Dual Cross-Linked Starch–Borax Double Network Hydrogels with Tough and Self-Healing Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Starch–Borax DC Hydrogels
2.3. Mechanical Properties
2.4. Rheological Measurements
2.5. Characterization
2.6. Self-Healing Assay
2.7. Statistical Analysis
3. Results and Discussion
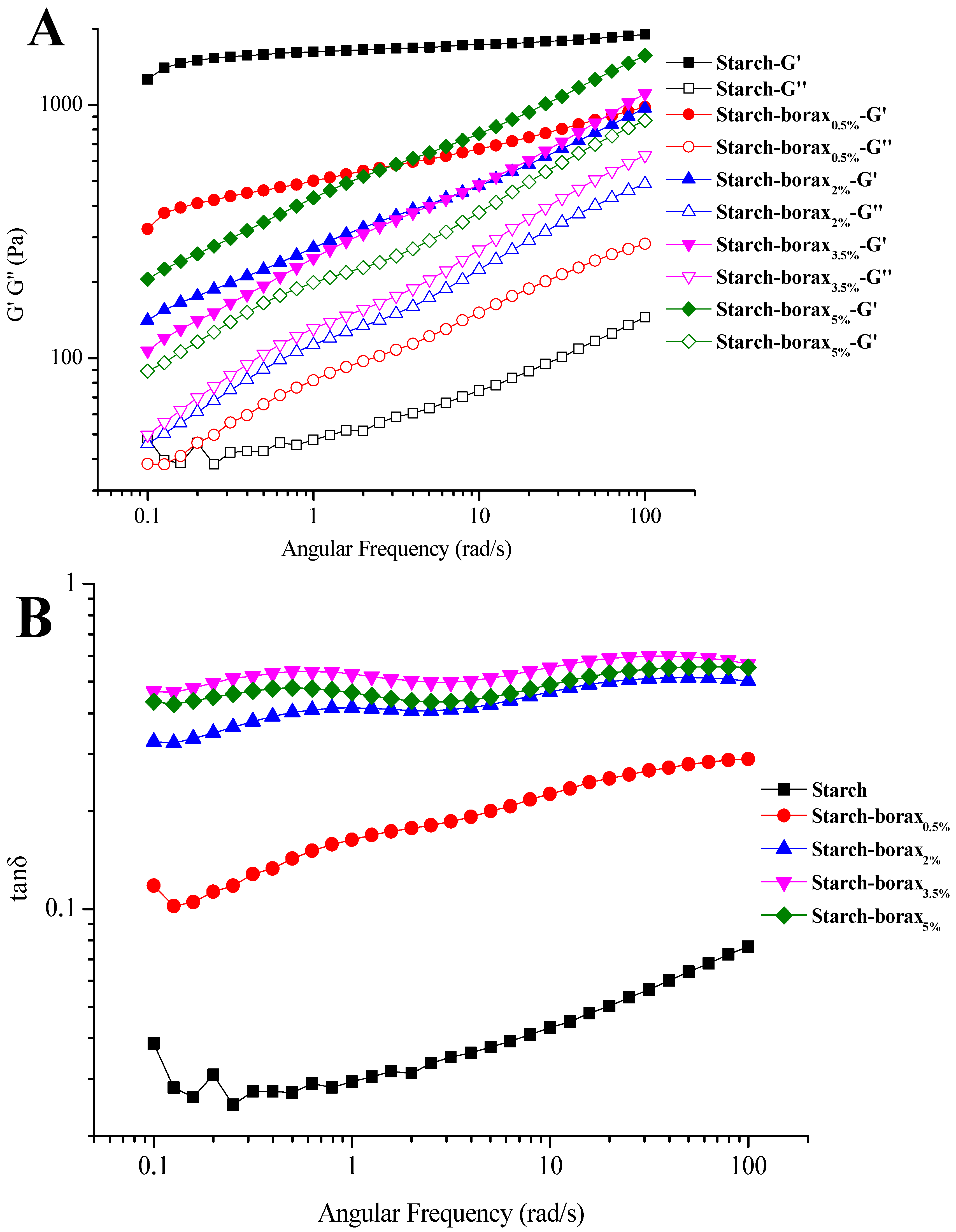
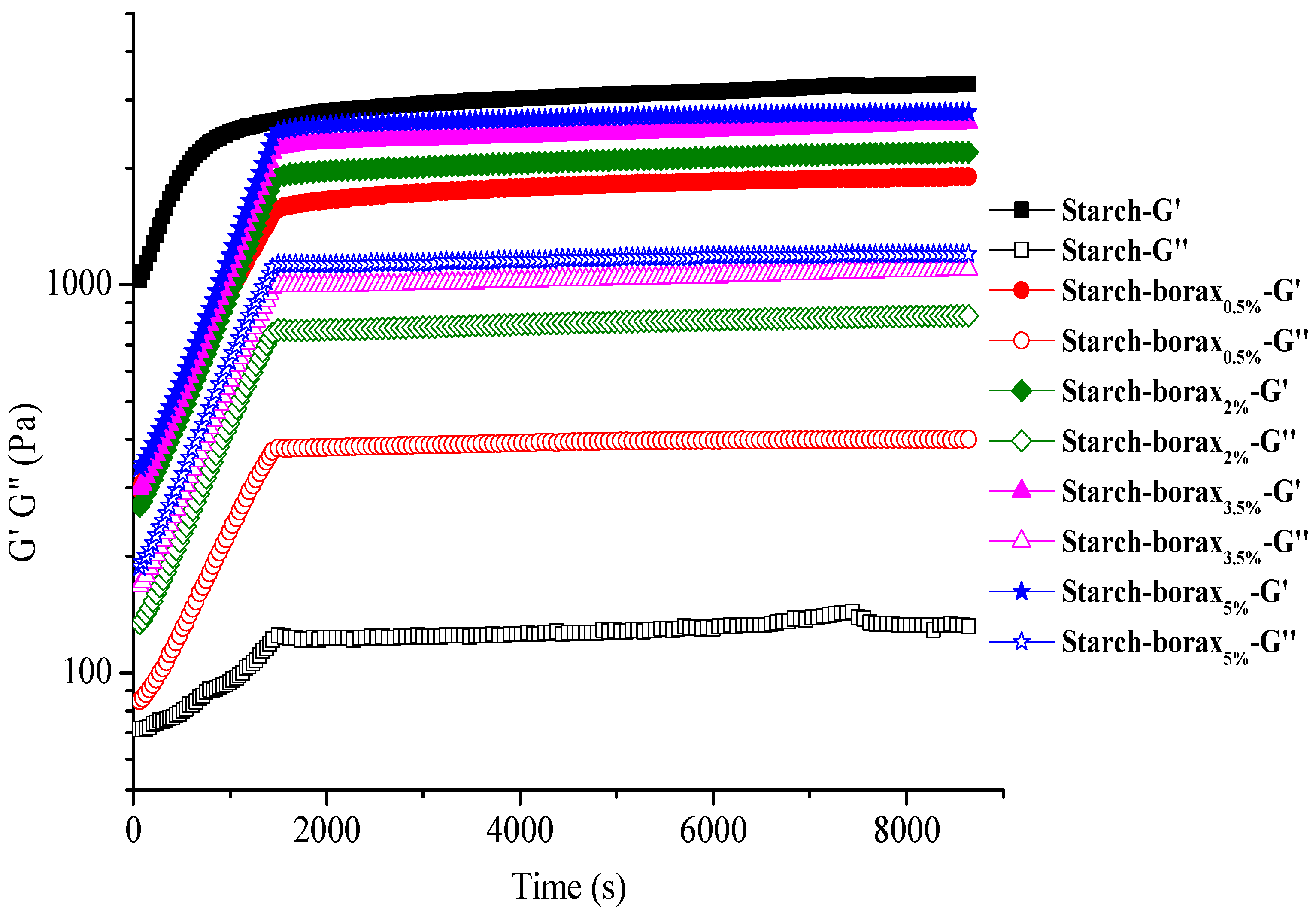
3.1. Rheological Properties
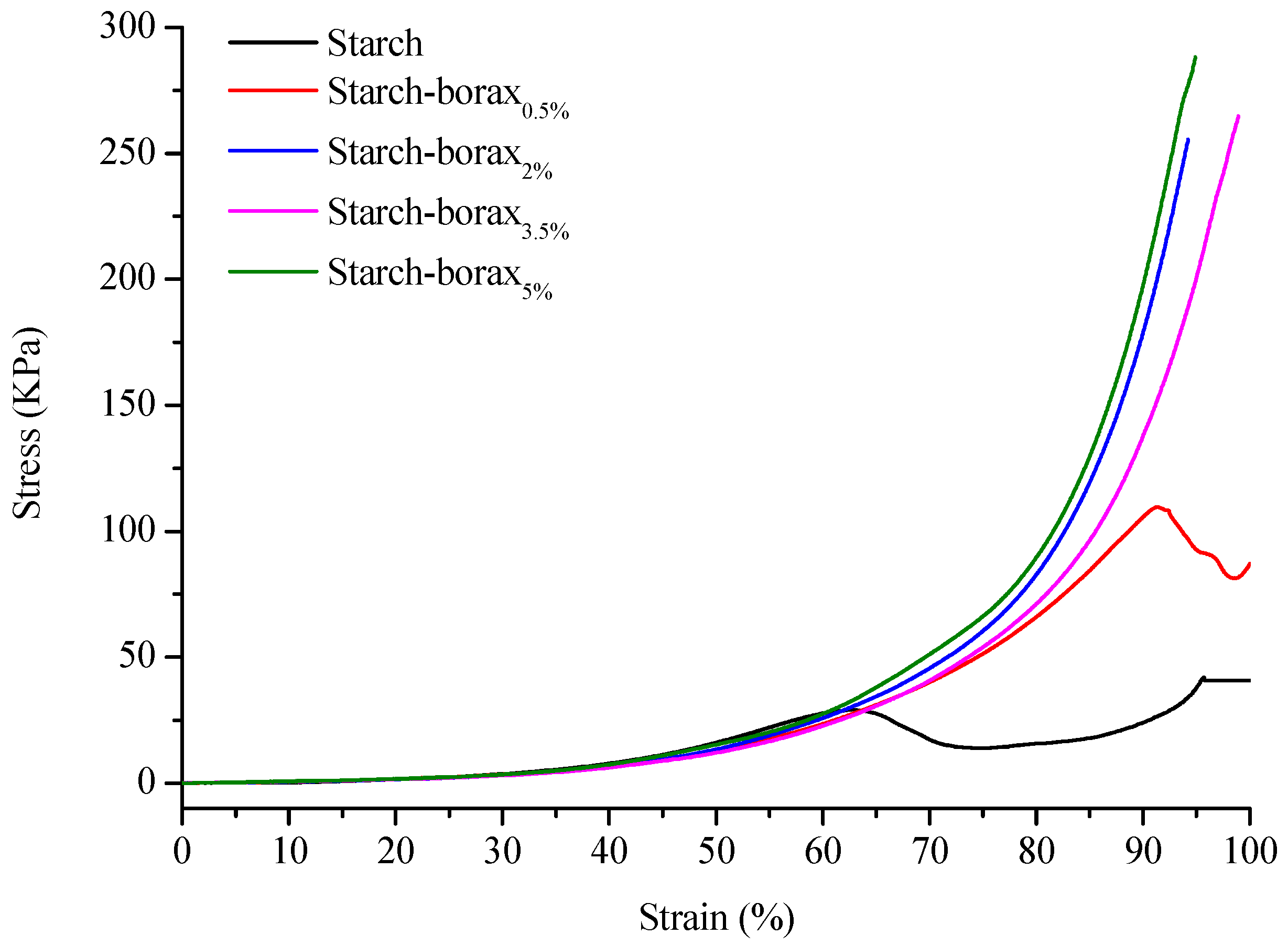
3.2. Textural Properties
3.3. SEM Analysis
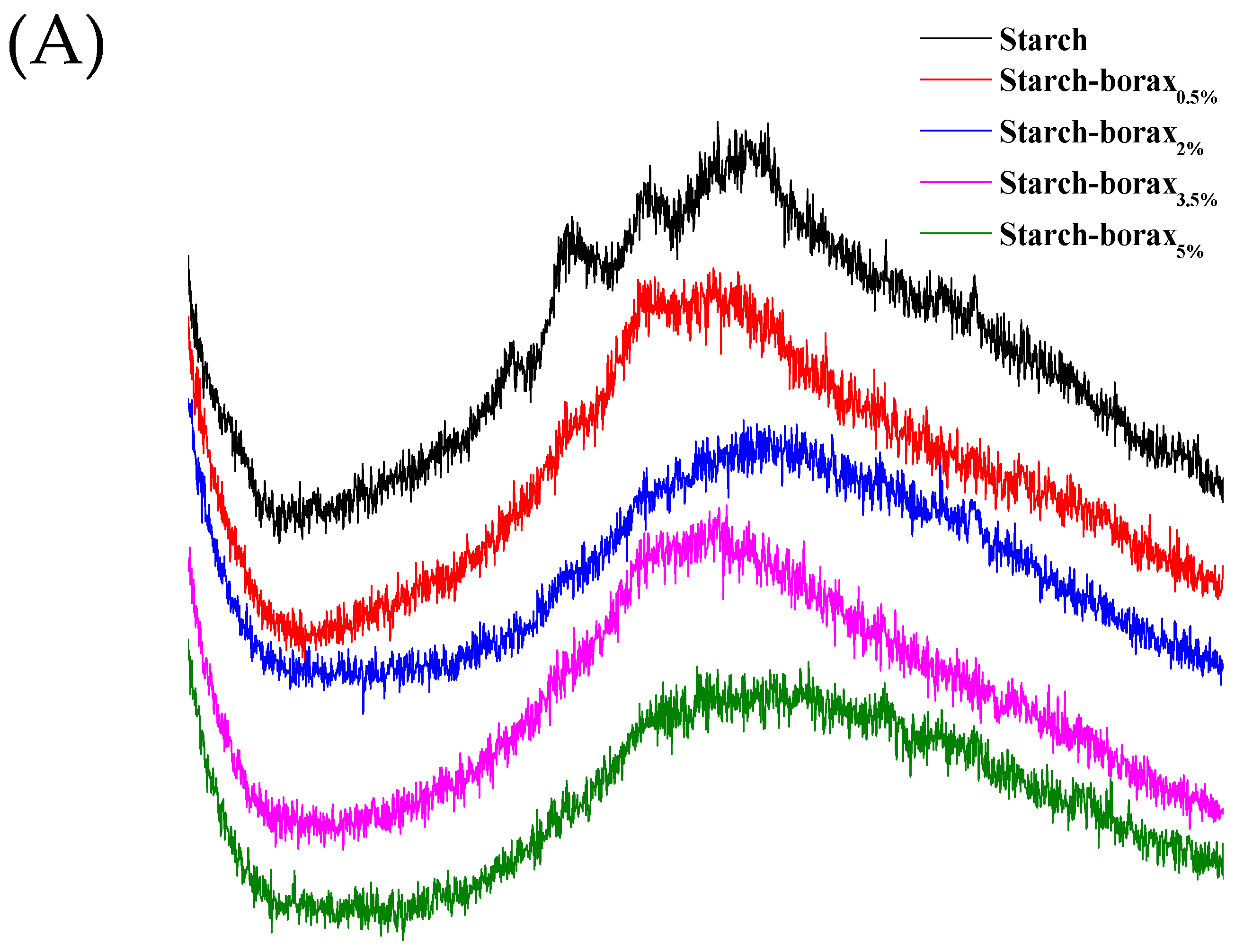
3.4. X-ray Diffraction (XRD)
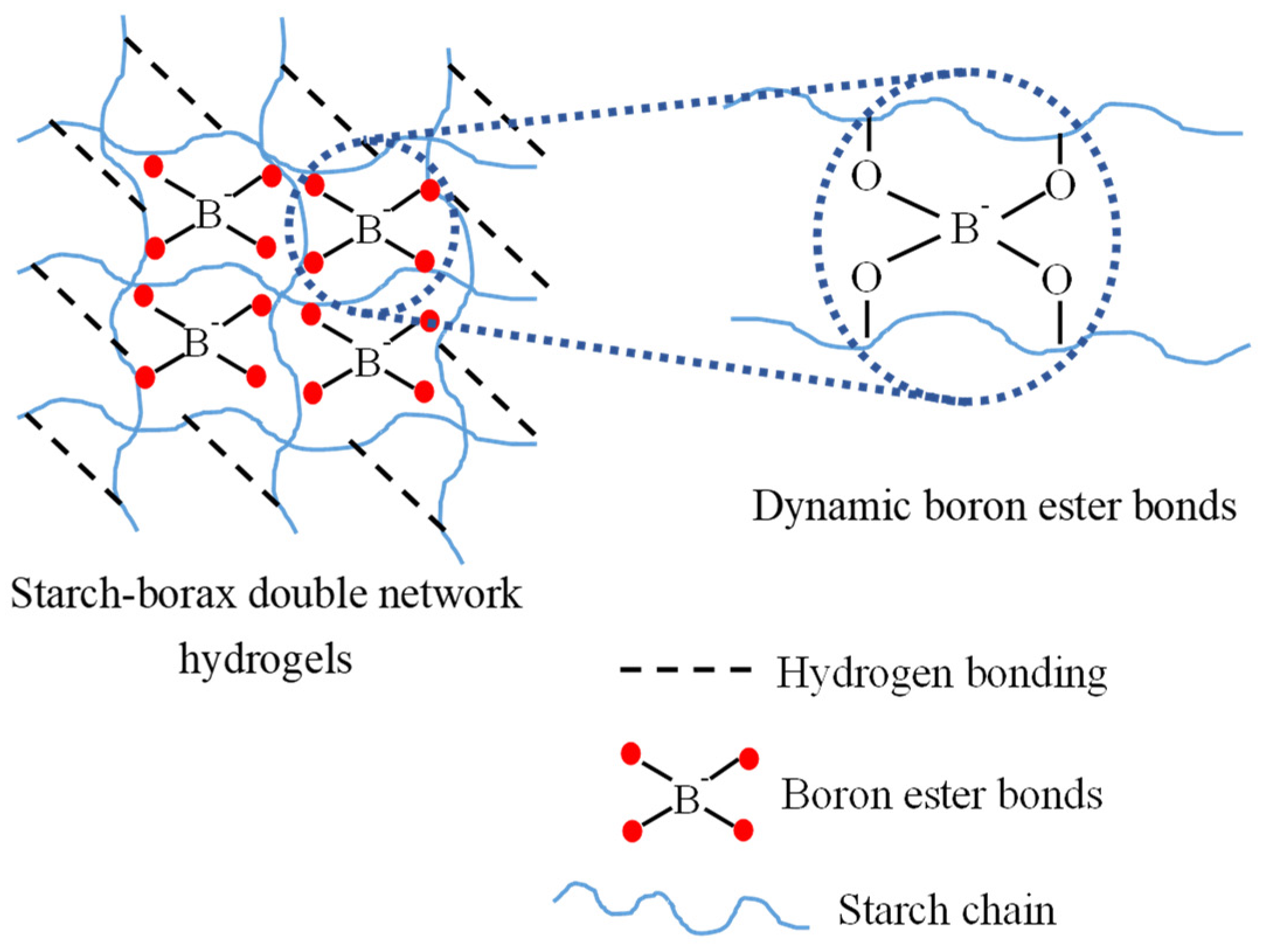
3.5. Fourier Transform Infrared (FTIR) Spectroscopy
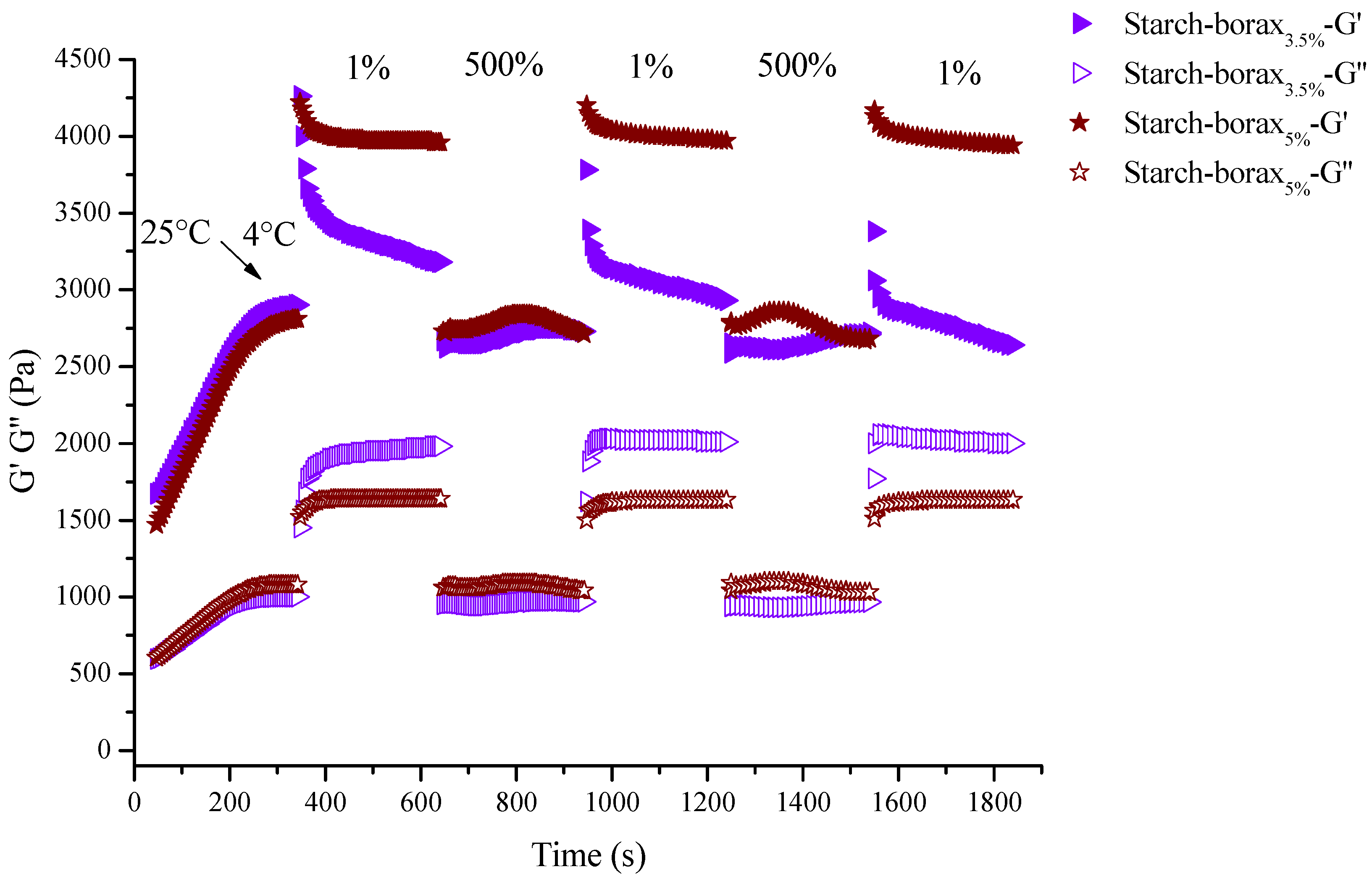
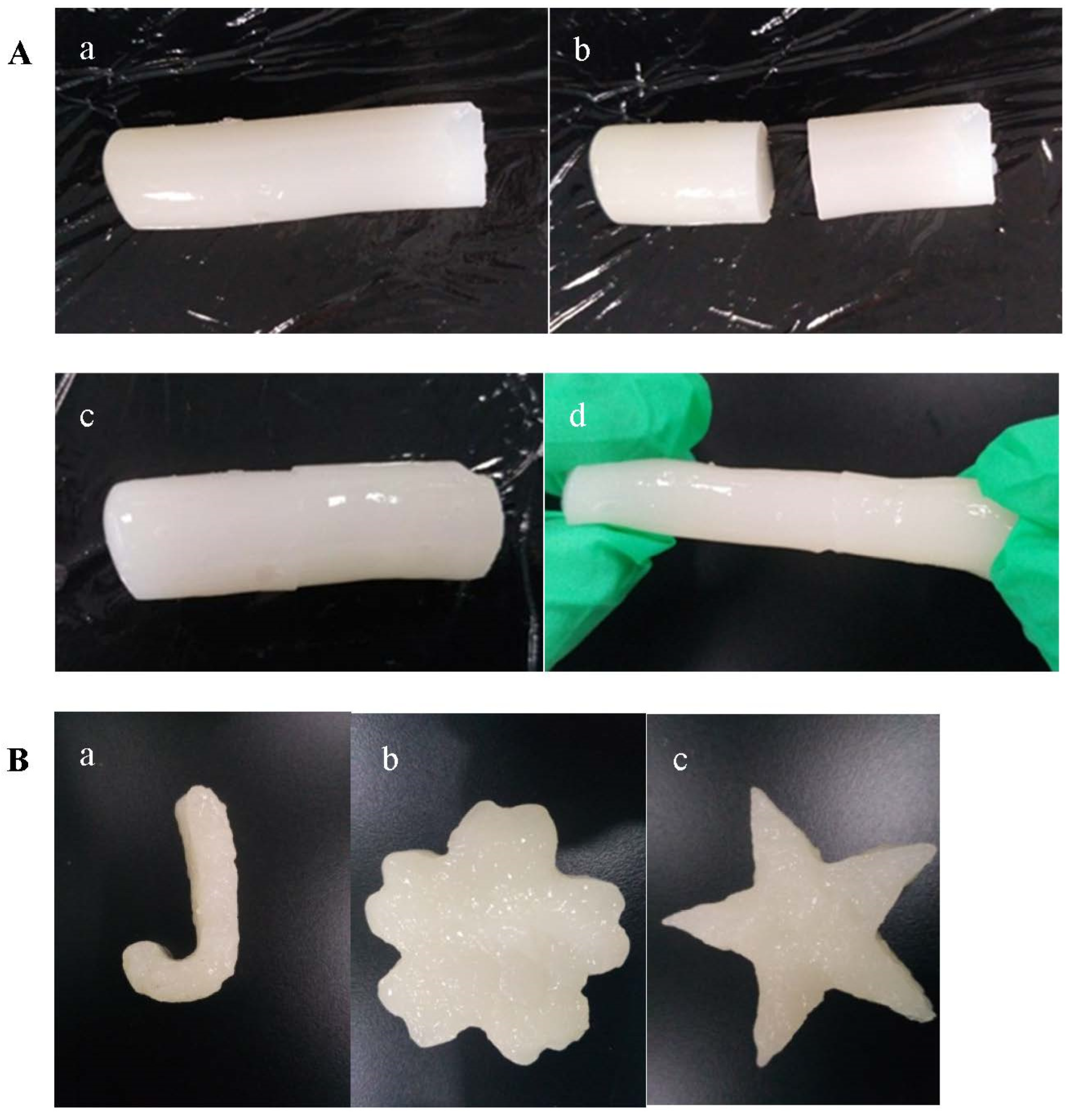
3.6. Self-Healing Behaviors
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, X.F.; Elsayed, I.; Navarathna, C.; Schueneman, G.T.; Hassan, E.I.B. Biohybrid hydrogel and aerogel from self-assembled nanocellulose and nanochitin as a high-efficiency adsorbent for water purification. ACS Appl. Mater. Interfaces 2019, 11, 46714–46725. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.Y.; Zhang, G.P.; Zeng, X.L.; Li, J.H.; Li, G.; Huang, W.P.; Sun, R.; Wong, C.P. High-Strength, Tough, Fatigue Resistant, and Self-Healing Hydrogel Based on Dual Physically Cross-Linked Network. ACS Appl. Mater. Interfaces 2016, 8, 24030–24037. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Huang, M.; Sun, D.; Hou, Y.; Li, Y.B.; Dong, T.S.; Wang, X.H.; Zhang, L.; Yang, W.Z. Dual Physically Cross-Linked kappa-Carrageenan-Based Double Network Hydrogels with Superior Self-Healing Performance for Biomedical Application. ACS Appl. Mater. Interfaces 2018, 10, 37544–37554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somaratne, G.; Nau, F.; Ferrua, M.J.; Singh, J.; Ye, A.Q.; Dupont, D.; Singh, R.P.; Floury, J. In-situ disintegration of egg white gels by pepsin and kinetics of nutrient release followed by time-lapse confocal microscopy. Food Hydrocoll. 2020, 98, 105228. [Google Scholar] [CrossRef]
- Li, X.; Yu, N.; Li, J.; Bai, J.A.; Xu, H.J. A novel “carrier-free” nanofiber co-delivery system with synergistic antitumor effect of Paclitaxel and Tetrandrine through the enhancement of mitochondria apoptosis. ACS Appl. Mater. Interfaces 2020, 12, 10096–10106. [Google Scholar] [CrossRef]
- Fan, W.X.; Yin, J.C.; Yi, C.L.; Xia, Y.Z.; Nie, Z.H.; Sui, K.Y. Nature-inspired sequential shape transformation of energy-patterned hydrogel sheets. ACS Appl. Mater. Interfaces 2020, 12, 4878–4886. [Google Scholar] [CrossRef] [PubMed]
- Olate-Moya, F.; Arens, L.; Wilhelm, M.; Mateos-Timoneda, M.A.; Engel, E.; Palza, H. Chondroinductive alginate-based hydrogels having graphene oxide for 3D printed scaffold fabrication. ACS Appl. Mater. Interfaces 2020, 12, 4343–4357. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.Z.; Li, H.; Ji, N.; Dai, L.; Xiong, L.; Sun, Q.J. Self-healing, stretchable, and freezing-resistant hydroxypropyl starch-based double-network hydrogels. Carbohydr. Polym. 2021, 251, 116982. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Shao, C.Y.; Yang, J. lonically cross-linked silk microfibers/alginate tough composite hydrogels with hierarchical structures. ACS Sustain. Chem. Eng. 2018, 6, 16788–16796. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of hydrogels and aerogels containing nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Ding, B.B.; Gao, H.C.; Song, J.H.; Li, Y.Y.; Zhang, L.N.; Cao, X.D.; Xu, M.; Cai, J. Tough and cell-compatible chitosan physical hydrogels for mouse bone mesenchymal stem cells in vitro. ACS Appl. Mater. Interfaces 2016, 8, 19739–19746. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.J.; Li, L. Recoverable and self-healing double network hydrogel based on kappa-carrageenan. ACS Appl. Mater. Interfaces 2016, 8, 29749–29758. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, A.; Kandi, R.; Rao, C.P. Injectable, self-healing, and stress sustainable hydrogel of BSA as a functional biocompatible material for controlled drug delivery in cancer cells. ACS Sustain. Chem. Eng. 2018, 6, 3321–3330. [Google Scholar] [CrossRef]
- Ge, S.; Ji, N.; Cui, S.; Xie, W.; Li, M.; Li, Y.; Xiong, L.; Sun, Q. Coordination of Covalent Cross-Linked Gelatin Hydrogels via Oxidized Tannic Acid and Ferric Ions with Strong Mechanical Properties. J. Agric. Food Chem. 2019, 67, 11489–11497. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.Q.; Chen, Q.; Zhu, L.; Chen, H.; Wei, D.D.; Chen, F.; Tang, Z.Q.; Yang, J.; Zheng, J. High strength and self-healable gelatin/polyacrylamide double network hydrogels. J. Mater. Chem. B 2017, 5, 7683–7691. [Google Scholar] [CrossRef] [PubMed]
- Lima-Tenorio, M.K.; Tenorio-Neto, E.T.; Guilherme, M.R.; Garcia, F.P.; Nakamura, C.V.; Pineda, E.A.G.; Rubira, A.F. Water transport properties through starch-based hydrogel nanocomposites responding to both pH and a remote magnetic field. Chem. Eng. J. 2015, 259, 620–629. [Google Scholar] [CrossRef]
- Zhu, T.X.; Mao, J.J.; Cheng, Y.; Liu, H.R.; Lv, L.; Ge, M.Z.; Li, S.H.; Huang, J.Y.; Chen, Z.; Li, H.Q.; et al. Recent progress of polysaccharide-based hydrogel interfaces for wound healing and tissue engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef] [Green Version]
- Konstantakos, S.; Marinopoulou, A.; Papaemmanouil, S.; Emmanouilidou, M.; Karamalaki, M.; Kolothas, E.; Saridou, E.; Papastergiadis, E.; Karageorgiou, V. Preparation of model starch complex hydrogels. Food Hydrocoll. 2019, 96, 365–372. [Google Scholar] [CrossRef]
- Ji, N.; Qin, Y.; Li, M.; Xiong, L.; Qiu, L.Z.; Bian, X.L.; Sun, Q.J. Fabrication and characterization of starch nanohydrogels via reverse emulsification and internal gelation. J. Agric. Food Chem. 2018, 66, 9326–9334. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, J.P.; Qiu, C.; Xu, X.M.; Jin, Z.Y. A dual cross-linked strategy to construct moldable hydrogels with high stretchability, good self-recovery, and self-healing capability. J. Agric. Food Chem. 2019, 67, 3966–3980. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ren, Y.; Zhu, Y.; Hao, L.; Chen, Y.; An, G.; Wu, H.; Shi, X.; Mao, C. A rapidly self-healing host-guest supramolecular hydrogel with high mechanical strength and excellent biocompatibility. Angew. Chem. Int. Ed. Engl. 2018, 57, 9008–9012. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, C.J.; Chen, W. Facile access to guar gum based supramolecular hydrogels with rapid self-healing ability and multistimuli responsive gel-sol transitions. J. Agric. Food Chem. 2019, 67, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S. Design and applications of interpenetrating polymer network hydrogels. A review. Chem. Eng. J. 2014, 243, 572–590. [Google Scholar] [CrossRef]
- Liu, C.J.; Lei, F.H.; Li, P.F.; Jiang, J.X.; Wang, K. Borax crosslinked fenugreek galactomannan hydrogel as potential water-retaining agent in agriculture. Carbohydr. Polym. 2020, 236, 116100. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.Q.; Su, S.Y.; Gan, H.B.; Wu, L.J.; Lin, C.H.; Xu, D.Y.; Zhou, H.F.; Lin, X.L.; Qin, Y.L. Facile fabrication and characterization of highly stretchable lignin-based hydroxyethyl cellulose self-healing hydrogel. Carbohydr. Polym. 2019, 223, 115080. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.K.; Pan, Z.; Song, C.Z.; Chen, Y.L.; Qian, X. Locust bean gum/gellan gum double-network hydrogels with superior self-healing and pH-driven shape-memory properties. Soft Matter. 2019, 15, 6171–6179. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Kong, Y.; Wang, X.; Shi, B. Novel one step preparation of a 3D alginate based MOF hydrogel for water treatment. New J. Chem. 2019, 19, 7202–7208. [Google Scholar] [CrossRef]
- Dassanayake, R.S.; Rajakaruna, E.; Abidi, N. Borax-cross-linked guar gum-manganese dioxide composites for oxidative decolorization of methylene blue. J. Nanomater. 2019, 2019, 7232715. [Google Scholar] [CrossRef]
- Spoljaric, S.; Salminen, A.; Luong, N.D.; Seppala, J. Stable, self-healing hydrogels from nanofibrillated cellulose, poly(vinyl alcohol) and borax via reversible crosslinking. Eur. Polym. J. 2014, 56, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Zhai, X.; Gao, S.; Xiang, Y.; Wang, A.; Li, Z.; Cui, B.; Wang, W. Cationized high amylose maize starch films reinforced with borax cross-linked nanocellulose. Int. J. Biol. Macromol. 2021, 193, 1421–1429. [Google Scholar] [CrossRef]
- Lu, H.; Ji, N.; Li, M.; Wang, Y.F.; Xiong, L.; Zhou, L.Y.; Qiu, L.Z.; Bian, X.L.; Sun, C.R.; Sun, Q.J. Preparation of borax cross-linked starch nanoparticles for improvement of mechanical properties of maize starch films. J. Agric. Food Chem. 2019, 67, 2916–2925. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.J.; Li, M.; Ji, N.; Liu, J.; Mu, H.Y.; Xiong, L.; Sun, Q.J. Preparation of a strong gelatin-short linear glucan nanocomposite hydrogel by an in situ self-assembly process. J. Agric. Food Chem. 2018, 66, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Zhuge, J.P.; Li, C.Q.; Jiang, L.B.; Yang, H. Self-healing quadruple shape memory hydrogels based on coordination, borate bonds and temperature with tunable mechanical properties. Iran. Polym. J. 2020, 29, 569–579. [Google Scholar] [CrossRef]
- Lu, B.; Lin, F.; Jiang, X.; Cheng, J.; Lu, Q.; Song, J.; Chen, C.; Huang, B. Engineering. one-pot assembly of microfibrillated cellulose reinforced PVA-borax hydrogels with self-healing and pH responsive properties. ACS Sustain. Chem. Eng. 2017, 5, 948–956. [Google Scholar] [CrossRef]
- Zhang, H.H.; Sun, B.H.; Zhang, S.K.; Zhu, Y.J.; Tian, Y.Q. Inhibition of wheat starch retrogradation by tea derivatives. Carbohydr. Polym. 2015, 134, 413–417. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Le, X.X.; Lu, W.; Jian, Y.K.; Zhang, J.W.; Chen, T. An “off-the-shelf” shape memory hydrogel based on the dynamic borax-diol ester bonds. Macromol. Mater. Eng. 2018, 303, 1800144. [Google Scholar] [CrossRef]
- Rezvan, G.; Pircheraghi, G.; Bagheri, R. Curcumin incorporated PVA-borax dual delivery hydrogels as potential wound dressing materials-Correlation between viscoelastic properties and curcumin release rate. J. Appl. Polym. Sci. 2018, 135, 46734. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, M.E.; Hernandez-Landaverde, M.A.; Delgado, J.M.; Ramirez-Gutierrez, C.F.; Ramirez-Cardona, M.; Millan-Malo, B.M.; Londoño-Restrepo, S.M. Crystalline structures of the main components of starch. Curr. Opin. Food Sci. 2021, 37, 107–111. [Google Scholar] [CrossRef]
- Sethi, S.; Saruchi; Kaith, B.S.; Kaur, M.; Sharma, N.; Kumar, V. Cross-linked xanthan gum-starch hydrogels as promising materials for controlled drug delivery. Cellulose 2020, 27, 4565–4589. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, M.C.; Ma, S.P.; Wang, H.S. Physicochemical characterization of rice, potato, and pea starches, each with different crystalline pattern, when incorporated with Konjac glucomannan. Food Hydrocoll. 2020, 101, 105499. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Borax cross-linked guar gum hydrogels as potential adsorbents for water purification. Carbohydr. Polym. 2017, 168, 274–281. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Fullenkamp, D.E.; Rivera, J.G.; Messersmith, P.B. pH responsive self-healing hydrogels formed by boronate-catechol complexation. Chem. Commun. 2011, 47, 7497–7499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, S.J.; Sun, P.P.; Wu, A.L.; Sun, N.; Sun, L.X.; Dong, B.; Zheng, L.Q. Ultra-fast self-healing PVA organogels based on dynamic covalent chemistry for dye selective adsorption. New J. Chem. 2019, 43, 7701–7707. [Google Scholar] [CrossRef]



| Samples | Hardness (g) | Springiness | Cohesiveness | Adhesiveness (g s) |
|---|---|---|---|---|
| Starch | 342.83 ± 18.75 d | 0.857 ± 0.034 b | 0.515 ± 0.021 d | 176.58 ± 7.54 c |
| Starch–borax0.5% | 611.26 ± 42.22 c | 0.867 ± 0.023 b | 0.523 ± 0.018 d | 445.72 ± 12.93 b |
| Starch–borax2% | 686.63 ± 65.26 a | 0.925 ± 0.010 a | 0.670 ± 0.009 c | 459.89 ± 38.27 ab |
| Starch–borax3.5% | 618.91 ± 30.64 c | 0.931 ± 0.009 a | 0.734 ± 0.009 b | 454.02 ± 22.49 ab |
| Starch–borax5% | 650.28 ± 29.97 b | 0.929 ± 0.002 a | 0.762 ± 0.006 a | 495.60 ± 20.28 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Ji, N.; Li, F.; Qin, Y.; Wang, Y.; Xiong, L.; Sun, Q. Dual Cross-Linked Starch–Borax Double Network Hydrogels with Tough and Self-Healing Properties. Foods 2022, 11, 1315. https://doi.org/10.3390/foods11091315
Chen X, Ji N, Li F, Qin Y, Wang Y, Xiong L, Sun Q. Dual Cross-Linked Starch–Borax Double Network Hydrogels with Tough and Self-Healing Properties. Foods. 2022; 11(9):1315. https://doi.org/10.3390/foods11091315
Chicago/Turabian StyleChen, Xiaoyu, Na Ji, Fang Li, Yang Qin, Yanfei Wang, Liu Xiong, and Qingjie Sun. 2022. "Dual Cross-Linked Starch–Borax Double Network Hydrogels with Tough and Self-Healing Properties" Foods 11, no. 9: 1315. https://doi.org/10.3390/foods11091315





