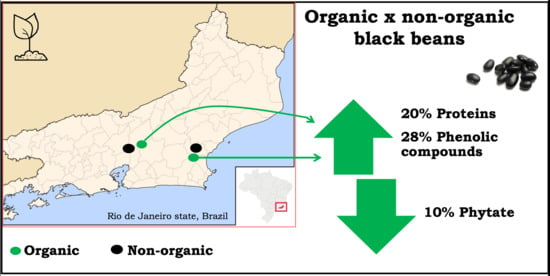
Organic Black Beans (Phaseolus vulgaris L.) from Rio de Janeiro State, Brazil, Present More Phenolic Compounds and Better Nutritional Profile Than Nonorganic
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Black Bean and Soil Samples
2.3. Soil Chemical Analysis
2.3.1. Mineral Composition
2.3.2. Nitrogen Content
2.4. Black Beans Chemical Analysis
2.4.1. Pesticides Residues
2.4.2. Proximate Composition
2.4.3. Phytate Content
2.4.4. Mineral Composition
2.4.5. Phenolic Compounds
2.5. Statistical Analyses
3. Results and Discussion
3.1. Pesticide Residues Were Not Detected in Any Sample
3.2. Organically Produced Black Beans Contain Approximately 20% More Proteins than Nonorganically Produced Ones
3.3. Lower Phytate Contents in Organic Black Beans May Increase Mineral Bioavailability
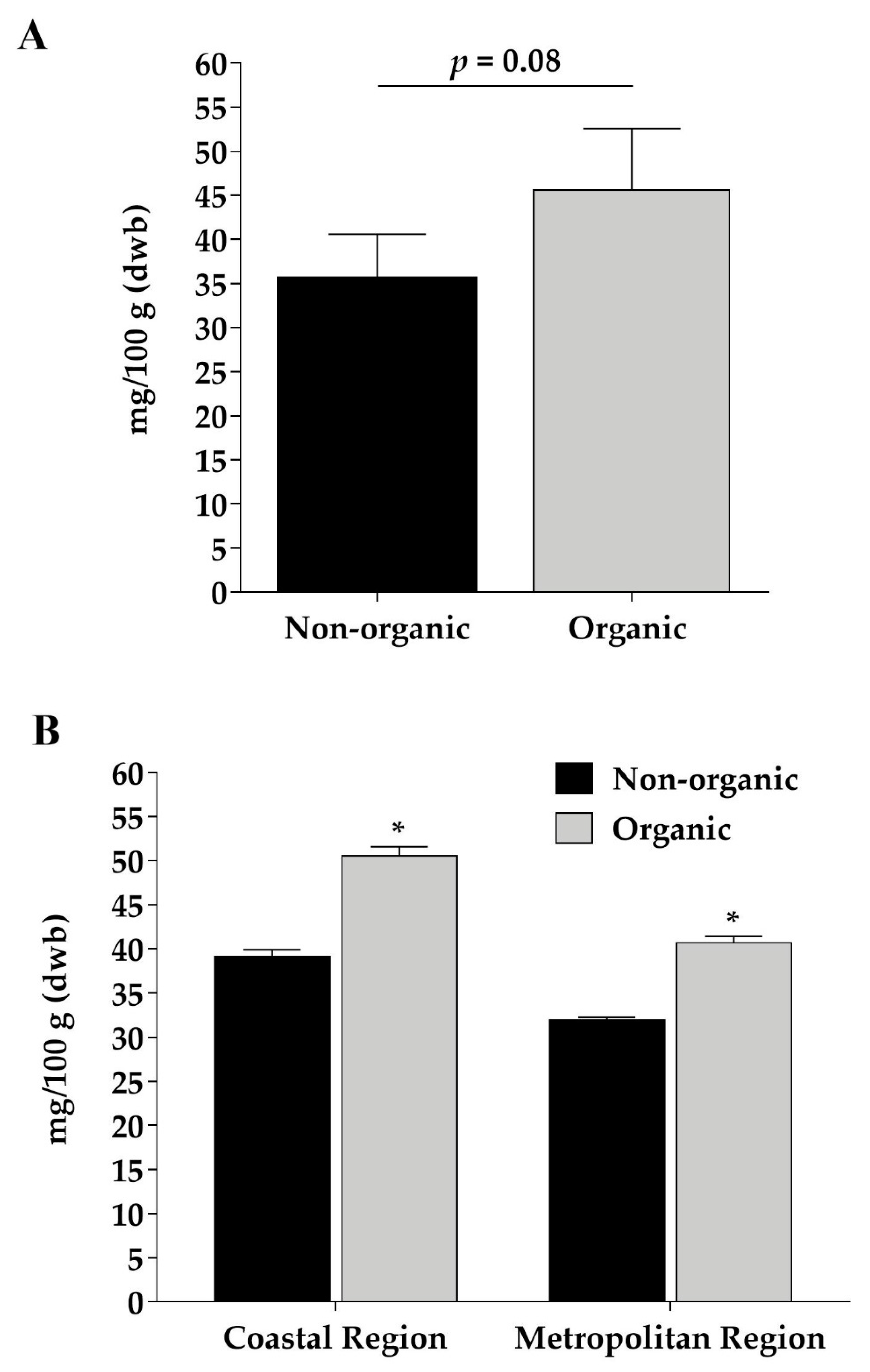
3.4. Organic Black Beans Present 28% Higher Phenolic Compounds Contents than Nonorganic Ones
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT, Countries by commodity (Dry beans). Available online: http://www.fao.org/faostat/en/#rankings/countries_by_commodity (accessed on 9 April 2021).
- Instituto Brasileiro de Geografia e Estatística (IBGE). Pesquisa de orçamentos familiares 2017-2018: Análise do Consumo Alimentar Pessoal no Brasil. 2020. Available online: https://biblioteca.ibge.gov.br/visualizacao/livros/liv101742.pdf. (accessed on 6 March 2021).
- Costa, G.E.D.A.; Queiroz-Monici, K.D.S.; Reis, S.M.P.M.; de Oliveira, A.C. Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chem. 2006, 94, 327–330. [Google Scholar] [CrossRef]
- Muzquiz, M.; Varela, A.; Burbano, C.; Cuadrado, C.; Guillamón, E.; Pedrosa, M.M. Bioactive compounds in legumes: Pronutritive and antinutritive actions. Implications for nutrition and health. Phytochem. Rev. 2012, 11, 227–244. [Google Scholar] [CrossRef]
- Landi, N.; Pacifico, S.; Piccolella, S.; Di Giuseppe, A.M.A.; Mezzacapo, M.C.; Ragucci, S.; Iannuzzi, F.; Zarrelli, A.; Di Maro, A. Valle Agricola lentil, an unknown lentil (Lens culinaris Medik.) seed from Southern Italy as a novel antioxidant and prebiotic source. Food Funct. 2015, 6, 3155–3164. [Google Scholar] [CrossRef] [PubMed]
- Cid-Gallegos, M.S.; Sánchez-Chino, X.M.; Álvarez-González, I.; Madrigal-Bujaidar, E.; Vásquez-Garzón, V.R.; Baltiérrez-Hoyos, R.; Villa-Treviño, S.; Dávila-Ortíz, G.; Jiménez-Martínez, C. Modification of In Vitro and In Vivo Antioxidant Activity by Consumption of Cooked Chickpea in a Colon Cancer Model. Nutrients 2020, 12, 2572. [Google Scholar] [CrossRef] [PubMed]
- Hayat, I.; Ahmad, A.; Masud, T.; Ahmed, A.; Bashir, S. Nutritional and Health Perspectives of Beans (Phaseolus vulgaris L.): An Overview. Crit. Rev. Food Sci. Nutr. 2013, 54, 580–592. [Google Scholar] [CrossRef]
- Chávez-Mendoza, C.; Sánchez, E. Bioactive Compounds from Mexican Varieties of the Common Bean (Phaseolus vulgaris): Implications for Health. Molecules 2017, 22, 1360. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Polyphenol-Rich Dry Common Beans (Phaseolus vulgaris L.) and Their Health Benefits. Int. J. Mol. Sci. 2017, 18, 2331. [Google Scholar] [CrossRef]
- Aparicio-Fernández, X.; García-Gasca, T.; Yousef, G.G.; Lila, M.A.; De Mejia, E.G.; Loarca-Piña, G. Chemopreventive Activity of Polyphenolics from Black Jamapa Bean (Phaseolus vulgaris L.) on HeLa and HaCaT Cells. J. Agric. Food Chem. 2006, 54, 2116–2122. [Google Scholar] [CrossRef]
- Guajardo-Flores, D.; Serna-Saldívar, S.O.; Gutiérrez-Uribe, J.A. Evaluation of the antioxidant and antiproliferative activities of extracted saponins and flavonols from germinated black beans (Phaseolus vulgaris L.). Food Chem. 2013, 141, 1497–1503. [Google Scholar] [CrossRef]
- Moreno-Jiménez, M.R.; Cervantes-Cardoza, V.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Estrella, I.; García-Gasca, T.D.J.; Herrera-Carrera, E.; Díaz-Rivas, J.O.; Rocha-Guzmán, N.E. Phenolic composition changes of processed common beans: Their antioxidant and anti-inflammatory effects in intestinal cancer cells. Food Res. Int. 2015, 76, 79–85. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Gan, R.-Y.; Ge, Y.-Y.; Zhang, D.; Corke, H. Polyphenols in Common Beans (Phaseolus vulgaris L.): Chemistry, Analysis, and Factors Affecting Composition. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1518–1539. [Google Scholar] [CrossRef] [PubMed]
- Koehnlein, E.A.; Bracht, A.; Nishida, V.S.; Peralta, R.M. Total antioxidant capacity and phenolic content of the Brazilian diet: A real scenario. Int. J. Food Sci. Nutr. 2014, 65, 293–298. [Google Scholar] [CrossRef]
- Dangour, A.D.; Dodhia, S.K.; Hayter, A.; Allen, E.; Lock, K.; Uauy, R. Nutritional quality of organic foods: A systematic review. Am. J. Clin. Nutr. 2009, 90, 680–685. [Google Scholar] [CrossRef]
- Barański, M.; Średnicka-Tober, D.; Volakakis, N.; Seal, C.; Sanderson, R.; Stewart, G.B.; Benbrook, C.; Biavati, B.; Markellou, E.; Giotis, C.; et al. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: A systematic literature review and meta-analyses. Br. J. Nutr. 2014, 112, 794–811. [Google Scholar] [CrossRef] [PubMed]
- Gershenzon, J. Secondary Metabolites and Plant Defense. In Plant Physiology, 3rd ed.; Taiz, L., Zeiger, E., Eds.; Sinauer Asso-ciates: Sunderland, MA, USA, 2002; pp. 283–308. [Google Scholar]
- Gomiero, T. Food quality assessment in organic vs. conventional agricultural produce: Findings and issues. Appl. Soil Ecol. 2018, 123, 714–728. [Google Scholar] [CrossRef]
- Baudry, J.; Assmann, K.E.; Touvier, M.; Allès, B.; Seconda, L.; Latino-Martel, P.; Ezzedine, K.; Galan, P.; Hercberg, S.; Lairon, D.; et al. Association of Frequency of Organic Food Consumption with Cancer Risk. JAMA Intern. Med. 2018, 178, 1597–1606. [Google Scholar] [CrossRef] [PubMed]
- Faller, A.; Fialho, E. Polyphenol content and antioxidant capacity in organic and conventional plant foods. J. Food Compos. Anal. 2010, 23, 561–568. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Method 3050B—Acid digestion of sediments, sludges, and soils. 1996. Available online: https://www.epa.gov/sites/production/files/2015-06/documents/epa-3050b.pdf (accessed on 18 March 2016).
- AOAC. Official Methods of Analysis, 17th ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Anastassiades, M.; Lehotay, S.J.; Štajnbaher, D.; Schenck, F.J. Fast and Easy Multiresidue Method Employing Acetonitrile Extraction/Partitioning and “Dispersive Solid-Phase Extraction” for the Determination of Pesticide Residues in Produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Frühbeck, G.; Alonso, R.; Marzo, F.; Santidrian, S. A Modified Method for the Indirect Quantitative Analysis of Phytate in Foodstuffs. Anal. Biochem. 1995, 225, 206–212. [Google Scholar] [CrossRef]
- Ellis, R.; Morris, E.R. Appropriate resin selection for rapid phytate analysis by ion-exchange chromatography. Cereal Chem. 1986, 63, 58–59. [Google Scholar]
- Mattila, P.; Kumpulainen, J. Determination of Free and Total Phenolic Acids in Plant-Derived Foods by HPLC with Diode-Array Detection. J. Agric. Food Chem. 2002, 50, 3660–3667. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Oliveira, A.A.; Revorêdo, T.B.; Martins, A.B.N.; Lacerda, E.C.Q.; Freire, A.S.; Braz, B.F.; Santelli, R.E.; Torres, A.G.; Perrone, D.; et al. Screening of the chemical composition and occurring antioxidants in jabuticaba (Myrciaria jaboticaba) and jussara (Euterpe edulis) fruits and their fractions. J. Funct. Foods 2015, 17, 422–433. [Google Scholar] [CrossRef]
- EURL DataPool website for Residues of Pesticides. Available online: https://www.eurl-pesticides-datapool.eu/ (accessed on 10 April 2021).
- ANVISA. Relatório das análises de amostras monitoradas no período de 2013 a 2015. Programa Análise Resíduos Agrotóxicos em Alimentos - PARA. 2016. Available online: https://www.gov.br/anvisa/pt-br/assuntos/agrotoxicos/programa-de-analise-de-residuos-em-alimentos/arquivos/3778json-file-1. (accessed on 29 June 2020).
- United States Department of Agriculture. Arugula, Raw. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/169387/nutrients (accessed on 9 December 2020).
- Balisteiro, D.M.; Rombaldi, C.V.; Genovese, M.I. Protein, isoflavones, trypsin inhibitory and in vitro antioxidant capacities: Comparison among conventionally and organically grown soybeans. Food Res. Int. 2013, 51, 8–14. [Google Scholar] [CrossRef]
- Bloom, A.J. Assimilation of mineral nutrientes. In Plant Physiology, 3rd ed.; Taiz, L., Zeiger, E., Eds.; Sinauer Associates: Sunderland, MA, USA, 2002; pp. 259–282. [Google Scholar]
- Ahemad, M.; Khan, M.S. Pesticides as Antagonists of Rhizobia and the Legume-Rhizobium Symbiosis: A Paradigmatic and Mechanistic Outlook. Biochem. Mol. Biol. 2013, 1, 63. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019. [Google Scholar]
- Lori, M.; Symnaczik, S.; Mäder, P.; De Deyn, G.; Gattinger, A. Organic farming enhances soil microbial abundance and activity—A meta-analysis and meta-regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef]
- Santos, M.S.; Nogueira, M.A.; Hungria, M. Microbial inoculants: Reviewing the past, discussing the present and previewing an outstanding future for the use of beneficial bacteria in agriculture. AMB Express 2019, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.; Foster, M.; McArthur, J.O.; Ojha, R.; Petocz, P.; Samman, S. Evaluation of the Micronutrient Composition of Plant Foods Produced by Organic and Conventional Agricultural Methods. Crit. Rev. Food Sci. Nutr. 2011, 51, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Hattab, S.; Bougattass, I.; Hassine, R.; Dridi-Al-Mohandes, B. Metals and micronutrients in some edible crops and their cultivation soils in eastern-central region of Tunisia: A comparison between organic and conventional farming. Food Chem. 2019, 270, 293–298. [Google Scholar] [CrossRef]
- Worthington, V. Nutritional Quality of Organic Versus Conventional Fruits, Vegetables, and Grains. J. Altern. Complement. Med. 2001, 7, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sinha, A.K.; Makkar, H.P.; Becker, K. Dietary roles of phytate and phytase in human nutrition: A review. Food Chem. 2010, 120, 945–959. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Oatway, L.; Vasanthan, T.; Helm, J.H. Phytic Acid. Food Rev. Int. 2001, 17, 419–431. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M.; Pastor-Corrales, M.S.; Luthria, D.L. The polyphenolic profiles of common bean (Phaseolus vulgaris L.). Food Chem. 2008, 107, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Luthria, D.L.; Pastor-Corrales, M.A. Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties. J. Food Compos. Anal. 2006, 19, 205–211. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Genovese, A.M.I.; Lajolo, F.M. Polyphenols and Antioxidant Capacity of Seed Coat and Cotyledon from Brazilian and Peruvian Bean Cultivars (Phaseolus vulgaris L.). J. Agric. Food Chem. 2007, 55, 90–98. [Google Scholar] [CrossRef]
- Giusti, F.; Caprioli, G.; Ricciutelli, M.; Torregiani, E.; Vittori, S.; Sagratini, G. Analysis of 17 polyphenolic compounds in organic and conventional legumes by high-performance liquid chromatography-diode array detection (HPLC-DAD) and evaluation of their antioxidant activity. Int. J. Food Sci. Nutr. 2017, 69, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Jakopic, J.; Slatnar, A.; Mikulic-Petkovsek, M.; Veberic, R.; Stampar, F.; Bavec, F.; Bavec, M. Effect of Different Production Systems on Chemical Profiles of Dwarf French Bean (Phaseolus vulgaris L. cv. Top Crop) Pods. J. Agric. Food Chem. 2013, 61, 2392–2399. [Google Scholar] [CrossRef]
- Oliveira, A.B.; Moura, C.F.H.; Gomes-Filho, E.; Marco, C.A.; Urban, L.; Miranda, M.R.A. The Impact of Organic Farming on Quality of Tomatoes Is Associated to Increased Oxidative Stress during Fruit Development. PLoS ONE 2013, 8, e56354. [Google Scholar] [CrossRef]


| Coastal Region | Metropolitan Region | |||
|---|---|---|---|---|
| Nonorganic | Organic | Nonorganic | Organic | |
| Proximate composition and phytate (g/100 g, dry weight basis) | ||||
| Lipid | 1.7 ± 0.1 | 1.4 ± 0.0 * | 1.9 ± 0.1 | 1.6 ± 0.1 * |
| Protein | 19.2 ± 0.4 | 25.2 ± 0.1 * | 22.4 ± 0.0 | 24.3 ± 0.3 * |
| Ash | 4.2 ± 0.0 | 3.8 ± 0.2 * | 4.4 ± 0.2 | 4.4 ± 0.1 |
| Carbohydrate | 26.8 | 27.7 | 25.8 | 23.1 |
| Total dietary fiber | 35.5 ± 2.6 | 30.8 ± 1.2 * | 31.2 ± 0.5 | 32.1 ± 0.9 |
| Insoluble dietary fiber | 31.9 ± 1.4 | 29.8 ± 0.5 * | 27.2 ± 0.7 | 27.0 ± 0.8 |
| Soluble dietary fiber | 3.60 | 1.00 | 3.95 | 5.11 |
| Phytate | 1.86 ± 0.02 | 1.76 ± 0.02 * | 2.08 ± 0.07 | 1.83 ± 0.11 * |
| Minerals (mg/100 g, dry weight basis) | ||||
| Ca | 158.6 ± 3.2 | 102.2 ± 11.4 * | 152.9 ± 2.4 | 176.4 ± 3.4 * |
| Cu | 1.01 ± 0.08 | 0.58 ± 0.05 * | 1.15 ± 0.03 | 0.83 ± 0.01 * |
| Fe | 4.13 ± 0.2 | 4.11 ± 0.4 | 4.56 ± 0.2 | 4.69 ± 0.3 |
| K | 1351.4 ± 25.6 | 1354.9 ± 3.8 | 1368.7 ± 11.7 | 1461.6 ± 21.5 * |
| Mg | 156.4 ± 4.3 | 146.2 ± 3.1 | 171.1 ± 2.6 | 169.1 ± 2.4 |
| Mn | 1.97 ± 0.15 | 1.01 ± 0.16 * | 1.46 ± 0.02 | 1.10 ± 0.08 * |
| Na | 15.1 ± 9.5 | 10.6 ± 4.1 | 20.9 ± 0.17 | 8.98 ± 2.4 * |
| P | 249.0 ± 56.4 | 318.2 ± 51.4 | 394.5 ± 14.4 | 372.9 ± 27.9 |
| Zn | 1.95 ± 0.10 | 1.61 ± 0.27 | 2.57 ± 0.08 | 2.31 ± 0.04 * |
| Coastal Region | Metropolitan Region | |||
|---|---|---|---|---|
| Nonorganic | Organic | Nonorganic | Organic | |
| Ca | 938.6 ± 1.8 | 131.8 ± 55.6 * | 941.5 ± 42.4 | 1322 ± 223 |
| Cu | 9.4 ± 0.07 | traces * | 8.75 ± 0.3 | 0.69 ± 0.47 * |
| Fe | 18,356 ± 725 | 1228 ± 239 * | 3625 ± 80 | 24,351 ± 2104 * |
| K | 2062 ± 57 | traces * | 184.4 ± 14.3 | 1306 ± 150 * |
| Mg | 3171 ± 45 | traces * | 61.1 ± 18 | 3311 ± 361 * |
| Mn | 219.7 ± 3.7 | 11.7 ± 1.9 * | 8.82 ± 2.9 | 275.4 ± 33.9 * |
| Na | traces | traces | traces | traces |
| P | 695.9 ± 2.6 | 81.5 ± 10.5 * | 938.4 ± 20.6 | 267.3 ± 35.1 * |
| Zn | 45.6 ± 1.0 | traces * | 12.46 ± 1.2 | 34.1 ± 5.2 * |
| N | 4801.1 ± 42.7 | 1048.2 ± 24.3 * | 6087.2 ± 199.1 | 2701.8 ± 14.9 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreto, N.M.B.; Pimenta, N.G.; Braz, B.F.; Freire, A.S.; Santelli, R.E.; Oliveira, A.C.; Bastos, L.H.P.; Cardoso, M.H.W.M.; Monteiro, M.; Diogenes, M.E.L.; et al. Organic Black Beans (Phaseolus vulgaris L.) from Rio de Janeiro State, Brazil, Present More Phenolic Compounds and Better Nutritional Profile Than Nonorganic. Foods 2021, 10, 900. https://doi.org/10.3390/foods10040900
Barreto NMB, Pimenta NG, Braz BF, Freire AS, Santelli RE, Oliveira AC, Bastos LHP, Cardoso MHWM, Monteiro M, Diogenes MEL, et al. Organic Black Beans (Phaseolus vulgaris L.) from Rio de Janeiro State, Brazil, Present More Phenolic Compounds and Better Nutritional Profile Than Nonorganic. Foods. 2021; 10(4):900. https://doi.org/10.3390/foods10040900
Chicago/Turabian StyleBarreto, Nathália M. B., Natália G. Pimenta, Bernardo F. Braz, Aline S. Freire, Ricardo E. Santelli, Angélica C. Oliveira, Lucia H. P. Bastos, Maria Helena W. M. Cardoso, Mariana Monteiro, Maria Eduarda L. Diogenes, and et al. 2021. "Organic Black Beans (Phaseolus vulgaris L.) from Rio de Janeiro State, Brazil, Present More Phenolic Compounds and Better Nutritional Profile Than Nonorganic" Foods 10, no. 4: 900. https://doi.org/10.3390/foods10040900
APA StyleBarreto, N. M. B., Pimenta, N. G., Braz, B. F., Freire, A. S., Santelli, R. E., Oliveira, A. C., Bastos, L. H. P., Cardoso, M. H. W. M., Monteiro, M., Diogenes, M. E. L., & Perrone, D. (2021). Organic Black Beans (Phaseolus vulgaris L.) from Rio de Janeiro State, Brazil, Present More Phenolic Compounds and Better Nutritional Profile Than Nonorganic. Foods, 10(4), 900. https://doi.org/10.3390/foods10040900