The Types of Polysaccharide Coatings and Their Mixtures as a Factor Affecting the Stability of Bioactive Compounds and Health-Promoting Properties Expressed as the Ability to Inhibit the α-Amylase and α-Glucosidase of Chokeberry Extracts in the Microencapsulation Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Extraction of Chokeberry Fruits
2.3. Microencapsulation Procedure
2.4. Analysis of Phenolic Compounds
2.5. Determination of Polymeric Procyanidin Content by the UPLC-PDA-FL
2.6. Determinations of Antioxidant Aapacity (ABTS, FRAP, and ORAC Methods) and Biological Activity
2.7. Color Measurement in the CIE L*a*b System
2.8. Optical Microscopy Analysis
2.9. Statistical Analysis
3. Results
3.1. Analysis of Phenolic Compounds and Polymeric Procyanidin Contents
3.1.1. Total Polyphenolic Compounds in Microspheres
3.1.2. Polyphenolic Profile of Chokeberry Microspheres
3.1.3. Microcapsules after Storage
3.1.4. Polyphenolic Compounds in Chokeberry Concentrate and Chokeberry Powder
3.2. Determinations of Antioxidants Capacity by ABTS, FRAP and ORAC Methods
3.3. Analysis of α-Amylase and α-Glucosidase Inhibition Assays
3.4. Color Measurement in the CIE L*a*b System
3.5. Optical Microscopy Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biesalski, H.K.; Dragsted, L.O.; Elmadfa, I.; Grossklaus, R.; Müller, M.; Schrenk, D.; Walter, P.; Weber, P. Bioactive compounds: Definition and assessment of activity. Nutrition 2009, 25, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denev, P.; Kratchanova, M.; Ciz, M.; Lojek, A.; Vasicek, O.; Nedelcheva, P.; Blazheva, D.; Toshkova, R.; Gardeva, E.; Yossifova, L.; et al. Biological activities of selected polyphenol-rich fruits related to immunity and gastrointestinal health. Food Chem. 2014, 157, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Reiss, R.; Johnston, J.; Tucker, K.; DeSesso, J.M.; Kenn, C.L. Estimation of cancer risks and benefits associated with a potential increased consumption of fruits and vegetables. Food Chem. Toxicol. 2012, 50, 4421–4427. [Google Scholar] [CrossRef]
- Gwóźdź, E.; Gębczyński, P. Prozdrowotne właściwości owoców, warzyw i ich przetworów. Post. Fitoter. 2015, 4, 268–271. [Google Scholar]
- Faienza, M.F.; Corbo, F.; Carocci, A.; Catalono, A.; Clodoveo, M.L.; Grano, M.; Wang, D.Q.-H.; D’Amato, G.; Muraglia, M.; Franchini, C.; et al. Novel insights in health-promoting properties of sweet cherries. J. Funct. Foods 2020, 69, 103945. [Google Scholar] [CrossRef]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Morello, S.; Peiretti, P.G. Bioactive Compounds and Antioxidant Capacity of Small Berries. Foods 2020, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Poiroux-Gonord, F.; Bidel, L.P.R.; Fanciullino, A.-L.; Gautier, H.; Lauri-Lopez, F. Health Benefits of Vitamins and Secondary Metabolites of Fruits and Vegetables and Prospects to Increase Their Concentrations by Agronomic Approaches. J. Agric. Food Chem. 2010, 58, 12065–12082. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Rodríguez, L.G.; Gasga, V.M.Z.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Burgos, J.A.S. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2020, 140, 109854. [Google Scholar] [CrossRef]
- Zielińska, A.; Siudem, P.; Paradowska, K.; Gralec, M.; Kaźmierski, S.; Wawer, I. Aronia melanocarpa Fruits as a Rich Dietary Source of Chlorogenic Acids and Anthocyanins: 1H-NMR, HPLC-DAD, and Chemometric Studies. Molecules 2020, 25, 3234. [Google Scholar] [CrossRef]
- Skupień, K.; Oszmiański, J. The effect of mineral ferilization on nutritive value and biological activity of chokeberry fruit. Agric. Food Sci. 2007, 16, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A Review on the Characteristic Components and Potential Health Effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikora, J.; Markowicz, M.; Mikiciuk-Olasik, E. Rola i właściwości lecznicze aronii czarnoowocowej w profilaktyce chorób cywilizacyjnych. Bromat. Chem. Toskykol. 2009, 1, 10–17. [Google Scholar]
- Oszmiański, J.; Lachowicz, S. Effect of the Production of Dried Fruits and Juice from Chokeberry (Aronia melanocarpa L.) on the Content and Antioxidative Activity of Bioactive Compounds. Molecules 2016, 21, 1098. [Google Scholar] [CrossRef]
- Gill, N.K.; Rios, D.; Osorio-Camacena, E.; Mojica, B.E.; Kaur, B.; Soderstrom, M.A.; Gonzalez, M.; Plaat, B.; Poblete, C.; Kaur, N.; et al. Anticancer Effects of Extracts from Three Different Chokeberry Species. Nutr. Cancer 2021, 73, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Kołodzieczyk-Czepas, J.; Szejk, M.; Pawlak, A.; Żbikowska, H.M. Właściwości przeciwutleniające kwasu kawowego i jego pochodnych. Żywn. Nauka. Technol. Jakość 2015, 3, 5–17. [Google Scholar] [CrossRef]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free. Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Munin, A.; Edwards-Lévy, F. Encapsulation of Natural Polyphenolic Compounds; a Review. Pharmaceutics 2011, 3, 793–829. [Google Scholar] [CrossRef] [Green Version]
- Korus, J.; Achremowicz, B.; Sikora, M. Mikrokapsułkowanie substancji spożywczych. Żywn. Technol. Jakość 1997, 1, 30–40. [Google Scholar]
- Bansode, S.S.; Banarjee, S.K.; Gaikwad, D.; Jadhav, S.L.; Thorat, R.M. Microencapsulation: A review. Int. J. Pharm. Sci. Rev. Res. 2010, 1, 38–43. [Google Scholar]
- Fangmeier, M.; Lehn, D.N.; Maciel, M.J.; Volken de Souza, C.F. Encapsulation of Bioactive Ingredients by Extrusion with Vibrating Technology: Advantages and Challenges. Food Bioprocess Technol. 2019, 12, 1472–1486. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and icroparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Suganya, V.; Anuradha, V. Microencapsulation and Nanoencapsulation: A Review. Int. J. Pharm. Clin. Res. 2017, 9, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Dumont, M.J.; Orsat, V. Encapsulation of phenolic compounds present in plants using protein matrices. Food Biosci. 2016, 15, 87–104. [Google Scholar] [CrossRef]
- Gierszewska-Drużyńska, M.; Ostrowska-Czubenko, J. Synthesis and properties of hydrogel memranes based on chitosan and sodium alginate. Polimery 2007, 52, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.W.; Hwang, S.J.; Park, J.B.; Park, H.J. Preparation and release characteristics of polimer-coated and blended alginate microspheres. J. Microencapsul. 2003, 20, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Piątkowski, M. Chemiczna modyfikacja chitozanu w polu promieniowania mikrofalowego. Czas. Tech. Chem. 2008, 105, 101–112. [Google Scholar]
- Harris, R.; Lecumberri, E.; Mateos-Aparicio, I.; Mengibar, M.; Heras, A. Chitosan nanoparticles and microspheres for the encapsulation of natural antioxidants extracted from Ilex paraguariensis. Carbohydr Polym. 2011, 84, 803–806. [Google Scholar] [CrossRef]
- Sánchez-Machado, D.I.; López-Cervantes, J.; Correa-Murrieta, M.M.; Sánchez-Duarte, R.G.; Cruz-Flores, P.; de la Mora-López, G.S.; Chitosan. Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Silva, A.S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 485–493. [Google Scholar] [CrossRef]
- Kosaraju, S.L.; D’ath, L.; Lawrance, A. Preparation and characterisation of chitosan microspheres for antioxidant delivery. Carbohydr. Polym. 2006, 64, 163–167. [Google Scholar] [CrossRef]
- Fathi, M.; Martin, A.; McClements, D.J. Nanoencapsulation of Food Ingredients using Carbohydrate Based Delivery Systems. Trends Food Sci. Technol. 2014, 39, 18–39. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2011, 51, 409–418. [Google Scholar] [CrossRef] [Green Version]
- Nowak, M.; Kijowska, K.; Kabziński, M.; Ptaszek, P. Wpływ stężenia hydrokoloidu na moc mieszania oraz parametry reologiczne wodnych roztworów gumy guar. Inżynieria Apar. Chem. 2018, 57, 31–33. [Google Scholar]
- Wojdyło, A.; Oszmiański, J.; Bielecki, P. Polyphenolic composition, antioxidant activity and polyphenol oxidase (PPO) activity of quince (Cydonia oblonga Miller) varieties. J. Agric. Food Chem. 2013, 61, 2762–2772. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Samoticha, J. Evaluation of phytochemicals, antioxidant capacity, and antidiabetic activity of novel smoothies from selected Prunus fruits. J. Funct. Foods 2016, 25, 397–407. [Google Scholar] [CrossRef]
- Bonarska-Kujawa, D.; Pruchnik, H.; Oszmiański, J.; Sarapuk, J.; Kieszczyńska, H. Changes Caused by Fruit Extracts in the Lipid Phase of Biological and Model Membranes. Food Biophys. 2011, 6, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Wang, S.Y. Oxygen Radical Absorbing Capacity of Phenolics in Bluberries, Cranberries, Chokeberries, and Lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Dembczyński, R.; Białas, W.; Olejnik, A.; Kowalczewski, P.; Drożdzyńska, A.; Jankowski, T. Separacja antocyjanów z owoców aronii, czarnego bzu, czarnej porzeczki i korzenia czarnej marchwi za pomocą chromatografii preparatywnej. Żywn. Nauka. Technol. Jakość 2015, 6, 41–52. [Google Scholar]
- Horszwald, A.; Julien, H.; Andlauer, W. Characterisation of Aronia powders obtained by different drying processes. Food Chem. 2013, 141, 2858–2863. [Google Scholar] [CrossRef] [PubMed]
- Sidor, A.; Gramza-Michałowska, A. Black Chokeberry Aronia Melanocarpa, L.—A Qualitative Composition, Phenolic Profile and Antioxidant Potential. Molecules 2019, 24, 3710. [Google Scholar] [CrossRef] [Green Version]
- Oszmiański, J.; Wojdyło, A. Aronia melanocarpa phenolics and their antioxidant activity. Eur. Food Res. Technol. 2005, 221, 809–813. [Google Scholar] [CrossRef]
- Pieczykolan, E.; Kurek, M.A. Use of guar gum, gum arabic, pectin, beta-glucan and inulin for microencapsulation of anthocyanins from chokeberry. Int. J. Biol. Macromol. 2019, 129, 665–671. [Google Scholar] [CrossRef]
- Worsztynowicz, P.; Napierała, M.; Białas, W.; Grajek, W.; Olkiewicz, M. Pancreatic α-amylase and lipase inhibitory activity of polyphenolic compounds present in the extract of black chokeberry (Aronia melanocarpa L.). Process. Biochem. 2014, 49, 1457–1463. [Google Scholar] [CrossRef]
- Xue, D.; Myracle, A.D. Fermentation alters the bioaccessible phenolic compounds and increases the alpha-glucosidase inhibitory effects of aronia juice in a dairy matrix following in-vitro digestion. Food Funct. 2018, 5, 2998–3007. [Google Scholar] [CrossRef]
- Kalisz, S.; Wolniak, M.; Mitek, M. Zmiany wybranych składników bioaktywnych w dżemach truskawkowych w trakcie ich przechowywania. Żywn. Nauka. Technol. Jakość. 2016, 3, 119–126. [Google Scholar]
- Ścibisz, I.; Gasik, A.; Mitek, M.; Cendrowski, A. Wpływ warunków przechowywania na barwę dżemów z owoców kolorowych. Żywn. Nauka. Technol. Jakość. 2011, 1, 99–111. [Google Scholar]
- Lachowicz, S.; Oszmiański, J.; Kalisz, S. Effects of various polysaccharide clarification agents and reaction time on content of polyphenolic compound, antioxidant activity, turbidity and colour of chokeberry juice. LWT 2018, 92, 347–360. [Google Scholar] [CrossRef]
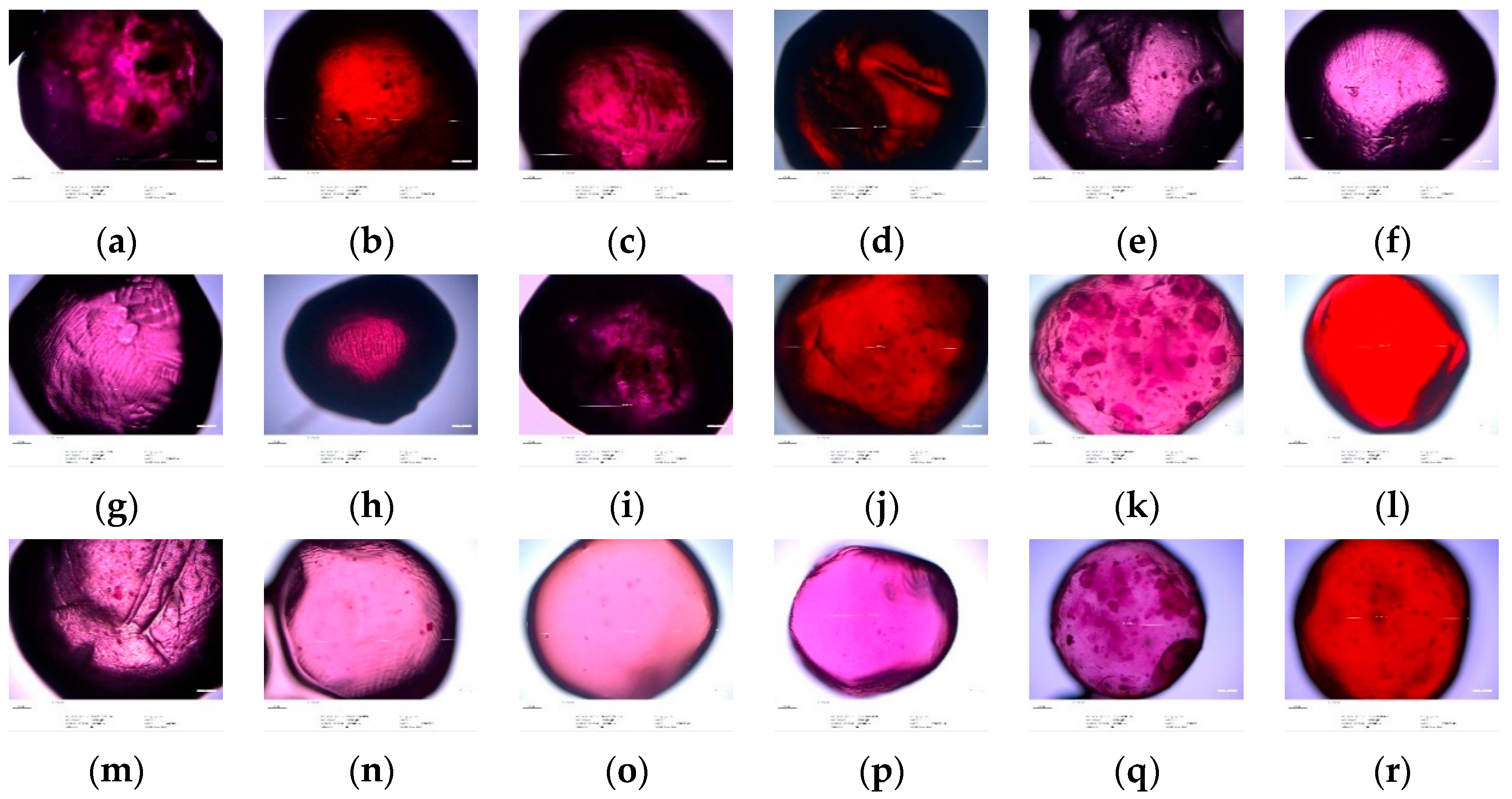
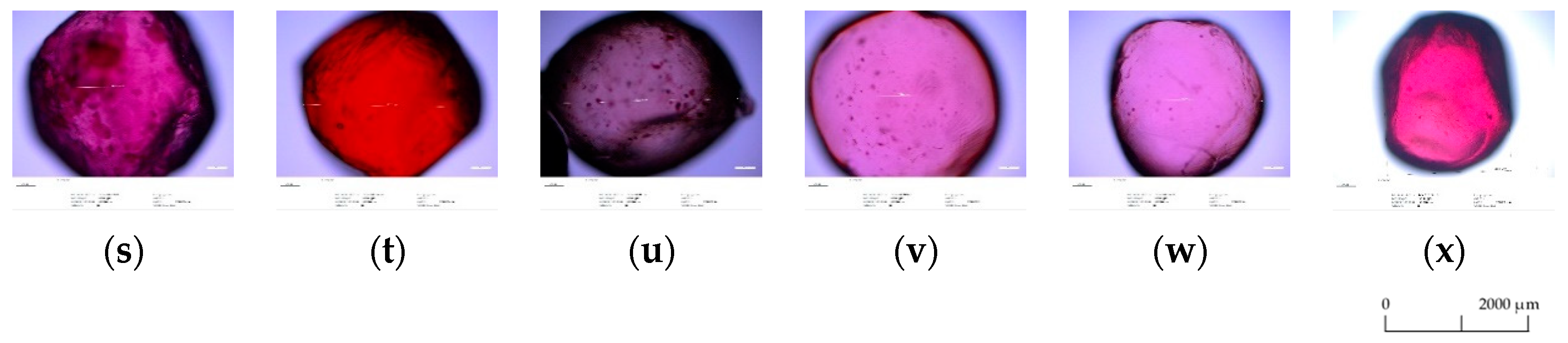

| Polyphenolic Compounds (mg/100 g of Products) | Storage Time | Types of Microspheres | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microspheres with Chokeberry Concentrate | Microspheres with Chokeberry Powder | ||||||||||||||||
| Alg: Chit: Gum (NF/K) | Alg: Gum (NF/K) | Alg: Chit: (NF/K) | Alg (NF/K) | Alg: Chit: Gum (F/K) | Alg: Gum (F/K) | Alg: Chit: (F/K) | Alg (F/K) | Alg: Chit: Gum (NF/P) | Alg: Gum (NF/P) | Alg: Chit: (NF/P) | Alg (NF/P) | Alg: Chit: Gum (F/P) | Alg: Gum (F/P) | Alg: Chit: (F/P) | Alg (F/P) | ||
| Total Anthocyanins | Zero times (non stored) | 32.90 a | 30.58 b | 29.53 b | 25.89 c | 10.65 g | 7.64 h | 12.19 f | 11.52 fg | 31.59 C | 38.90 AB | 21.58 D | 41.47 A | 5.00 I | 11.53 F | 9.53 FG | 10.17 FG |
| After 2 weeks of storage | 22.29 cd | 30.31 b | 15.88 e | 25.70 c | 6.68 hi | 6.00 hi | 10.68 g | 7.98 h | 16.85 E | 37.26 B | 15.07 E | 33.61 BC | 2.83 J | 8.72 G | 6.37 H | 8.81 G | |
| After 4 weeks of storage | 20.47 d | 30.20 b | 15.59 e | 21.07 d | 1.93 j | 5.26 i | 10.32 g | 7.34 h | 12.31 F | 35.84 B | 7.29 GH | 32.29 C | 0.00 L | 1.44 K | 6.29 H | 5.57 H | |
| Total Phenolic acid | Zero times (non stored) | 12.06 ab | 12.93 a | 11.59 bc | 12.32 a | 7.10 e | 9.50 cd | 6.22 f | 10.48 c | 17.17 B | 17.76 B | 10.32 E | 22.84 A | 4.18 G | 17.97 B | 7.63 F | 13.70 CD |
| After 2 weeks of storage | 10.54 c | 12.26 a | 8.10 de | 12.68 a | 4.09 h | 8.79 d | 6.35 f | 8.98 d | 12.94 D | 15.63 C | 8.06 F | 22.21 A | 2.98 H | 15.24 C | 6.65 G | 13.15 D | |
| After 4 weeks of storage | 4.96 g | 3.26 h | 1.33 j | 1.87 i | 0.00 m | 0.75 l | 0.96 k | 8.59 d | 6.64 G | 2.92 H | 1.24 K | 10.78 E | 0.00 L | 2.23 I | 1.38 J | 11.44 DE | |
| Total Flavonols | Zero times (non stored) | 4.99 a | 4.60 ab | 5.17 a | 4.14 b | 2.57 de | 2.41 e | 2.70 d | 2.91 d | 6.31 BC | 5.86 C | 5.02 D | 7.58 A | 1.22 J | 5.17 D | 3.23 G | 3.99 F |
| After 2 weeks of storage | 3.95 b | 3.83 bc | 3.44 c | 3.74 bc | 0.93 hi | 1.61 f | 1.54 f | 2.20 e | 4.39 E | 5.03 D | 3.55 G | 6.67 B | 0.19 K | 4.05 EF | 2.44 I | 3.65 FG | |
| After 4 weeks of storage | 2.68 de | 1.19 g | 0.37 j | 0.90 i | 0.00 k | 0.00 k | 1.10 h | 1.90 ef | 1.53 J | 3.01 H | 1.11 J | 2.44 I | 0.00 L | 1.54 J | 0.80 J | 2.74 HI | |
| Total Flavan-3-ols (monomers & dimers) | Zero times (non stored) | 4.20 b | 3.58 c | 4.34 b | 4.26 b | 2.07 f | 2.79 d | 2.14 ef | 3.13 cd | 4.98 B | 4.49 B | 3.30 D | 7.39 A | 3.29 DE | 4.02 C | 2.65 E | 3.76 CD |
| After 2 weeks of storage | 3.49 c | 2.55 de | 3.77 c | 4.09 b | 1.33 i | 2.46 e | 1.76 g | 2.84 d | 4.16 C | 3.83 C | 2.23 F | 6.03 AB | 0.56 H | 3.23 DE | 2.20 F | 3.06 E | |
| After 4 weeks of storage | 5.00 a | 2.53 de | 3.18 cd | 3.59 c | 0.30 j | 2.29 e | 1.61 h | 1.78 g | 3.49 D | 3.40 D | 1.52 G | 4.92 B | 0.34 H | 3.11 E | 2.08 FG | 2.97 E | |
| Total Flavan-3-ols (Procyanidin polymers) | Zero times (non stored) | 54.14 a | 50.44 b | 10.25 h | 6.85 i | 24.43 e | 25.36 e | 28.39 de | 3.94 j | 98.03 B | 58.04 E | 8.79 O | 11.81 N | 24.28 J | 50.63 F | 28.96 I | 4.58 S |
| After 2 weeks of storage | 9.76 h | 17.83 fg | 49.85 b | 32.42 d | 4.14 j | 3.27 k | 28.90 de | 29.46 d | 7.30 P | 16.69 L | 81.63 C | 72.13 D | 2.13 T | 6.31 R | 29.73 I | 31.53 HI | |
| After 4 weeks of storage | 39.70 c | 54.28 a | 45.72 b | 30.27 d | 15.53 g | 19.85 f | 24.29 e | 30.89 d | 12.97 M | 106.01 A | 46.83 G | 80.89 C | 16.59 L | 34.16 H | 31.01 I | 22.21 K | |
| Total content of polyphenols | Zero times (non stored) | 108.29 a | 102.13 b | 60.88 g | 53.46 h | 46.82 j | 47.70 ij | 51.64 hi | 31.98 l | 158.08 A | 125.05 D | 49.01 I | 91.09 F | 37.97 KL | 89.32 F | 52.00 I | 36.20 L |
| After 2 weeks of storage | 50.03 i | 66.78 f | 81.04 d | 78.63 d | 17.17 p | 22.13 n | 49.23 i | 51.46 i | 45.64 J | 78.44 G | 110.54 E | 140.65 C | 8.69 N | 37.55 L | 47.39 IJ | 60.20 H | |
| After 4 weeks of storage | 72.81 e | 91.46 c | 66.19 f | 57.70 g | 17.76 o | 28.15 m | 38.28 k | 50.50 i | 146.94 B | 151.18 AB | 57.99 HI | 131.32 D | 16.93 M | 42.48 K | 41.56 K | 44.93 J | |
| Components and Properties | Chokeberry Powder | Chokeberry Concentrate | |||
|---|---|---|---|---|---|
| Polyphenolic Compounds | Retention Time (Rt) | [H-M]− (m/z) | MS/MS (m/z) | [mg/100 g] | [mg/100 g] |
| Cyanidin-3-O-galactoside | 4.27 | 449+ | 287 | 10,940.10 ± 121.45 b | 2836.88 ± 52.73 b |
| Cyanidin-3-O-glucoside | 4.48 | 449+ | 287 | 647.42 ± 6.84 i | 134.83 ± 4.28 i |
| Cyanidin-3-O-arabinoside | 4.68 | 419+ | 287 | 3823.07 ± 46.46 c | 918.34 ± 20.07 e |
| Cyanidin-3-O-xyloside | 4.99 | 419+ | 287 | 900.20 ± 21.61 g | 156.57 ± 2.11 h |
| Pelargonidin-3-O-arabinoside | 5.17 | 403+ | 271 | 143.82 ± 0.39 p | 16.89 ± 0.59 r |
| Σ Anthocyanins | 16,454.46 ± 196.75 A | 4063.51 ± 79.78 B | |||
| Neochlorogenic acid | 3.45 | 353 | 191 | 3697.23 ± 86.32 d | 1287.54 ± 19.36 d |
| 3-O-p-Coumaroylquinic acid | 3.61 | 337 | 191 | 3.39 ± 0.92 t | 0.68 ± 0.04 t |
| Chlorogenic acid | 4.13 | 353 | 191 | 3169.39 ± 59.06 e | 1478.55 ± 37.11 c |
| Cryptochlorogenic acid | 4.18 | 353 | 191 | 14.39 ± 1.43 s | 2.99 ± 0.01 s |
| Σ Phenolic acid | 6883.78 ± 147.73 C | 2769.76 ± 56.52 C | |||
| Quercetin-3-O-vicianoside | 5.80 | 595 | 432/301 | 462.60 ± 2.93 k | 84.47 ± 1.93 n |
| Quercetin-3-O-robinobioside | 6.07 | 609 | 463/301 | 498.91 ± 15.30 j | 86.11 ± 1.36 n |
| Quercetin-3-rutinoside | 6.14 | 609 | 463/301 | 688.17 ± 16.17 h | 115.34 ± 0.95 j |
| Quercetin-3-galactoside | 6.30 | 463 | 301 | 2147.48 ± 39.70 f | 462.81 ± 3.75 f |
| Quercetin-3-glucoside | 6.39 | 463 | 301 | 500.31 ± 11.78 j | 100.27 ± 2.05 l |
| Isorhamnetin pentosylhexoside | 6.76 | 609 | 315 | 437.63 ± 12.12 l | 108.81 ± 0.52 k |
| Isorhamnetin rhamnosyl hexoside isomer | 6.83 | 623 | 463/315 | 225.98 ± 2.72 o | 53.59 ± 0.72 o |
| Σ Flavonols | 4961.04 ± 100.72 D | 1011.38 ± 10.28 D | |||
| (+)-catechin | 4.26 | 289 | 289 | 62.38 ± 3.29 r | 41.55 ± 0.11 p |
| Procyanidin B2 | 4.48 | 577 | 234.34 ± 6.25 n | 41.55 ± 0.84 p | |
| (−)-epicatechin | 4.56 | 289 | 577/289 | 230.51 ± 4.35 no | 93.33 ± 0.35 m |
| A-type PA-trimer | 5.01 | 866 | 287 | 350.62 ± 4.16 m | 100.59 ± 3.49 l |
| Eriodictynol-glucuronide | 6.28 | 463 | 655.72 ± 6.46 i | 296.49 ± 3.03 g | |
| Σ Flawan-3-ols (mono and dimers) | 1533.57 ± 24.51 E | 573.50 ± 7.82 E | |||
| Procyanidin polymers | 15,607.24 ± 99.32 a B | 5197.38 ± 48.73 a A | |||
| Antioxidant activity | [mmol TE/100 g] | [mmol TE/100 g] | |||
| ABTS | 357.62 ± 9.023 | 209.60 ± 6.718 | |||
| FRAP | 254.16 ± 1.189 | 169.06 ± 4.683 | |||
| ORAC | 500.60 ± 27.880 | 405.45 ± 16.255 | |||
| Enzyme inhibitory activity α-amylase α-glucosidase | [mg/mL] 0.94 ± 0.01 0.29 ± 0.04 | [mg/mL] 2.24 ± 0.04 1.14 ± 0.08 | |||
| Types of Microspheres | Zero Time (Non Stored) | Time after 2 Weeks of Storage | Time after 4 Weeks of Storage | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ABTS (mmol TE/100 g) | FRAP (mmol TE/100 g) | ORAC (mmol TE/100 g) | ABTS (mmol TE/100 g) | FRAP (mmol TE/100 g) | ORAC (mmol TE/100 g) | ABTS (mmol TE/100 g) | FRAP (mmol TE/100 g) | ORAC (mmol TE/100 g) | |
| Alg:Chit:Gum (NF/K) | 0.712 ± 0.045 ab | 0.655 ± 0.013 b | 2.038 ± 0.055 c | 0.677 ± 0.050 b | 0.650 ± 0.005 b | 2.042 ± 0.031 c | 0.607 ± 0.004 c | 0.542 ± 0.008 d | 1.240 ± 0.048 kl |
| Alg:Gum (NF/K) | 0.713 ± 0.038 ab | 0.652 ± 0.016 b | 2.398 ± 0.001 b | 0.729 ± 0.009 a | 0.678 ± 0.005 a | 2.628 ± 0.038 a | 0.601 ± 0.008 c | 0.595 ± 0.000 c | 1.229 ± 0.064 l |
| Alg:Chit (NF/K) | 0.570 ± 0.019 cd | 0.542 ± 0.003 d | 1.857 ± 0.060 d | 0.567 ± 0.017 cd | 0.474 ± 0.015 e | 1.322 ± 0.053 j | 0.588 ± 0.016 c | 0.460 ± 0.003 e | 1.308 ± 0.071 kj |
| Alg (NF/K) | 0.446 ± 0.021 f | 0.402 ± 0.018 g | 1.433 ± 0.008 hi | 0.541 ± 0.042 cd | 0.463 ± 0.031 e | 1.378 ± 0.047 ij | 0.522 ± 0.008 e | 0.434 ± 0.005 f | 0.997 ± 0.025 m |
| Alg: Chit: Gum (F/K) | 0.324 ± 0.006 h | 0.254 ± 0.021 ij | 1.729 ± 0.023 e | 0.267 ± 0.026 cd | 0.207 ± 0.003 k | 1.346 ± 0.034 j | 0.277 ± 0.008 ij | 0.188 ± 0.018 k | 0.867 ± 0.022 n |
| Alg: Gum (F/K) | 0.315 ± 0.021 hi | 0.243 ± 0.004 ij | 1.627 ± 0.059 f | 0.315 ± 0.009 j | 0.244 ± 0.016 ij | 1.515 ± 0.064 g | 0.310 ± 0.021 hi | 0.235 ± 0.002 ij | 0.710 ± 0.037 o |
| Alg: Chit (F/K) | 0.332 ± 0.024 h | 0.261 ± 0.023 i | 1.511 ± 0.030 g | 0.280 ± 0.025 hij | 0.231 ± 0.019 j | 1.432 ± 0.020 hi | 0.304 ± 0.009 hij | 0.243 ± 0.019 ij | 0.732 ± 0.053 o |
| Alg (F/K) | 0.309 ± 0.021 hi | 0.242 ± 0.013 ij | 1.464 ± 0.013 gh | 0.383 ± 0.003 ij | 0.310 ± 0.011 h | 1.030 ± 0.055 m | 0.403 ± 0.007 g | 0.324 ± 0.008 h | 0.699 ± 0.019 o |
| Alg:Chit:Gum (NF/P) | 0.840 ± 0.043 AB | 0.763 ± 0.005 B | 2.100 ± 0.068 AB | 0.811 ± 0.014 BC | 0.729 ± 0.030 C | 2.026 ± 0.021 BC | 0.733 ± 0.021 D | 0.609 ± 0.010 D | 1.172 ± 0.024 IJ |
| Alg:Gum (NF/P) | 0.823 ± 0.031 AB | 0.771 ± 0.023 B | 2.083 ± 0.073 AB | 0.870 ± 0.048 A | 0.808 ± 0.016 A | 2.154 ± 0.028 A | 0.808 ± 0.039 BC | 0.814 ± 0.001 A | 1.283 ± 0.060 GH |
| Alg:Chit (NF/P) | 0.578 ± 0.011 E | 0.540 ± 0.007 E | 1.854 ± 0.017 D | 0.582 ± 0.017 E | 0.465 ± 0.020 F | 1.124 ± 0.053 JK | 0.374 ± 0.004 HI | 0.273 ± 0.004 KL | 0.868 ± 0.057 M |
| Alg (NF/P) | 0.809 ± 0.032 BC | 0.763 ± 0.001 B | 1.989 ± 0.066 C | 0.829 ± 0.027 AB | 0.838 ± 0.003 A | 1.362 ± 0.044 G | 0.773 ± 0.004 CD | 0.823 ± 0.000 A | 1.630 ± 0.137 E |
| Alg: Chit: Gum (F/P) | 0.202 ± 0.010 LM | 0.151 ± 0.009 O | 1.457 ± 0.077 F | 0.219 ± 0.005 L | 0.163 ± 0.003 NO | 1.237 ± 0.045 HI | 0.170 ± 0.008 M | 0.163 ± 0.003 NO | 0.641 ± 0.033 O |
| Alg: Gum (F/P) | 0.565 ± 0.030 E | 0.428 ± 0.037 G | 1.868 ± 0.024 D | 0.462 ± 0.015 G | 0.362 ± 0.013 I | 2.056 ± 0.040 BC | 0.513 ± 0.004 F | 0.396 ± 0.009 H | 1.055 ± 0.031 KL |
| Alg: Chit (F/P) | 0.320 ± 0.019 JK | 0.244 ± 0.026 LM | 1.340 ± 0.041 G | 0.325 ± 0.023 JK | 0.253 ± 0.008 LM | 0.847 ± 0.032 M | 0.302 ± 0.010 K | 0.232 ± 0.023 M | 0.725 ± 0.019 NO |
| Alg (F/P) | 0.337 ± 0.067 IJK | 0.297 ± 0.039 JK | 1.579 ± 0.002 E | 0.363 ± 0.011 IJ | 0.284 ± 0.018 K | 0.987 ± 0.033 L | 0.417 ± 0.006 H | 0.322 ± 0.010 J | 0.749 ± 0.034 N |
| Types of Microspheres | Enzyme Inhibitory Activity of α-Amylase IC50 (mg/mL) | Enzyme Inhibitory Activity of α-Glucosidase IC50 (mg/mL) | |||||
|---|---|---|---|---|---|---|---|
| Zero Time (Non Stored) | after 2 Weeks of Storage | after 4 Weeks of Storage | Zero Time (Non Stored) | After 2 Weeks of Storage | after 4 Weeks of Storage | ||
| Microspheres with chokeberry concentrate (K) | Alg: Chit: Gum (NF) | 84.15 ± 2.72 e | 12.06 ± 0.11 a | 74.96 ± 1.85 c | 112.84 ± 2.86 a | 111.22 ± 2.85 a | 121.49 ± 2.70 b |
| Alg: Gum (NF) | 70.22 ± 2.81 c | 137.01 ± 3.72 ij | 93.19 ± 1.17 f | 133.34 ± 3.05 c | 120.06 ± 3.75 b | 198.94 ± 3.71 e | |
| Alg: Chit (NF) | 108.98 ± 0.99 g | 87.77 ± 1.63 e | 140.51 ± 2.01 j | 137.09 ± 2.96 c | 123.97 ± 1.85 b | 117.75 ± 3.21 ab | |
| Alginian (NF) | 127.63 ± 1.38 h | 146.66 ± 2.05 k | 154.97 ± 1.97 l | 189.68 ± 3.11 d | 212.30 ± 4.40 f | 275.81 ± 5.80 h | |
| Alg: Chit: Gum (F) | 152.52 ± 2.01 l | 81.25 ± 1.53 d | 165.02 ± 1.57 m | 452.81 ± 10.54 k | 494.56 ± 7.23 m | 360.46 ± 7.77 i | |
| Alg: Gum (F) | 140.45 ± 1.69 j | 23.12 ± 0.07 b | 126.89 ± 2.00 h | 401.14 ± 7.97 j | 474.50 ± 8.05 l | 906.28 ± 17.75 | |
| Alg: Chit (F) | 160.37 ± 4.12 lm | 154.28 ± 3.11 l | 134.45 ± 2.63 i | 413.05 ± 8.15 j | 223.50 ± 3.49 g | 623.24 ± 19.46 o | |
| Alginian (F) | 136.37 ± 1.12 i | 78.94 ± 1.95 d | 79.55 ± 0.63 d | 414.43 ± 9.17 j | 228.57 ± 5.28 g | 520.53 ± 10.53 n | |
| Microspheres with chokeberry powder (P) | Alg: Chit: Guma (NF) | 85.82 ± 1.03 F | 76.71 ± 1.27 E | 69.80 ± 1.02 CD | 115.63 ± 3.13 C | 113.69 ± 2.00 C | 108.05 ± 2.18 B |
| Alg: Guma (NF) | 85.49 ± 0.59 F | 54.34 ± 0.03 B | 315.68 ± 5.93 M | 142.94 ± 1.52 F | 114.75 ± 1.99 C | 125.23 ± 2.84 D | |
| Alg: Chit (NF) | 67.17 ± 1.73 C | 102.82 ± 1.53 G | 109.04 ± 2.03 H | 131.66 ± 2.07 E | 100.57 ± 2.21 A | 170.87 ± 1.53 G | |
| Alginian (NF) | 113.04 ± 1.93 H | 114.36 ± 1.73 H | 85.84 ± 0.45 F | 122.92 ± 2.39 D | 131.64 ± 1.73 E | 185.75 ± 3.05 H | |
| Alg: Chit: Guma (F) | 213.94 ± 3.21 K | 111.69 ± 1.99 H | 127.69 ± 2.74 I | 608.30 ± 16.32 P | 626.79 ± 10.10 P | 390.51 ± 7.00 N | |
| Alg: Guma (F) | 71.13 ± 1.03 D | 48.54 ± 0.24 A | 130.40 ± 1.06 I | 169.33 ± 4.02 G | 194.97 ± 1.11 I | 261.62 ± 1.53 K | |
| Alg: Chit (F) | 250.23 ± 1.85 L | 127.52 ± 1.62 I | 87.50 ± 2.63 F | 930.16 ± 21.54 S | 520.50 ± 13.09 O | 683.48 ± 14.71 R | |
| Alginian (F) | 151.46 ± 2.00 J | 112.97 ± 2.03 H | 216.32 ± 4.01 K | 208.76 ± 4.61 J | 334.25 ± 2.22 L | 363.32 ± 4.41 M | |
| Types of Microspheres | L* | a* | b* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Zero Times (Non Stored) | after 2 Weeks of Storage | after 4 Weeks of Storage | Zero Times (Non Stored) | after 2 Weeks of Storage | after 4 Weeks of Storage | Zero Times (Non Stored) | after 2 Weeks of Storage | after 4 Weeks of Storage | |
| Alg:Chit:Gum (NF/K) | 27.16 ± 0.74 h | 21.23 ± 0.25 f | 15.86 ± 0,68 cd | 19.96 ± 0.83 k | 16.72 ± 0.25 n | 18.19 ± 1.00l m | 2.53 ± 0.31 l | 2.66 ± 0.68 l | 3.39 ± 0.73 kl |
| Alg:Gum (NF/K) | 12.83 ± 0.69 b | 15.18 ± 0.38 c | 11.27 ± 0.62 a | 17.15 ± 1.18 mn | 35.15 ± 0.21 c | 33.49 ± 0.15 d | 5.17 ± 0.35 ih | 17.72 ± 0.22 b | 15.91 ± 0.34 c |
| Alg:Chit (NF/K) | 16.84 ± 0.70 d | 22.97 ± 0.40 g | 13.57 ±0.58 b | 18.36 ± 1.76 p | 20.53 ± 0.70 jk | 10.60 ± 0.49 o | 3.78 ± 0.40 k | 4.05 ± 0.49 jk | 1.46 ±0.37 m |
| Alg (NF/K) | 19.38 ± 0.48 e | 21.99 ± 0.86 fg | 15.29 ± 0.65 c | 8.49 ± 2.05 lm | 28.36 ± 0.28 f | 39.08 ± 0.61 b | 3.32 ± 0.52 lk | 12.52 ± 0.27 d | 23.98 ± 0.67 a |
| Alg: Chit: Gum (F/K) | 29.51 ± 0.59 i | 22.94 ± 0.63 g | 35.67 ± 0.33 k | 21.91 ± 0.8 1ij | 8.44 ± 0.96 p | 19.17 ± 0.60 kl | 4.29± 0.02 ijk | −2.54 ± 0.93 n | 11.46 ± 0.95 e |
| Alg: Gum (F/K) | 33.09 ± 0.57 j | 47.77 ± 0.96 m | 15.91 ± 0.71 k | 22.74 ± 0.55 hi | 24.24 ± 0.15 g | 33.28 ± 1.03 d | 5.40 ± 0.21 h | 5.96 ± 0.52 h | 11.62 ± 1.02 de |
| Alg: Chit (F/K) | 22.61 ± 0,51 g | 37.07 ± 0.55 l | 28.56 ± 0.38 i | 23.52 ± 0.7 gh | 30.8 ± 0.61e | 41.46 ± 0.48 a | 4.99± 0.47 hij | 7.58 ± 0.54 g | 17.56 ± 1.26 b |
| Alg (F/K) | 21.90 ± 0,65 fg | 26.74 ± 0.41 h | 21.82 ± 0.93 fg | 19.60 ± 0.75 ik | 29.37 ± 0.67 f | 24.63 ± 0.17 g | 2.39 ± 0.21 m | 8.63 ± 0.59 f | 5.88 ± 0.63 h |
| Alg:Chit:Gum (NF/P) | 11.77 ± 0.55 A | 34.43 ± 0.71 G | 10.55 ± 0.62 A | 7.71 ± 1.44 L | 4.88 ± 0.62 N | 8.05 ± 0.39 L | 0.29 ± 0.55 l | −2.31 ± 0.85 N | −0.76 ± 0.81 M |
| Alg:Gum (NF/P) | 15.46 ± 0.37 B | 15.96 ± 0.88 B | 12.11 ± 0.79 A | 8.23 ± 1.23 L | 36.31 ± 0.58 C | 18.99 ± 0.46 I | 1.79 ± 0.45 k | 19.72 ± 0.51 A | 6.70 ± 0.11 F |
| Alg:Chit (NF/P) | 19.14 ± 0.14 C | 24.97 ± 0.82 E | 10.84 ± 0.66 A | 21.02 ± 1.59 GH | 27.51 ± 0.64 E | 14.88 ± 1.08 J | 4.12 ± 0.75 m | 7.29 ± 0.97 F | 2.41 ± 0.96 JK |
| Alg (NF/P) | 12.08 ± 0.83 A | 23.76 ± 0.72 DE | 10.40 ± 0.34 A | 11.05 ± 1.39 K | 22.08 ± 0.45 G | 22.04 ± 0.76 G | 2.96 ± 0.7 1ij | 9.52 ± 0.97 E | 8.76 ± 0.41 E |
| Alg: Chit: Gum (F/P) | 34.89 ± 0.80 G | 60.67 ± 0.66 I | 47.68 ± 0.38 H | 10.93 ± 0.55 K | 13.49 ± 0.81 J | 14.72 ± 0.40 J | 2.85 ± 0.14 ijk | 8.83 ± 0.79 E | 13.93 ± 0.68 C |
| Alg: Gum (F/P) | 21.31 ± 0.12 CD | 34.98 ± 0.82 G | 16.42 ± 0.32 CD | 19.01 ± 0.72 I | 42.71 ± 0.33 A | 39.22 ± 1.17 B | 5.39 ± 0.45 g | 16.44 ± 0.72 B | 20.65 ± 0.58 A |
| Alg: Chit (F/P) | 29.53 ± 0.35 F | 45.50 ± 0.57 H | 2.41 ± 0.55 F | 27.06 ± 0.81 E | 25.20 ± 0.25 F | 39.50 ± 0.61 B | 6.79 ± 0.58 f | 7.13 ± 0.55 F | 15.58 ± 0.22 B |
| Alg (F/P) | 25.35 ± 0.09 E | 33.78 ± 0.76 G | 19.30 ± 0.73 C | 19.69 ± 0.44 HI | 34.32 ± 0.51 D | 33.04 ± 0.72 D | 3.65 ± 0.86 hi | 11.30 ± 0.60 D | 11.50 ± 0.59 D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haładyn, K.; Tkacz, K.; Wojdyło, A.; Nowicka, P. The Types of Polysaccharide Coatings and Their Mixtures as a Factor Affecting the Stability of Bioactive Compounds and Health-Promoting Properties Expressed as the Ability to Inhibit the α-Amylase and α-Glucosidase of Chokeberry Extracts in the Microencapsulation Process. Foods 2021, 10, 1994. https://doi.org/10.3390/foods10091994
Haładyn K, Tkacz K, Wojdyło A, Nowicka P. The Types of Polysaccharide Coatings and Their Mixtures as a Factor Affecting the Stability of Bioactive Compounds and Health-Promoting Properties Expressed as the Ability to Inhibit the α-Amylase and α-Glucosidase of Chokeberry Extracts in the Microencapsulation Process. Foods. 2021; 10(9):1994. https://doi.org/10.3390/foods10091994
Chicago/Turabian StyleHaładyn, Kamil, Karolina Tkacz, Aneta Wojdyło, and Paulina Nowicka. 2021. "The Types of Polysaccharide Coatings and Their Mixtures as a Factor Affecting the Stability of Bioactive Compounds and Health-Promoting Properties Expressed as the Ability to Inhibit the α-Amylase and α-Glucosidase of Chokeberry Extracts in the Microencapsulation Process" Foods 10, no. 9: 1994. https://doi.org/10.3390/foods10091994






