A practical solution to improve the field of view in circular scanning-based photoacoustic tomography. J. Biophotonics 2024, 17, e202400125. [Google Scholar] [CrossRef] [PubMed]
- Ranjbaran, S.M.; Kratkiewicz, K.; Manwar, R.; Avanaki, K. Line Illumination in Linear Array Photoacoustic Imaging Using a Powell Lens: A Proof-of-Concept Study. Photonics 2024, 11, 288. [Google Scholar] [CrossRef]
- Kratkiewicz, K.; Manwar, R.; Zafar, M.; Ranjbaran, S.M.; Mozaffarzadeh, M.; de Jong, N.; Ji, K.; Avanaki, K. Development of a stationary 3D photoacoustic imaging system using sparse single-element transducers: Phantom study. Appl. Sci. 2019, 9, 4505. [Google Scholar] [CrossRef]
- Kratkiewicz, K.; Manwar, R.; Zhou, Y.; Mozaffarzadeh, M.; Avanaki, K. Technical considerations in the Verasonics research ultrasound platform for developing a photoacoustic imaging system. Biomed. Opt. Express 2021, 12, 1050–1084. [Google Scholar] [CrossRef]
- Manwar, R.; Lara, J.B.; Prakash, R.; Ranjbaran, S.M.; Avanaki, K. Randomized multi-angle illumination for improved linear array photoacoustic computed tomography in brain. J. Biophotonics 2022, 15, e202200016. [Google Scholar] [CrossRef] [PubMed]
- Manwar, R.; Li, X.; Mahmoodkalayeh, S.; Asano, E.; Zhu, D.; Avanaki, K. Deep learning protocol for improved photoacoustic brain imaging. J. Biophotonics 2020, 13, e202000212. [Google Scholar] [CrossRef]
- Mozaffarzadeh, M.; Mahloojifar, A.; Orooji, M.; Adabi, S.; Nasiriavanaki, M. Double-stage delay multiply and sum beamforming algorithm: Application to linear-array photoacoustic imaging. IEEE Trans. Biomed. Eng. 2017, 65, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Nasiriavanaki, M.; Xia, J.; Wan, H.; Bauer, A.Q.; Culver, J.P.; Wang, L.V. High-resolution photoacoustic tomography of resting-state functional connectivity in the mouse brain. Proc. Natl. Acad. Sci. USA 2013, 111, 21–26. [Google Scholar] [CrossRef]
- Saint-Martin, L.; Prakash, R.; Ranjbaran, S.M.; Manwar, R.; Avanaki, K. Study of Aberrations in Transcranial Photoacoustic Imaging Using μCT images of Skull: A Simulation Study. IEEE Sens. J. 2025. [Google Scholar] [CrossRef]
- Wang, L.V. Tutorial on photoacoustic microscopy and computed tomography. IEEE J. Sel. Top. Quantum Electron. 2008, 14, 171–179. [Google Scholar] [CrossRef]
- Hariri, A.; Fatima, A.; Mohammadian, N.; Mahmoodkalayeh, S.; Ansari, M.A.; Bely, N.; Avanaki, M.R.N. Development of low-cost photoacoustic imaging systems using very low-energy pulsed laser diodes. J. Biomed. Opt. 2017, 22, 075001. [Google Scholar] [CrossRef] [PubMed]
- Kruger, R.A.; Kuzmiak, C.M.; Lam, R.B.; Reinecke, D.R.; Del Rio, S.P.; Steed, D. Dedicated 3D photoacoustic breast imaging. Med Phys. 2013, 40, 113301. [Google Scholar] [CrossRef]
- Ku, G.; Wang, L.V. Deeply penetrating photoacoustic tomography in biological tissues enhanced with an optical contrast agent. Opt. Lett. 2005, 30, 507–509. [Google Scholar] [CrossRef]
- Chitgupi, U.; Nyayapathi, N.; Kim, J.; Wang, D.; Sun, B.; Li, C.; Carter, K.; Huang, W.-C.; Kim, C.; Xia, J.; et al. Surfactant-Stripped Micelles for NIR-II Photoacoustic Imaging through 12 cm of Breast Tissue and Whole Human Breasts. Adv. Mater. 2019, 31, e1902279. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Wang, K.; Oraevsky, A.A.; Anastasio, M.A. Impact of nonstationary optical illumination on image reconstruction in optoacoustic tomography. J. Opt. Soc. Am. A 2016, 33, 2333–2347. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic tomography: In vivo imaging from organelles to organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef]
- Gamelin, J.K.; Aguirre, A.; Maurudis, A.; Huang, F.; Castillo, D.; Wang, L.V.; Zhu, Q. Curved array photoacoustic tomographic system for small animal imaging. J. Biomed. Opt. 2008, 13, 024007. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Chatni, M.R.; Maslov, K.I.; Guo, Z.; Wang, K.; Anastasio, M.A.; Wang, L.V. Whole-body ring-shaped confocal photoacoustic computed tomography of small animals in vivo. J. Biomed. Opt. 2012, 17, 050506. [Google Scholar] [CrossRef]
- Xia, J.; Guo, Z.; Maslov, K.I.; Wang, L.V.; Aguirre, A.; Zhu, Q.; Percival, C. Three-dimensional photoacoustic tomography based on the focal-line concept. J. Biomed. Opt. 2011, 16, 090505. [Google Scholar] [CrossRef]
- Deán-Ben, X.L.; Fehm, T.F.; Ford, S.J.; Gottschalk, S.; Razansky, D. Spiral volumetric optoacoustic tomography visualizes multi-scale dynamics in mice. Light. Sci. Appl. 2017, 6, e16247. [Google Scholar] [CrossRef]
- Li, L.; Zhu, L.; Ma, C.; Lin, L.; Yao, J.; Wang, L.; Maslov, K.; Zhang, R.; Chen, W.; Shi, J.; et al. Single-impulse panoramic photoacoustic computed tomography of small-animal whole-body dynamics at high spatiotemporal resolution. Nat. Biomed. Eng. 2017, 1, 0071. [Google Scholar] [CrossRef] [PubMed]
- Merčep, E.; Herraiz, J.L.; Deán-Ben, X.L.; Razansky, D. Transmission–reflection optoacoustic ultrasound (TROPUS) computed tomography of small animals. Light. Sci. Appl. 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, L.; Behnam, H.; Tavakkoli, J.; Avanaki, M.R.N. Skull’s Photoacoustic Attenuation and Dispersion Modeling with Deterministic Ray-Tracing: Towards Real-Time Aberration Correction. Sensors 2019, 19, 345. [Google Scholar] [CrossRef] [PubMed]
- Ranjbaran, S.M.; Aghamiry, H.S.; Gholami, A.; Operto, S.; Avanaki, K. Quantitative Photoacoustic Tomography Using Iteratively Refined Wavefield Reconstruction Inversion: A Simulation Study. IEEE Trans. Med Imaging 2023, 43, 874–885. [Google Scholar] [CrossRef]
- Prakash, R.; Manwar, R.; Avanaki, K. Evaluation of 10 current image reconstruction algorithms for linear array photoacoustic imaging. J. Biophotonics 2024, 17, e202300117. [Google Scholar] [CrossRef]
- Wang, B.; Xiang, L.; Jiang, M.S.; Yang, J.; Zhang, Q.; Carney, P.R.; Jiang, H. Photoacoustic tomography system for noninvasive real-time three-dimensional imaging of epilepsy. Biomed. Opt. Express 2012, 3, 1427–1432. [Google Scholar] [CrossRef]
- Wang, B.; Xiao, J.; Jiang, H. Simultaneous real-time 3D photoacoustic tomography and EEG for neurovascular coupling study in an animal model of epilepsy. J. Neural Eng. 2014, 11, 046013. [Google Scholar] [CrossRef]
- Ephrat, P.; Roumeliotis, M.; Prato, F.S.; Carson, J.J. Four-dimensional photoacoustic imaging of moving targets. Opt. Express 2008, 16, 21570–21581. [Google Scholar] [CrossRef]
- Ephrat, P.; Keenliside, L.; Seabrook, A.; Prato, F.S.; Carson, J.J.L. Three-dimensional photoacoustic imaging by sparse-array detection and iterative image reconstruction. J. Biomed. Opt. 2008, 13, 054052. [Google Scholar] [CrossRef]
- Toi, M.; Asao, Y.; Matsumoto, Y.; Sekiguchi, H.; Yoshikawa, A.; Takada, M.; Kataoka, M.; Endo, T.; Kawaguchi-Sakita, N.; Kawashima, M.; et al. Visualization of tumor-related blood vessels in human breast by photoacoustic imaging system with a hemispherical detector array. Sci. Rep. 2017, 7, 41970. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Asao, Y.; Yoshikawa, A.; Sekiguchi, H.; Takada, M.; Furu, M.; Saito, S.; Kataoka, M.; Abe, H.; Yagi, T.; et al. Label-free photoacoustic imaging of human palmar vessels: A structural morphological analysis. Sci. Rep. 2018, 8, 786. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kajita, H.; Konishi, N.; Oh, A.; Urano, M.; Watanabe, S.; Asao, Y.; Imanishi, N.; Tsuji, T.; Jinzaki, M.; et al. Subcutaneous lymphatic vessels in the lower extremities: Comparison between photoacoustic lymphangiography and near-infrared fluorescence lymphangiography. Radiology 2020, 295, 469–474. [Google Scholar] [CrossRef]
- Nagae, K.; Asao, Y.; Sudo, Y.; Murayama, N.; Tanaka, Y.; Ohira, K.; Ishida, Y.; Otsuka, A.; Matsumoto, Y.; Saito, S.; et al. Real-time 3D photoacoustic visualization system with a wide field of view for imaging human limbs. F1000Research 2019, 7, 1813. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, Y.; Asao, Y.; Sekiguchi, H.; Yoshikawa, A.; Ishii, T.; Nagae, K.-I.; Kobayashi, S.; Tsuge, I.; Saito, S.; Takada, M.; et al. Visualising peripheral arterioles and venules through high-resolution and large-area photoacoustic imaging. Sci. Rep. 2018, 8, 14930. [Google Scholar] [CrossRef]
- Zafar, M.; McGuire, L.S.; Ranjbaran, S.M.; Matchynski, J.I.; Manwar, R.; Conti, A.C.; Perrine, S.A.; Avanaki, K. Spiral laser scanning photoacoustic microscopy for functional brain imaging in rats. Neurophotonics 2024, 11, 015007. [Google Scholar] [CrossRef]
- Zafar, M.; Kratkiewicz, K.; Manwar, R.; Avanaki, M. Development of Low-Cost Fast Photoacoustic Computed Tomography: System Characterization and Phantom Study. Appl. Sci. 2019, 9, 374. [Google Scholar] [CrossRef]
- Kirchner, T.; Villringer, C.; Laufer, J. Evaluation of ultrasound sensors for transcranial photoacoustic sensing and imaging. Photoacoustics 2023, 33, 100556. [Google Scholar] [CrossRef] [PubMed]
- Laser Institute of America. American National Standard for Safe Use of Lasers; Laser Institute of America: Orlando, FL, USA, 2007. [Google Scholar]
- Bossy, E.; Daoudi, K.; Boccara, A.-C.; Tanter, M.; Aubry, J.-F.; Montaldo, G.; Fink, M. Time reversal of photoacoustic waves. Appl. Phys. Lett. 2006, 89, 184108. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.V. Time reversal in photoacoustic or thermoacoustic tomography. In Photoacoustic Imaging and Spectroscopy; CRC Press: Boca Raton, FL, USA, 2017; pp. 117–120. [Google Scholar]
- Treeby, B.E.; Cox, B.T. k-Wave: MATLAB toolbox for the simulation and reconstruction of photoacoustic wave fields. J. Biomed. Opt. 2010, 15, 021314. [Google Scholar] [CrossRef]
- Lusk, J.F.; Miranda, C.; Smith, B.S. Ovarian Cancer Detection Using Photoacoustic Flow Cytometry. JoVE 2020, 155, e60279. [Google Scholar] [CrossRef]
- Manwar, R.; Islam, T.; Ranjbaran, S.M.; Avanaki, K. Transfontanelle photoacoustic imaging: Ultrasound transducer selection analysis. Biomed. Opt. Express 2022, 13, 676–693. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Hartanto, J.; Joseph, R.; Wu, C.-H.; Zhao, Y.; Chen, Y.-S. Hybrid photoacoustic and fast super-resolution ultrasound imaging. Nat. Commun. 2023, 14, 2191. [Google Scholar] [CrossRef] [PubMed]


| Ref. | Data Acquisition System | No. of Channels | Resolution (Bits) | Sampling Rate (MSamples·s−1) | Cost (USD) |
| [54] | PXIe-5170 | 8 | 14 | 250 | ~15 k |
| [16] | Vantage 64 | 64 | 14 | 62.5 | ~30 k |
| [55] | Legion ADC | 128–256 | 12 | 40 | ~50 k |
| [56] | Vantage 256 | 256 | 14 | 62.5 | ~70 k |
| This report | AFE5832 + TSW1400 | 32 | 12 | 40 | <2 k |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, M.; Manwar, R.; Ranjbaran, S.M.; Avanaki, K. Utility of Low-Cost Multichannel Data Acquisition System for Photoacoustic Computed Tomography. Photonics 2025, 12, 385. https://doi.org/10.3390/photonics12040385
Zafar M, Manwar R, Ranjbaran SM, Avanaki K. Utility of Low-Cost Multichannel Data Acquisition System for Photoacoustic Computed Tomography. Photonics. 2025; 12(4):385. https://doi.org/10.3390/photonics12040385
Chicago/Turabian StyleZafar, Mohsin, Rayyan Manwar, Seyed Mohsen Ranjbaran, and Kamran Avanaki. 2025. "Utility of Low-Cost Multichannel Data Acquisition System for Photoacoustic Computed Tomography" Photonics 12, no. 4: 385. https://doi.org/10.3390/photonics12040385
APA StyleZafar, M., Manwar, R., Ranjbaran, S. M., & Avanaki, K. (2025). Utility of Low-Cost Multichannel Data Acquisition System for Photoacoustic Computed Tomography. Photonics, 12(4), 385. https://doi.org/10.3390/photonics12040385





