The Influence of Annealing Temperature on the Microstructure and Electrical Properties of Sputtered ZnO Thin Films
Abstract
1. Introduction
2. Results and Discussion
2.1. Surface Morphology
2.1.1. Atomic Force Microscopy (AFM) Analysis
2.1.2. Scanning Electron Microscopy (SEM) Analysis
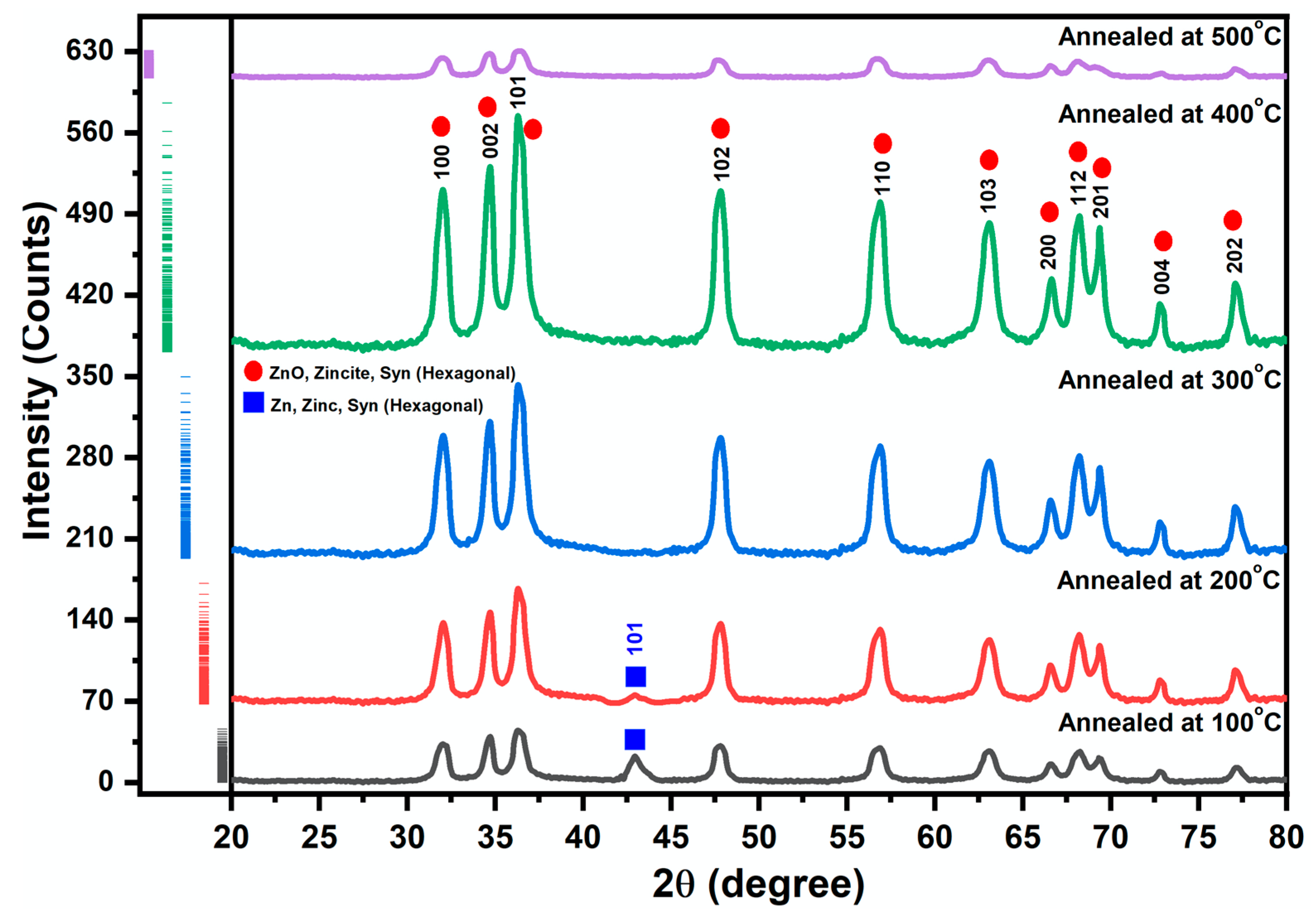
2.2. Structural Analysis
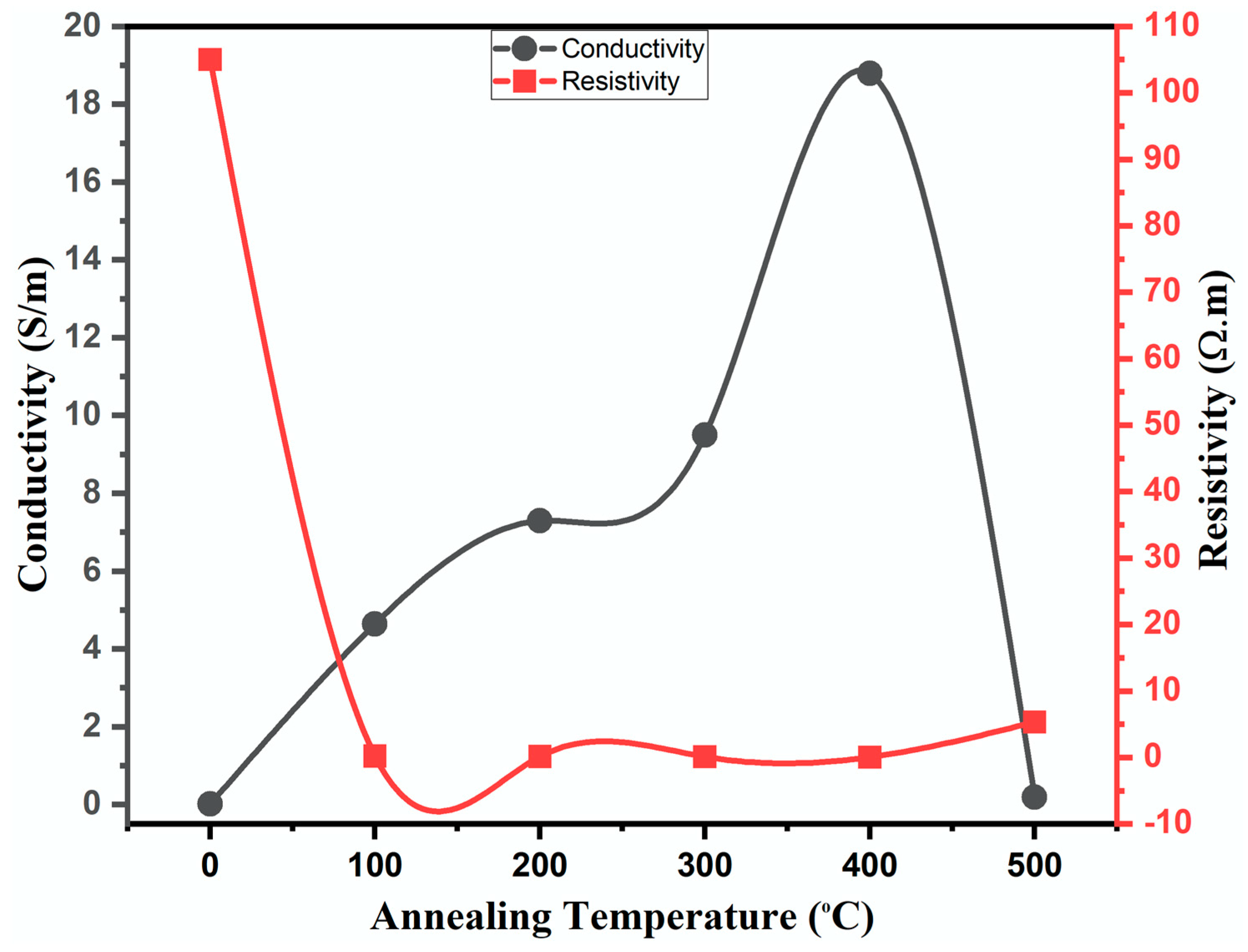
2.3. Electrical Properties
3. Conclusions
4. Experimental
4.1. Films Deposition
4.1.1. Substrate Preparation
4.1.2. Sputtering System and Film Deposition Process
4.2. Characterization Tools
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, M.; Islam, M. Deposition and Characterization of Molybdenum Thin Films Using DC Plasma Magnetron Sputtering 1. Semiconductors 2013, 47, 1610–1615. [Google Scholar] [CrossRef]
- Hussein, A.K. Applications of nanotechnology in renewable energies—A comprehensive overview and understanding. Renew. Sustain. Energy Rev. 2015, 42, 460–476. [Google Scholar] [CrossRef]
- Ali, S.; Saleem, S.; Salman, M.; Khan, M. Synthesis, structural and optical properties of ZnS–ZnO nanocomposites. Mater. Chem. Phys. 2020, 248, 122900. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today Proc. 2020, 49, 3028–3035. [Google Scholar] [CrossRef]
- Kumar, V.P.S.; Manikandan, N.; Nagaprasad, N.; Letatesfaye, J.; Krishnaraj, R. Analysis of the Performance Characteristics of ZnO Nanoparticles’ Dispersed Polyester Oil. Adv. Mater. Sci. Eng. 2022, 2022, 4844979. [Google Scholar] [CrossRef]
- Liu, C.; Burghaus, U.; Besenbacher, F.; Wang, Z.L. Preparation and Characterization of Nanomaterials for Sustainable Energy Production. ACS Nano 2010, 4, 5517–5526. [Google Scholar] [CrossRef]
- Sanchez, C.; Lebeau, B.; Chaput, F.; Boilot, J. Optical Properties of Functional Hybrid Organic-Inorganic Nanocomposites. Adv. Mater. 2004, 15, 1969–1994. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, F.; Liu, L.; Gao, Y.; Xing, L.; Li, Q.; Fu, C.; Zhong, Z.; Zhang, X. Improvement of bond strength between ZnO nanorods and carbon fibers using magnetron sputtered ZnO films as the interphase. CrystEngComm 2017, 19, 868–875. [Google Scholar] [CrossRef]
- Ferhati, H.; Djeffal, F.; Kacha, K. Optimizing the optical performance of ZnO/Si-based solar cell using metallic nanoparticles and interface texturization. Optik 2018, 153, 43–49. [Google Scholar] [CrossRef]
- Klingshirn, C.; Priller, H.; Decker, M.; Bruckner, J.; Kalt, H.; Hauschild, R.; Zeller, J.; Waag, A.; Bakin, A.; Wehmann, H.; et al. Excitonic properties of ZnO. Adv. Solid State Phys. 2005, 45, 275–287. [Google Scholar]
- Kou, L.Z.; Guo, W.L.; Li, C. Piezoelectricity of ZNO and its nanostructures. In 2008 Symposium on Piezoelectricity, Acoustic Waves, and Device Applications; IEEE: New York, NY, USA, 2008; pp. 354–359. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Wu, H.; Yang, J.; Wang, Y.; Zhang, J.; Bao, Q.; Wang, M.; Ma, Z.; Tress, W.; et al. A Transparent Electrode Based on Solution-Processed ZnO for Organic Optoelectronic Devices. Nat. Commun. 2022, 13, 4387. [Google Scholar] [CrossRef]
- Blom, F.R.; Bauhuis, G. 365 RF Planar Magnetron Sputtered ZnO Films II: Electrical Properties. Thin Solid Film. 1991, 204, 365–376. [Google Scholar] [CrossRef]
- Mahmood, F.S.; Gould, R.D.; Hassan, A.K.; Salih, H.M. DC properties of ZnO thin films prepared by rf magnetron sputtering. Thin Solid Film. 1995, 770, 95–98. [Google Scholar]
- Ponja, S.D.; Sathasivam, S.; Parkin, I.P.; Carmalt, C.J. Highly conductive and transparent gallium doped zinc oxide thin films via chemical vapor deposition. Sci. Rep. 2020, 10, 1–7. [Google Scholar]
- Sharma, R.; Yoo, S. ZnO in organic electronics. In Nanostructured Zinc Oxide: Synthesis, Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Saitou, M.; Makabe, A.; Tomoyose, T. AFM study of surface roughening in sputter-deposited nickel films on ITO glasses. Europhys. Lett. 2000, 52, 185–188. [Google Scholar] [CrossRef]
- Hasan, S.A.; Gibson, D.; Song, S.; Wu, Q.; Ng, W.P.; McHale, G.; Dean, J.; Fu, Y.Q. ZnO thin film based flexible temperature sensor. Proc. IEEE Sens. 2017, 2017, 1–3. [Google Scholar]
- Xuan, J.; Zhao, G.; Sun, M.; Jia, F.; Wang, X.; Zhou, T.; Yin, G.; Liu, B. Low-temperature operating ZnO-based NO2 sensors: A review. RSC Adv. 2020, 10, 39786–39807. [Google Scholar] [CrossRef]
- Nomura, K.; Ohta, H.; Ueda, K.; Kamiya, T.; Hirano, M.; Hosono, H. Thin-Film Transistor Fabricated in Single-Crystalline Transparent Oxide Semiconductor. Nanotechnology 2003, 300, 1269–1272. [Google Scholar] [CrossRef]
- Bel Hadj Tahar, R.; Ban, T.; Ohya, Y.; Takahashi, Y. Tin doped indium oxide thin films: Electrical properties. J. Appl. Phys. 1998, 83, 2631–2645. [Google Scholar] [CrossRef]
- Kind, B.H.; Yan, H.; Messer, B.; Law, M.; Yang, P. Nanowire Ultraviolet Photodetectors and Optical. Adv. Mater. 2002, 14, 200–202. [Google Scholar] [CrossRef]
- Zaier, A. Annealing effects on the structural, electrical and optical properties of ZnO thin films prepared by thermal evaporation technique. J. King Saud Univ. Sci. 2015, 27, 356–360. [Google Scholar] [CrossRef]
- Husna, J.; Aliyu, M.M.; Islam, M.A.; Chelvanathan, P. Influence of Annealing Temperature on the Properties of ZnO Thin Films Grown by Sputtering. Energy Procedia 2012, 25, 55–61. [Google Scholar] [CrossRef]
- Shivaraj, B.W.; Murthy, H.N.N.; Krishna, M.; Satyanarayana, B.S. Effect of Annealing Temperature on Structural and Optical properties of Dip and Spin coated ZnO Thin Films. Procedia Mater. Sci. 2015, 10, 292–300. [Google Scholar] [CrossRef]
- Films, T.S.; Engineering, E.; Enschede, A.E. 1.1. Sputtered thin film ZnO. Thin Solid Film. 1991, 204, 349–364. [Google Scholar]
- Soci, C.; Zhang, A.; Xiang, B.; Dayeh, S.A.; Aplin, D.P.R.; Park, J.; Bao, X.Y.; Lo, Y.H.; Wang, D. ZnO Nanowire UV Photodetectors with High Internal Gain. Nano Lett. 2007, 7, 1003–1009. [Google Scholar] [CrossRef]
- Gurylev, V.; Perng, T.P. Defect engineering of ZnO: Review on oxygen and zinc vacancies. J. Eur. Ceram. Soc. 2021, 41, 4977–4996. [Google Scholar] [CrossRef]
- Heremans, J.P. The ugly duckling. Nature 2014, 508, 327–328. [Google Scholar] [CrossRef]
- McCluskey, M.D. Defects in ZnO. In Defects in Advanced Electronic Materials and Novel Low Dimensional Structures; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Gorla, C.R.; Emanetoglu, N.W.; Liang, S.; Mayo, W.E.; Lu, Y.; Wraback, M.; Shen, H.; Gorla, C.R.; Emanetoglu, N.W.; Liang, S.; et al. Structural, optical, and surface acoustic wave properties of epitaxial ZnO films grown on (0112) sapphire by metalorganic chemical vapor deposition. J. Appl. Phys. 2009, 85, 2595–2602. [Google Scholar] [CrossRef]
- Huang, P.; Huang, C.; Lin, M.; Chou, C.; Hsu, C.; Kuo, C. The Effect of Sputtering Parameters on the Film Properties of Molybdenum Back Contact for CIGS Solar Cells. Int. J. Photoenergy 2013, 2013, 390824. [Google Scholar] [CrossRef]
- Kumar, S.; Gupta, V.; Sreenivas, K. Synthesis of photoconducting ZnO nano-needles using an unbalanced magnetron sputtered ZnO/Zn/ZnO multilayer structure. Nanotechnology 2005, 16, 1167. [Google Scholar] [CrossRef]
- McCluskey, M.D.; Jokela, S.J. Defects in ZnO. J. Appl. Phys. 2009, 106, 071101. [Google Scholar] [CrossRef]
- Mohamed, D. Influence of Low Ag Doping on Structural, Morphological and Optical Properties of Sol-Gel Dip-Coated Nanostructured ZnO Thin Films. Int. J. Nat. Sci. 2016, 4, 15–28. [Google Scholar]
- Chen, W.-T.; Fang, P.-C.; Chen, Y.-W.; Chiu, S.-J.; Ku, C.-S.; Brahma, S.; Lo, K.-Y. Zn dots coherently grown as the seed and buffer layers on Si(111) for ZnO thin film: Mechanism, in situ analysis, and simulation. J. Vac. Sci. Technol. A 2022, 40, 063403. [Google Scholar] [CrossRef]
- Fu, Z.; Lin, B.; Liao, G.; Wu, Z. The effect of Zn buffer layer on growth and luminescence of ZnO films deposited on Si substrates. J. Cryst. Growth 1998, 193, 316–321. [Google Scholar] [CrossRef]
- Seto, J.Y.W. The electrical properties of polycrystalline silicon films. J. Appl. Phys. 1975, 46, 5247–5254. [Google Scholar] [CrossRef]
- Bhuvana, K.P.; Elanchezhiyan, J.; Gopalakrishnan, N.; Balasubramanian, T. Influence of grain size on the properties of AlN doped ZnO thin film. Mater. Sci. Semicond. Processing. 2011, 14, 84–88. [Google Scholar] [CrossRef]
- Zhu, M.W.; Wang, Z.J.; Chen, Y.N.; Wang, H.L.; Zhang, Z.D. Effect of grain boundary on electrical properties of polycrystalline lanthanum nickel oxide thin films. Appl. Phys. A 2013, 112, 1011–1018. [Google Scholar] [CrossRef]
- Imajo, T.; Suemasu, T.; Toko, K. Strain effects on polycrystalline germanium thin films. Sci. Rep. 2021, 11, 8333. [Google Scholar] [CrossRef]
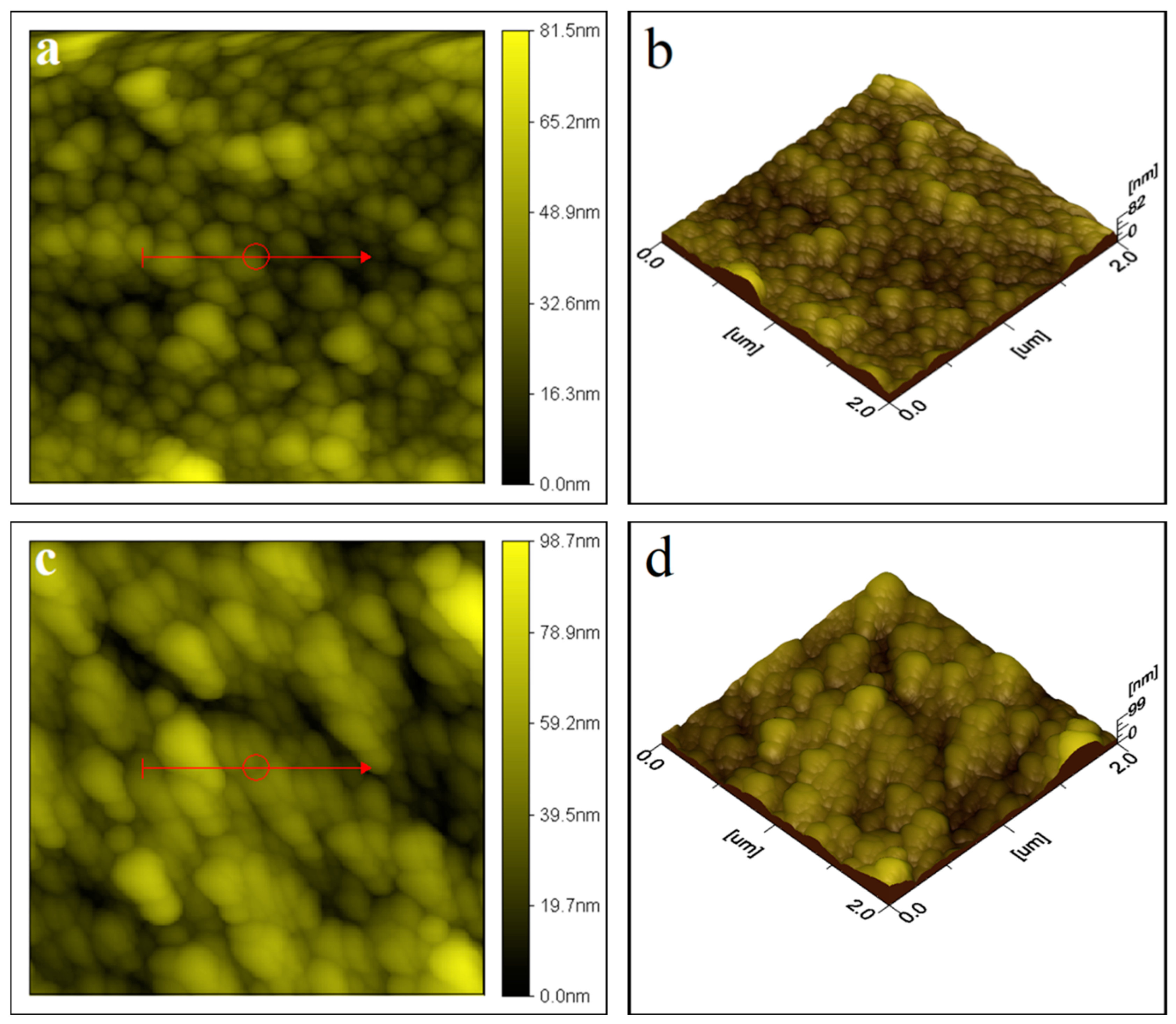
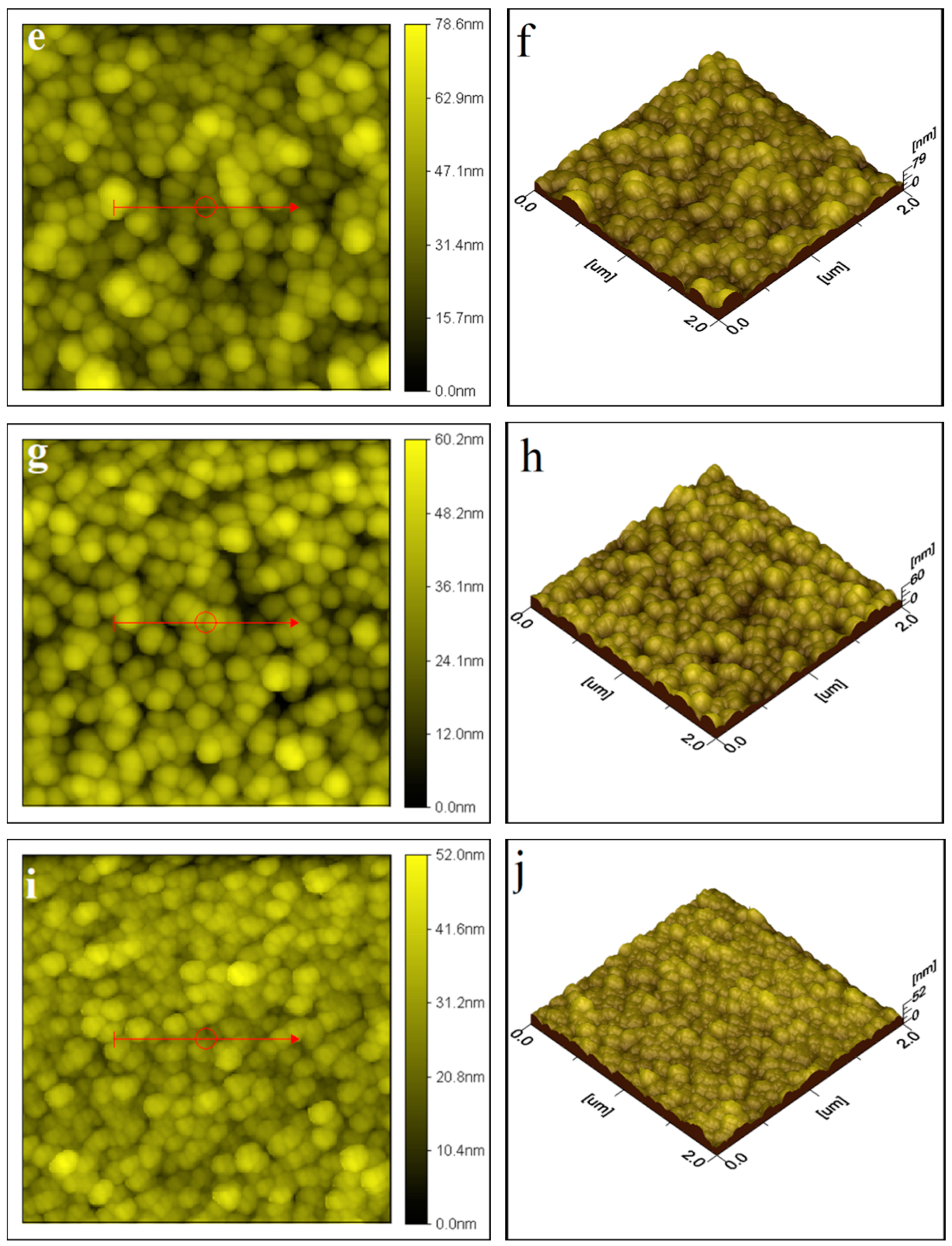
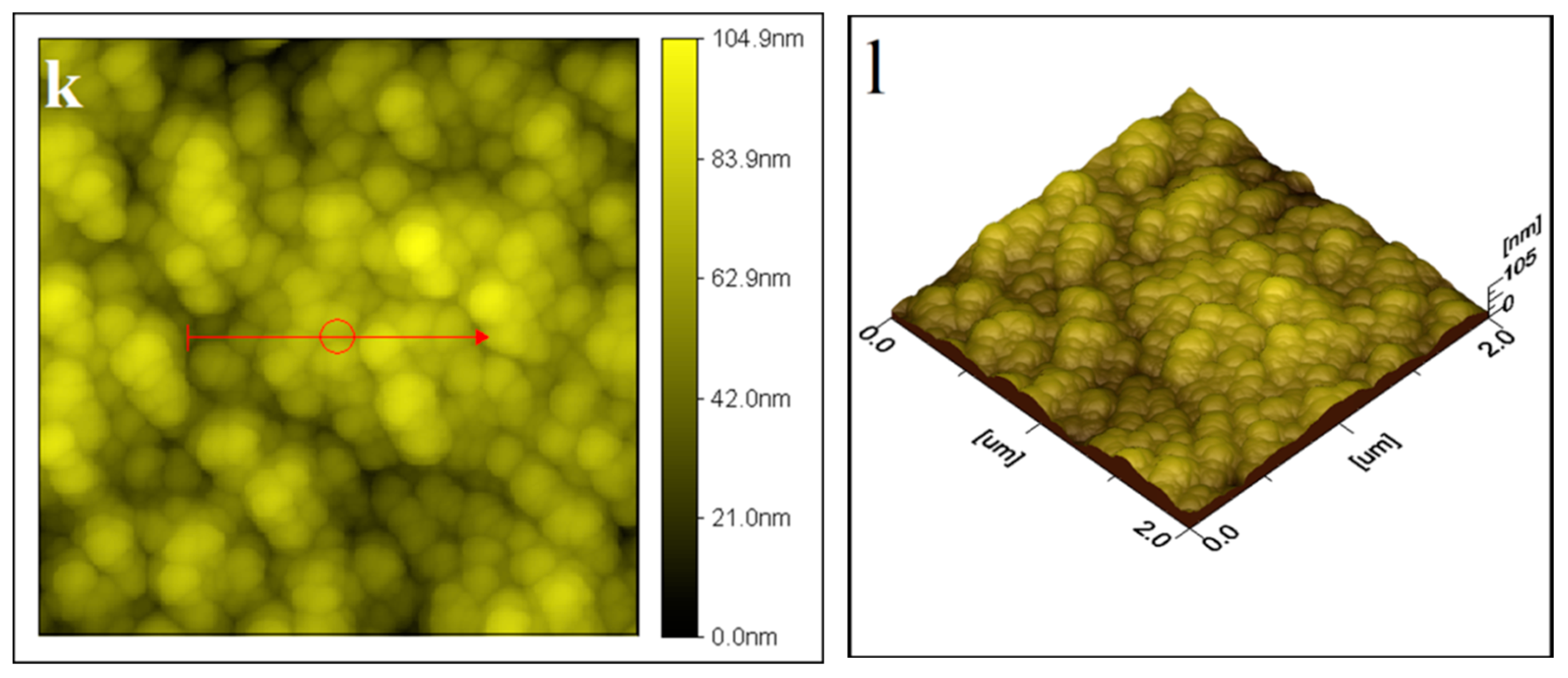
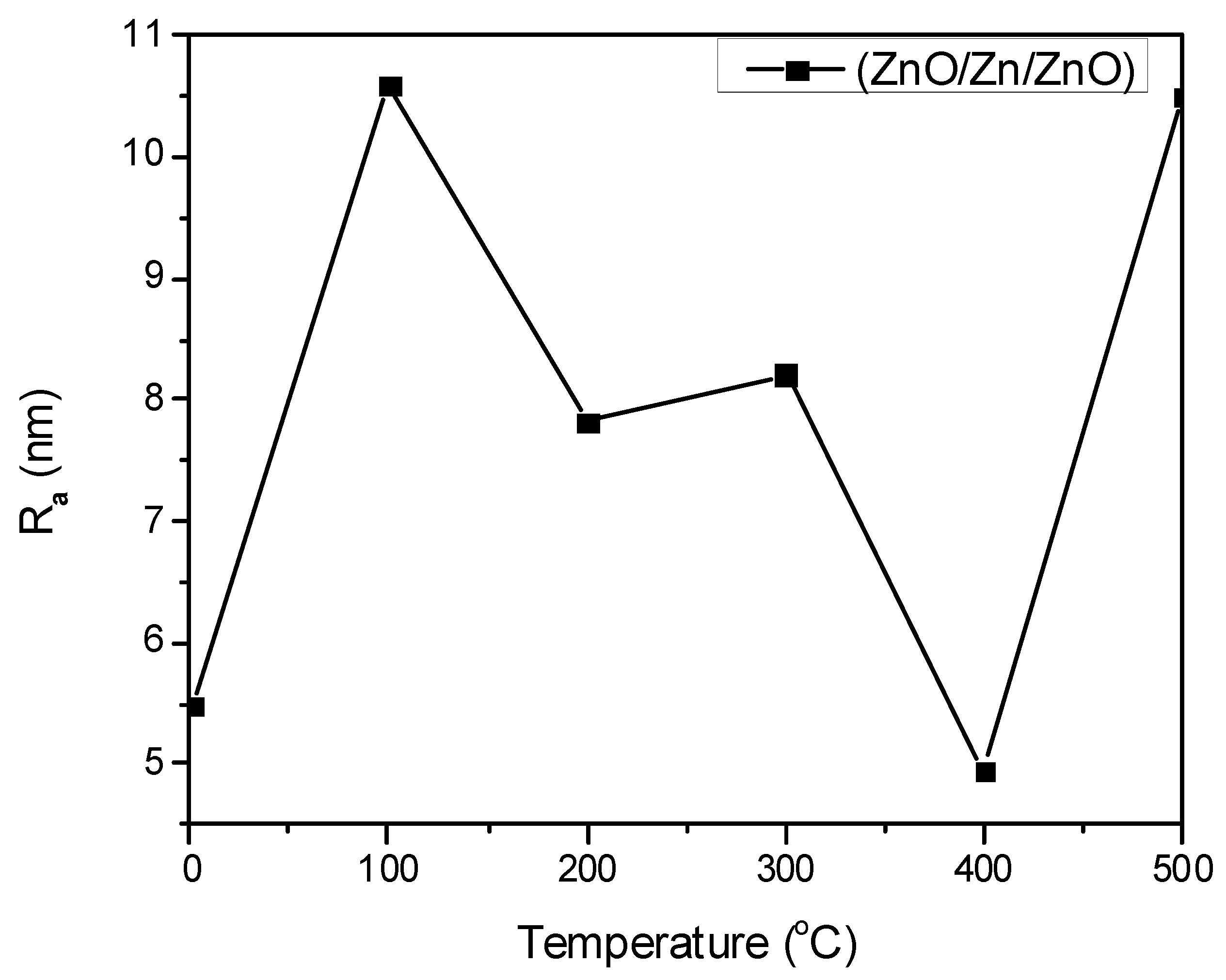
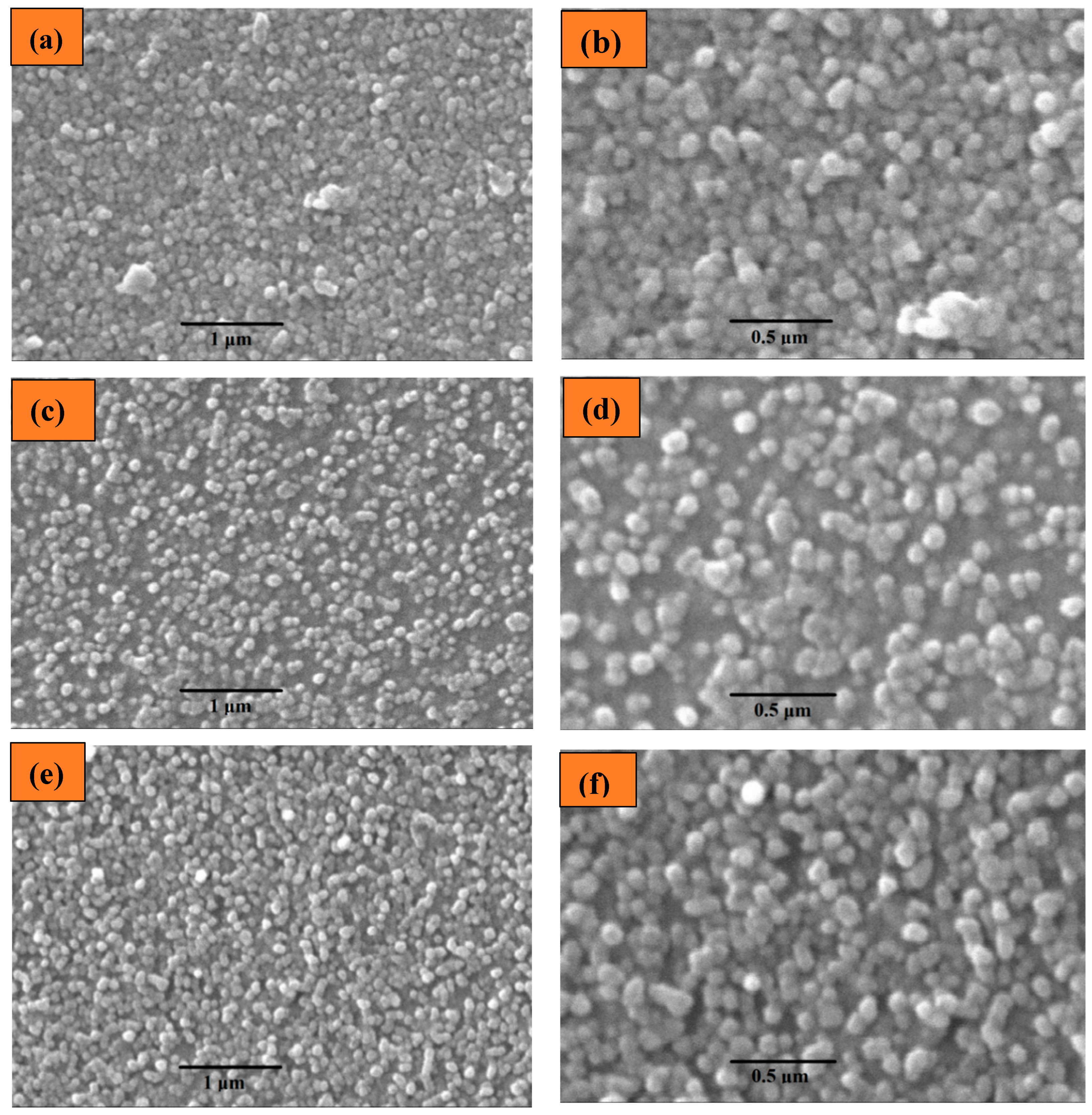
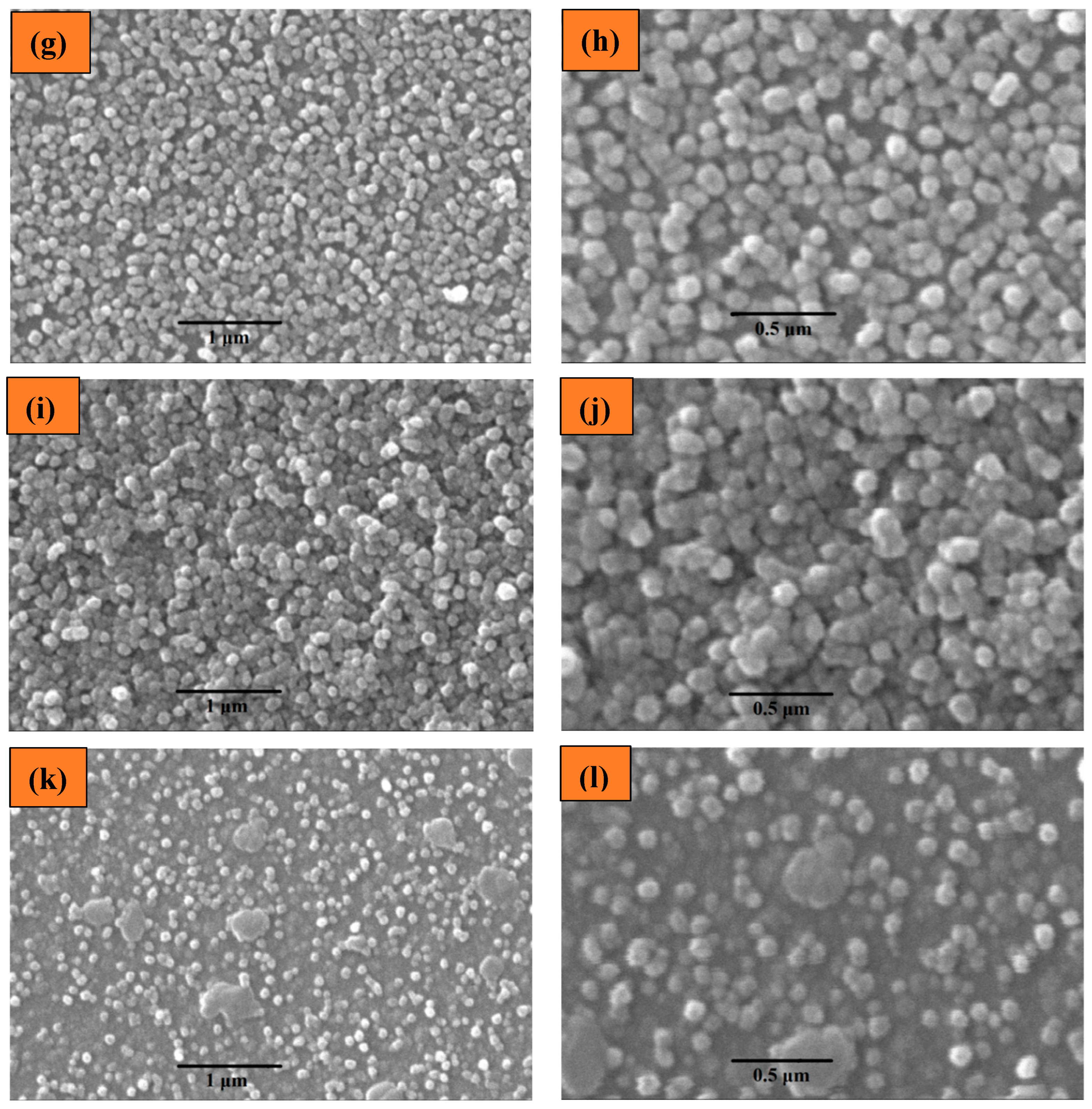
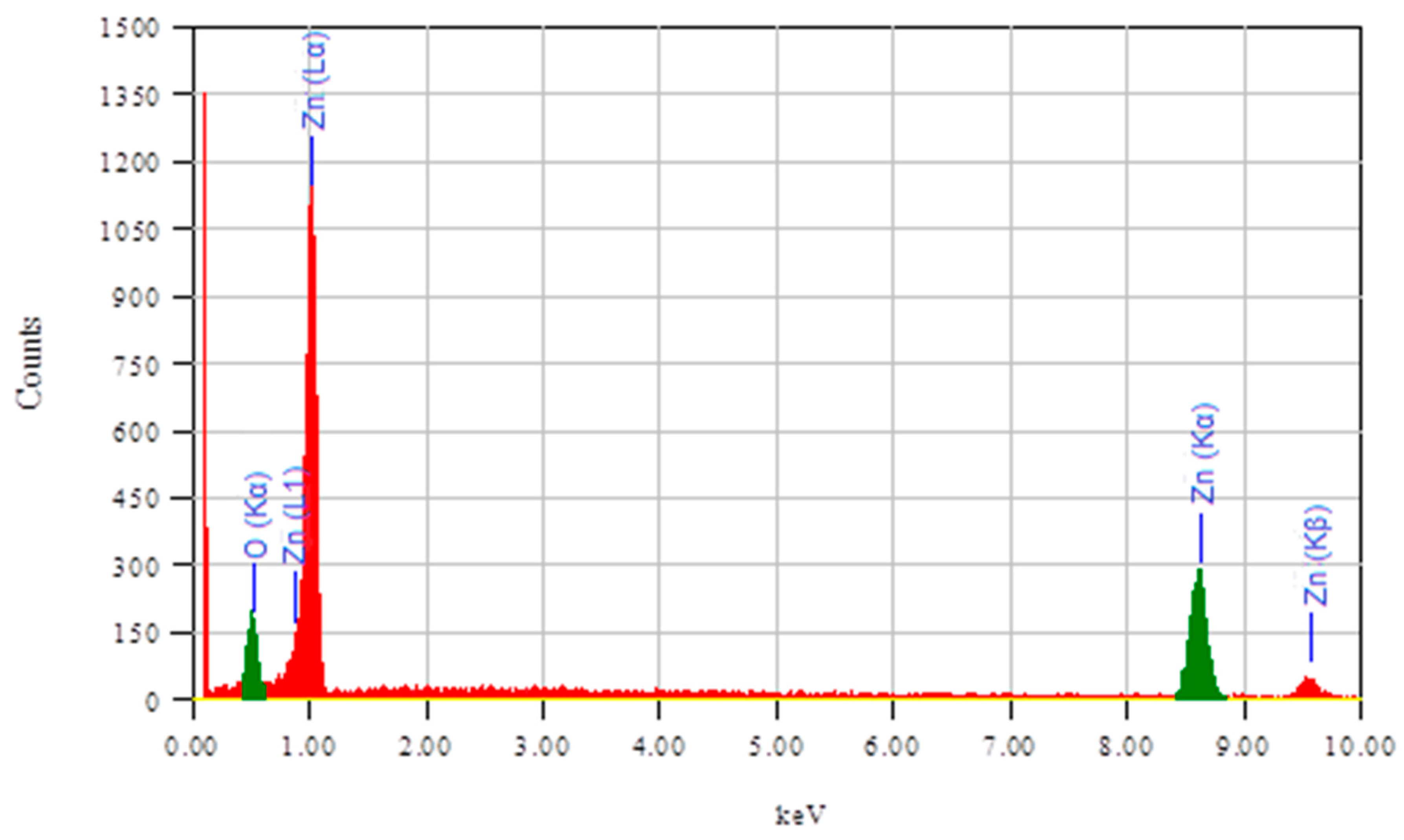
| S. No. | Thin Film | Average Particle Size |
|---|---|---|
| 1 | As deposited | 74.41 ± 1.75 nm |
| 2 | 100 °C | 78.08 ± 1.83 nm |
| 3 | 200 °C | 66.85 ± 1.62 nm |
| 4 | 300 °C | 54.46 ± 1.23 nm |
| 5 | 400 °C | 50.67 ± 1.02 nm |
| 6 | 500 °C | 163.00 ± 2.51 nm |
| S. No. | Thin Film | Electrical Conductivity (S/m) | Electrical Resistivity (Ω.m) |
|---|---|---|---|
| 1 | As deposited | 0.0095 | 105 |
| 2 | 100 °C | 4.637 | 0.2157 |
| 3 | 200 °C | 7.292 | 0.1371 |
| 4 | 300 °C | 9.49 | 0.105 |
| 5 | 400 °C | 18.79 | 0.05321 |
| 6 | 500 °C | 0.187 | 5.348 |
| Deposition Parameters | ZnO | Zn | ZnO | |
|---|---|---|---|---|
| Pressure (mTorr) | 25 | 50 | 50 | |
| Power (Watt) | 300 | 300 | 300 | |
| Thickness | 1.5 µm | 30 nm | 75 nm | |
| Annealing Temperature (°C) | ||||
| 100 | 200 | 300 | 400 | 500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshoaibi, A. The Influence of Annealing Temperature on the Microstructure and Electrical Properties of Sputtered ZnO Thin Films. Inorganics 2024, 12, 236. https://doi.org/10.3390/inorganics12090236
Alshoaibi A. The Influence of Annealing Temperature on the Microstructure and Electrical Properties of Sputtered ZnO Thin Films. Inorganics. 2024; 12(9):236. https://doi.org/10.3390/inorganics12090236
Chicago/Turabian StyleAlshoaibi, Adil. 2024. "The Influence of Annealing Temperature on the Microstructure and Electrical Properties of Sputtered ZnO Thin Films" Inorganics 12, no. 9: 236. https://doi.org/10.3390/inorganics12090236
APA StyleAlshoaibi, A. (2024). The Influence of Annealing Temperature on the Microstructure and Electrical Properties of Sputtered ZnO Thin Films. Inorganics, 12(9), 236. https://doi.org/10.3390/inorganics12090236







