MoS2/MgAl-LDH Composites for the Photodegradation of Rhodamine B Dye
Abstract
1. Introduction
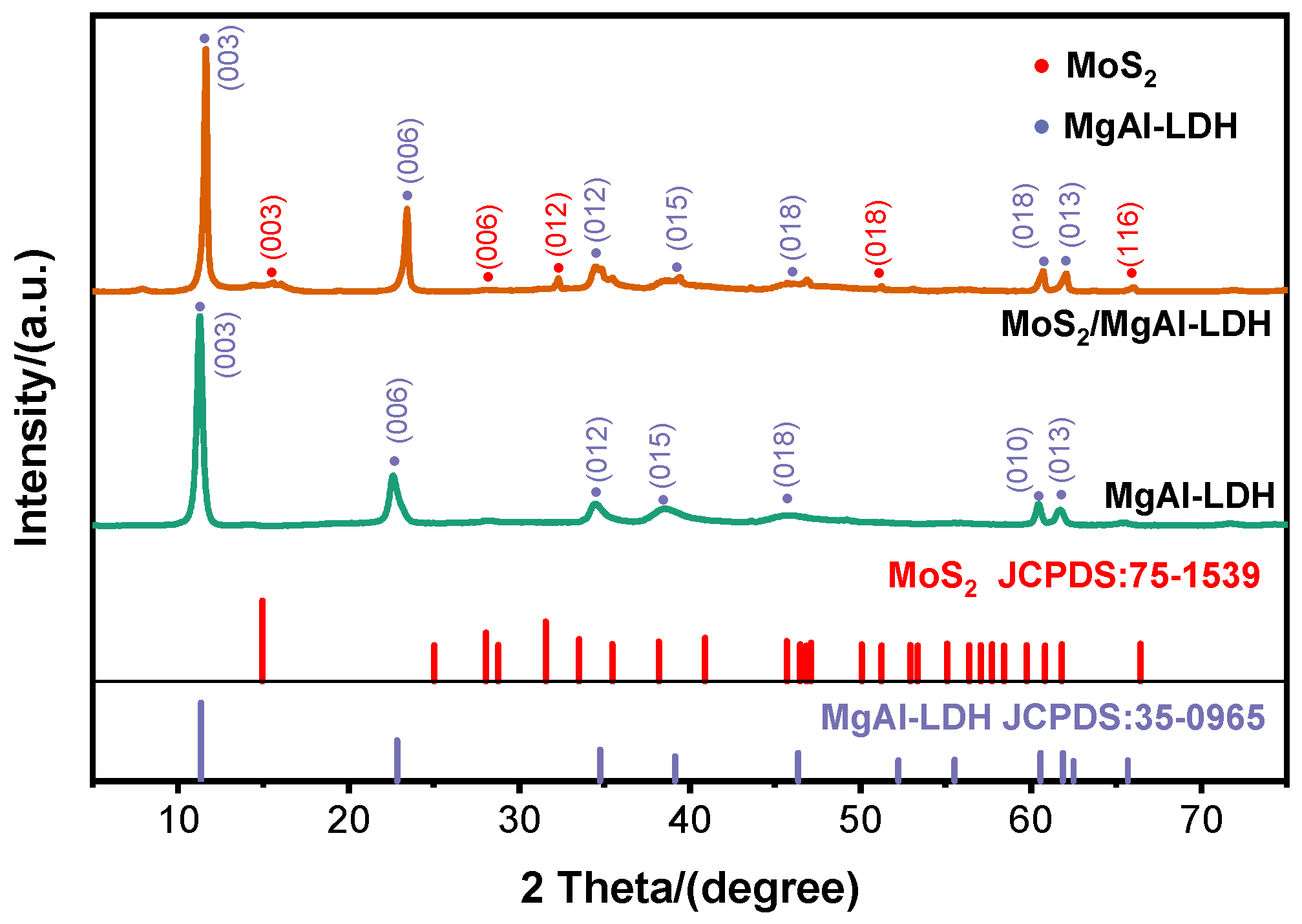
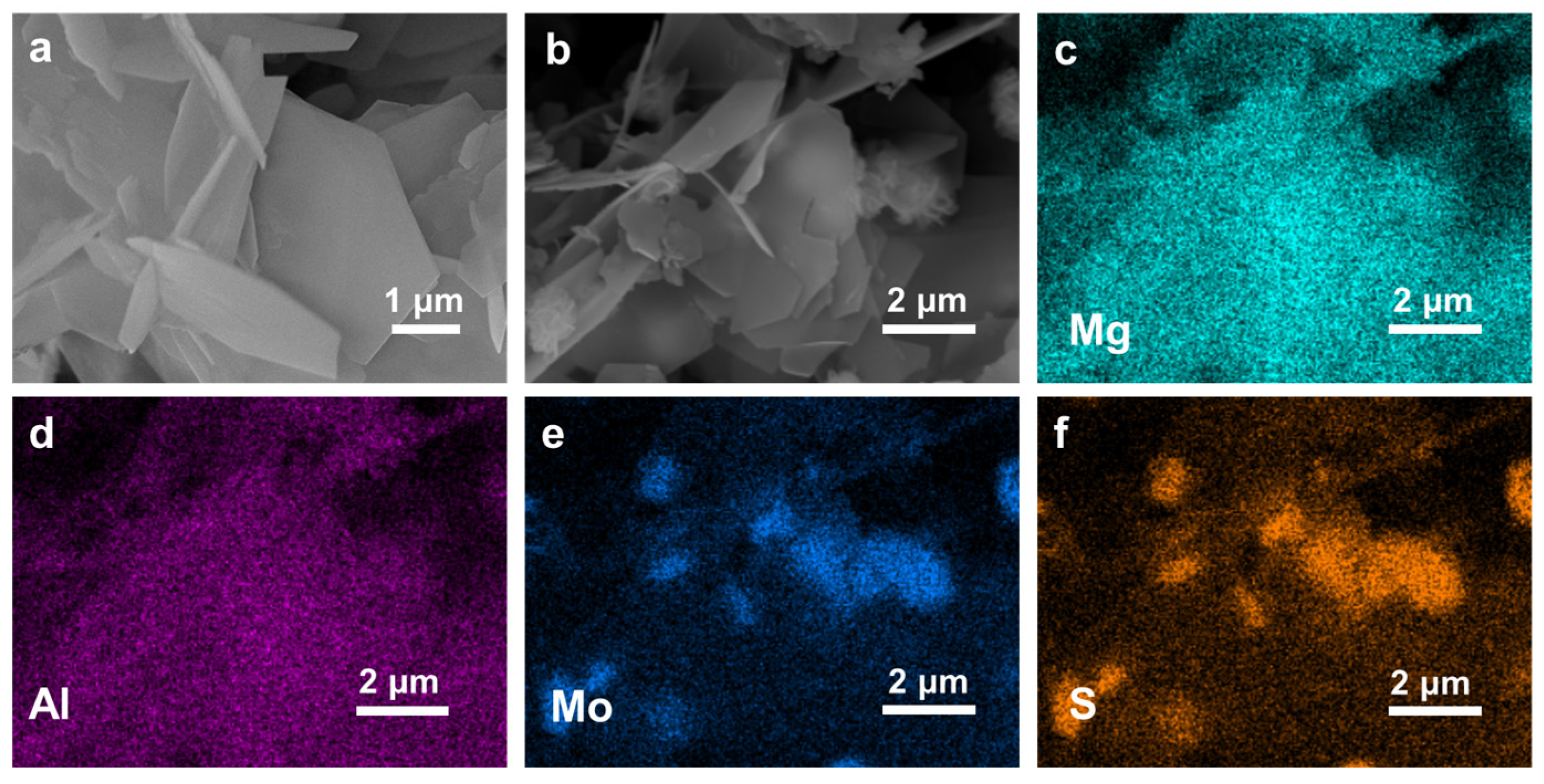
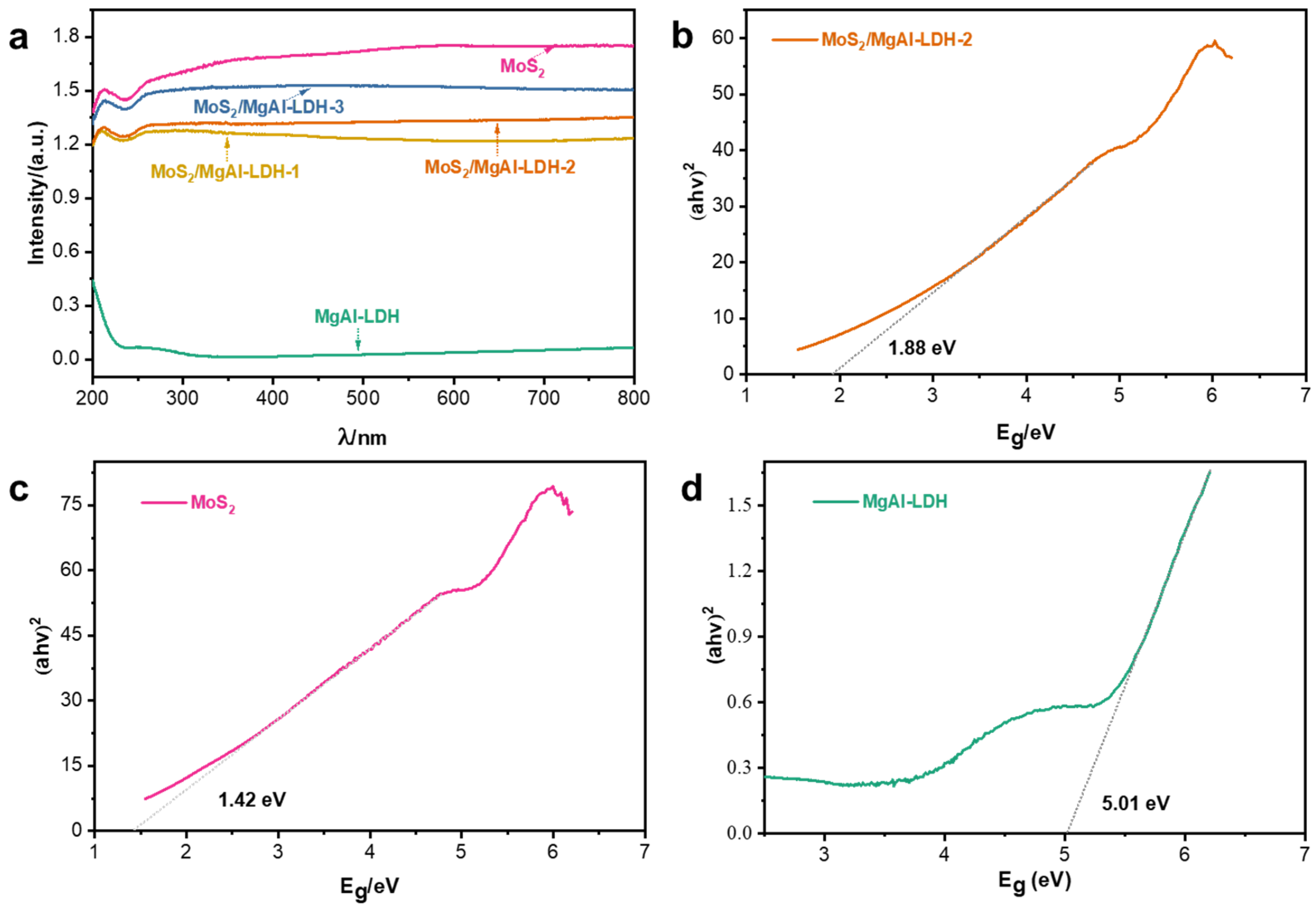
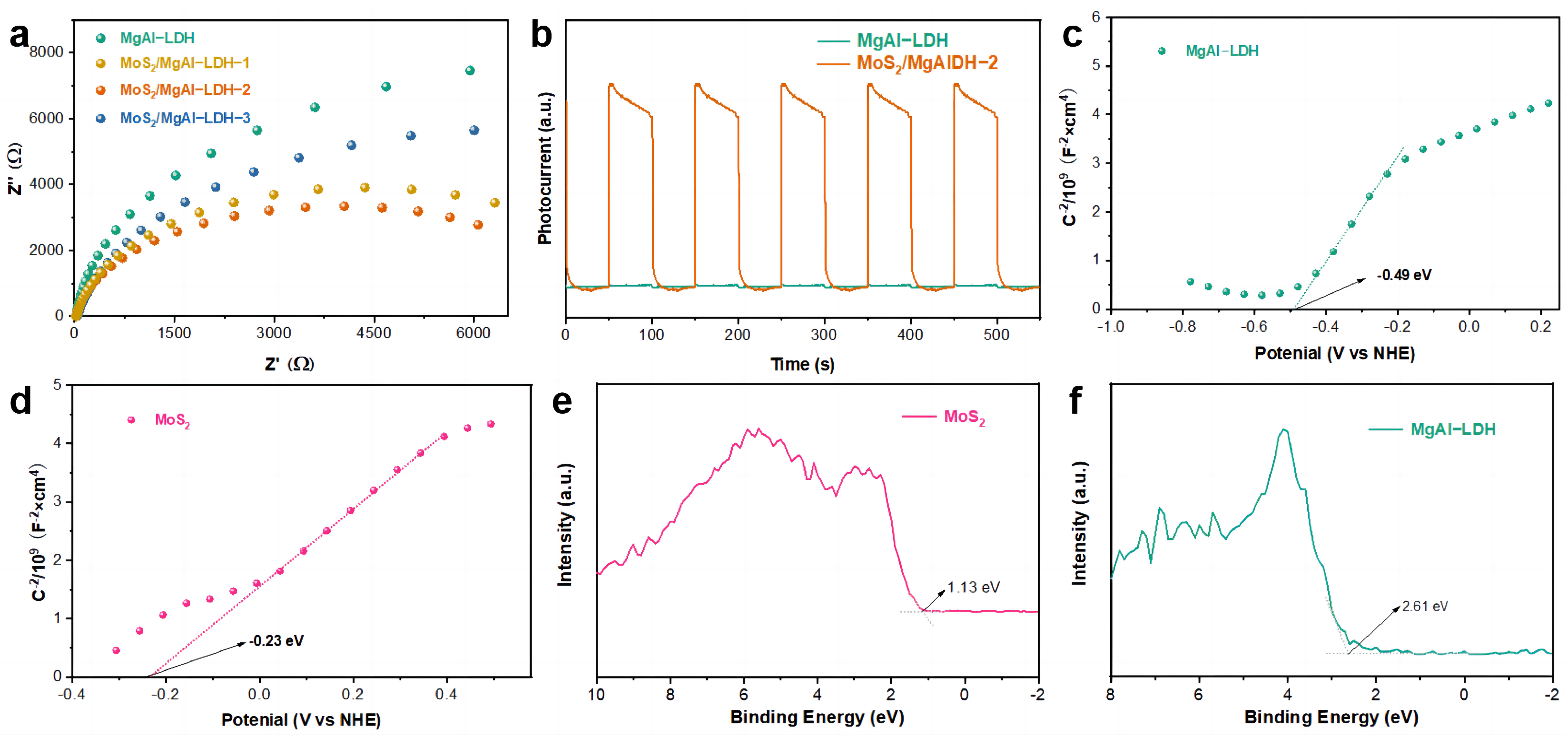
2. Results and Discussion
3. Materials and Methods
3.1. Preparation of Photocatalysts
3.2. Characterizations
3.3. Photocatalystic Degration of RhB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, H.; Tan, P.; Liu, Y.; Zhai, H.; Du, W.; Liu, X.; Pan, J. Ultrafast interfacial charge evolution of the Type-II cadmium Sulfide/Molybdenum disulfide heterostructure for photocatalytic hydrogen production. J. Colloid Interface Sci. 2022, 619, 246–256. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, T.; Che, G.; Liu, C. A synergism between Schottky junction and interfacial P-Ni bond for improving the hydrogen evolution of 2D/2D NiS/Phosphorus-doped g-C3N4 photocatalyst. Appl. Surf. Sci. 2022, 578, 152004. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Cai, X.; Wu, J. Adsorption and Visible Photocatalytic Synergistic Removal of a Cationic Dye with the Composite Material BiVO4/MgAl-LDHs. Materials 2023, 16, 6879. [Google Scholar] [CrossRef]
- You, C.; Wang, C.; Cai, M.; Liu, Y.; Zhu, B.; Li, S. Improved Photo-Carrier Transfer by an Internal Electric Field in BiOBr/N-rich C3N5 3D/2D S-Scheme Heterojunction for Efficiently Photocatalytic Micropollutant Removal. Acta Phys.-Chim. Sin. 2024, 40, 2407014. [Google Scholar] [CrossRef]
- Athar, M.S.; Rasool, Z.; Muneer, M.; Altass, H.M.; Althagafi, I.I.; Ahmed, S.A. Fabrication of Direct Z-Scheme CoNiWO4/Ph-gC3N4 Heterocomposites: Enhanced Photodegradation of Bisphenol A and Anticancer Activity. ACS Omega 2023, 8, 38272–38287. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Feng, Q.; Fang, C.; Chen, R.; Wang, Y.; Xu, L.; Liu, C. A novel ZnCo2O4/BiOBr p-n/Z-scheme heterojunction photocatalyst for enhancing photocatalytic activity. Environ. Sci. Pollut. Res. Int. 2024, 31, 26839–26854. [Google Scholar] [CrossRef] [PubMed]
- Mittal, H.; Kumar, A.; Sharma, D.; Khanuja, M. Z-Scheme Enabled 1D/2D Nanocomposite of ZnO Nanorods and Functionalized g-C3 N4 Nanosheets for Sustainable Degradation of Terephthalic Acid. ChemSusChem 2024, 18, e202401408. [Google Scholar] [CrossRef]
- Aboraia, A.M.; Al-Omoush, M.; Solayman, M.; Saad, H.M.H.; Khabiri, G.; Saad, M.; Alsulaim, G.M.; Soldatov, A.V.; Ismail, Y.A.M.; Gomaa, H. A heterostructural MoS2 QDs@UiO-66 nanocomposite for the highly efficient photocatalytic degradation of methylene blue under visible light and simulated sunlight. RSC Adv. 2023, 13, 34598–34609. [Google Scholar] [CrossRef]
- Dai, H.; Yang, X.; Tang, F. Ag2S Nanoparticles Supported on 3D Flower-Shaped Bi2WO6 Enhanced Visible Light Catalytic Degradation of Tetracycline. ACS Omega 2023, 8, 42647–42658. [Google Scholar] [CrossRef]
- Hu, H.; He, Y.; Yu, H.; Li, D.; Sun, M.; Feng, Y.; Zhang, C.; Chen, H.; Deng, C. Constructing a noble-metal-free 0D/2D CdS/SnS2heterojunction for efficient visible-light-driven photocatalytic pollutant degradation and hydrogen generation. Nanotechnology 2023, 34, 505712. [Google Scholar] [CrossRef]
- Wang, C.; You, C.; Rong, K.; Shen, C.; Yang, F.; Li, S. An S-Scheme MIL-101(Fe)-on-BiOCl Heterostructure with Oxygen Vacancies for Boosting Photocatalytic Removal of Cr(VI). Acta Phys.-Chim. Sin. 2024, 40, 2307045. [Google Scholar] [CrossRef]
- Chen, L.; Chuang, Y.; Nguyen, T.; Chen, C.; Dong, C. Enhanced photocatalytic activity of tin oxide-doped molybdenum disulfide nanohybrids under visible light irradiation: Antibiotics elimination, heavy metal reduction and antibacterial behavior. Environ. Res. 2023, 238, 117259. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.; Li, F.; Evans, D.G.; Duan, X. Catalytic applications of layered double hydroxides: Recent advances and perspectives. Chem. Soc. Rev. 2014, 43, 7040–7066. [Google Scholar] [CrossRef] [PubMed]
- Han, J.B.; Lu, J.; Wei, M.; Wang, Z.L.; Duan, X. Heterogeneous ultrathin films fabricated by alternate assembly of exfoliated layered double hydroxides and polyanion. Chem. Commun. 2008, 41, 5188–5190. [Google Scholar] [CrossRef]
- Bai, S.; Wang, Z.; Tan, L.; Waterhouse, G.I.N.; Zhao, Y.; Song, Y.-F. 600 nm Irradiation-Induced Efficient Photocatalytic CO2 Reduction by Ultrathin Layered Double Hydroxide Nanosheets. Ind. Eng. Chem. Res. 2020, 59, 5848–5857. [Google Scholar] [CrossRef]
- Portillo-Vélez, N.; Velásquez-Torres, M.; Pérez-Hernández, R.; Ibarra, I.; Peralta, R.; Tzompantzi, F. Boosted Photocatalytic Activity of Zn/Al-Based Layered Double Hydroxides Through Cobalt Incorporation for Phenol Degradation. ChemistrySelect 2024, 9, e202304860. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Ma, L.; Liu, Y.; Hu, L. Nano-flower S-scheme heterojunction NiAl-LDH/MoS2 for enhancing photocatalytic hydrogen production. New J. Chem. 2022, 46, 228–238. [Google Scholar] [CrossRef]
- Song, B.; Zeng, Z.; Zeng, G.; Gong, J.; Xiao, R.; Ye, S.; Chen, M.; Lai, C.; Xu, P.; Tang, X. Powerful combination of g-C3N4 and LDHs for enhanced photocatalytic performance: A review of strategy, synthesis, and applications. Adv. Colloid Interface Sci. 2019, 272, 101999. [Google Scholar] [CrossRef]
- Zhang, K.; Wan, T.; Wang, H.; Luo, Y.; Shi, Y.; Zhang, Z.; Liu, G.; Li, J. Decorated Oxidation-resistive deficient Titanium oxide nanotube supported NiFe-nanosheets as high-efficiency electrocatalysts for overall water splitting. J. Colloid Interface Sci. 2023, 645, 66–75. [Google Scholar] [CrossRef]
- Yu, F.; Duan, X.; Jiang, R.; Ren, J.; Zhang, J.; Feng, C.; Li, C.; Hu, K.; Hou, X. Study on the regulation mechanism of Al doping on the controllable photocatalytic performance of the ZnO@MgAl-LDH nanocomposite. Appl. Surf. Sci. 2025, 680, 161386. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, W.; Li, Z.; Zhang, Z.; Zhou, G. A new strategy: Fermi level control to realize 3D pyramidal NiCo-LDH/ReS2/n-PSi as a high-performance photoanode for the oxygen evolution reaction. J. Mater. Chem. C 2022, 10, 3848–3855. [Google Scholar] [CrossRef]
- Wu, B.; Zeng, H.; Xiong, J.; Peng, J.; Liu, F.; Yang, Z. Engineering S-scheme Bi2WO6/CoAl-LDH heterostructure for enhancing photocatalytic redox ability. J. Alloys Compd. 2024, 1002, 175224. [Google Scholar] [CrossRef]
- Sun, M.; Liu, Y.; Na, Y.; Li, Z.; Chen, M.; Dai, S.; Guo, X.; Li, P.; Zhao, T.; Zheng, R. Mechanochemical preparation of Z-scheme CdIn2S4/Zn-Al LDH heterojunction with enhanced photocatalytic performance for SIPX degradation. J. Alloys Compd. 2025, 1010, 177247. [Google Scholar] [CrossRef]
- Hao, T.; Xu, H.; Sun, S.; Yu, H.; Qin, Q.; Song, B.; Li, M.; Shao, G.; Fan, B.; Wang, H.; et al. Assembling flower-like MgAl-LDH nanospheres and g-C3N4 nanosheets for high efficiency removal of methyl orange. Ceram. Int. 2024, 50, 10724–10734. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Ma, L.; Liu, Y.; Hu, L. High efficiency hydrogen production with visible light layered MgAl-LDH coupled with CoSx. Chem. Phys. Lett. 2021, 784, 139124. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, W.; Yu, J.; Jiao, F. Effective photocatalytic degradation of methylene blue by Cu2O/MgAl layered double hydroxides. React. Kinet. Mech. Catal. 2015, 115, 581–596. [Google Scholar] [CrossRef]
- Tran, V.-T.; Chen, D.-H. CuS@MoS2 p–n heterojunction photocatalyst integrating photothermal and piezoelectric enhancement effects for tetracycline degradation. J. Environ. Chem. Eng. 2024, 12, 113158. [Google Scholar] [CrossRef]
- Govinda, R.; Mahalingam, S.; Gnanarani, S.; Jayashree, C.; Ganeshraja, A.; Pugazhenthiran, N.; Rahaman, M.; Abinaya, S.; Senthil, B.; Kim, J. TiO2 nanorod decorated with MoS2 nanospheres: An efficient dual-functional photocatalyst for antibiotic degradation and hydrogen production. Chemosphere 2024, 357, 142033. [Google Scholar] [CrossRef]
- Hu, Q.; Chen, L.; Xie, X.; Qin, Z.; Ji, H.; Su, T. Construction of Electron Bridge and Activation of MoS2 Inert Basal Planes by Ni Doping for Enhancing Photocatalytic Hydrogen Evolution. Acta Phys.-Chim. Sin. 2024, 40, 2406024. [Google Scholar] [CrossRef]
- Vennapoosa, C.; Shelake, S.; Jaksani, B.; Jamma, A.; Moses, A.; Sesha Sainath, A.; Ahmadipour, M.; Pal, U. Surface engineering of a 2D CuFe-LDH/MoS2 photocatalyst for improved hydrogen generation. Mater. Adv. 2024, 5, 4159–4171. [Google Scholar] [CrossRef]
- Zheng, G.; Wu, C.; Wang, J.; Mo, S.; Wang, Y.; Zou, Z.; Zhou, B.; Long, F. Facile synthesis of few-layer MoS2 in MgAl-LDH layers for enhanced visible-light photocatalytic activity. RSC Adv. 2019, 9, 24280–24290. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, C.; Zhang, Y.; Guo, Z.; Luo, Y.; Mao, C. Engineering ultrafine NiS cocatalysts as active sites to boost photocatalytic hydrogen production of MgAl layered double hydroxide. Appl. Surf. Sci. 2020, 506, 144999. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, C.; Jiang, L.; Lin, H.; An, Y.; Zhou, D.; Leung, M.; Yang, S. Nanohybridization of MoS2 with Layered Double Hydroxides Efficiently Synergizes the Hydrogen Evolution in Alkaline Media. Joule 2017, 1, 383–393. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, R.; Xu, Z.; Zhang, S.; Zhang, C.; Li, G. Electrical and luminescence properties, and energy band structure of SrBi2-xErxNb2O9 multifunctional ceramics. Ceram. Int. 2021, 47, 30938–30946. [Google Scholar] [CrossRef]
- Jia, L.; Ma, N.; Shao, P.; Ge, Y.; Liu, J.; Dong, W.; Song, H.; Lu, C.; Zhou, Y.; Xu, X. Incorporating ReS2 Nanosheet into ZnIn2S4 Nanoflower as Synergistic Z-Scheme Photocatalyst for Highly Effective and Stable Visible-Light-Driven Photocatalytic Hydrogen Evolution and Degradation. Small 2024, 20, e2404622. [Google Scholar] [CrossRef]
- Xue, C.; An, H.; Shao, G.; Yang, G. Accelerating directional charge separation via built-in interfacial electric fields originating from work-function differences. Chin. J. Catal. 2021, 42, 583–594. [Google Scholar] [CrossRef]
- Cai, J.; Xia, Y.; Gang, R.; He, S.; Komarneni, S. Activation of MoS2 via tungsten doping for efficient photocatalytic oxidation of gaseous mercury. Appl. Catal. B. Environ. 2022, 314, 121486. [Google Scholar] [CrossRef]
- Yang, F.; Hu, P.; Yang, F.; Chen, B.; Yin, F.; Hao, K.; Sun, R.; Gao, L.; Sun, Z.; Wang, K.; et al. CNTs Bridged Basal-Plane-Active 2H-MoS2 Nanosheets for Efficient Robust Electrocatalysis. Small 2023, 19, e2301468. [Google Scholar] [CrossRef]
- Shang, X.; Hu, W.; Li, X.; Dong, B.; Liu, Y.; Han, G.; Chai, Y.; Liu, C. Oriented Stacking along Vertical (002) Planes of MoS2, A Novel Assembling Style to Enhance Activity for Hydrogen Evolution. Electrochim. Acta 2017, 224, 25–31. [Google Scholar] [CrossRef]
- Ovezmyradov, B.; Chen, H.; Duan, S.; Zhu, M.; Zhang, D.; Xue, C.; Ovezmyradov, M.; Yang, G. Construction of N-doped 2D TiO2/MoS2 S-scheme heterojunction for enhanced photodegradation activity by rhodamine B. React. Kinet. Mech. Catal. 2024, 138, 471–484. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, S.; Zheng, J.; Liu, J.; Huang, M. Construction of Ag-modified ZnO/g-C3N4 heterostructure for enhanced photocatalysis performance. J. Chem. Phys. 2024, 161, 154707. [Google Scholar] [CrossRef] [PubMed]
- Sarngan, P.; Sasi, S.; Mukherjee, P.; Mitra, K.; Sivalingam, Y.; Swami, A.; Ghorai, U.; Sarkar, D. Unveiling efficient S-scheme charge carrier transfer in hierarchical BiOBr/TiO2 heterojunction photocatalysts. Nanoscale 2024, 16, 19006–19020. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, X.; He, J.; Chen, T.; Han, L.; Zhou, Q.; Zhao, D.; Wang, Y.; Wang, S. Effect of Size Tuning of Hexagonal SnS2 Nanosheet on the Efficiency of Photocatalytic Degradation Processes. Small 2024, 20, e2406002. [Google Scholar] [CrossRef]
- Mei, Z.; Wang, G.; Yan, S.; Wang, J. Rapid Microwave-Assisted Synthesis of 2D/1D ZnIn2S4/TiO2 S-scheme Heterojunction for Catalyzing Photocatalytic Hydrogen Evolution. Acta Phys. Chim. Sin. 2020, 37, 2009097. [Google Scholar] [CrossRef]
- Miao, Z.; Wang, Q.; Zhang, Y.; Meng, L.; Wang, X. In situ construction of S-scheme AgBr/BiOBr heterojunction with surface oxygen vacancy for boosting photocatalytic CO2 reduction with H2O. Appl. Catal. B. Environ. 2022, 301, 120802. [Google Scholar] [CrossRef]
- Yang, F.; Cao, Z.; Wang, J.; Wang, S.; Zhong, H. In situ self-assembly of molybdenum disulfide/Mg–Al layered double hydroxide composite for enhanced photocatalytic activity. J. Alloys Compd. 2020, 817, 153308. [Google Scholar] [CrossRef]



| Samples | Kobs/min−1 | R2 |
|---|---|---|
| MgAl-LDH | 0.0008 | 0.9669 |
| MoS2/MgAl-LDH-1 | 0.0023 | 0.9572 |
| MoS2/MgAl-LDH-2 | 0.0077 | 0.9936 |
| MoS2/MgAl-LDH-3 | 0.0073 | 0.9922 |
| Samples | MgAl-LDH | MoS2 |
|---|---|---|
| CB | −0.49 eV | −0.23 eV |
| VB | 4.52 eV | 1.19 eV |
| Band gap | 5.01 eV | 1.42 eV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, J.; Li, G.; Wang, Y.; Zhang, C.; Nan, H.; Yang, G. MoS2/MgAl-LDH Composites for the Photodegradation of Rhodamine B Dye. Inorganics 2025, 13, 88. https://doi.org/10.3390/inorganics13030088
Dai J, Li G, Wang Y, Zhang C, Nan H, Yang G. MoS2/MgAl-LDH Composites for the Photodegradation of Rhodamine B Dye. Inorganics. 2025; 13(3):88. https://doi.org/10.3390/inorganics13030088
Chicago/Turabian StyleDai, Jingjing, Guofei Li, Yuanyuan Wang, Cancan Zhang, Hui Nan, and Guijun Yang. 2025. "MoS2/MgAl-LDH Composites for the Photodegradation of Rhodamine B Dye" Inorganics 13, no. 3: 88. https://doi.org/10.3390/inorganics13030088
APA StyleDai, J., Li, G., Wang, Y., Zhang, C., Nan, H., & Yang, G. (2025). MoS2/MgAl-LDH Composites for the Photodegradation of Rhodamine B Dye. Inorganics, 13(3), 88. https://doi.org/10.3390/inorganics13030088







