Extraction of Polyphenols and Valorization of Fibers from Istrian-Grown Pomegranate (Punica granatum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Sample Preparation
2.3. Preparation of the Pomegranate Water and Ethanol Extracts
2.4. Total Phenolics
2.5. Total Flavonoids
2.6. Antioxidant Capacity
2.7. Quantitative Determination of Catechin with TLC
2.8. Hydrolysis of Pomegranate Extracts for TLC Screening of Phenolic Acids
2.9. TLC Screening of Phenolic Acids
2.10. Solid-Phase Extraction of Pomegranate Extracts before LC-MS Analysis and HPLC
2.11. Liquid Chromatography-Mass Spectrometry
2.12. Quantification of Individual Anthocyanins
2.13. Thermal Treatment of Pomegranate Juice
2.14. Color Measurement
2.15. Chemical Composition
2.16. Delignification, Fiber Characterization, and Paper Production
2.17. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Compounds, Flavonoids, and Antioxidant Capacity of Pomegranate Extracts
3.2. Quantitative Determination of Catechin and Qualitative Identification of Phenolic Compounds with TLC
3.3. LC-MS Analysis of Pomegranate Extracts
3.4. HPLC Determination of Individual Anthocyanins in Pomegranate Extracts
3.5. Thermal Treatment of Pomegranate Juice
3.6. Color Measurements
3.7. Fibrous Compounds in Pomegranate Peel
3.8. Test Production of Paper
4. Conclusions
Supplementary Materials
 ) and 70% ethanol pomegranate extracts (
) and 70% ethanol pomegranate extracts ( pomegranate seeds;
pomegranate seeds;  pomegranate juice;
pomegranate juice;  pomegranate membrane;
pomegranate membrane;  pomegranate mesocarp;
pomegranate mesocarp;  pomegranate peel) scanned on the HPTLC cellulose plate at 655 nm after derivatization with DMACA reagent. Additionally to described fractions (Section 2.2) pomegranate membrane is shown herein as the thin mesocarp layer directly in contact with the arils; Figure S2: Separation of anthocyanins in pomegranate juice by HPLC. Peeks: (1) Del-3,5-diGly; (2) Cy-3,5-diGly; (3) Del-3-Gly; (4) Pel-3,5-diGly; (5) Cy-3-Gly; (6) Pel-3-Gly, (7) non-determined with HPLC; Figure S3: Laboratory samples of paper produced from commercial cellulose fibers (left) and a mixture of cellulose fibers with pomegranate peels as a filler (right).
pomegranate peel) scanned on the HPTLC cellulose plate at 655 nm after derivatization with DMACA reagent. Additionally to described fractions (Section 2.2) pomegranate membrane is shown herein as the thin mesocarp layer directly in contact with the arils; Figure S2: Separation of anthocyanins in pomegranate juice by HPLC. Peeks: (1) Del-3,5-diGly; (2) Cy-3,5-diGly; (3) Del-3-Gly; (4) Pel-3,5-diGly; (5) Cy-3-Gly; (6) Pel-3-Gly, (7) non-determined with HPLC; Figure S3: Laboratory samples of paper produced from commercial cellulose fibers (left) and a mixture of cellulose fibers with pomegranate peels as a filler (right).Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nath Prasad, R.; Chandra, R.; Teixeira da Silva, J.A. Postharvest Handling and Processing of Pomegranate Fruit, Vegetable and Cereal Science and Biotechnology Postharvest Handling and Processing of Pomegranate. In Fruit, Vegetable and Cereal Science and Biotechnology; Global Science Books: Isleworth, UK, 2010; pp. 88–95. [Google Scholar]
- Bandini, M.; Ban, D.; Bandelj, D.; Baruca Arbeiter, A.; Bučar-Miklavčič, M.; Butinar, B.; Cigič, B.; Ferenac, M.; Godena, S.; Gunde-Cimerman, N.; et al. Granatno Jabolko (Punica granatum L.), Žižola (Ziziphus Jujuba Mill.), Madelj (Prunus Dulcijs (Mill.) D.A. Webb), Navadna Jagodičnica (Arbutus unedo L.) in Navadni Koprivovec (Cetis australis L.) v Istri; Ban, D., Bandelj, D., Bučar-Miklavčič, M., Poklar Ulrih, N., Eds.; Univerzitetna založba Annales: Koper, Slovenia, 2015; ISBN 978-961-6964-41-8. [Google Scholar]
- Giménez-Bastida, J.A.; Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. Evidence for Health Properties of Pomegranate Juices and Extracts beyond Nutrition: A Critical Systematic Review of Human Studies. Trends Food Sci. Technol. 2021, 114, 410–423. [Google Scholar] [CrossRef]
- Olvera-Sandoval, C.; Enrique Fabela-Illescas, H.; Fernández-Martínez, E.; Araceli Ortiz-Rodríguez, M.; Cariño-Cortés, R.; Alberto Ariza-Ortega, J.; Carlos Hernández-González, J.; Olivo, D.; Valadez-Vega, C.; Belefant-Miller, H.; et al. Potential Mechanisms of the Improvement of Glucose Homeostasis in Type 2 Diabetes by Pomegranate Juice. Antioxidants 2022, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Syed, D.N.; Chamcheu, J.-C.; Adhami, V.M.; Mukhtar, H. Pomegranate Extracts and Cancer Prevention: Molecular and Cellular Activities. Anticancer. Agents Med. Chem. 2013, 13, 1149. [Google Scholar] [CrossRef]
- Ming, D.S.; Pham, S.; Deb, S.; Chin, M.Y.; Kharmate, G.; Adomat, H.; Beheshti, E.H.; Locke, J.; Guns, E.T. Pomegranate Extracts Impact the Androgen Biosynthesis Pathways in Prostate Cancer Models in Vitro and in Vivo. J. Steroid Biochem. Mol. Biol. 2014, 143, 19–28. [Google Scholar] [CrossRef]
- Turrini, E.; Ferruzzi, L.; Fimognari, C. Potential Effects of Pomegranate Polyphenols in Cancer Prevention and Therapy. Oxid. Med. Cell. Longev. 2015, 2015, 938475. [Google Scholar] [CrossRef] [PubMed]
- Keta, O.; Deljanin, M.; Petković, V.; Zdunić, G.; Janković, T.; Živković, J.; Ristić-Fira, A.; Petrović, I.; Šavikin, K. Pomegranate (Punica granatum L.) Peel Extract: Potential Cytotoxic Agent against Different Cancer Cell Lines. Rec. Nat. Prod. 2020, 14, 326–339. [Google Scholar] [CrossRef]
- Sharma, P.; Mcclees, S.F.; Afaq, F. Molecules Pomegranate for Prevention and Treatment of Cancer: An Update. Molecules 2017, 22, 177. [Google Scholar] [CrossRef]
- Russo, V.; Continella, A.; Drago Id, C.; Gentile, A.; La Malfa, S.; Leotta, C.G.; Pulvirenti, L.; Ruberto, G.; Pitari, G.M.; Siracusaid, L. Secondary Metabolic Profiles and Anticancer Actions from Fruit Extracts of Immature Pomegranates. PLoS ONE 2021, 16, e0255831. [Google Scholar] [CrossRef]
- Pagliarulo, C.; De Vito, V.; Picariello, G.; Colicchio, R.; Pastore, G.; Salvatore, P.; Volpe, M.G. Inhibitory Effect of Pomegranate (Punica granatum L.) Polyphenol Extracts on the Bacterial Growth and Survival of Clinical Isolates of Pathogenic Staphylococcus Aureus and Escherichia Coli. Food Chem. 2016, 190, 824–831. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Assessment of Polyphenolic Profile and Antibacterial Activity of Pomegranate Peel (Punica granatum) Flour Obtained from Co-Product of Juice Extraction. Food Control 2016, 59, 94–98. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial Activity of Pomegranate Peel Extracts Performed by High Pressure and Enzymatic Assisted Extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef]
- Sorrenti, V.; Lucia Randazzo, C.; Caggia, C.; Ballistreri, G.; Valeria Romeo, F.; Fabroni, S.; Timpanaro, N.; Raffaele, M.; Vanella, L. Beneficial Effects of Pomegranate Peel Extract and Probiotics on Pre-Adipocyte Differentiation. Front. Microbiol. 2019, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Burgos, E.C.; Burgos-Hernández, A.; Noguera-Artiaga, L.; Kačániová, M.; Hernández-García, F.; Cárdenas-López, J.L.; Carbonell-Barrachina, Á.A. Antimicrobial Activity of Pomegranate Peel Extracts as Affected by Cultivar. J. Sci. Food Agric. 2016, 97, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, D.; Bhat, Z.F.; Kumar, S. Pomegranate (Punica granatum) Rind Extract as a Novel Preservative in Cheese. Food Biosci. 2015, 12, 47–53. [Google Scholar] [CrossRef]
- Ruan, J.-H.; Li, J.; Adili, G.; Sun, G.-Y.; Abuduaini, M.; Abdulla, R.; Maiwulanjiang, M.; Aisa, H.A. Phenolic Compounds and Bioactivities from Pomegranate (Punica granatum L.) Peels. Cite This J. Agric. Food Chem 2022, 2022, 3686. [Google Scholar] [CrossRef]
- Sentandreu, E.; Cerdán-Calero, M.; Sendra, J.M. Phenolic Profile Characterization of Pomegranate (Punica granatum) Juice by High-Performance Liquid Chromatography with Diode Array Detection Coupled to an Electrospray Ion Trap Mass Analyzer. J. Food Compos. Anal. 2013, 30, 32–40. [Google Scholar] [CrossRef]
- Fischer, U.A.; Carle, R.; Kammerer, D.R. Identification and Quantification of Phenolic Compounds from Pomegranate (Punica granatum L.) Peel, Mesocarp, Aril and Differently Produced Juices by HPLC-DAD-ESI/MS(N). Food Chem. 2011, 127, 807–821. [Google Scholar] [CrossRef]
- Fischer, U.A.; Jaksch, A.V.; Carle, R.; Kammerer, D.R. Influence of Origin Source, Different Fruit Tissue and Juice Extraction Methods on Anthocyanin, Phenolic Acid, Hydrolysable Tannin and Isolariciresinol Contents of Pomegranate (Punica granatum L.) Fruits and Juices. Eur. Food Res. Technol. 2013, 237, 209–221. [Google Scholar] [CrossRef]
- Montefusco, A.; Durante, M.; Migoni, D.; De Caroli, M.; Ilahy, R.; Pék, Z.; Helyes, L.; Fanizzi, F.P.; Mita, G.; Piro, G.; et al. Analysis of the Phytochemical Composition of Pomegranate Fruit Juices, Peels and Kernels: A Comparative Study on Four Cultivars Grown in Southern Italy. Plants 2021, 10, 2521. [Google Scholar] [CrossRef]
- Gutfinger, T. Polyphenols in Olive Oils. J. Am. Oil Chem. Soc. 1981, 58, 966–968. [Google Scholar] [CrossRef]
- Yang, J.; Meyers, K.J.; van der Heide, J.; Liu, R.H. Varietal Differences in Phenolic Content and Antioxidant and Antiproliferative Activities of Onions. J. Agric. Food Chem. 2004, 52, 6787–6793. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Vovk, I.; Simonovska, B.; Vuorela, H. Separation of Eight Selected Flavan-3-Ols on Cellulose Thin-Layer Chromatographic Plates. J. Chromatogr. A 2005, 1077, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Glavnik, V.; Simonovska, B.; Vovk, I. Densitometric Determination of (+)-Catechin and (-)-Epicatechin by 4-Dimethylaminocinnamaldehyde Reagent. J. Chromatogr. A 2009, 1216, 4485–4491. [Google Scholar] [CrossRef]
- Nuutila, A.; Kammiovirta, K.; Oksman-Caldentey, K.-M. Comparison of Methods for the Hydrolysis of Flavonoids and Phenolic Acids from Onion and Spinach for HPLC Analysis. Food Chem. 2002, 76, 519–525. [Google Scholar] [CrossRef]
- Simonovska, B.; Vovk, I.; Andrenšek, S.; Valentová, K.; Ulrichová, J. Investigation of Phenolic Acids in Yacon (Smallanthus sonchifolius) Leaves and Tubers. J. Chromatogr. A 2003, 1016, 89–98. [Google Scholar] [CrossRef]
- Lätti, A.K.; Riihinen, K.R.; Kainulainen, P.S. Analysis of Anthocyanin Variation in Wild Populations of Bilberry (Vaccinium myrtillus L.) in Finland. J. Agric. Food Chem. 2008, 56, 190–196. [Google Scholar] [CrossRef]
- Moze, S.; Polak, T.; Gasperlin, L.; Koron, D.; Vanzo, A.; Poklar Ulrih, N.; Abram, V. Phenolics in Slovenian Bilberries (Vaccinium myrtillus L.) and Blueberries (Vaccinium corymbosum L.). J. Agric. Food Chem. 2011, 59, 6998–7004. [Google Scholar] [CrossRef]
- Bartl, P.; Albreht, A.; Skrt, M.; Tremlová, B.; Ošťádalová, M.; Šmejkal, K.; Vovk, I.; Ulrih, N.P. Anthocyanins in Purple and Blue Wheat Grains and in Resulting Bread: Quantity, Composition, and Thermal Stability. Int. J. Food Sci. Nutr. 2015, 66, 514–519. [Google Scholar] [CrossRef]
- Gombač, Z.; Črnivec, I.G.O.; Skrt, M.; Istenič, K.; Knafelj, A.K.; Pravst, I.; Ulrih, N.P. Stabilisation of Lutein and Lutein Esters with Polyoxyethylene Sorbitan Monooleate, Medium-Chain Triglyceride Oil and Lecithin. Foods 2021, 10, 500. [Google Scholar] [CrossRef]
- Bremer, M.; Fischer, S.; Nguyen, T.C.; Wagenführ, A.; Phuong, L.X.; Dai, V.H. Effects of Thermal Modification on the Properties of Two Vietnamese Bamboo Species. Part II: Effects on Chemical Composition. BioResources 2013, 8, 981–993. [Google Scholar] [CrossRef]
- TAPPI Test Method T222. Acid-Insoluble Lignin in Wood and Pulp. 2006. Available online: https://www.tappi.org/content/SARG/T222.pdf (accessed on 1 September 2022).
- Kapun, T.; Zule, J.; Fabjan, E.; Hočevar, B.; Grilc, M.; Likozar, B. Engineered Invasive Plant Cellulose Fibers as Resources for Papermaking. Eur. J. Wood Wood Prod. 2022, 80, 501–514. [Google Scholar] [CrossRef]
- Osojnik Črnivec, I.G.; Skrt, M.; Šeremet, D.; Sterniša, M.; Farčnik, D.; Štrumbelj, E.; Poljanšek, A.; Cebin, N.; Pogačnik, L.; Smole Možina, S.; et al. Waste Streams in Onion Production: Bioactive Compounds, Quercetin and Use of Antimicrobial and Antioxidative Properties. Waste Manag. 2021, 126, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Hasnaoui, N.; Jbir, R.; Mars, M.; Trifi, M.; Kamal-Eldin, A.; Melgarejo, P.; Hernandez, F. Organic Acids, Sugars, and Anthocyanins Contents in Juices of Tunisian Pomegranate Fruits. Int. J. Food Prop. 2011, 14, 741–757. [Google Scholar] [CrossRef]
- Tabaraki, R.; Heidarizadi, E.; Benvidi, A. Optimization of Ultrasonic-Assisted Extraction of Pomegranate (Punica granatum L.) Peel Antioxidants by Response Surface Methodology. Sep. Purif. Technol. 2012, 98, 16–23. [Google Scholar] [CrossRef]
- Gözlekçi, Ş.; Saraçoǧlu, O.; Onursal, E.; Özgen, M. Total Phenolic Distribution of Juice, Peel, and Seed Extracts of Four Pomegranate Cultivars. Pharmacogn. Mag. 2011, 7, 161–164. [Google Scholar] [CrossRef]
- Ardekani, M.R.S.; Hajimahmoodi, M.; Oveisi, M.R.; Sadeghi, N.; Jannat, B.; Ranjbar, A.M.; Gholam, N.; Moridi, T. Comparative Antioxidant Activity and Total Flavonoid Content of Persian Pomegranate (Punica granatum L.) Cultivars. Iran. J. Pharm. Res. IJPR 2011, 10, 519. [Google Scholar]
- Orak, H.H.; Yagar, H.; Isbilir, S.S. Comparison of Antioxidant Activities of Juice, Peel, and Seed of Pomegranate (Punica granatum L.) and Inter-Relationships with Total Phenolic, Tannin, Anthocyanin, and Flavonoid Contents. Food Sci. Biotechnol. 2012, 21, 373–387. [Google Scholar] [CrossRef]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant Activity of Pomegranate Juice and Its Relationship with Phenolic Composition and Processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef]
- Makalesi, A.; Karakaplan, M.; Özcan, M. Determination of Phenolic Acids in Pomegranate Juices by HPLC-DAD. Eur. J. Sci. Technol. 2017, 6, 32–37. [Google Scholar]
- Pala, Ç.U.; Toklucu, A.K. Effect of UV-C Light on Anthocyanin Content and Other Quality Parameters of Pomegranate Juice. J. Food Compos. Anal. 2011, 24, 790–795. [Google Scholar] [CrossRef]
- Mena, P.; Vegara, S.; Martí, N.; García-Viguera, C.; Saura, D.; Valero, M. Changes on Indigenous Microbiota, Colour, Bioactive Compounds and Antioxidant Activity of Pasteurised Pomegranate Juice. Food Chem. 2013, 141, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Passafiume, R.; Perrone, A.; Sortino, G.; Gianguzzi, G.; Saletta, F.; Gentile, C.; Farina, V. Chemical–Physical Characteristics, Polyphenolic Content and Total Antioxidant Activity of Three Italian-Grown Pomegranate Cultivars. NFS J. 2019, 16, 9–14. [Google Scholar] [CrossRef]
- Ferrari, G.; Maresca, P.; Ciccarone, R. The Application of High Hydrostatic Pressure for the Stabilization of Functional Foods: Pomegranate Juice. J. Food Eng. 2010, 100, 245–253. [Google Scholar] [CrossRef]
- Valero, M. Clarification of Pomegranate Juice at Industrial Scale. J. Food Process. Technol. 2014, 5, 1–6. [Google Scholar] [CrossRef]
- Ahmad, Z.; Al Dajani, W.W.; Paleologou, M.; Xu, C. Sustainable Process for the Depolymerization/Oxidation of Softwood and Hardwood Kraft Lignins Using Hydrogen Peroxide under Ambient Conditions. Molecules 2020, 25, 2329. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Ververis, C.; Georghiou, K.; Danielidis, D.; Hatzinikolaou, D.G.; Santas, P.; Santas, R.; Corleti, V. Cellulose, Hemicelluloses, Lignin and Ash Content of Some Organic Materials and Their Suitability for Use as Paper Pulp Supplements. Bioresour. Technol. 2007, 98, 296–301. [Google Scholar] [CrossRef]
- Belle, J.; Odermatt, J. Initial Wet Web Strength of Paper. Cellulose 2016, 23, 2249–2272. [Google Scholar] [CrossRef]
- Ferdous, T.; Ni, Y.; Quaiyyum, M.A.; Uddin, M.N.; Jahan, M.S. Non-Wood Fibers: Relationships of Fiber Properties with Pulp Properties. ACS Omega 2021, 6, 21613–21622. [Google Scholar] [CrossRef]
- Sha, J.; Nikbakht, A.; Wang, C.; Zhang, H.; Olson, J. The Effect of Consistency and Freeness on the Yield Stress of Chemical Pulp Fibre Suspensions. BioResources 2015, 10, 4287–4299. [Google Scholar] [CrossRef][Green Version]
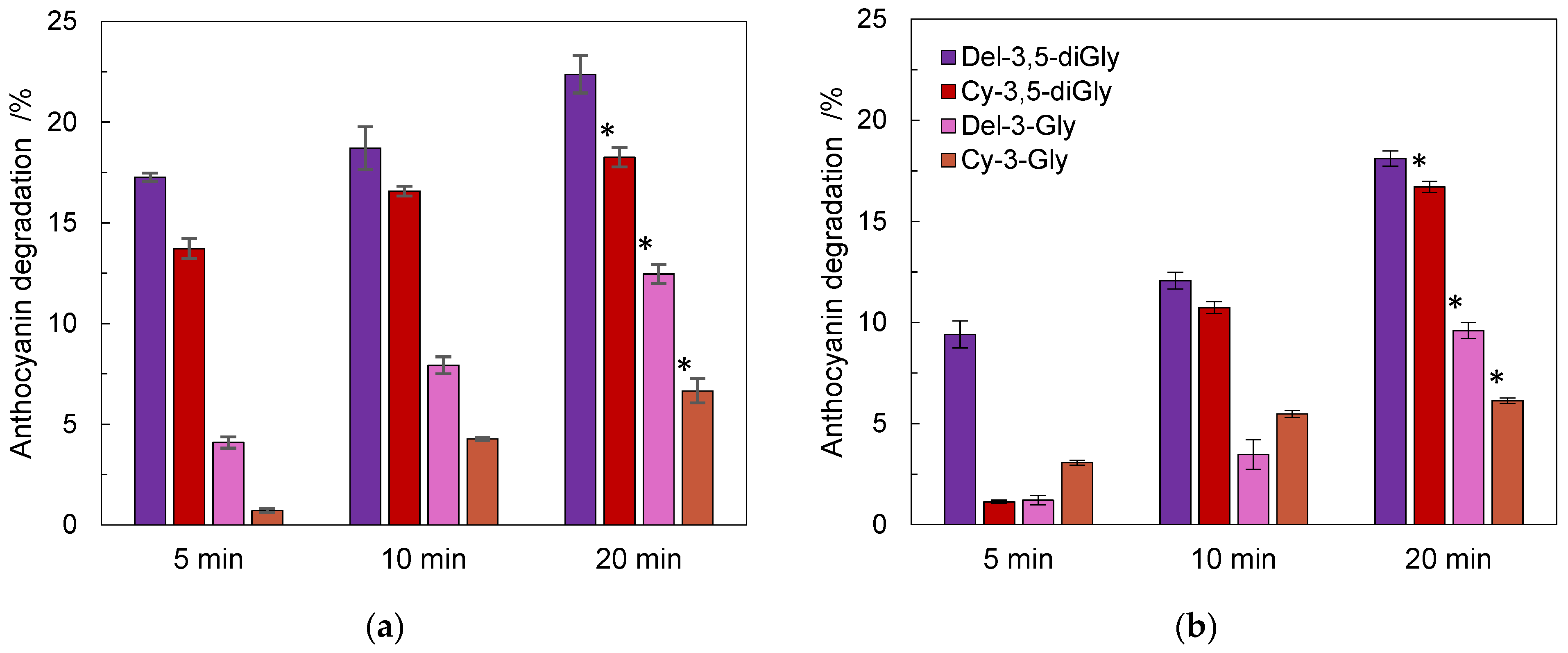
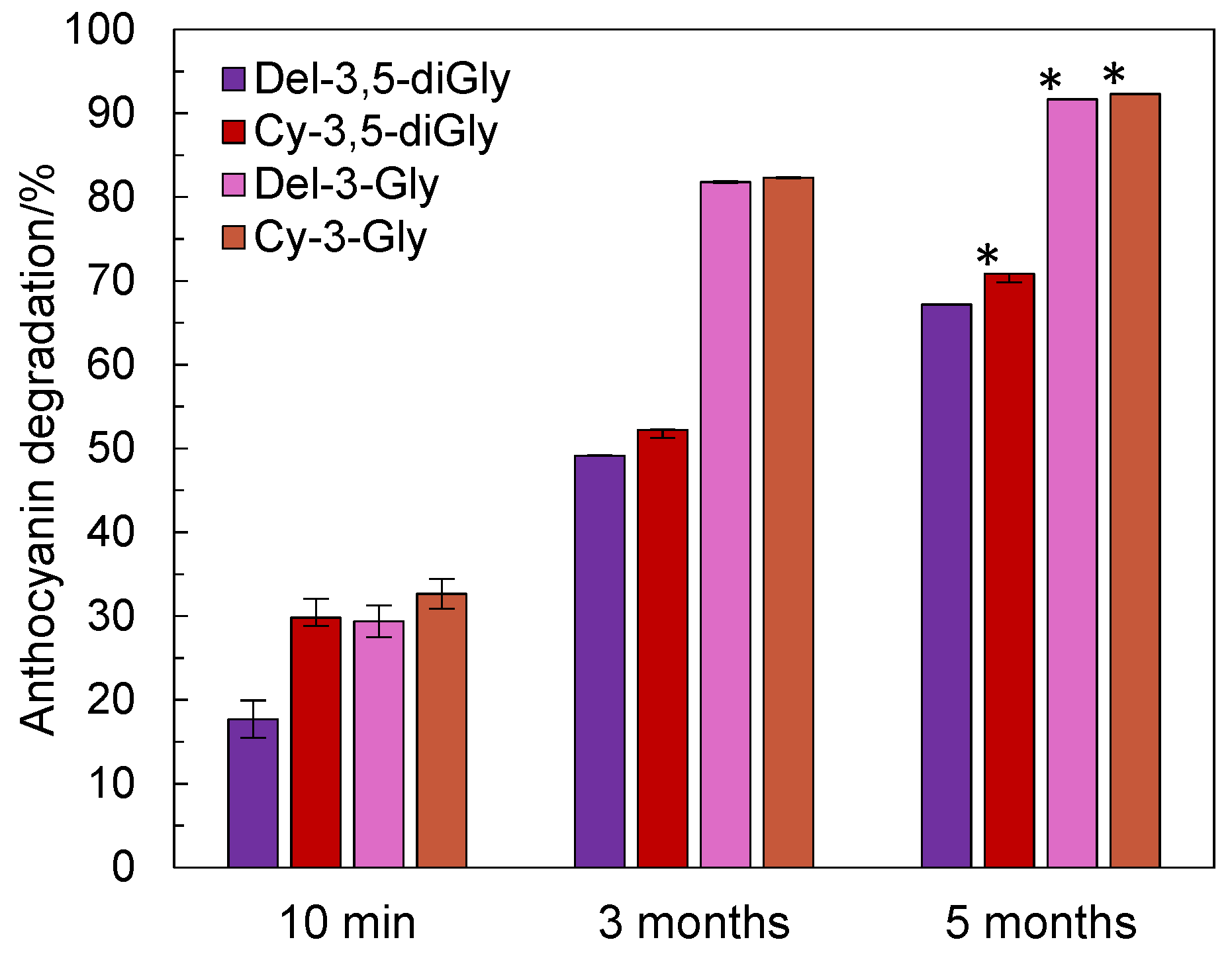
| Compound | Regression Equation | Correlation Factor, R2 | LOD (µg/mL) | LOQ (µg/mL) |
|---|---|---|---|---|
| Cy-3-Gly | y = 72.79·x − 42.09 | 0.9999 | 0.585 | 1.77 |
| Cy-3,5-diGly | y = 79.05·x − 24.20 | 0.9997 | 1.38 | 4.16 |
| Del-3-Gly | y = 138.2·x − 116.2 | 0.9997 | 0.90 | 2.73 |
| Del-3,5-diGly | y = 77.38·x − 25.05 | 0.9997 | 1.35 | 4.09 |
| Pel-3-Gly | y = 122.9·x − 126.7 | 0.9979 | 2.52 | 7.62 |
| Pel-3,5-diGly | y = 35.10·x − 9.443 | 0.9984 | 1.95 | 5.93 |
| Source | Fresh Mass Fraction | Water Content | Lyophilized Water Extract | Lyophilized Ethanol Extract |
|---|---|---|---|---|
| % (FM/FM) | % (DM/FM) | |||
| Peel | 18.9 | 72.6 | 43.2 | 62.8 |
| Mesocarp | 30.8 | 73.9 | 64.9 | 75.4 |
| Arils | 50.2 | n.d. | n.d. | n.d. |
| - seeds and pulp | 18.1 | n.d. | 80.5 | 82.3 |
| - juice * | 32.1 | 80.1 | 20.6 | 28.6 |
| Source | Water Extracts | Ethanol Extracts | ||||||
|---|---|---|---|---|---|---|---|---|
| Total Phenolic Content (mg GAE/g DM) | Flavonoids (mg CE/g DM) | Antioxidant Capacity (µg TE/g DM) | Flavonoids: Phenolics Ratio (/) | Total Phenolic Content (mg GAE/g DM) | Flavonoids (mg CE/g DM) | Antioxidant Capacity (µg TE/g DM) | Flavonoids: Phenolics Ratio (/) | |
| Peel | 8.80 ± 0.40 a1 | 2.80 ± 0.20 a2 | 128 ± 2 a3 | 0.32 | 30.5 ± 0.6 a4 | 4.25 ± 0.05 a5 | 17.0 ± 6.0 a6 | 0.14 |
| Mesocarp | 8.00 ± 0.20 a1 | 1.98 ± 0.01 a2 | 69.7 ± 0.7 b3 | 0.25 | 26.3 ± 0.0 b4 | 2.50 ± 0.04 b5 | 5.50 ± 0.20 ab6 | 0.095 |
| Juice | 1.74 ± 0.02 b1 | 0.083 ± 0.009 b2 | 23.0 ± 1.0 c3 | 0.048 | 1.12 ± 0.04 c4 | 0.100 ± 0.006 c5 | 2.75 ± 0.03 ac6 | 0.084 |
| Seeds | 0.420 ± 0.010 c1 | 0.031 ± 0.001 b2 | 0.266 ± 0.003 d3 | 0.074 | 1.48 ± 0.02 d4 | 0.220 ± 0.020 c5 | 4.90 ± 0.20 ab6 | 0.015 |
| Origin | Pomegranate Part | Pretreatment and Extraction Conditions | Total Phenolic Content | Source |
|---|---|---|---|---|
| Peru, unknown cultivar | Peel | Pre-steamed, 80% MeOH, 0.1% HCl | 101.86 ± 12.8 mg GAE/g DM extract | [19] |
| Mesocarp | 198.17 ± 2.9 mg GAE/g DM extract | |||
| Juice 1 | Juicing at 10 bar | 2015.2 ± 21.66 mg GAE/L | ||
| Juice 2 | Juicing at 150 bar | 5186.0 ± 172.5 mg GAE/L | ||
| Juice 3 | Juicing at 250 bar | 2122.0 ± 0.0 mg GAE/L | ||
| Tunisia, 12 cultivars | Peel + mesocarp | Pre-homogenized, 80% ETOH + 70% ACE | 205.07 ± 0.0 to 276.35 ± 0.07 mg GAE/g extracted | [37] |
| Iran, local markets | Peel − undefined | sonicated in 70% EtOH, 60 °C, 30 min | 86.78 mg GAE/g DM extract | [38] |
| 70% EtOH, 30 °C, 30 min | 76.22 mg GAE/g DM extract | |||
| 70% EtOH, 30 °C, 10 min | 70.95 mg GAE/g DM extract | |||
| 30% EtOH, 30 °C, 10 min | 49.35 mg GAE/g DM extract | |||
| Turkey, 4 cultivars | Peel − undefined | Pre-homogenized, 50% EtOH | 1.78 to 3.55 mg GAE/g fresh weight | [39] |
| Seeds | 1.31 to 1.55 mg GAE/g fresh weight | |||
| Juice | 0.121 to 0.177 mg GAE/g fresh weight | |||
| Iran, 9 cultivars | Peel − undefined | Soxhlet extraction in ACE, EtOAc, MeOH, and H20 | 18.61 ± 0.53 to 36.40 ± 1.34 mg GAE/g extract | [40] |
| Pulp − undefined | 11.62 ± 0.63 to 21.03 ± 1.51 mg GAE/g extract |
| Source | Catechin (mg/g DM) | Quercetin (RF = 0.43) | o-Coumaric Acid (RF = 0.61) * | Gallic Acid (RF = 0.27) * | Caffeic Acid (RF = 0.43) * | Chlorogenic Acid (RF = 0.41) * |
|---|---|---|---|---|---|---|
| Peel | 0.429 | + | + | + | + | + |
| Mesocarp | 0.083 | + | + | + | + | + |
| Juice | 0.024 | + | + | + | + | + |
| Seeds | 0.088 | + | + | + | + | + |
| Source Peak Number | Retention Time (min) | Molecular Ion [M]+ (m/z) | MS/MS Fragment Ions (m/z) | Identification |
|---|---|---|---|---|
| Peel | ||||
| 1 | 7.98 | 611 | 449, 287 | Cyanidin + 2 hexoses |
| 2 | 8.62 | 465 | 303 | Delphinidin 3-glucoside |
| 3 | 9.27 | 595 | 433, 271 | Pelargonidin + 2 hexoses |
| 4 | 9.96 | 449 | 287 | Cyanidin 3-glucoside |
| 5 | 11.37 | 433 | 271 | Pelargonidin 3-glucoside |
| 6 | 12.65 | 419 | 287 | Cyanidin + arabinose or Cyanidin + xylose |
| Mesocarp | ||||
| 1 | 9.97 | 449 | 287 | Cyanidin 3-glucoside |
| 2 | 11.41 | 433 | 271 | Pelargonidin 3-glucoside |
| Juice | ||||
| 1 | 6.66 | 627 | 465, 303 | Delphinidin + 2 hexoses |
| 2 | 8.00 | 611 | 449, 287 | Cyanidin + 2 hexoses |
| 3 | 8.65 | 465 | 303 | Delphinidin 3-glucoside |
| 4 | 9.28 | 595 | 433, 271 | Pelargonidin + 2 hexoses |
| 5 | 10.00 | 449 | 287 | Cyanidin 3-glucoside |
| 6 | 11.40 | 433 | 271 | Pelargonidin 3-glucoside |
| 7 | 12.68 | 419 | 287 | Cyanidin + arabinose or Cyanidin + xylose |
| Anthocyanin | Water Extracts | Ethanol Extracts | ||
|---|---|---|---|---|
| Peel (µg/g DM) | Juice (µg/g DM) | Peel (µg/g DM) | Juice (µg/g DM) | |
| Del-3,5-diGly | n.d. | 22.07 ± 0.09 a1 | n.d. | 16.2 ± 0.1 b1 |
| Cy-3,5-diGly | 378.3 ± 0.1 a2 | 31.1 ± 0.1 b2 | 23.45 ± 0.02 b2 | 28.9 ± 0.3 b2 |
| Del-3-Gly | 9.03 ± 0.01 a3 | 7.10 ± 0.04 a3 | 9.83 ± 0.04 a3 | 7.49 ± 0.05 a3 |
| Pel-3,5-diGly | 270.88 ± 0.02 a4 | 1.35 ± 0.03 b4 | 23.51 ± 0.03 a4 | 0.80 ± 0.03 b4 |
| Cy-3-Gly | 1029.0 ± 0.3 a5 | 21.52 ± 0.03 bc5 | 127.13 ± 0.08 b5 | 23.0 ± 0.1 bc5 |
| Pel-3-Gly | 490.27 ± 0.03 a6 | 1.08 ± 0.01 bc6 | 58.84 ± 0.01 b6 | 1.03 ± 0.05 bc6 |
| Sum | 2177.5 ± 0.3 | 84.3 ± 0.1 | 242.76 ± 0.01 | 77.4 ± 0.3 |
| Parameter | Initial | 3 Months Storage | 5 Months Storage |
|---|---|---|---|
| L* | 26.1 ± 0.7 a1 | 19.1 ± 0.6 b1 | 18.1 ± 0.7 b1 |
| a* | 13.0 ± 0.5 a2 | 1.4 ± 0.1 b2 | 1.2 ± 0.2 b2 |
| b* | 3.7 ± 0.2 a3 | 1.0 ± 0.2 b3 | 0.8 ± 0.1 b3 |
| ΔE | 0 | 13.9 | 14.6 |
| ΔC | 0 | 11.9 | 12.2 |
| Parameter | Pomegranate Peels |
|---|---|
| Cellulose (% DM) | 11.0 |
| Hemicellulose (% DM) | 11.6 |
| Lignin (% DM) | 12.2 |
| Extract in 70% ethanol (% DM) | 68.2 |
| Fiber width (µm) | 39.82 |
| Curl (%) | 9.91 |
| Fibrillation (%) | 4.45 |
| Lc(l) ISO (µm) | 112 |
| Parameter | Samples | |
|---|---|---|
| Pomegranate Peels/Cellulose (15/85%) | Cellulose (100%) | |
| Grammage (g/m2) | 63.6 | 65.0 |
| Thickness (um) | 161 | 116 |
| Tensile index (Nm/g) | 41.4 | 53.3 |
| Breaking length (km) | 4.222 | 5.334 |
| Bendtsen roughness (mL/min) | 1677 | 342 |
| ISO whiteness (%) | 41.4 | 77.0 |
| Opacity (%) | 96.8 | 86.6 |
| Tear index (mNm2/g) | 7.35 | 7.85 |
| Burst index (KNm/g) | 2.65 | 3.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skrt, M.; Albreht, A.; Vovk, I.; Constantin, O.E.; Râpeanu, G.; Sežun, M.; Osojnik Črnivec, I.G.; Zalar, U.; Poklar Ulrih, N. Extraction of Polyphenols and Valorization of Fibers from Istrian-Grown Pomegranate (Punica granatum L.). Foods 2022, 11, 2740. https://doi.org/10.3390/foods11182740
Skrt M, Albreht A, Vovk I, Constantin OE, Râpeanu G, Sežun M, Osojnik Črnivec IG, Zalar U, Poklar Ulrih N. Extraction of Polyphenols and Valorization of Fibers from Istrian-Grown Pomegranate (Punica granatum L.). Foods. 2022; 11(18):2740. https://doi.org/10.3390/foods11182740
Chicago/Turabian StyleSkrt, Mihaela, Alen Albreht, Irena Vovk, Oana Emilia Constantin, Gabriela Râpeanu, Mija Sežun, Ilja Gasan Osojnik Črnivec, Uroš Zalar, and Nataša Poklar Ulrih. 2022. "Extraction of Polyphenols and Valorization of Fibers from Istrian-Grown Pomegranate (Punica granatum L.)" Foods 11, no. 18: 2740. https://doi.org/10.3390/foods11182740
APA StyleSkrt, M., Albreht, A., Vovk, I., Constantin, O. E., Râpeanu, G., Sežun, M., Osojnik Črnivec, I. G., Zalar, U., & Poklar Ulrih, N. (2022). Extraction of Polyphenols and Valorization of Fibers from Istrian-Grown Pomegranate (Punica granatum L.). Foods, 11(18), 2740. https://doi.org/10.3390/foods11182740











