Abstract
The siliceous precursor was hydrolyzed from tetraethylorthosilicate (TEOS) under acidic conditions, followed by the addition of sodium aluminate and sodium hydroxide. Y zeolite was subsequently obtained through hydrothermal crystallization under alkaline conditions. Key synthesis parameters, including reactant molar ratios, crystallization temperature, and time, were systematically varied to optimize the synthesis conditions. The synthesized products were characterized using X-ray diffraction (XRD), scanning electron microscopy (SEM), nitrogen adsorption analysis, and inductively coupled plasma (ICP) elemental analysis. Well-crystallized Y zeolite with a silica-alumina ratio (SAR) of 5.55 was successfully synthesized via TEOS hydrolysis catalyzed by sulfuric acid at a low crystallization temperature of 85 °C. The catalytic performance of benzyl phenyl ether, a lignin model compound, over NiY catalyst was evaluated in a high-pressure reactor. The results indicate that the catalytic efficiency of Y zeolite synthesized using TEOS as the silicon source under acidic hydrolysis conditions is significantly superior to Y zeolite prepared using alkaline silica sol as the silicon source.
1. Introduction
Zeolite, a crystalline aluminum silicate with a well-defined micro-porous structure, plays a crucial role in energy development, chemical production, and application [1]. In particular, zeolites are mainly used for heterogeneous catalysis and adsorption separation. The discovery of zeolite brought breakthrough innovations to both areas [2]. Among these, Y zeolite is one of the most important zeolites, which belongs to the Faujasite (FAU) family and has 3D twelve-membered ring (MR) channels and spherical supercages. Due to the large pore opening and strong acidity of Y zeolite, its discovery and application have fundamentally changed the fluid catalytic cracking (FCC) and hydrotreating processes in oil refineries [3]. In recent years, FAU zeolites have also shown great potential as an efficient catalyst for converting biomass into fuels and related chemicals [4]. A high frame silica/alumina ratio (SAR) has been proven to be the decisive factor for strong acidity and good thermal stability of Y zeolite, both of which are two of the most important properties for catalytic applications [5]. In the past decades of research, people have been trying to improve the SAR of Y zeolite. Post-synthesis dealumination is a common method for the preparation of high silicon Y zeolite, which usually uses steam/acid leaching method to remove Al atoms from the skeleton [6]. However, in the post-treatment process, the decrease in crystallinity, the loss of mass, and the generation of dealumination gradient are always unavoidable. Therefore, the direct synthesis of high-silicon Y zeolite is undoubtedly the most attractive method compared with the post-treatment method [7].
High-silicon Y zeolite synthesis can be accomplished through various methods, with hydrothermal conditions being the most common approach [8,9,10]. For example, in the production of high-silica Y zeolite via hydrothermal synthesis, a range of templates has been utilized to synthesize FAU zeolite that exhibit exceptional performance. These templates include both cost-effective and more expensive options such as crown ethers, silylated polymers, N-methylpyridinium iodide, modified carbon materials, and amphiphilic organosilanes [11,12,13,14]. Despite this, many of these templates are commercially unavailable or costly, and many template agents not only cannot be recycled but also cause harm to the environment. Therefore, it is of great significance to use effective green template-free direct synthesis of Y zeolite. In a notable study, Wang et al. [15] researched the introduction of hydroxyl radicals (•OH) in the synthesis system can significantly enhance the formation of Si–O–Si bond in the nucleation stage, to synthesize NaY zeolite with SAR of 6.35. Later, Ren et al. [16] studied the introduction of Co2+ into the synthesis system, and the Y zeolite with a small crystal size and a SAR of 6.15 was obtained. Additionally, Delprato et al. [17], directly synthesized two pure and well-crystallized zeolites with a framework Si/Al ratio close to 5 using the cubic phase with 15-crown-5 and the hexagonal phase with 18-crown-6.
Dissolving silica precursors is a critical factor in zeolite synthesis, as it facilitates the formation of silicate intermediates that subsequently undergo nucleation and crystallization. Various silica sources have been employed for Y zeolite synthesis, such as water glass, fumed silica, tetraethyl orthosilicate (TEOS), colloidal silica, and sodium metasilicate. TEOS is known to hydrolyze effectively in both alkaline and acidic environments. Recent studies have demonstrated that MCM-22, ZSM-5, and Silicalite-1 can be successfully synthesized via acid-catalyzed hydrolysis of TEOS.
In light of the reduction in fossil energy reserves and the growing severity of climate change, there has been increasing attention to the development and utilization of lignin [18,19]. Currently, both domestic and international researchers are exploring various methods to degrade lignin, including physical, biological, and chemical approaches [20,21]. However, due to the complex chemical composition and structure of lignin, significant breakthroughs have yet to be achieved [22,23].
In a recent study, Zhu Chang et al. [24] evaluated the performance of different metals (Ni, Pd, Co, and Cu) loaded onto an activated carbon (AC) catalyst for the selective C-O bond cleavage of lignin model compound BPE. The results showed that Ni/AC exhibited the highest catalytic activity, with phenol yield reaching 85% [25]. Liu et al. also found that during the lignin degradation process, compared with ZSM-5 and SBA-15, the metal-modified composite zeolite catalysts (FeZS, CoZS, NiZS, and CuZS) produced more liquid products and promoted the formation of hydrocarbons. The catalytic activity of nickel used for modification was higher than that of cobalt and iron [26]. Furthermore, Zhang et al. synthesized MCM-41 mesoporous zeolite with different metals by a novel microwave irradiation method, among which the nickel zeolite exhibited a larger specific surface area and a more uniform pore size distribution [27]. These findings suggest that loading nickel onto Y zeolite is a promising option worth further investigation.
In this study, a high-crystallinity Y zeolite was synthesized at 85 °C via acid-catalyzed hydrolysis of tetraethyl orthosilicate (TEOS). The effects of gel compositions and crystallization conditions were thoroughly investigated. Compared to the conventional hydrothermal synthesis route, the acid-catalyzed hydrolysis of TEOS offers advantages such as a lower crystallization temperature and a higher SiO2/Al2O3 ratio in the solid phase. Subsequently, nickel (Ni) was loaded onto the Y zeolite through an impregnation-reduction method, and the influence of temperature, pressure, and time on the degradation of benzyl phenyl ether by the NiY zeolite was evaluated using a high-pressure reactor. Additionally, the performance differences between Y zeolites prepared via acid-catalyzed hydrolysis of TEOS and those prepared using basic silica sol were compared.
2. Results and Discussion
2.1. Effect of Crystallization Temperature and Crystallization Time
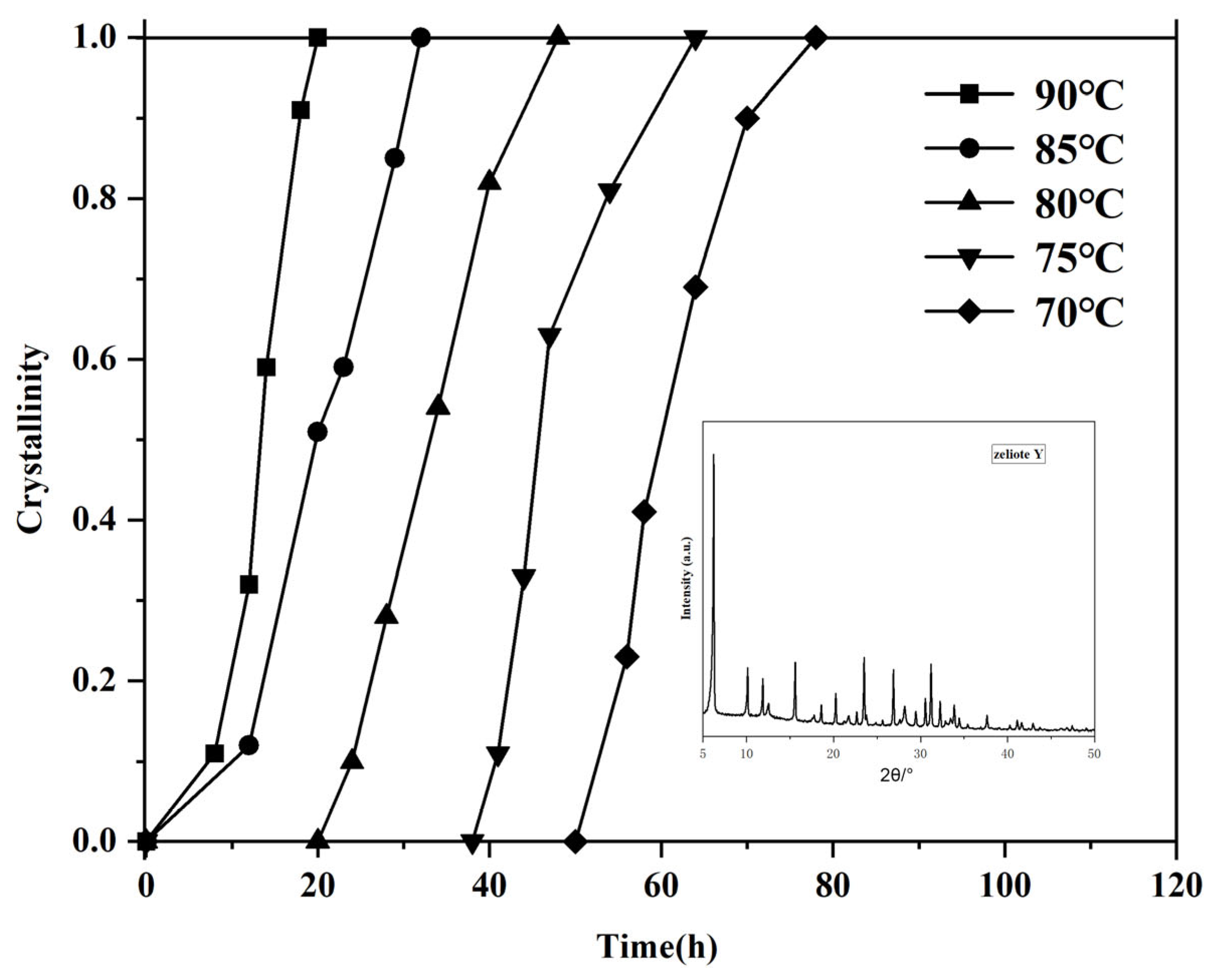
The influence of hydrothermal temperature on the crystallization of zeolite Y was evaluated by synthesizing samples at temperatures ranging from 70 to 95 °C without using any template. The gel compositions were maintained at a mole oxide ratio of 7 Na2O: 1 Al2O3: 10 SiO2: 300 H2O. The prepared gel was stirred and aged at 30 °C for 24 h, followed by crystallization for an additional 24 h. Figure 1 presents the crystallization rate curves of the products synthesized at different temperatures. As shown in the crystallization curve, varying temperatures significantly impact the nucleation stage of crystal formation. Specifically, as the crystallization temperature increases, the induction period for nucleation is shortened. However, the slopes of the crystallization curves remain largely consistent, indicating that different crystallization temperatures have minimal effect on the crystal growth rate. Consequently, higher crystallization temperatures result in shorter overall crystal growth times. Zeolite Y in Figure 1 is a sample that crystallizes for 32 h at a crystallization temperature of 85 °C. Strong characteristic diffraction peaks attributed to Y zeolite molecular sieves were observed at 2θ = 6.19°, 10.12°, 11.87°, 15.62°, 18.65°, 20.32°, 23.60°, 26.99°, 30.69°, 31.33°, 32.39°, and 34.01°, with no impurity peaks. This indicates that the synthesized molecular sieve has the topological structure of Y zeolite molecular sieves and has good crystallinity without impurity crystals [28]. The sample with the largest sum of the peak areas of the ten main diffraction peaks is set as the standard sample (with a crystallinity of 100%).
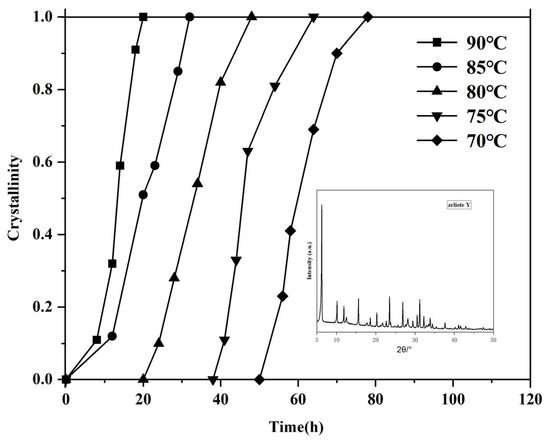
Figure 1.
Calculated crystallinity of the prepared samples crystalized at various temperatures and times.
It is worth noting that highly crystalline Y-type zeolites can be synthesized when the temperature is above 70 °C. Under a low-temperature condition of 70 °C, highly crystalline Y-type zeolites can be obtained by extending the crystallization time to 80 h, while at 90 °C, it only takes 20 h to achieve the same result. Early efforts under hydrothermal conditions revealed that Y zeolite was created mostly at 100 °C, and impurities like P zeolite were observed at a lower temperature, 80 °C [29]. The present result convinced us that the synthesis method by acid-catalyzed hydrolysis of tetraethylorthosilicate allows the generation of pure Y zeolite at a very low crystallization temperature, 70 °C, although needing a longer crystallization time.
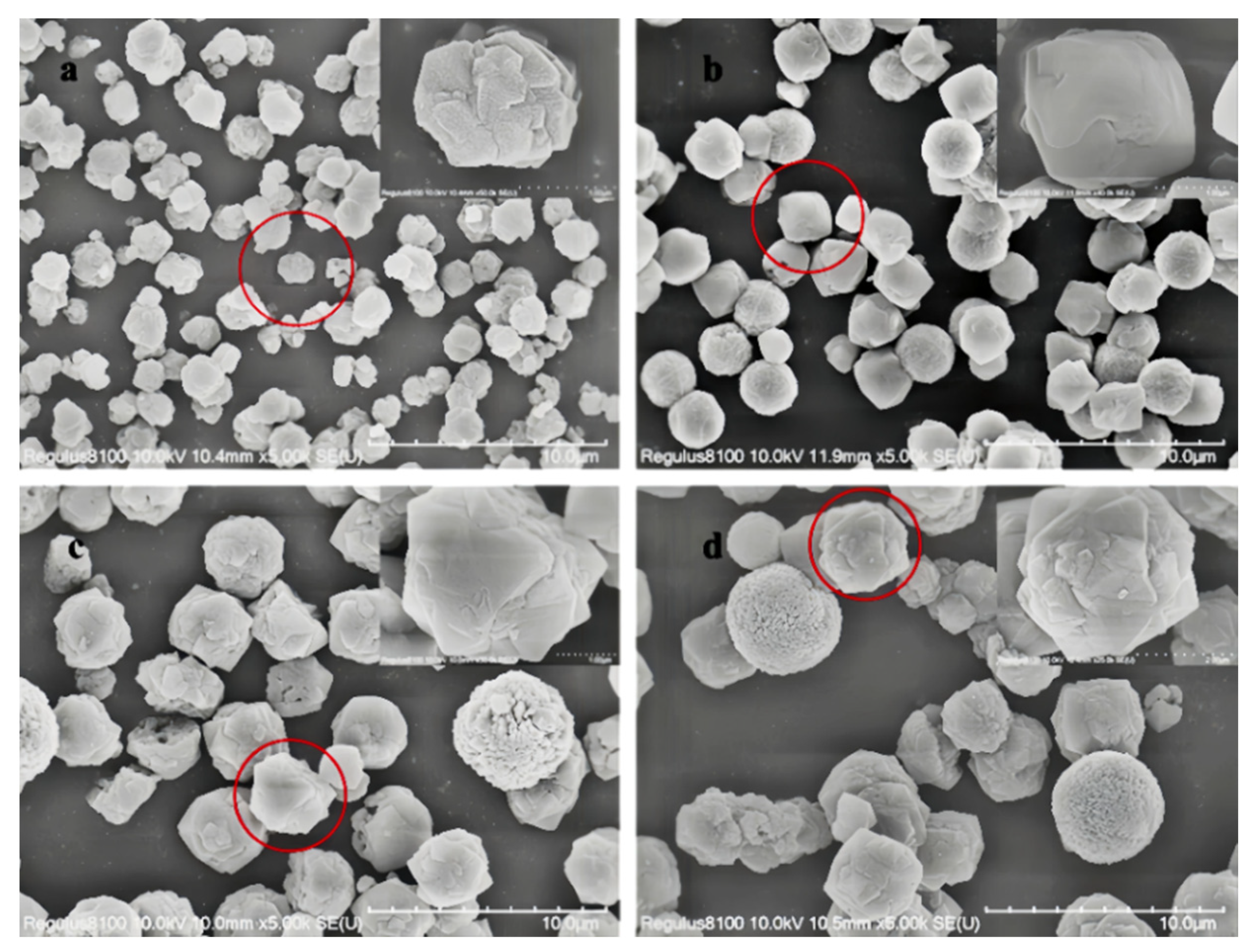
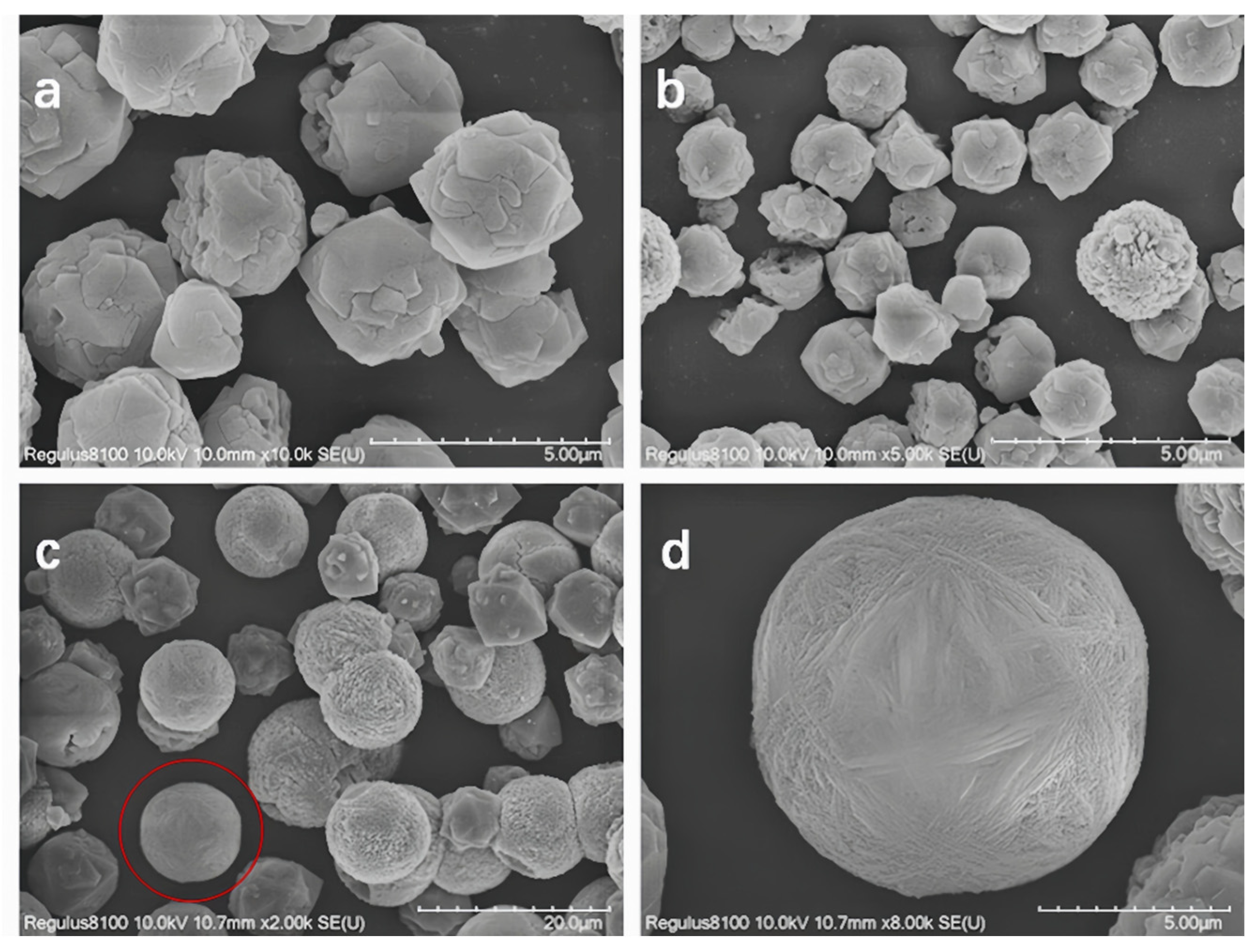
The SEM images of Y zeolite products at different crystallization stages are presented in Figure 2. It can be observed that the particle size of Y zeolite synthesized at various crystallization temperatures exhibits significant differences. At a constant crystallization temperature, the particle size of the product increases with extended crystallization time. Specifically, when the temperature increases from 80 °C to 85 °C, the particle size grows from 1.6 μm to 2.4 μm after 32 h of crystallization and from 3.6 μm to 4.0 μm after 48 h. This phenomenon can be attributed to the dependence of zeolite particle size on both nucleation and growth rates. At lower temperatures, conditions favor the formation of crystal nuclei while controlling the grain growth rate. As the temperature rises, the growth process of crystal nuclei accelerates significantly, while the nucleation process is suppressed, leading to an increase in the particle size of the final product [30,31].
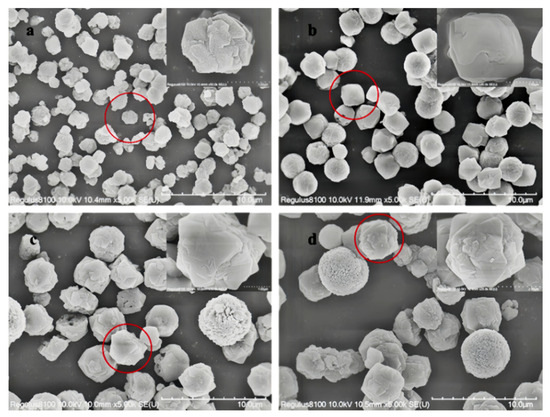
Figure 2.
SEM images of Y zeolite with seeding prepared at different temperatures and times (the images in the red circles will be magnified in the upper right corner) (a) 80 °C 32 h, (b) 80 °C 48 h, (c) 85 °C 32 h, and (d) 85 °C 48 h.
2.2. Effect of Initial SiO2/Al2O3 Ratio Amount
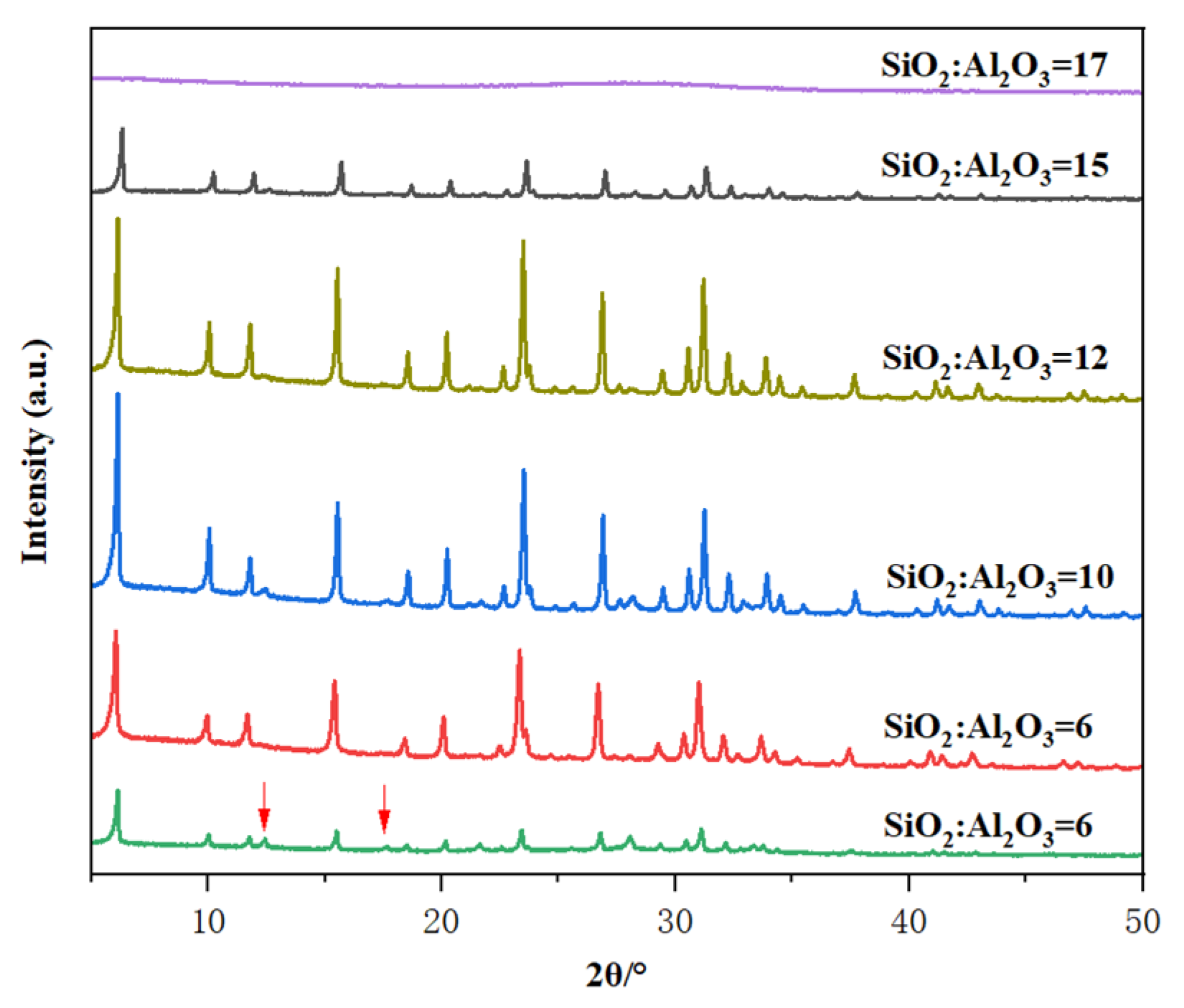
The effect of the SiO2/Al2O3 ratio on the crystallization of Y-type zeolite was investigated under the conditions of crystallization temperature of 85 °C and crystallization time of 32 h. When the mole oxide compositions of the gels were 7 Na2O: 1 Al2O3: X SiO2: 300 H2O, in which X varied from 6.0 to 17.0. Figure 3 shows the XRD pattern and crystallinity of zeolite prepared with different SiO2/Al2O3 ratios of initial gels. As shown in Figure 3, when SiO2/Al2O3 = 6, the product obtained contains not only the Y crystal phase but also P zeolite, indicating that under the condition of a low silica-to-alumina ratio, it is easy to synthesize P zeolite. Pure Y-type zeolite was obtained when the SiO2/Al2O3 ratio was 9 to 12, among which the sample with SiO2/Al2O3 of 10 had the highest crystallinity. Therefore, as the aluminum content increases, the ratio of nucleation to crystal growth rate decreases, ultimately resulting in large grains. When the SiO2/Al2O3 ratio was up to 17, the XRD showed that the synthesized sample is an amorphous product. When the SiO2/Al2O3 ratio increases from 6 to 10, the lower silicon-to-aluminum ratio may affect the polymerization state of each species in the reaction system and the reaction rate, thereby influencing the crystallinity. When the SiO2/Al2O3 ratio increases from 10 to 15, the higher silicon-to-aluminum ratio is due to the fact that aluminum has a promoting effect on crystallization in the reaction system. Therefore, the higher the silicon-to-aluminum ratio, the lower the aluminum content, which leads to a slower crystallization rate and lower crystallinity of the samples under the same crystallization time and temperature. This indicates that there is an appropriate range for the synthesis of this molecular sieve, and both too high and too low silicon-to-aluminum ratios are not conducive to synthesis [32,33]. Table 1 shows the physical and chemical properties of Y zeolite. According to the ICP analysis, SiO2/Al2O3 ratios for final Y solids obtained with initial gel SiO2/Al2O3 ratios of 6, 9, 10, 12, and 15 were 2.47, 3.89, 4.56, 5.55, and 5.73, respectively. The BET surface areas were 494, 641, 743, 672, and 567 m2/g, respectively. The lower surface areas at SiO2/ Al2O3 ratios of 6 and 15 correspond to their lower crystallinities.
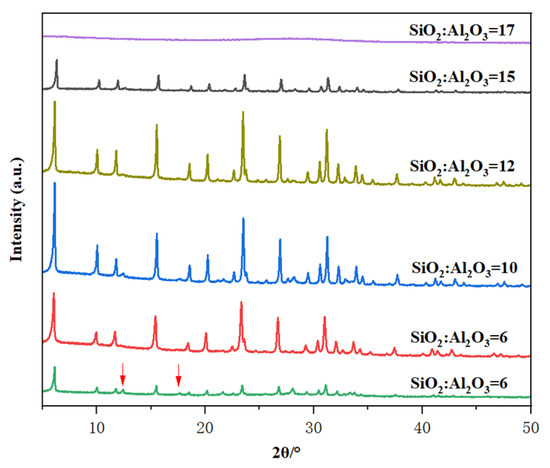
Figure 3.
The effect of SiO2/Al2O3 ratio in the initial gel on the XRD patterns of the prepared samples.

Table 1.
Textural parameters and of the samples obtained at different Si/Al ratios.
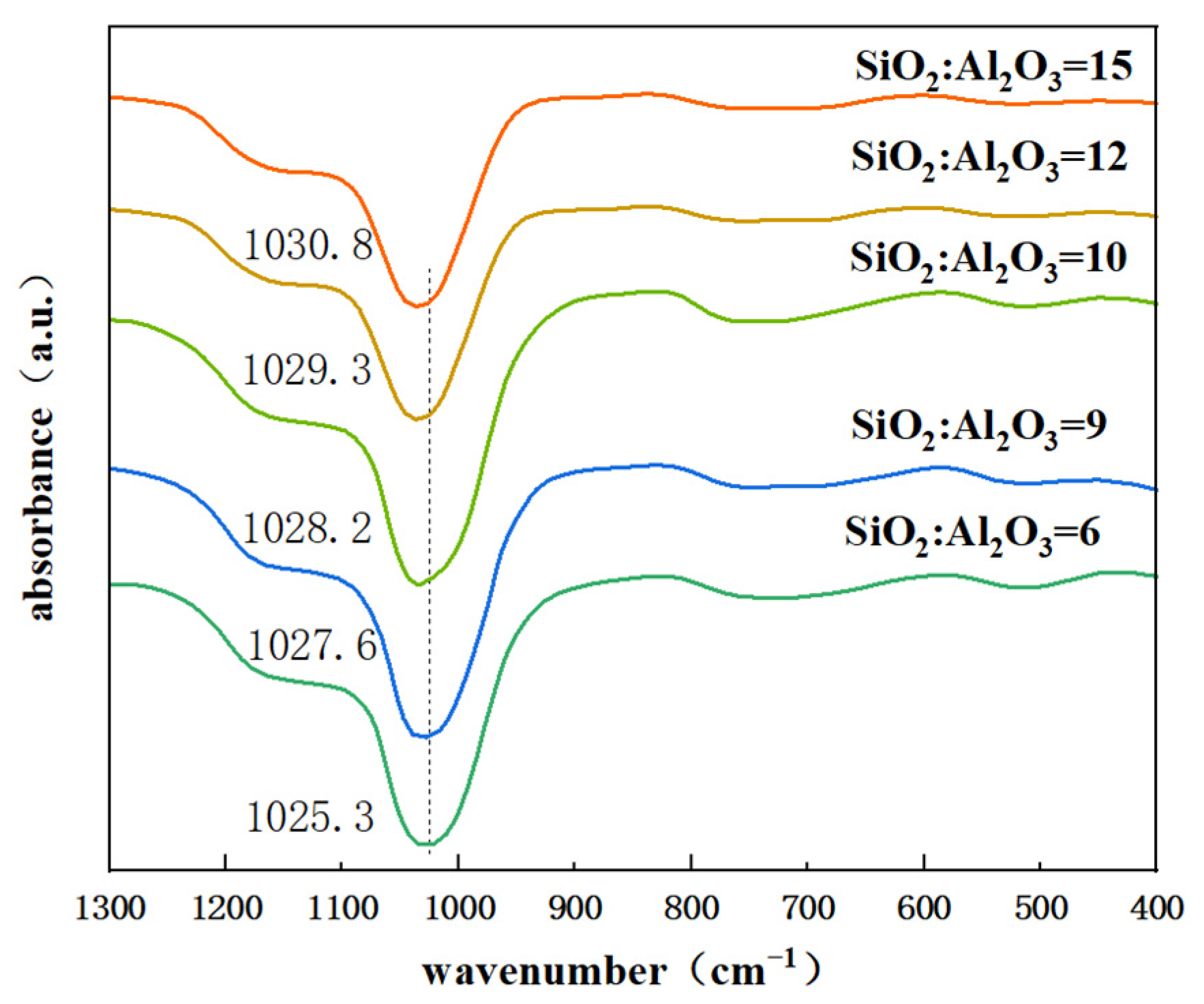
Figure 4 shows the infrared spectra of different SiO2/Al2O3 ratios of Y zeolite. The peak at 1025.3 cm−1 is attributed to the internal antisymmetric stretching vibration. It is well established that the external bonding vibrations of TO4 (T = Si, Al) tetrahedra originate from framework vibrations and are highly sensitive to structural changes in zeolites. With the increase of SiO2/Al2O3 ratio, the absorption peak at 1025.3 cm−1 gradually shifts to higher wavenumbers (1030.8cm−1). This shift occurs because the Al-O bond length (0.175 nm) exceeds the Si-O bond length (0.161 nm), and aluminum has a lower electronegativity than silicon, while their masses are comparable. Consequently, an increase in the Si/Al ratio results in a reduced force constant, leading to an increase in the observed wavenumber [34].
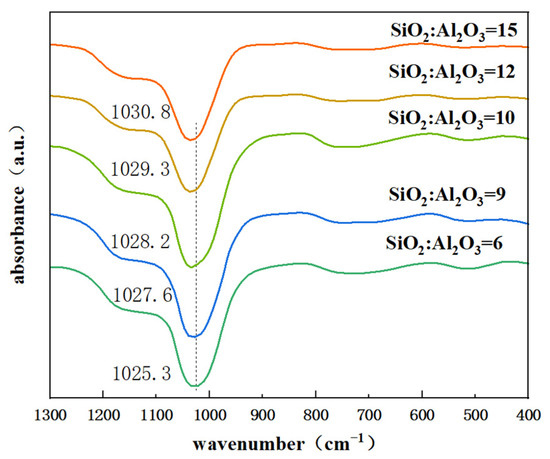
Figure 4.
FTIR of zeolite Y with different SiO2/Al2O3 ratios in the initial gel.
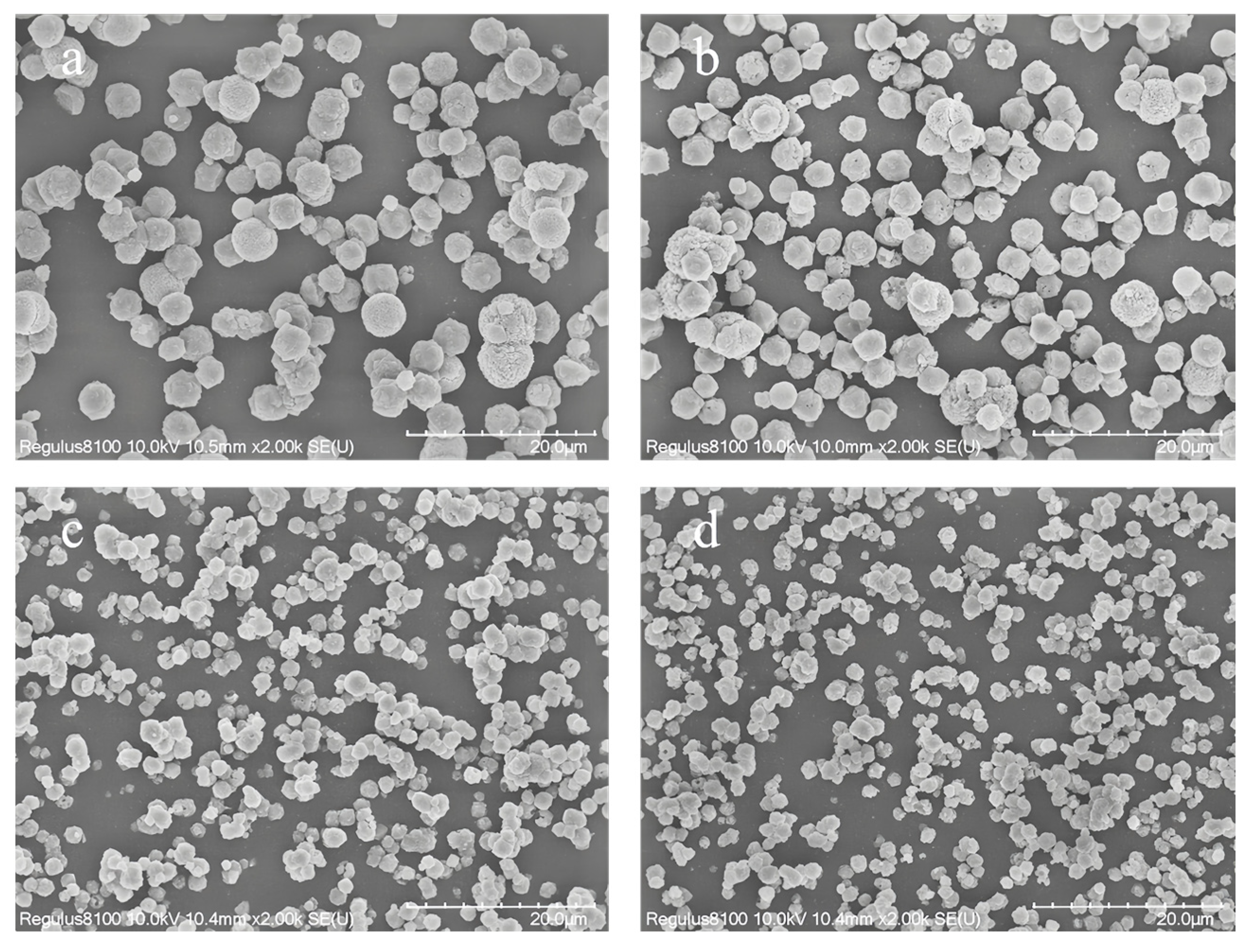
Figure 5 presents the SEM images of samples with varying SiO2/Al2O3 ratios in the initial gel. As shown in the figure, none of the samples exhibit significant amorphous material. When the gel SiO2/Al2O3 ratio is 6 (Figure 5a) and the crystallization time is 24 h, the resulting Y zeolite particles have an average size of approximately 3.5 μm. For a gel SiO2/Al2O3 ratio of 10 (Figure 5b), with a crystallization time of 32 h, the Y zeolite particle size decreases to about 1.3 μm. Extending the crystallization time to 72 h for the same SiO2/Al2O3 ratio (Figure 5c) results in Y zeolite particles with a size of approximately 10 μm. In summary, increasing the SiO2/Al2O3 ratio in the gel generally leads to a decrease in Y zeolite particle size, while extending the crystallization time promotes particle growth. Further magnification of the sample synthesized with a gel SiO2/Al2O3 ratio of 12 (Figure 5d) reveals that the sample exhibits both the characteristic inverted octahedral morphology of Y zeolite and a spherical structure. Typically, the grain size of zeolites is determined by the balance between nucleation and crystal growth rates during synthesis. In the case of Y zeolite synthesis, higher aluminum content in the initial gel can inhibit nucleation due to excessive aluminum ions. Additionally, the influx of chloride ions into the zeolite framework necessitates more cations to balance the negative skeletal charge, thereby reducing the overall crystallization rate. Consequently, as the aluminum content increases, the ratio of nucleation to crystal growth rate decreases, leading to the formation of larger grains.
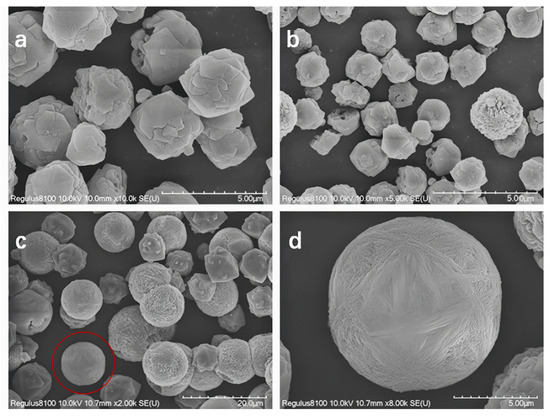
Figure 5.
SEM spectra of samples synthesized with different SiO2/Al2O3 ratios in the initial gel: (a) SiO2/Al2O3 = 6, crystallization time 32 h, (b) SiO2/Al2O3 = 10, crystallization time 32 h, (c) SiO2/Al2O3 = 10, crystallization time 72 h, and (d) is the local magnification circled in (c).
2.3. Effect of Initial Na2O Amount
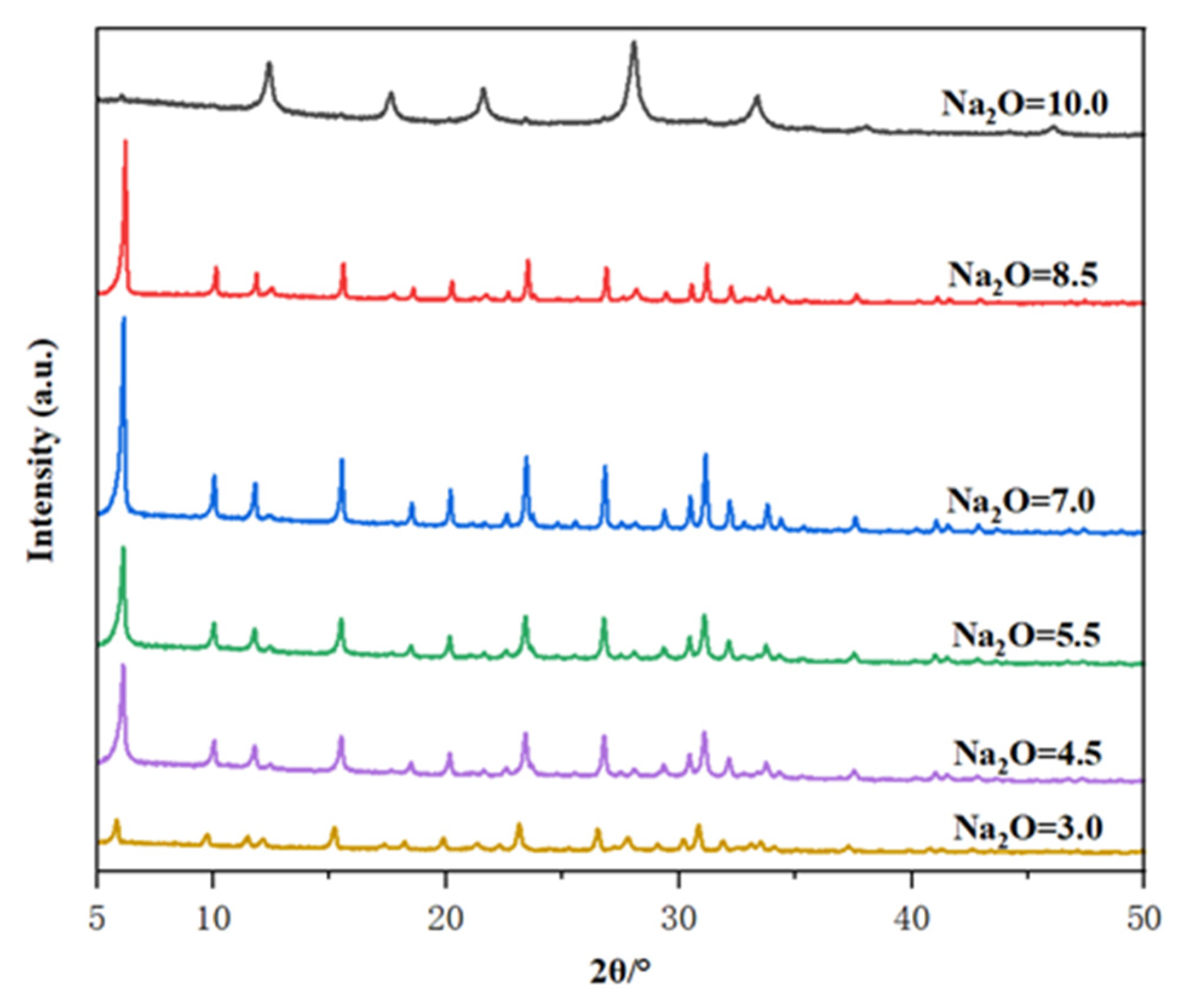
It is well known that the alkalinity of the initial gel is an important parameter affecting the crystallization of zeolite. Therefore, the molar composition of the oxide in the gel is X Na2O: 1 Al2O3: 10 SiO2: 300 H2O, where the value of X varies between 3.0 and 10.0. The crystallization temperature is controlled at 85 °C and the crystallization time is 32 h to evaluate the effect of the gel’s alkalinity. Figure 6 shows the XRD patterns of the resultant as-calcined samples. When the Na2O content ranged from 3 to 8.5, all characteristic peak positions were consistent with those of zeolite, indicating that varying the dosage of Na2O did not induce structural changes in Y zeolite framework, thereby preventing the formation of amorphous substances and ensuring the production of pure Y products. Within this range, the crystallinity increased with the rise in Na2O content, attributed to the accelerated dissolution of silicon in highly alkaline media. At low alkalinity (Na2O content was 3), a poorly crystallized Y phase was observed because the low concentration of hydroxyl ions could not depolymerize the silica source to provide sufficient solubilized aluminosilicate species for nucleation [35,36,37,38]. In contrast, high alkalinity (Na2O content was 8.5) reduces crystallinity, which may be due to the presence of excessive hydroxyl ions leading to dissolution of the already formed zeolite nuclei. A higher Na2O content up to 10 caused the occurrence of P zeolite crystals. Accordingly, the Na2O content ranges from 4.5 to 8.5 is optimal for producing the pure phase of Y zeolite.
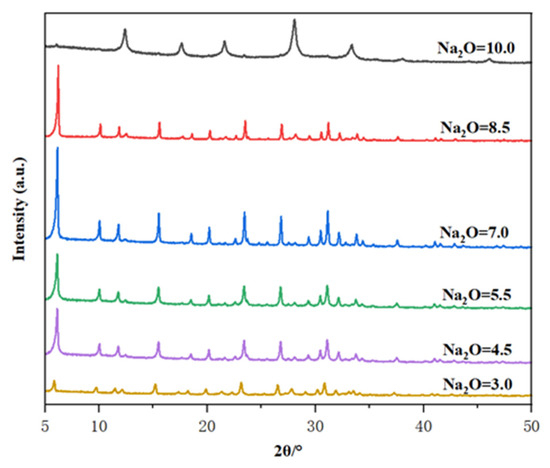
Figure 6.
The effect of Na2O ratio in the initial gel on the XRD patterns of the prepared samples.
The morphology of Y-type zeolite is also affected by the content of Na2O. Figure 7 shows the scanning electron microscope (SEM) images of the synthesized samples with different Na2O contents. It can be seen that there is basically no amorphous material in all samples, which is consistent with the conclusion about the XRD images in Figure 6. In addition, the Na2O content has an impact on the particle size of Y-type zeolite. As the Na2O content increases, the particle size of the product will significantly decrease. This is because silicon is more easily dissolved in a strongly alkaline medium, so its combination with the crystal framework becomes less and less. This change leads to a reduction in the particle size of the obtained Y-type zeolite.
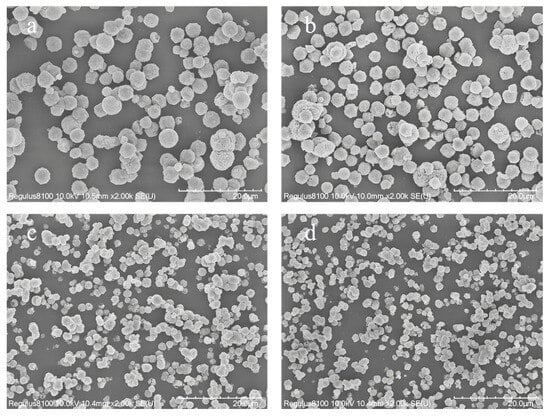
Figure 7.
SEM images of zeolite Y samples synthesized with different Na2O contents: (a) Na2O/Al2O3 = 4.5, (b) Na2O /Al2O3 = 5.5, (c) Na2O/Al2O3 = 7, and (d) Na2O/Al2O3 = 8.5.
2.4. Effect of Initial H2O Amount
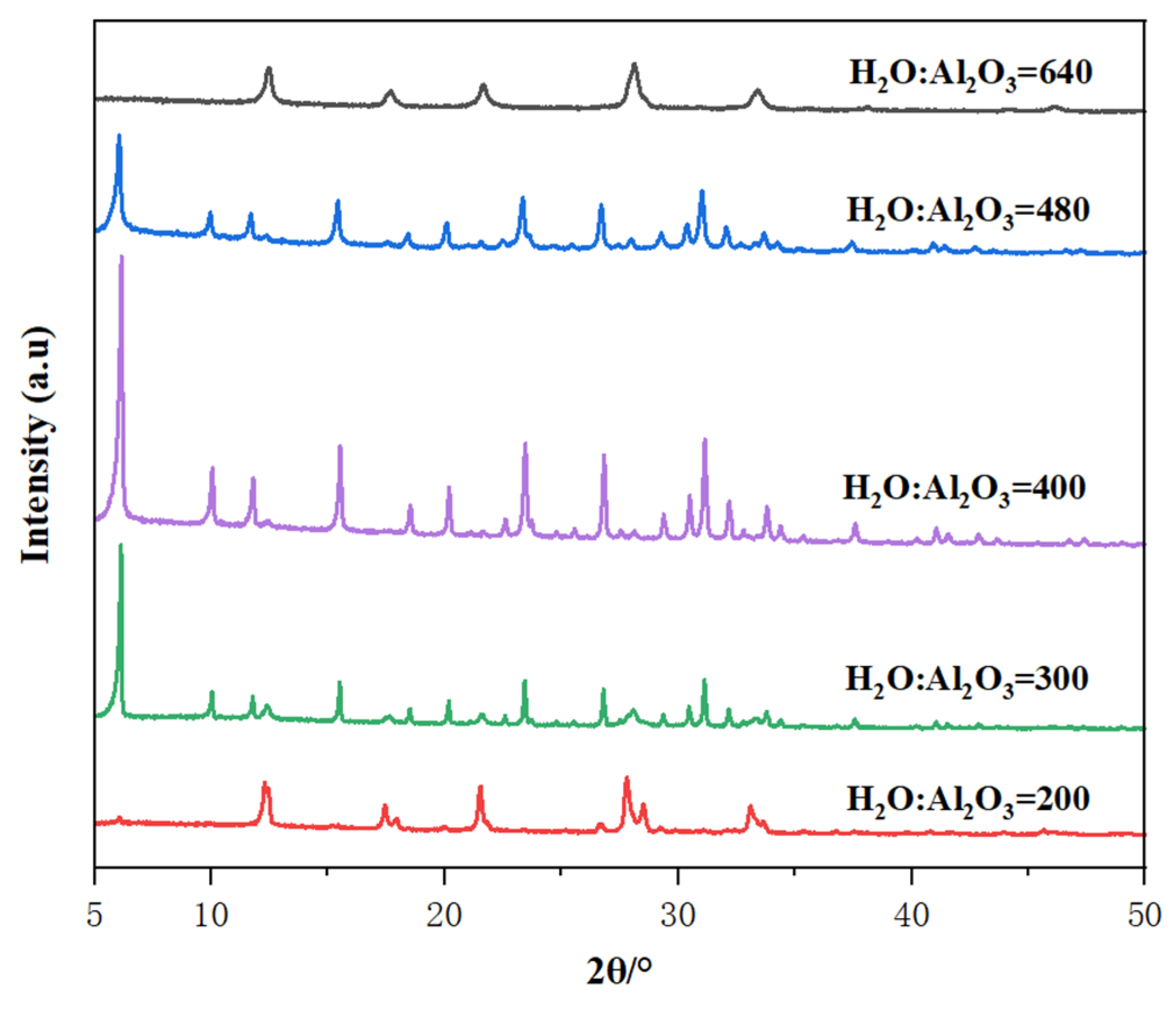
The water content in the synthetic system directly influences the concentration of each component in the gel, thereby affecting the crystallization process of zeolite. Figure 8 presents the XRD patterns of samples synthesized with varying H2O content, where the mole oxide compositions of the gels were 7 Na2O: 1 Al2O3: 10 SiO2: X H2O, and X ranged from 200 to 640. At a very low H2O content of 200, the highly concentrated gel produced only P zeolite crystals. The low water content shortened the induction period and accelerated both nucleation and crystal growth rates, promoting rapid crystallization. However, insufficient water content prevented the formation of a uniform gel, making it difficult to produce Y zeolite and leading to the formation of impurity crystals. Conversely, at a much diluted gel mixture (H2O content of 600), only P zeolite crystals were formed due to the significantly slower nucleation rate caused by the greater distance between reactants in the dilute solution. When the H2O content was within the range of 300 to 480, pure and highly crystalline Y zeolite solids were produced. However, at the higher end of this range (H2O content of 480), the slightly diluted gel resulted in lower crystallinity of Y zeolite, as the high water content reduced the concentration of reactants, thereby slowing the crystallization rate. Therefore, an optimal H2O content range of 300 to 480 is most suitable for synthesizing high-quality Y zeolite [39].
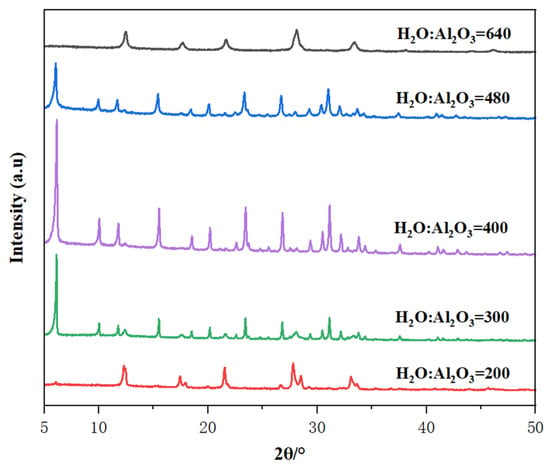
Figure 8.
The effect of H2O/Al2O3 ratio in the initial gel on the XRD patterns of the prepared samples.
2.5. Comparison of Nickel Content in Two Types of Zeolite
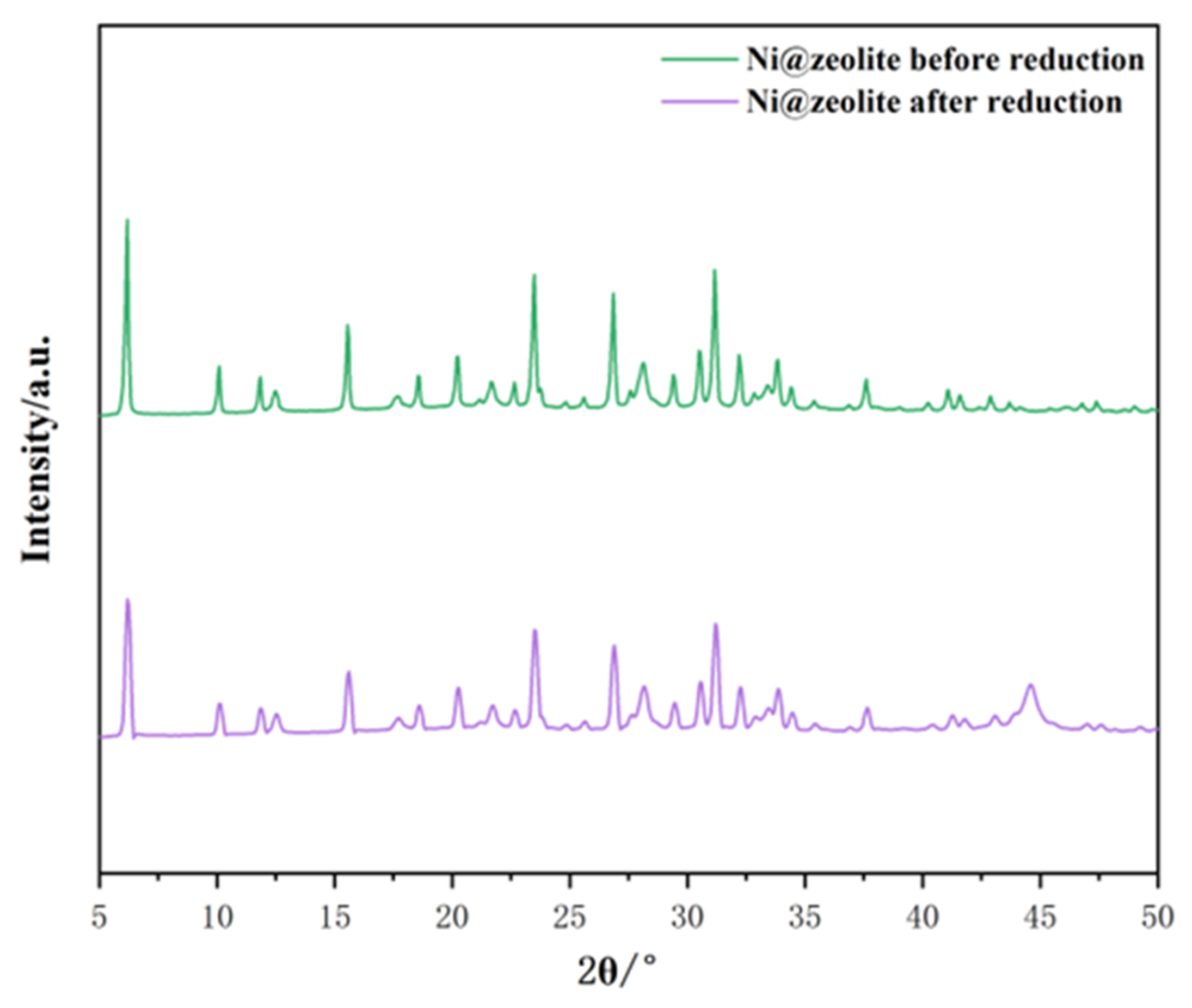
Zeolite was selected at a ratio of 7 Na2O:1 Al2O3:10 SiO2:300 H2O, and then nickel was loaded onto it and characterized. As can be seen from Figure 9, all the characteristic diffraction peaks of the nickel-modified Y zeolites are completely retained before and after nickel reduction, indicating that the zeolite unit structure remains intact after nickel is introduced by the impregnation method. In addition, no other phases related to nickel species were observed before re-reduction, which may suggest that the Ni introduced by the impregnation method is highly dispersed on the surface of Y zeolite [40]. After reduction, the characteristic peak that appears at 44.5° is attributed to the (111) crystal plane of metallic nickel [41]. As shown in Table 2, the zeolite samples made by acid hydrolysis of tetraethyl orthosilicate have a larger specific surface area, which allows for more active sites, and this is one of the reasons why they have a higher nickel content. Therefore, theoretically, the NiY zeolite made by acid hydrolysis of tetraethyl orthosilicate should have stronger catalytic activity.
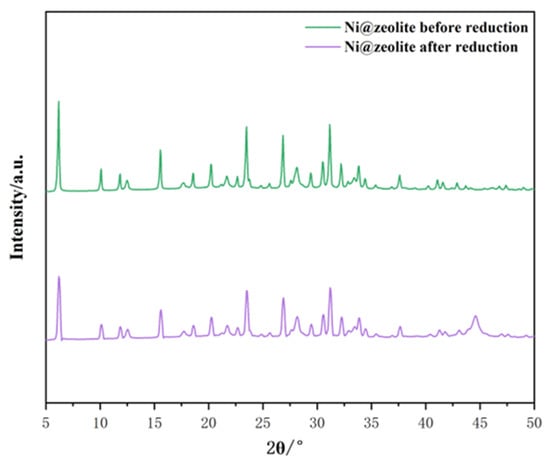
Figure 9.
Comparison of two catalysts before and after loading nickel.

Table 2.
A comparison of BET and Ni contents of two types of Y zeolite.
2.6. Catalytic Performance
The performance test of degrading benzyl phenyl ether was conducted using NiY zeolite with a composition ratio of 7 Na2O: 1 Al2O3: 10 SiO2: 300 H2O.
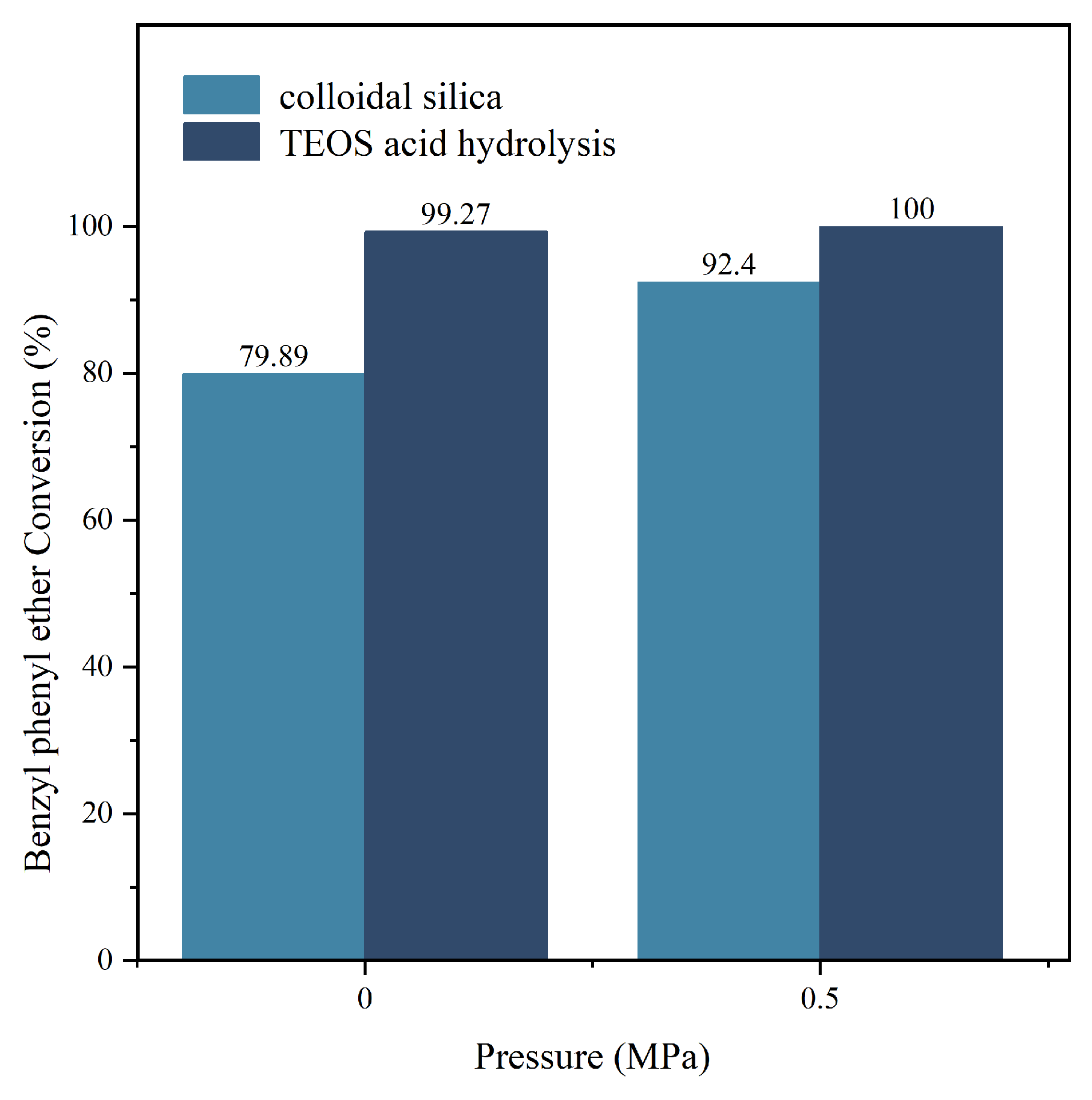
In the experiment shown in Figure 10, we studied the difference in the catalytic performance of two catalysts for the benzyl phenyl ether synthesis. The reaction time was 2 h, and the temperature was 200 °C. Using two different catalysts (one with acid-hydrolyzed ethyl orthosilicate as the silica source and the other with basic silica sol as the silica source), we studied the effect of the catalyst on the conversion rate of benzyl phenyl ether under two pressures (0 MPa and 0.5 MPa). The results showed that the Y zeolite catalyst with acid-hydrolyzed ethyl orthosilicate as the silica source had significantly better catalytic performance than the Y zeolite catalyst with basic silica sol as the silica source. The experimental results once again confirmed the previous hypothesis: the catalyst prepared by acid-hydrolyzing orthosilicic acid has a larger specific surface area and higher metal loading, which not only provides more extensive reaction active sites but also effectively improves the adsorption and conversion efficiency of reactant molecules, thereby significantly enhancing its catalytic activity.
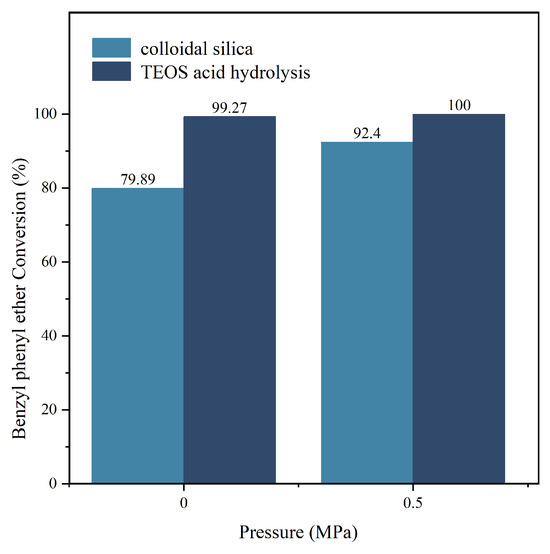
Figure 10.
Comparison of the catalytic performance between two catalysts.
3. Materials and Methods
3.1. Zeolite Synthesis
The following is a typical synthesis procedure. A total of 10.0 g TEOS (98%, Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China) was mixed with 32.5 g deionized water in a beaker. Under vigorous stirring, sulphuric acid (99.3%, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) was slowly dropped into the TEOS solution to obtain a pH value of 1.0. The obtained mixture A was stirred at 20 °C for 20 h to obtain a complete hydrolysis of TEOS. Solution B was prepared by successively adding 2.695 g of sodium hydroxide (NaOH, 98%, Merck KGaA, Darmstadt, Germany) and 1.084 g sodium aluminate (14% Al2O3, 25% Na2O and 61% H2O, Macklin, Shanghai Macklin Biochemical Co., Ltd., Shanghai, China) into 12 g of deionized water under stirring. Then, solution B was added into solution A to make the gel mixture. Aging was conducted by stirring at room temperature for 12 h. The molar composition of the synthesis gel was 7 Na2O:1 Al2O3:10 SiO2:300 H2O. Finally, the slurry was transferred into a Teflon-lined stainless-steel autoclave and left to crystallize statically at 85 °C. Products from the synthesis were separated by centrifugation, washed with deionized water and air-dried at 100 °C. The obtained as-synthesized samples were calcined at 550 °C for 5 h in an air stream to produce the as-calcined Y.
The traditional method for the synthesis of Y zeolite is as follows: 2.73 g NaAlO2 and 6.7 g NaOH are dissolved in 20 g deionized water to form solution A. Meanwhile, 25g 40% basic silica sol–gel is dissolved in 20g deionized water to obtain solution B. Solution A and solution B are mixed thoroughly, and then 35g deionized water is added. The final mother liquid composition ratio is 7 Na2O: 1 Al2O3: 10 SiO2: 300 H2O. The obtained mother liquid is stirred at room temperature for 24 h, transferred to a reaction kettle lined with Teflon, and subjected to static crystallization at 100 °C and self-generated pressure for 32 h to obtain the crystallized product. The product is separated by centrifugation and washed with deionized water until the pH value is close to neutral (about 8) before being dried at 120 °C.
3.2. Synthesis of NiY Zeolite
Mix 0.5504 g of Ni(NO3)2·6 H2O (99.7%, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) with 10 mL of deionized water in a flask and dissolve it. Then, add 1 g of Y zeolite and mix well. Stir the mixture at room temperature of 30 °C for 48 h to obtain a pale green slurry. After drying the slurry by rotary evaporation, place the zeolite in a tube furnace and heat it at a rate of 5 °C/min to 450 °C. Reduce the zeolite with hydrogen gas for 5 h at this temperature to obtain NiY zeolite.
3.3. Zeolite Characterization
The X-ray diffraction (XRD) patterns of the prepared zeolites were determined using a Philips PW1730 X-ray diffractometer (Cu Kα radiation, 40 kV and 40 mA) from Eindhoven, North Brabant, The Netherlands. By this method, the prepared sample with the highest peak intensity was assumed to have 100% crystallinity, and the crystallinities of the other prepared samples were normalized by considering the ratio of their peak intensities (corrected for half-width) to that of the sample with the highest crystallinity.
The specific surface area and pore volume of the prepared zeolite were determined by nitrogen physical adsorption using the Micromeritics ASAP 2010 automated system from Norcross, GA, USA.
The nickel content was detected using the Thermo Fisher iCAP PRO (OES, Waltham, MA, USA) from the United States.
The Vertex80 Fourier Transform Infrared Spectrometer (FTIR) produced by Bruker in Karlsruhe, Germany was used for the measurement, with transmittance as the measurement mode. The resolution was 4.0 cm−1, the range was 4000–400 cm−1, and it was scanned 32 times.
3.4. Testing of NiY Catalytic Performance
Using a 100 mL high-pressure reactor, the performance of the NiY zeolite loaded with 10 wt% was tested with the substrate of benzyl phenyl ethers. Amounts of 30 mL of isopropanol, 20 mg of NiY samples, and 184.2 mg of benzyl phenyl ethers (0.001 mol) were added to the high-pressure reactor. The catalytic performance of the NiY zeolite was evaluated by adjusting parameters such as temperature, pressure, and reaction time. The formula for calculating the removal rate of the benzyl phenyl ether is (C0 − C)/C0, where C0 represents the initial concentration of the benzyl phenyl ether solution, and C represents the concentration of benzyl phenyl ether solution after degradation.
The specific experimental steps are as follows:
- First, 20 mg of the catalyst sample and 184.2 mg of the benzyl phenyl ether were weighed and added to a 100 mL high-pressure reactor with isopropanol as the solvent (30 mL), and the lid of the high-pressure reactor was tightened.
- After exhausting the air three times with hydrogen, the reactor was filled with a certain pressure of hydrogen. The reactor program was adjusted to a certain temperature, pressure, and reaction time, and the stirring speed inside the reactor was set to stir the reaction.
- After a certain period of reaction, the reactor was taken out and cooled to room temperature. The hydrogen was slowly vented out until it was exhausted, and the reactor was opened to take samples. The solid and liquid products were separated by magnetic separation under an external magnetic field, and the liquid product was analyzed by Gas chromatography (GC) for conversion rate, and the solid was recovered and preserved.
4. Conclusions
This study aims to synthesize Y-type zeolites using tetraethyl orthosilicate (TEOS) as the silicon source. The effects of crystallization temperature, time, and Na2O content in the seed on the crystal size and the SiO2/Al2O3 ratio of the NaY zeolite were systematically investigated. The results indicate that increasing the crystallization temperature and extending the crystallization time promote crystal growth over nucleation, thereby yielding larger Y-type zeolite crystals. When the temperature exceeds 70 °C, with a SiO2/Al2O3 ratio of 9–12 and a Na2O/Al2O3 ratio of 4.5–8.5, as well as water dosage controlled between 300 and 480 parts, highly crystalline Y-type zeolite can be synthesized. Additionally, catalytic degradation experiments of benzyl phenyl ethers conducted in a high-pressure reactor demonstrated that NiY zeolites prepared via the acidic hydrolysis of TEOS significantly outperformed zeolites synthesized from alkaline silica sol. Under conditions of 200 °C and 0.5 MPa for 2 h, the conversion rate reached 100%; even under normal pressure, the conversion rate remained as high as 99.27%, far surpassing the conversion rates of traditional catalysts (92.4% and 79.89%, respectively). This indicates that NiY zeolites prepared by TEOS hydrolysis maintains high catalytic activity at lower pressures and temperatures, demonstrating significant practical application potential. This method provides a novel approach for preparing Y-type zeolites with high catalytic activity and excellent stability.
Author Contributions
Conceptualization, B.Z., Z.L. and H.Z.; methodology, B.Z., Z.L. and H.Z.; investigation, B.Z., Z.L, Y.L., H.Z., Y.T., Y.Z. and M.X.; resources, H.Z. and M.X.; data curation, B.Z., Z.L. and H.Z.; writing—original draft preparation, B.Z., Z.L. and Y.L.; writing—review and editing, B.Z., Z.L., Y.T. and H.Z.; formal analysis, B.Z., Z.L., Y.L., H.Z., Y.T., Y.Z. and M.X.; supervision, Y.Z. and M.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shi, J.; Wang, Y.; Yang, W.; Tang, Y.; Xie, Z. Recent advances of pore system construction in zeolite-catalyzed chemical industry processes. Chem. Soc. Rev. 2015, 44, 8877–8903. [Google Scholar] [CrossRef] [PubMed]
- Martínez, C.; Corma, A. Inorganic molecular sieves: Preparation, modification and industrial application in catalytic processes. Coord. Chem. Rev. 2011, 255, 1558–1580. [Google Scholar] [CrossRef]
- Van der Wal, L.I.; Oenema, J.; Smulders, L.C.J.; Samplonius, N.J.; Nandpersad, K.R.; Zečević, J.; de Jong, K.P. Control and Impact of Metal Loading Heterogeneities at the Nanoscale on the Performance of Pt/Zeolite Y Catalysts for Alkane Hydroconversion. ACS Catal. 2021, 11, 3842–3855. [Google Scholar] [CrossRef] [PubMed]
- West, R.M.; Holm, M.S.; Saravanamurugan, S.; Xiong, J.; Beversdorf, Z.; Taarning, E.; Christensen, C.H. Zeolite H-USY for the production of lactic acid and methyl lactate from C3-sugars. J. Catal. 2010, 269, 122–130. [Google Scholar] [CrossRef]
- Vermeiren, W.; Gilson, J.-P. Impact of Zeolites on the Petroleum and Petrochemical Industry. Top. Catal. 2009, 52, 1131–1161. [Google Scholar] [CrossRef]
- Agostini, G.; Lamberti, C.; Palin, L.; Milanesio, M.; Danilina, N.; Xu, B.; Janousch, M.; van Bokhoven, J.A. In Situ XAS and XRPD Parametric Rietveld Refinement To Understand Dealumination of Y Zeolite Catalyst. J. Am. Chem. Soc. 2009, 132, 667–678. [Google Scholar] [CrossRef]
- Silaghi, M.-C.; Chizallet, C.; Raybaud, P. Challenges on molecular aspects of dealumination and desilication of zeolites. Microporous Mesoporous Mater. 2014, 191, 82–96. [Google Scholar] [CrossRef]
- Sun, B.; Kang, Y.; Shi, Q.; Arowo, M.; Luo, Y.; Chu, G.; Zou, H. Synthesis of ZSM-5 by hydrothermal method with pre-mixing in a stirred-tank reactor. Can. J. Chem. Eng. 2019, 97, 3063–3073. [Google Scholar] [CrossRef]
- Moosavifar, M.; Heidari, S.M.; Fathyunes, L.; Ranjbar, M.; Wang, Y.; Arandiyan, H. Photocatalytic Degradation of Dye Pollutant Over FeTPP/NaY Zeolite Nanocomposite. J. Inorg. Organomet. Polym. 2019, 30, 1621–1628. [Google Scholar] [CrossRef]
- Ghebache, Z.; Hamidouche, F.; Safidine, Z.; Trari, M.; Bellal, B. Synthesis and Electrical Conducting Properties of Poly(aniline) Doped with Zeolite HY Nanocomposites Containing SnO2 for High-Performance Supercapacitor Electrode. J. Inorg. Organomet. Polym. 2019, 29, 1548–1558. [Google Scholar] [CrossRef]
- Tang, T.; Zhang, L.; Dong, H.; Fang, Z.; Fu, W.; Yu, Q.; Tang, T. Organic template-free synthesis of zeolite Y nanoparticle assemblies and their application in the catalysis of the Ritter reaction. RSC Adv. 2017, 7, 7711–7717. [Google Scholar] [CrossRef]
- Ferdov, S.; Tsuchiya, K.; Tsunoji, N.; Sano, T. Comparative study between high-silica faujasites (FAU) from organic-free system and the commercial zeolite Y. Microporous Mesoporous Mater. 2019, 276, 154–159. [Google Scholar] [CrossRef]
- Li, R.; Linares, N.; Sutjianto, J.G.; Chawla, A.; Garcia-Martinez, J.; Rimer, J.D. Ultrasmall Zeolite L Crystals Prepared from Highly Interdispersed Alkali-Silicate Precursors. Angew. Chem. Int. Ed. 2018, 57, 11283–11288. [Google Scholar] [CrossRef] [PubMed]
- Mintova, S.; Gilson, J.-P.; Valtchev, V. Advances in nanosized zeolites. Nanoscale 2013, 5, 6693. [Google Scholar] [CrossRef]
- Wu, Q.; Hong, X.; Zhu, L.; Meng, X.; Han, S.; Zhang, J.; Liu, X.; Jin, C.; Xiao, F.-S. Generalized ionothermal synthesis of silica-based zeolites. Microporous Mesoporous Mater. 2019, 286, 163–168. [Google Scholar] [CrossRef]
- Han, S.; Tang, X.; Wang, L.; Ma, Y.; Chen, W.; Wu, Q.; Zhang, L.; Zhu, Q.; Meng, X.; Zheng, A.; et al. Potassium-directed sustainable synthesis of new high silica small-pore zeolite with KFI structure (ZJM-7) as an efficient catalyst for NH3-SCR reaction. Appl. Catal. B Environ. 2021, 281, 119480. [Google Scholar] [CrossRef]
- Delprato, F.; Delmotte, L.; Guth, J.L.; Huve, L. Synthesis of new silica-rich cubic and hexagonal faujasites using crown-etherbased supramolecules as templates. Zeolites 1990, 10, 546–552. [Google Scholar] [CrossRef]
- Yu, O.; Kim, K.H. Lignin to Materials: A Focused Review on Recent Novel Lignin Applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, Y.; Wu, T.; Deng, Y.; Shen, K.; Fang, G. Research Progress on the Application of Lignin Functional Materials. Appl. Chem. Ind. 2022, 51, 491–495+500. [Google Scholar]
- Mukherjee, A.; Mandal, T.; Ganguly, A.; Chatterjee, P.K. Lignin Degradation in the Production of Bioethanol—A Review. ChemBioEng Rev. 2016, 3, 86–96. [Google Scholar] [CrossRef]
- Yadav, V.K.; Gupta, N.; Kumar, P.; Dashti, M.G.; Tirth, V.; Khan, S.H.; Yadav, K.K.; Islam, S.; Choudhary, N.; Algahtani, A.; et al. Recent Advances in Synthesis and Degradation of Lignin and Lignin Nanoparticles and Their Emerging Applications in Nanotechnology. Materials 2022, 15, 953. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Liu, Y.; Wu, S.; Zhuang, J. A review for lignin valorization: Challenges and perspectives in catalytic hydrogenolysis. Ind. Crops Prod. 2021, 172, 114008. [Google Scholar] [CrossRef]
- Torres, L.A.Z.; Woiciechowski, A.L.; de Andrade Tanobe, V.O.; Karp, S.G.; Lorenci, L.C.G.; Faulds, C.; Soccol, C.R. Lignin as a potential source of high-added value compounds: A review. J. Clean. Prod. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Zhu, C.; Cao, J.-P.; Zhao, X.-Y.; Xie, T.; Ren, J.; Wei, X.-Y. Mechanism of Ni-catalyzed selective C O cleavage of lignin model compound benzyl phenyl ether under mild conditions. J. Energy Inst. 2019, 92, 74–81. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Xu, J. Hydrogenolysis of lignosulfonate into phenols over heterogeneous nickel catalysts. Chem. Commun. 2012, 48, 7019. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Hu, J.; Zhong, W.; Hu, Z.; Wang, H. Comprehensive study of alkali lignin pyrolysis catalyzed by composite metal-modified molecular sieves for the preparation of hydrocarbon liquid fuels. J. Anal. Appl. Pyrolysis 2024, 181, 106608. [Google Scholar] [CrossRef]
- Jiang, T.; Zhao, Q.; Chen, K.; Tang, Y.; Yu, L.; Yin, H. Synthesis and characterization of Co (Ni or Cu)-MCM-41 mesoporous molecular sieves with different amount of metal obtained by using microwave irradiation method. Appl. Surf. Sci. 2008, 254, 2575–2580. [Google Scholar] [CrossRef]
- Mendoza, C.; Echavarría, A. A systematic study on the synthesis of nanosized Y zeolite without using organic structure-directing agents: Control of Si/Al ratio. J. Porous Mater. 2022, 29, 907–919. [Google Scholar] [CrossRef]
- Moteki, T.; Okubo, T. From Charge Density Mismatch to a Simplified, More Efficient Seed-Assisted Synthesis of UZM-4. Chem. Mater. 2013, 25, 2603–2609. [Google Scholar] [CrossRef]
- Inayat, A.; Knoke, I.; Spiecker, E.; Schwieger, W. Assemblies of Mesoporous FAU-Type Zeolite Nanosheets. Angew. Chem. Int. Ed. 2012, 51, 1962–1965. [Google Scholar] [CrossRef]
- Valtchev, V.; Tosheva, L. Porous Nanosized Particles: Preparation, Properties, and Applications. Chem. Rev. 2013, 113, 6734–6760. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.L.S.; Monteiro, J.L.F.; Pastore, H.O. Static crystallization of zeolites MCM-22 and MCM-49. Microporous Mesoporous Mater. 1999, 32, 131–145. [Google Scholar] [CrossRef]
- Cheng, M.; Tan, D.; Liu, X.; Han, X.; Bao, X.; Lin, L. Effect of aluminum on the formation of zeolite MCM-22 and kenyaite. Microporous Mesoporous Mater. 2001, 42, 307–316. [Google Scholar] [CrossRef]
- Ma, Y.-K.; Rigolet, S.; Michelin, L.; Paillaud, J.; Mintova, S.; Khoerunnisa, F.; Daou, T.; Ng, E. Facile and fast determination of Si/Al ratio of zeolites using FTIR spectroscopy technique. Microporous Mesoporous Mater. 2021, 311, 110683. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, J.; Dai, W.; Hunger, M. Solid-state nuclear magnetic resonance investigations of the nature, property, and activity of acid sites on solid catalysts. Solid State Nucl. Magn. Reson. 2011, 39, 116–141. [Google Scholar] [CrossRef]
- Mu, L.; Feng, W.; Zhang, H.; Hu, X.; Cui, Q. Synthesis and catalytic performance of a small crystal NaY zeolite with high SiO2/Al2O3 ratio. RSC Adv. 2019, 9, 20528–20535. [Google Scholar] [CrossRef]
- Meng, B.; Ren, S.; Li, Z.; Nie, S.; Zhang, X.; Song, W.; Guo, Q.; Shen, B. A facile organic-free synthesis of high silica zeolite Y with small crystal in the presence of Co2+. Microporous Mesoporous Mater. 2021, 323, 111248. [Google Scholar] [CrossRef]
- Eapen, M.J.; Reddy, K.S.N.; Shiralkar, V.P. Hydrothermal crystallization of zeolite beta using tetraethylammonium bromide. Zeolites 1994, 14, 295–302. [Google Scholar] [CrossRef]
- Nakhaei Pour, A.; Mohammadi, A. Effects of Synthesis Parameters on Organic Template-Free Preparation of Zeolite Y. J. Inorg. Organomet. Polym. 2021, 31, 2501–2510. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, P.; Liu, X.; Huang, W.; Fan, X.; Yan, Y.; Zhang, R.; Wang, L.; Zhou, Y. Synthesis of Ni-Modified ZSM-5 Zeolites and Their Catalytic Performance in n-Octane Hydroconversion. Front. Chem. 2020, 8, 586445. [Google Scholar] [CrossRef]
- Richardson, J. X-Ray Diffraction Study of Nickel Oxide Reduction by Hydrogen. Appl. Catal. A Gen. 2003, 246, 137–150. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).