Effect of In Vitro Digestion on the Antioxidant and Angiotensin-Converting Enzyme Inhibitory Potential of Buffalo Milk Processed Cheddar Cheese
Abstract
:1. Introduction
2. Materials and Methods
2.1. Procurement of Raw Material for Cheddar Cheese Manufacturing
2.2. Manufacturing of Processed Cheddar Cheese
2.3. Preparation of Freeze-Dried Water Soluble and Ethanol Soluble Extracts (pH 4.6 Soluble Fraction) of Processed Cheddar Cheese
2.4. In Vitro Enzymatic Digestion of Processed Cheddar Cheese
2.5. ACE-Inhibition Assay
2.6. Determination of Total Phenolic Content of Processed Cheddar Cheese
2.7. Determination of Total Antioxidant Capacity (TAC) of Processed Cheddar Cheese
2.8. Determination of DPPH Radical Scavenging Activity of Processed Cheddar Cheese
2.9. Reducing Power Ability of Processed Cheddar Cheese
2.10. Sensory Evaluation
2.11. Statistical Analysis
3. Results
3.1. Total Antioxidant Activity (TAA) of Processed Cheddar Cheese (PCS), Orally Digested (PODC), and Duodenal Digested Cheddar Cheese (PDC)
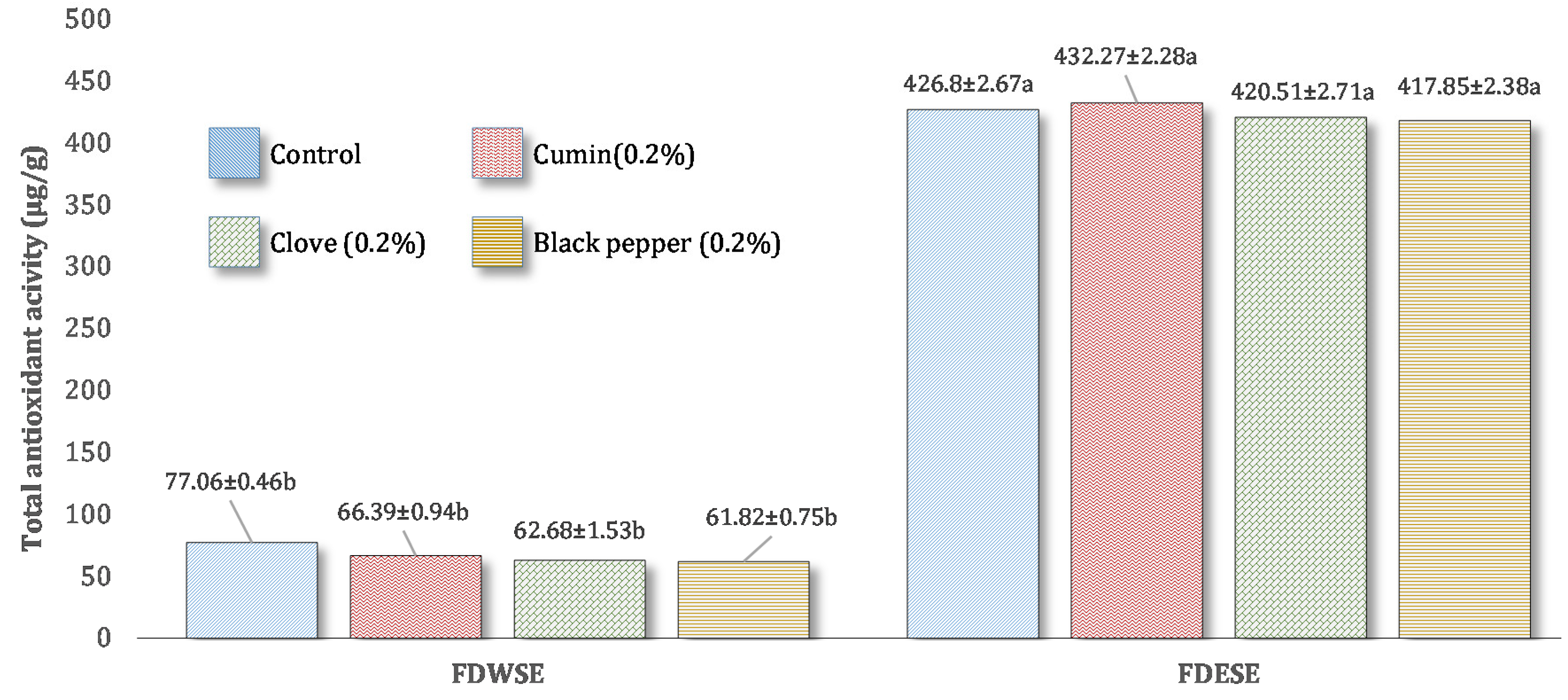
Total Antioxidant Activity (TAA) of Freeze-Dried Water Soluble and Ethanol Soluble Extract of Processed Cheddar Cheese
3.2. Total Phenolic Content (TPC) of Processed Cheddar Cheese (PCS), Orally Digested (PODC), and Duodenal Digested Cheddar Cheese (PDC)
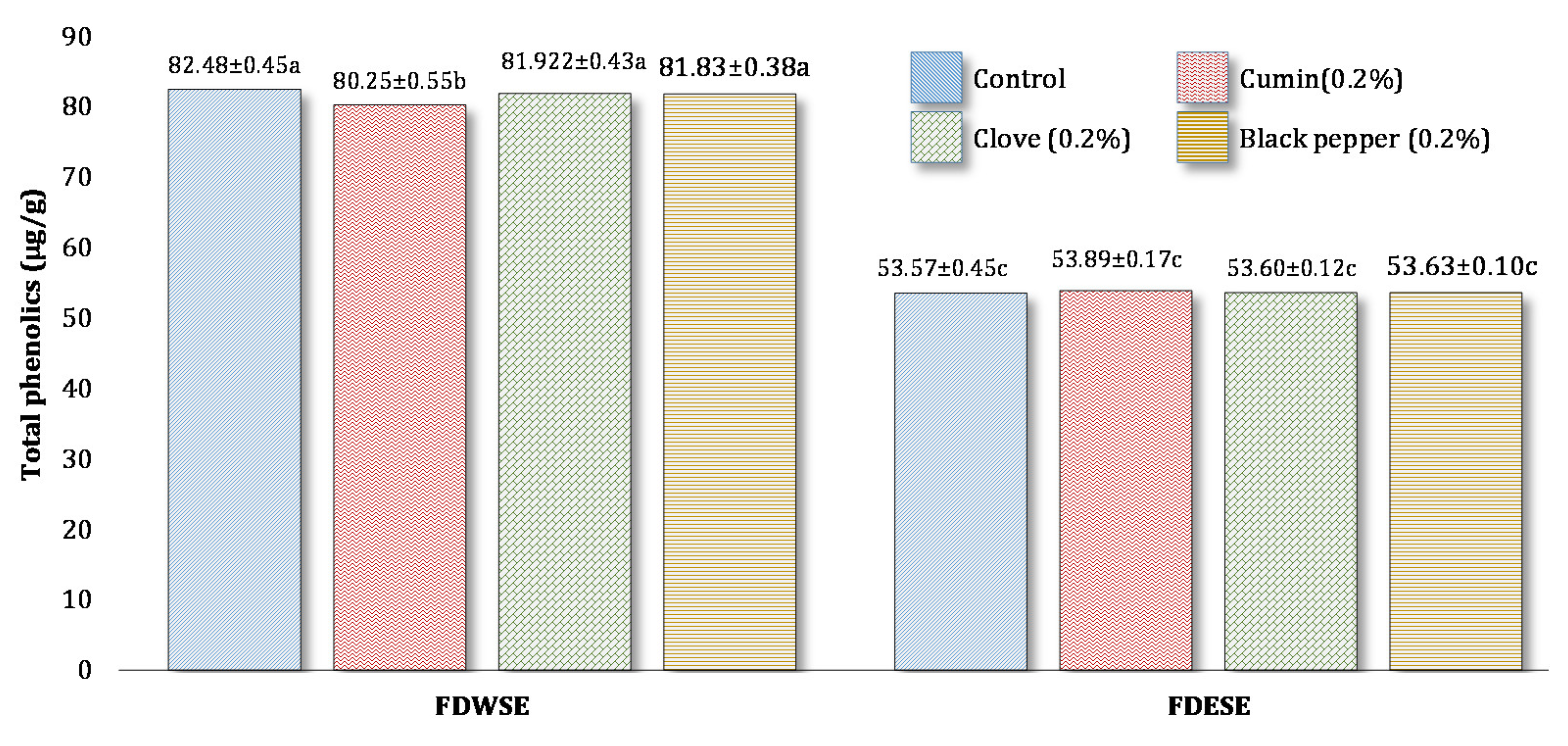
Total Phenolic Content (TPC) of Freeze-Dried Water Soluble and Ethanol Soluble Extract of Processed Cheddar Cheese xxx
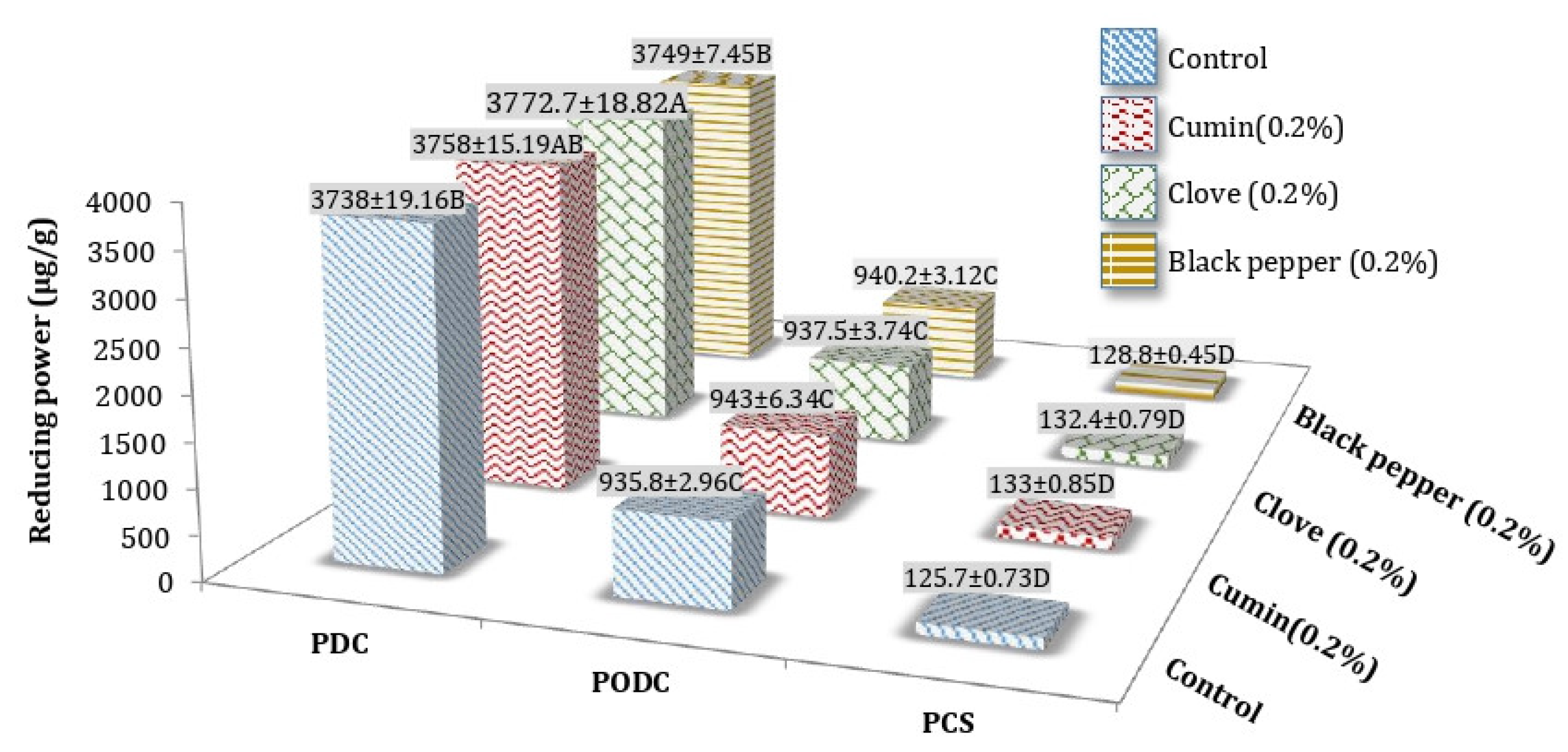
3.3. Reducing Power (RP) of Processed Cheddar Cheese (PCS), Orally Digested (PODC), and Duodenal Digested Cheddar Cheese (PDC)
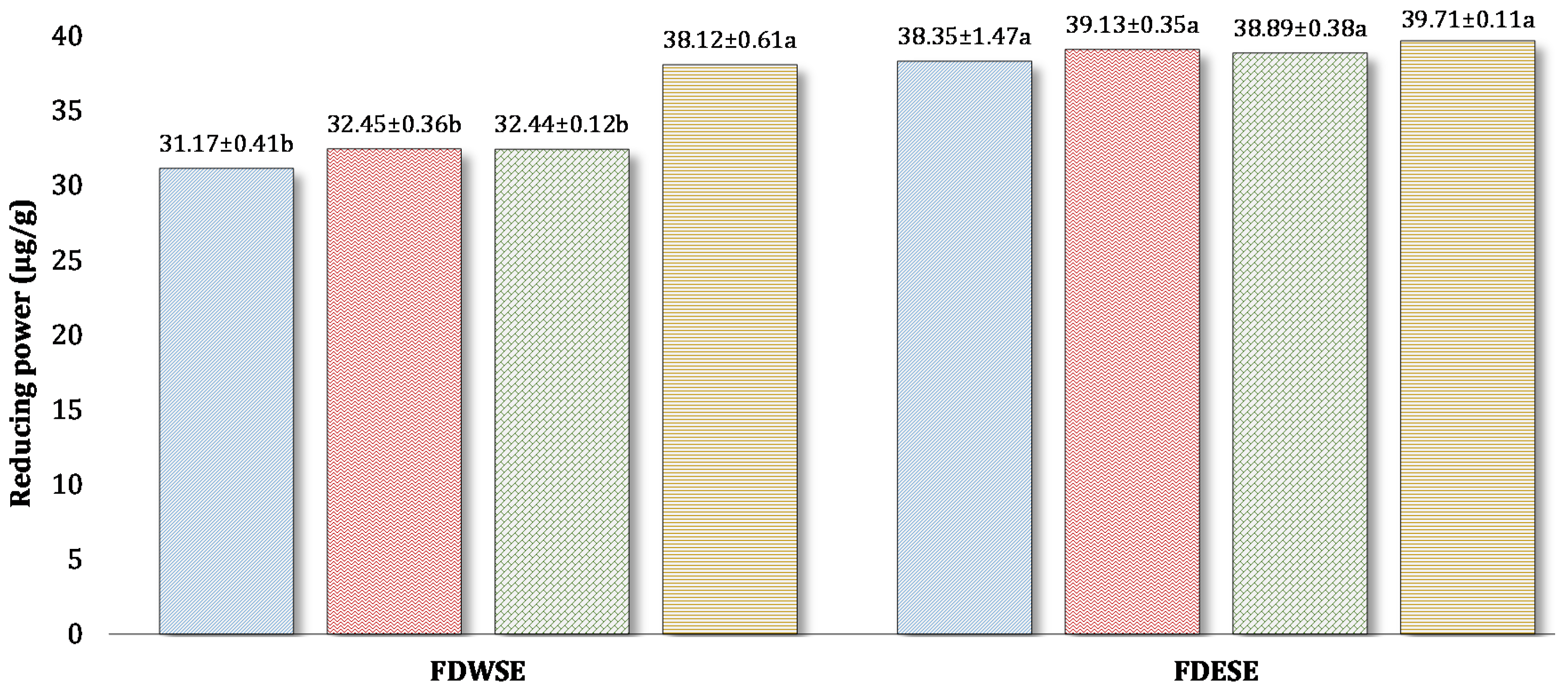
Reducing Power (RP) of Freeze-Dried Water-Soluble (WSE) and Ethanol Soluble Extract (ESE) of Processed Cheddar Cheese
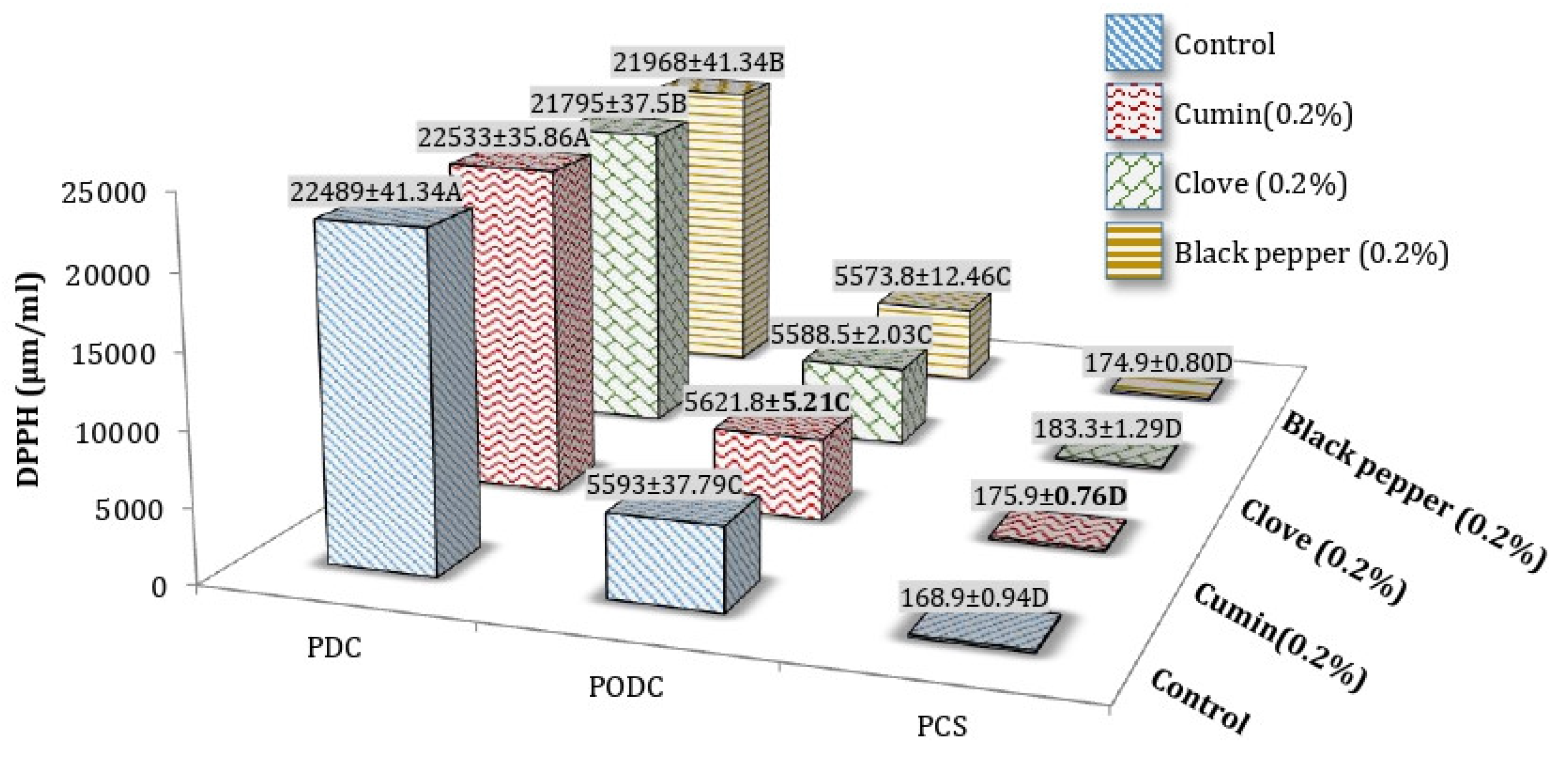
3.4. DPPH (Radical Scavenging Activity) of Processed Cheddar Cheese (PCS), Orally Digested (PODC), and Duodenal Digested Cheddar Cheese (PDC)
DPPH (Radical Scavenging Activity) of Freeze-Dried Water-Soluble (WSE) and Ethanol Soluble Extract (ESE) of Processed Cheddar Cheese
3.5. Determination of ACE-Inhibition (%) and IC50 Values (mg/mL) of Processed Cheddar Cheese
Determination of ACE-Inhibition % and IC50 (mg/mL) Values of Freeze-Dried Water (WSE) and Ethanol Soluble Extract (ESE) of Processed Cheddar Cheese
3.6. Sensory Score of Processed Cheddar Cheese
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation 2002, 106, 2747–2757. [Google Scholar] [CrossRef]
- Korhonen, H. Milk-derived bioactive peptides: From science to applications. J. Funct. Foods 2009, 1, 177–187. [Google Scholar] [CrossRef]
- López-Fandiño, R.; Otte, J.; Van Camp, J. Physiological, chemical and technological aspects of milk-protein-derived peptides with antihypertensive and ACE-inhibitory activity. Int. Dairy J. 2006, 16, 1277–1293. [Google Scholar] [CrossRef]
- Iwaniak, A.; Mogut, D. Metabolic Syndrome-Preventive Peptides Derived from Milk Proteins and Their Presence in Cheeses: A Review. Appl. Sci. 2020, 10, 2772. [Google Scholar] [CrossRef]
- Jäkälä, P.; Vapaatalo, H. Antihypertensive peptides from milk proteins. Pharm. Res. 2010, 3, 251–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliakbarian, B.; Dehghani, F.; Perego, P. The effect of citric acid on the phenolic contents of olive oil. Food Chem. 2009, 116, 617–623. [Google Scholar] [CrossRef]
- Aloğlu, H.Ş.; Öner, Z. Determination of antioxidant activity of bioactive peptide fractions obtained from yogurt. J. Dairy Sci. 2011, 94, 5305–5314. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.R.; Chen, M.J.; Lin, C.W. Antimutagenic and antioxidant properties of milk−kefir and soymilk−kefir. J. Agric. Food Chem. 2005, 53, 2467–2474. [Google Scholar] [CrossRef]
- Shihabudeen, H.M.S.; Priscilla, D.H.; Thirumurugan, K. Cinnamon extract inhibits α-glucosidase activity and dampens postprandial glucose excursion in diabetic rats. Nutr. Metab. 2011, 8, 46. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.A.; Broadhurst, C.L.; Polansky, M.M.; Schmidt, W.F.; Khan, A.; Flanagan, V.P.; Schoene, N.W.; Graves, D.J. Isolation and characterization of polyphenol type-A polymers from cinnamon with insulin-like biological activity. J. Agric. Food Chem. 2004, 52, 65–70. [Google Scholar] [CrossRef]
- Machado, M.; Dinis, A.M.; Salgueiro, L.; Custódio, J.B.; Cavaleiro, C.; Sousa, M.C. Anti-Giardia activity of Syzygium aromaticum essential oil and eugenol: Effects on growth, viability, adherence and ultrastructure. Exp. Parasitol. 2011, 127, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Pramod, P.K.; Sajeevan, T.P.; Ramachandran, A.; Thampy, S.; Pai, S.S. Effects of two anesthetics on water quality during simulated transport of a tropical ornamental fish, the Indian tiger barb Puntius filamentosus. N. Am. J. Aquac. 2010, 72, 290–297. [Google Scholar] [CrossRef]
- Bettaieb, I.; Bourgou, S.; Wannes, W.A.; Hamrouni, I.; Limam, F.; Marzouk, B. Essential oils, phenolics, and antioxidant activities of different parts of cumin (Cuminum cyminum L.). J. Agric. Food Chem. 2010, 58, 10410–10418. [Google Scholar] [CrossRef]
- Randhawa, M.A.; Alghamdi, M.S. Anticancer activity of Nigella sativa (black seed)—A review. Am. J. Chin. Med. 2011, 39, 1075–1091. [Google Scholar] [CrossRef] [Green Version]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Foda, M.I. Sensory evaluation and antioxidant activity of new Mudaffara cheese with spices under different storage temperatures. Res. J. Appl. Sci. 2012, 3143–3150. [Google Scholar]
- Singh, R.R.; Singh, R.; Shakya, B.R. Impact of turmeric addition on the properties of paneer, prepared from different types of milk. Int. J. Res. Eng. Technol. 2014, 4, 1874–1883. [Google Scholar]
- Josipović, R.; Medverec Knežević, Z.; Frece, J.; Markov, K.; Kazazić, S.; Mrvčić, J. Improved properties and microbiological safety of novel cottage cheese containing spices. Food Technol. Biotechnol. 2015, 53, 454–462. [Google Scholar] [CrossRef]
- Lawrence, R.C.; Gilles, J.; Creamer, L.K.; Crow, V.L.; Heap, H.A.; Honoré, C.G.; Johnston, K.A.; Samal, P.K. Cheddar cheese and related dry-salted cheese varieties. In Cheese Chemistry, Physics and Microbiology; Elsevier Academic Press: New York, NY, USA, 2004; Volume 2, pp. 72–82. [Google Scholar]
- Pripp, A.H.; Sørensen, R.; Stepaniak, L.; Sørhaug, T. Relationship between proteolysis and angiotensin-I-converting enzyme inhibition in different cheeses. LWT-Food Sci. Technol. 2006, 39, 677–683. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.O.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, T.M.; Vegarud, G.E.; Abrahamsen, R.K.; Skeie, S. Characterization of the Norwegian autochthonous cheese Gamalost and its angiotensin I-converting enzyme (ACE) inhibitory activity during ripening. Dairy Sci. Technol. 2012, 92, 613–625. [Google Scholar] [CrossRef] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: New Yor, NY, USA, 1999; Volume 299, pp. 152–178. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Yi, Z.; Yu, Y.; Liang, Y.; Zeng, B. In vitro antioxidant and antimicrobial activities of the extract of Pericarpium Citri Reticulatae of a new Citrus cultivar and its main flavonoids. LWT-Food Sci. Technol. 2008, 41, 597–603. [Google Scholar] [CrossRef]
- Hegazy, A.E.; Ibrahium, M.I. Antioxidant activities of orange peel extracts. World Appl. Sci. J. 2012, 18, 684–688. [Google Scholar] [CrossRef]
- Srinivas, S.; Prakash, V. Bioactive Peptides from Bovine Milk a-Casein: Isolation, Characterization and Multifunctional properties. Int. J. Pept. Res. Ther. 2010, 16, 15. [Google Scholar] [CrossRef]
- Barac, M.; Vucic, T.; Zilic, S.; Pesic, M.; Sokovic, M.; Petrovic, J.; Kostic, A.; Sredovic Ignjatovic, I.; Milincic, D. The Effect of In Vitro Digestion on Antioxidant, ACE-Inhibitory and Antimicrobial Potentials of Traditional Serbian White-Brined Cheeses. Foods 2019, 8, 94. [Google Scholar] [CrossRef] [Green Version]
- Schmelzer, C.E.H.; Schops, R.; Reynell, L.; Ulbrich-Hofmann, R.; Neubert, H.H.R.; RaithJ, K. Peptic digestion of -casein Time course and fate of possible bioactive peptides. J. Chromatogr. A 2007, 1166, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Roufik, S.; Gauthier, F.S.; Turgeon, L.S. In vitro digestibility of bioactive peptides derived from bovine b-lactoglobulin. Int. Dairy J. 2006, 16, 294–302. [Google Scholar] [CrossRef]
- Vučić, T.; Milinčić, D.; Žilić, S.; Ignjatović-Sredović, I.; Sarić, Z.; Ećim-Đurić, O.; Kostić, A.; Barać, M. The effect of in vitro digestion on antioxidant properties of water-soluble and insoluble protein fractions of traditional Serbian white- brined cheeses. Mljekarstvo 2020, 70, 253–265. [Google Scholar] [CrossRef]
- Sieber, R.; Bütikofer, U.; Egger, C.; Portmann, R.; Walther, B.; Wechsler, D. ACE-inhibitory activity and ACE-inhibiting peptides in different cheese varieties. Dairy Sci. Technol. 2010, 90, 47–73. [Google Scholar] [CrossRef]
- Pepe, G.; Sommella, E.; Ventre, G.; Carmina, S.M.; Adesso, S.; Ostacolo, C.; Marzocco, S.; Novellino, E.; Campiglia, P. Antioxidant peptides released from gastrointestinal digestion of “Stracchino” soft cheese: Characterization, in vitro intestinal protection and bioavailability. J. Funct. Foods 2016, 26, 494–505. [Google Scholar] [CrossRef]
- Yang, W.; Hao, X.; Zhang, X.; Zhang, G.; Li, X.; Liu, L.; Sun, Y.; Pan, Y. Identification of antioxidant peptides from cheddar cheese made with Lactobacillus helveticus. LWT 2021, 141, 110866. [Google Scholar] [CrossRef]
- Tenore, C.G.; Alberto Ritieni, A.; Campiglia, P.; Stiuso, P.; Maro, D.S.; Sommella, E.; Pepe, G.; D’Urso, E.; Novellino, E. Antioxidant peptides from “Mozzarella di Bufala Campana DOP” after simulated gastrointestinal digestion: In vitro intestinal protection, bioavailability, and anti-haemolytic capacity. J. Funct. Foods 2015, 15, 365–375. [Google Scholar] [CrossRef]
- Solieri, L.; Baldaccini, A.; Martini, S.; Bianchi, A.; Pizzamiglio, V.; Tagliazucchi, D. Peptide Profiling and Biological Activities of 12-Month Ripened Parmigiano Reggiano Cheese. Biology 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Mann, B.; Kumar, R.; Sangwan, R.B. ACE-Inhibitory Activity of Cheddar Cheeses Made with Adjunct Cultures at Different Stages of Ripening. Adv. Dairy Res. 2013, 1, 102. [Google Scholar] [CrossRef] [Green Version]
- Erkaya, T.; Şengül, M. Bioactivity of water soluble extracts and some characteristics of white cheese during the ripening period as effected by packaging type and probiotic adjunct cultures. J. Dairy Res. 2015, 82, 47–55. [Google Scholar] [CrossRef]
- Chen, P.; Liu, L.; Zhang, X.; Bora, A.F.M.; Li, X.; Zhao, M.; Hao, X.; Wang, Y. Antioxidant activity of Cheddar cheese during its ripening time and after simulated gastrointestinal digestion as affected by probiotic bacteria. Int. J. Food Prop. 2019, 22, 218–229. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Lee, S.J.; Gadiyaram, B.L.; Park, Y.W. Tocopherol concentration and their changes in caprine milk cheese during extended refrigeration and frozen storage. J. Dairy Sci. 2003, 86, 71–72. [Google Scholar]
- Aqil, F.; Ahmad, I.; Mehmood, Z. Antioxidant and free radical scavenging properties of twelve traditionally used Indian medicinal plants. Turk. J. Biol. 2006, 30, 177–183. [Google Scholar]
- Karthikeyan, J.; Rani, P. Enzymatic and non-enzymatic antioxidants in selected Piper species. Indian J. Exp. Biol. 2003, 41, 135–140. [Google Scholar]
- Choi, E.M.; Hwang, J.K. Effect of some medicinal plants on plasma antioxidant system and lipid levels in rats. An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. Phytother. Res. 2005, 19, 382–386. [Google Scholar] [CrossRef]
- Hossain, M.; Brunton, N.; Barry-Ryan, C.; Martin-Diana, A.B.; Wilkinson, M. Antioxidant activity of spice extracts and phenolics in comparison to synthetic antioxidants. Rasayan J. Chem. 2008, 1, 751–756. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Everett, D.W. Antioxidant activity and recovery of green tea catechins in full-fat cheese following gastrointestinal simulated digestion. J. Food Compos. Anal. 2016, 48, 13–24. [Google Scholar] [CrossRef]
- Shaiban, M.; Al-Mamary, M.; Al-Habori, M. Total antioxidant activity and total phenolic contents in Yemeni smoked cheese. Malays J. Nutri. 2006, 12, 87–92. [Google Scholar]
- Hala, M.F.; Ebtisam, E.D.; Sanaa, I.; Badran, M.A.; Marwa, A.S.; Said, M.E. Manufacture of low fat UF-soft cheese supplemented with rosemary extract (as natural antioxidant). J. Am. Sci. 2010, 6, 570–579. [Google Scholar]
- Hilario, M.C.; Puga, C.D.; Ocana, A.N.; Romo, F.P.G. Antioxidant activity, bioactive polyphenols in Mexican goats’ milk cheeses on summer grazing. J. Dairy Res. 2010, 77, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Levkov, V.; Gad Ovska, S.; Tuševski, O.; Gjorgovska, N.; Mateva, N. Preliminary study of total phenolic content in traditional sheep cheese (Bieno Sirenje). Maced. J. Anim. Sci. 2014, 4, 31–35. [Google Scholar]
- Liu, L.; Qu, X.; Xia, Q.; Wang, H.; Chen, P.; Li, X.; Wang, L.; Yang, W. Effect of Lactobacillus rhamnosus on the antioxidant activity of Cheddar cheese during ripening and under simulated gastrointestinal digestion. LWT 2018, 95, 99–106. [Google Scholar] [CrossRef]
- Abadía-García, L.; Cardador, A.; del Campo, S.T.M.; Arvízu, S.M.; Castaño-Tostado, E.; Regalado-González, C.; García-Almendarez, B.; Amaya-Llano, S.L. Influence of probiotic strains added to cottage cheese on generation of potentially antioxidant peptides, anti-listerial activity, and survival of probiotic microorganisms in simulated gastrointestinal conditions. Int. Dairy J. 2013, 33, 191–197. [Google Scholar] [CrossRef]
- Cumby, N.; Zhong, Y.; Naczk, M.; Shahidi, F. Antioxidant activity and water-holding capacity of canola protein hydrolysates. Food Chem. 2008, 109, 144–148. [Google Scholar] [CrossRef]
- Burits, M.; Bucar, F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000, 14, 323–328. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, J.; Tang, X.; Xiong, Y.L. Reducing, radical scavenging, and chelation properties of in vitro digests of alcalase-treated zein hydrolysate. J. Agric. Food Chem. 2008, 56, 2714–2721. [Google Scholar] [CrossRef]
- Gupta, A.; Mann, B.; Kumar, R.; Sangwan, R.B. Antioxidant activity of Cheddar cheeses at different stages of ripening. Int. J. Dairy Technol. 2009, 62, 339–347. [Google Scholar] [CrossRef]
- Chaudhry, N.M.; Tariq, P. Bactericidal activity of black pepper, bay leaf, aniseed and coriander against oral isolates. Pak. J. Pharm. Sci. 2006, 19, 214–218. [Google Scholar]
- Park, I.K.; Lee, S.G.; Shin, S.C.; Park, J.D.; Ahn, Y.J. Larvicidal activity of isobutylamides identified in Piper nigrum fruits against three mosquito species. J. Agric. Food Chem. 2002, 50, 1866–1870. [Google Scholar] [CrossRef]
- Qureshi, T.M.; Vegarud, G.E.; Abrahamsen, R.K.; Skeie, S. Angiotensin I-converting enzyme-inhibitory activity of the Norwegian autochthonous cheeses Gamalost and Norvegia after in vitro human gastrointestinal digestion. J. Dairy Sci. 2013, 96, 838–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamo, A. Cheddar cheese characterization and its biochemical change during ripening. Int. J. Ad. Sci. Res. Manag. 2017, 2, 53–59. [Google Scholar]
- Lucey, J.A.; Singh, H. Acid coagulation of milk. In Advanced Dairy Chemistry; Fox, P.F., McSweeney, P.L.H., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2003; Volume 1, pp. 1001–1025. [Google Scholar]
- Goosen, C. Consumer acceptance of Cheddar Cheese: Intrinsic, Extrinsic and Socio-Demographic Influences. Ph.D. Thesis, Stellenbosch University, Stellenbosch, South Africa, 2014. [Google Scholar]
- Murtaza, M.A.; Huma, N.; Sameen, A.; Murtaza, M.S.; Mahmood, S.; Mueen-ud-Din, G.; Meraj, A. Texture, flavor, and sensory quality of buffalo milk Cheddar cheese as influenced by reducing sodium salt content. J. Dairy Sci. 2014, 97, 6700–6707. [Google Scholar] [CrossRef] [PubMed]




| Cheddar Cheese Samples | Treatments | ACE-Inhibition Activity | |
|---|---|---|---|
| ACE-Inhibition (%) | IC50 Values mg/mL | ||
| Orally Digested processed cheddar cheese samples | CPODC LPODC BPPODC | 66.2 c ± 1.00 67.5 b ± 0.47 69.1 a ± 0.55 | 0.95 a ± 0.01 0.86 b ± 0.01 0.76 c ± 0.01 |
| Duodenal digested processed cheddar cheese samples | CPDC LPDC BPPDC | 89.5 b ± 0.55 90.3 ab ± 0.33 91.2 a ± 0.75 | 0.75 a ± 0.03 0.67 b ± 0.01 0.56 c ± 0.01 |
| Cheddar Cheese Samples (Buffalo Milk) | ACE-Inhibition Activity | ||
|---|---|---|---|
| ACE-Inhibition (%) | IC50 Values mg/mL | ||
| Water Soluble Extract of processed cheddar cheese samples | CFDWSE | 82.3 e ± 0.47 | 0.26 a ± 0.01 |
| LFDWSE | 85.5 cd ± 0.43 | 0.24 b ± 0.02 | |
| BPFDWSE | 88.4 b ± 0.32 | 0.21 e ± 0.01 | |
| Ethanol Soluble extract of processed cheddar cheese | CFDESE | 84.1 de ± 0.43 | 0.23 c ± 0.01 |
| LFDESE | 87.2 bc ± 0.33 | 0.22 d ± 0.03 | |
| BPFDESE | 90.3 a ± 0.49 | 0.19 f ± 0.02 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaukat, A.; Nadeem, M.; Qureshi, T.M.; Kanwal, R.; Sultan, M.; Kashongwe, O.B.; Shamshiri, R.R.; Murtaza, M.A. Effect of In Vitro Digestion on the Antioxidant and Angiotensin-Converting Enzyme Inhibitory Potential of Buffalo Milk Processed Cheddar Cheese. Foods 2021, 10, 1661. https://doi.org/10.3390/foods10071661
Shaukat A, Nadeem M, Qureshi TM, Kanwal R, Sultan M, Kashongwe OB, Shamshiri RR, Murtaza MA. Effect of In Vitro Digestion on the Antioxidant and Angiotensin-Converting Enzyme Inhibitory Potential of Buffalo Milk Processed Cheddar Cheese. Foods. 2021; 10(7):1661. https://doi.org/10.3390/foods10071661
Chicago/Turabian StyleShaukat, Amal, Muhammad Nadeem, Tahir Mahmood Qureshi, Rabia Kanwal, Muhammad Sultan, Olivier Basole Kashongwe, Redmond R. Shamshiri, and Mian Anjum Murtaza. 2021. "Effect of In Vitro Digestion on the Antioxidant and Angiotensin-Converting Enzyme Inhibitory Potential of Buffalo Milk Processed Cheddar Cheese" Foods 10, no. 7: 1661. https://doi.org/10.3390/foods10071661
APA StyleShaukat, A., Nadeem, M., Qureshi, T. M., Kanwal, R., Sultan, M., Kashongwe, O. B., Shamshiri, R. R., & Murtaza, M. A. (2021). Effect of In Vitro Digestion on the Antioxidant and Angiotensin-Converting Enzyme Inhibitory Potential of Buffalo Milk Processed Cheddar Cheese. Foods, 10(7), 1661. https://doi.org/10.3390/foods10071661









