Biodegradable Chitosan-Based Films as an Alternative to Plastic Packaging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
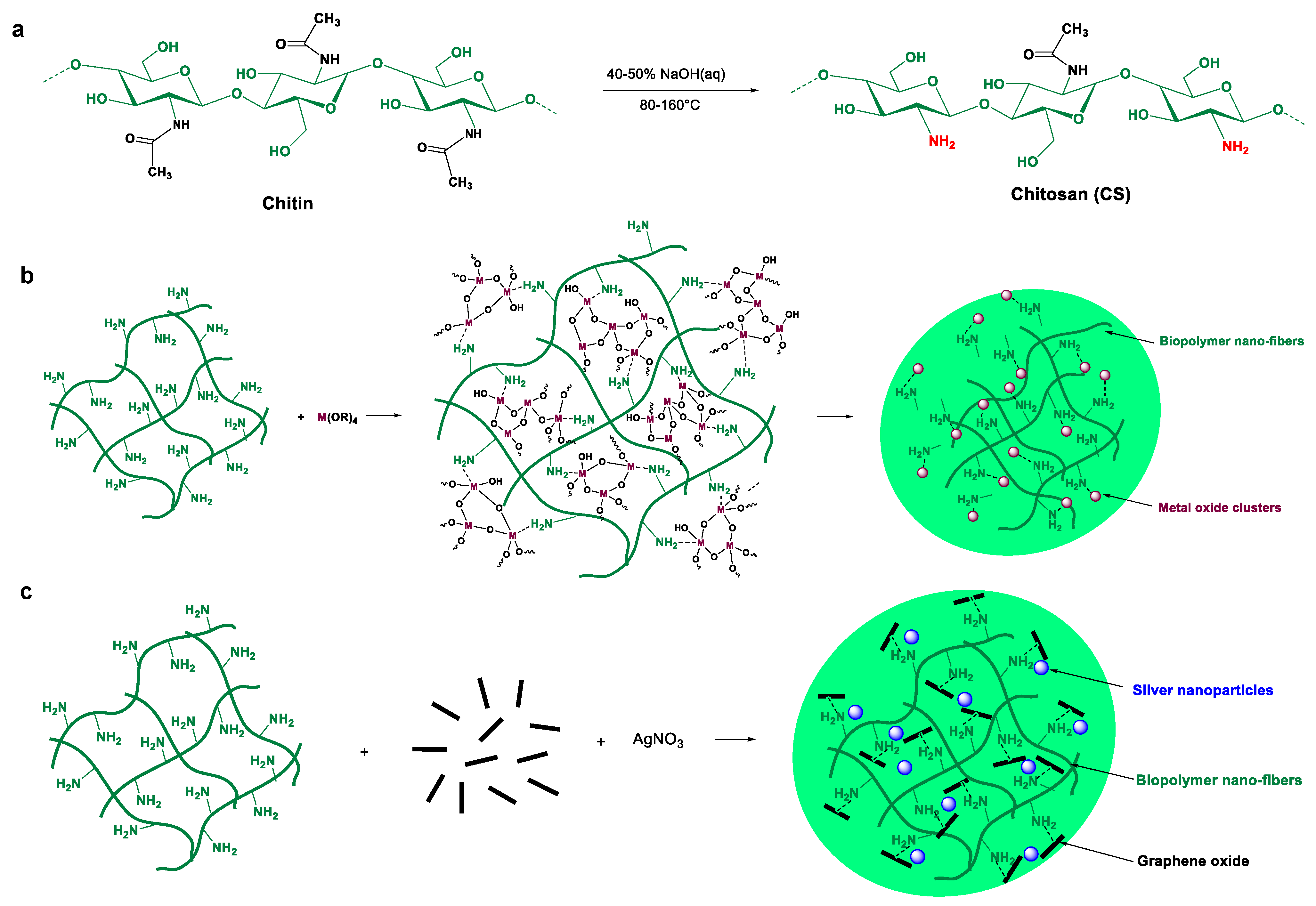
2.2. Preparation of Chitosan–Metal Oxide Films
2.3. Preparation of Chitosan–Graphene Film
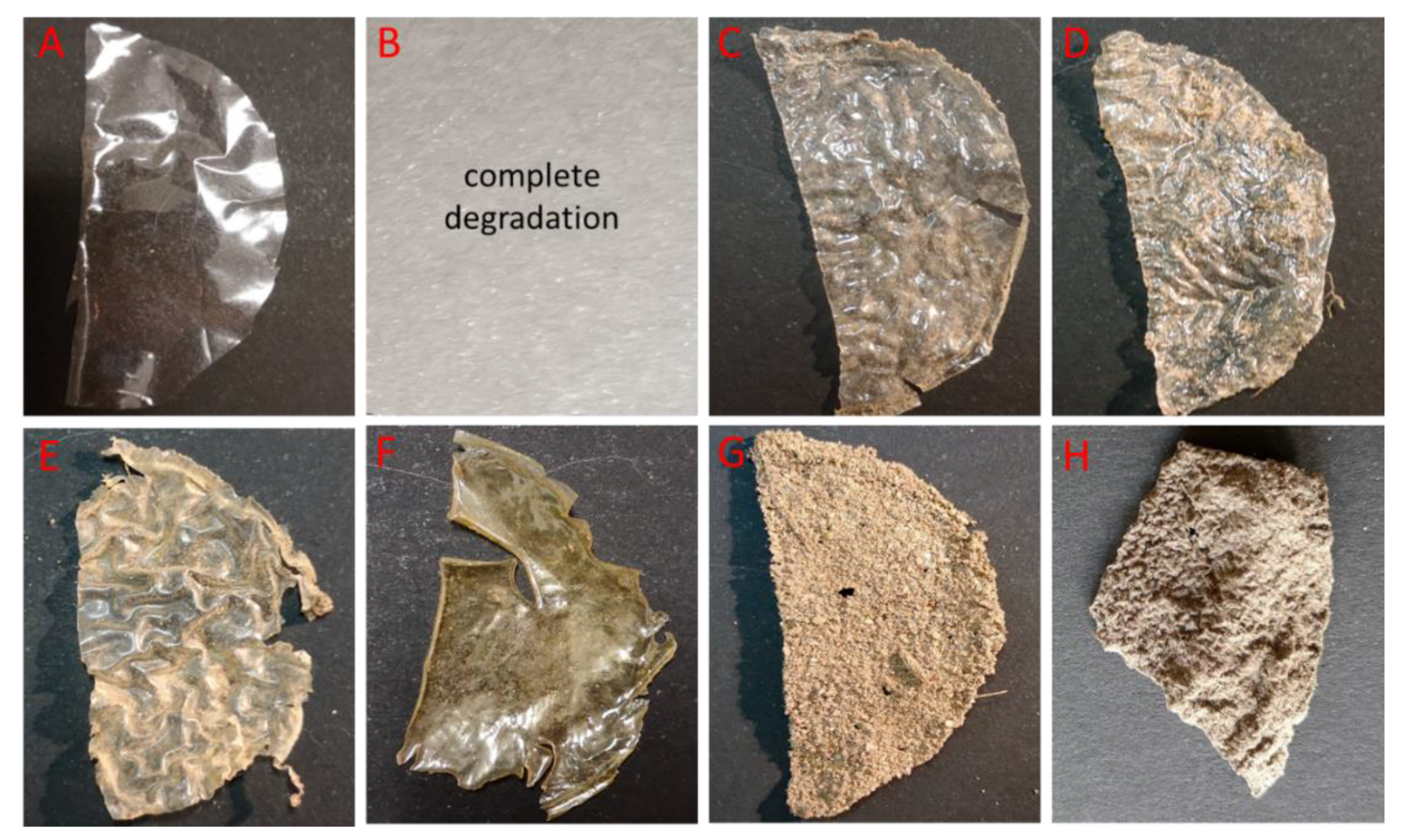
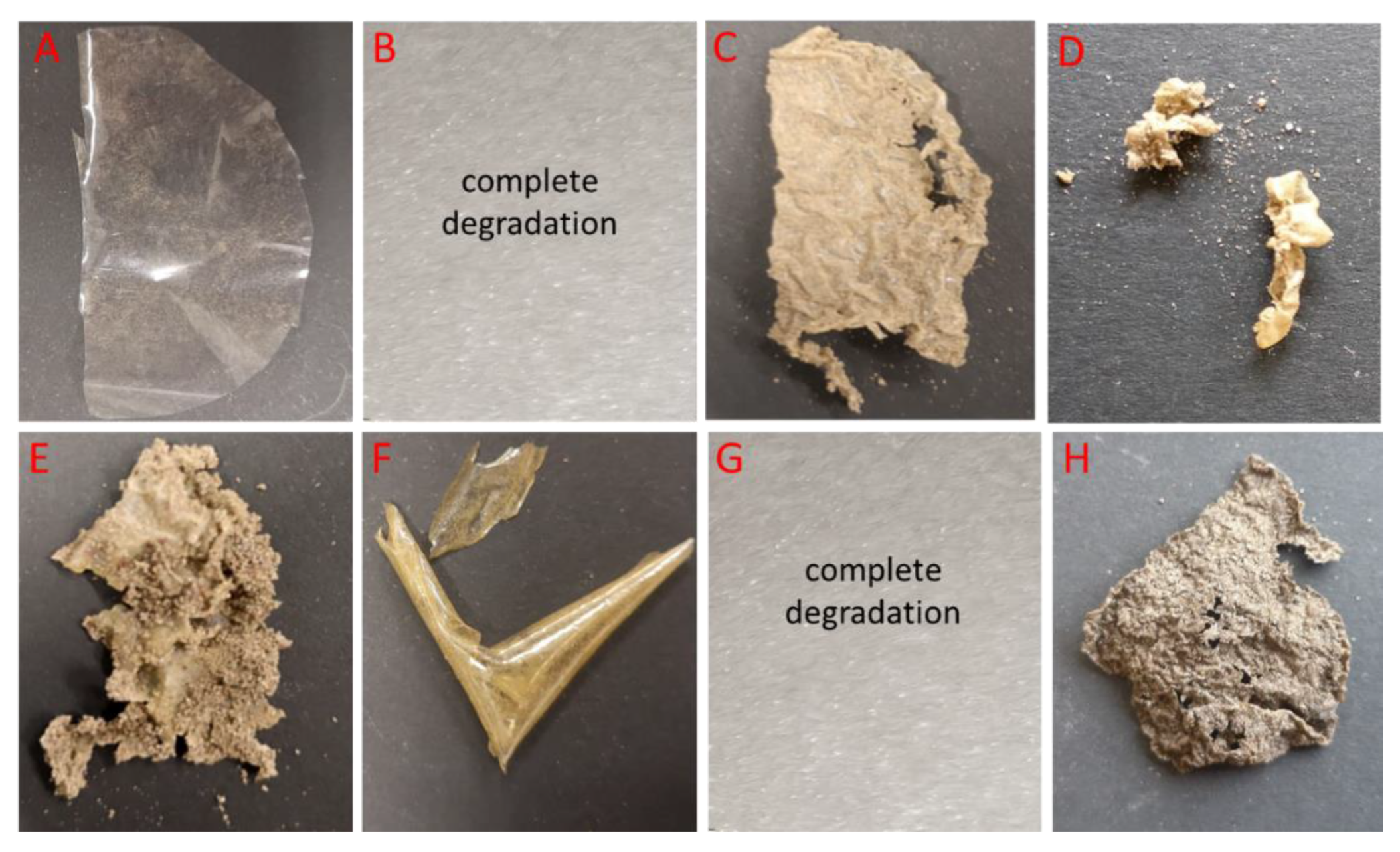
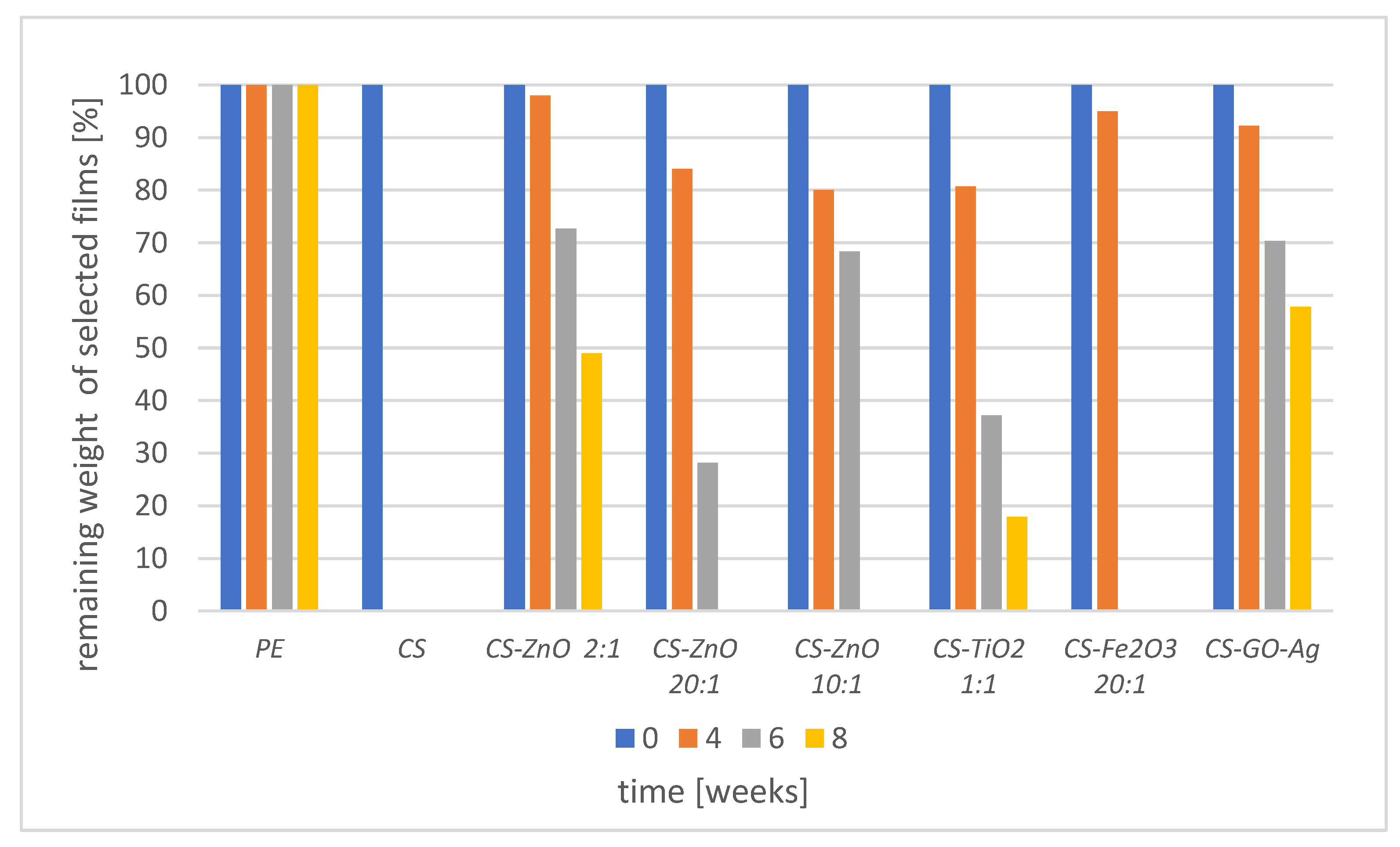
2.4. Biodegradability of Chitosan Films
2.5. Solubility in Water
3. Results and Discussion
3.1. Preparation of Metal (Oxide)-Containing Chitosan Films
3.2. Biodegradation of Chitosan Films in Soil
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sadeghizadeh-Yazdi, J.; Habibi, M.; Kamali, A.A.; Banaei, M. Application of Edible and Biodegradable Starch-Based Films in Food Packaging: A Systematic Review and Meta-Analysis. Curr. Res. Nutr. Food Sci. 2019, 7, 624–637. [Google Scholar] [CrossRef]
- Yao, Z.; Seong, H.J.; Jang, Y.-S. Environmental Toxicity and Decomposition of Polyethylene. Ecotoxicol. Environ. Saf. 2022, 242, 113933. [Google Scholar] [CrossRef]
- Singh, N.; Ogunseitan, O.A.; Wong, M.H.; Tang, Y. Sustainable Materials Alternative to Petrochemical Plastics Pollution: A Review Analysis. Sustain. Horiz. 2022, 2, 100016. [Google Scholar] [CrossRef]
- Madera-Santana, T.J.; Freile-Pelegrín, Y.; Encinas, J.C.; Ríos-Soberanis, C.R.; Quintana-Owen, P. Biocomposites Based on Poly(Lactic Acid) and Seaweed Wastes from Agar Extraction: Evaluation of Physicochemical Properties. J. Appl. Polym. Sci. 2015, 132, 42320. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.; Chen, L.; Niu, P.; Yang, X.; Guo, Y. Preparation and Characterization of Chitosan Film Incorporated with Thinned Young Apple Polyphenols as an Active Packaging Material. Carbohydr. Polym. 2017, 163, 81–91. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for Food Packaging: Recent Advances in Active and Intelligent Films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Mourak, A.; Hajjaji, M.; Alagui, A.; Martin, P.; Joly, N. Effects of Geomaterial-Originated Fillers on Microstructure and Mechanical/Physical Properties of α- and β-Chitosan-Based Films. Molecules 2021, 26, 7514. [Google Scholar] [CrossRef]
- Chabbi, J.; Jennah, O.; Katir, N.; Lahcini, M.; Bousmina, M.; El Kadib, A. Aldehyde-Functionalized Chitosan-Montmorillonite Films as Dynamically-Assembled, Switchable-Chemical Release Bioplastics. Carbohydr. Polym. 2018, 183, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-Based Biodegradable Functional Films for Food Packaging Applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Yilmaz Atay, H. Antibacterial Activity of Chitosan-Based Systems. In Functional Chitosan; Jana, S., Jana, S., Eds.; Springer: Singapore, 2019; pp. 457–489. [Google Scholar] [CrossRef]
- Suo, B.; Li, H.; Wang, Y.; Li, Z.; Pan, Z.; Ai, Z. Effects of ZnO Nanoparticle-Coated Packaging Film on Pork Meat Quality during Cold Storage: ZnO Nanoparticles on Chilled Pork. J. Sci. Food Agric. 2017, 97, 2023–2029. [Google Scholar] [CrossRef] [PubMed]
- Maruthupandy, M.; Rajivgandhi, G.; Muneeswaran, T.; Anand, M.; Quero, F. Highly Efficient Antibacterial Activity of Graphene/Chitosan/Magnetite Nanocomposites against ESBL-Producing Pseudomonas Aeruginosa and Klebsiella Pneumoniae. Colloids Surf. B Biointerfaces 2021, 202, 111690. [Google Scholar] [CrossRef]
- Ediyilyam, S.; George, B.; Shankar, S.S.; Dennis, T.T.; Wacławek, S.; Černík, M.; Padil, V.V.T. Chitosan/Gelatin/Silver Nanoparticles Composites Films for Biodegradable Food Packaging Applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Kaewklin, P. Fabrication and Characterization of Chitosan-Titanium Dioxide Nanocomposite Film as Ethylene Scavenging and Antimicrobial Active Food Packaging. Food Hydrocoll. 2018, 84, 125–134. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of Multifunctional Food Packaging Films Based on Chitosan, TiO2 Nanoparticles and Anthocyanin-Rich Black Plum Peel Extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Y.; Yang, Y.; Qiu, W.; Wang, X.; Liu, B.; Wang, Y.; Sun, G. Synthesis, Characterization, and Antibacterial Activity of Chitosan/TiO 2 Nanocomposite against Xanthomonas Oryzae Pv. Oryzae. Carbohydr. Polym. 2016, 152, 825–831. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.; Rachtanapun, C.; Rachtanapun, P. Biodegradable Rice Starch/Carboxymethyl Chitosan Films with Added Propolis Extract for Potential Use as Active Food Packaging. Polymers 2018, 10, 954. [Google Scholar] [CrossRef]
- Molaee Aghaee, E.; Kamkar, A.; Akhondzadeh Basti, A.; Khanjari, A.; Kontominas, M.G. Effect of Packaging with Chitosan Biodegradable Films Formulated with Garlic Essential Oil (Allium sativum L.) on the Chemical Properties of Chicken Fillet. Int. J. Hydrog. Energy 2015, 8, 379–390. [Google Scholar]
- Adiletta, G.; Zampella, L.; Coletta, C.; Petriccione, M. Chitosan Coating to Preserve the Qualitative Traits and Improve Antioxidant System in Fresh Figs (Ficus carica L.). Agriculture 2019, 9, 84. [Google Scholar] [CrossRef]
- Martínez-González, M.D.C.; Bautista-Baños, S.; Correa-Pacheco, Z.N.; Corona-Rangel, M.L.; Ventura-Aguilar, R.I.; Del Río-García, J.C.; Ramos-García, M.D.L. Effect of Nanostructured Chitosan/Propolis Coatings on the Quality and Antioxidant Capacity of Strawberries During Storage. Coatings 2020, 10, 90. [Google Scholar] [CrossRef]
- Wrońska, N.; Anouar, A.; El Achaby, M.; Zawadzka, K.; Kędzierska, M.; Miłowska, K.; Katir, N.; Draoui, K.; Różalska, S.; Piwoński, I.; et al. Chitosan-Functionalized Graphene Nanocomposite Films: Interfacial Interplay and Biological Activity. Materials 2020, 13, 998. [Google Scholar] [CrossRef]
- Hammi, N.; Wrońska, N.; Katir, N.; Lisowska, K.; Marcotte, N.; Cacciaguerra, T.; Bryszewska, M.; El Kadib, A. Supramolecular Chemistry-Driven Preparation of Nanostructured, Transformable, and Biologically Active Chitosan-Clustered Single, Binary, and Ternary Metal Oxide Bioplastics. ACS Appl. Bio Mater. 2019, 2, 61–69. [Google Scholar] [CrossRef]
- Wrońska, N.; Katir, N.; Miłowska, K.; Hammi, N.; Nowak, M.; Kędzierska, M.; Anouar, A.; Zawadzka, K.; Bryszewska, M.; El Kadib, A.; et al. Antimicrobial Effect of Chitosan Films on Food Spoilage Bacteria. Int. J. Mol. Sci. 2021, 22, 5839. [Google Scholar] [CrossRef]
- Hamodin, A.G.; Elgammal, W.E.; Eid, A.M.; Ibrahim, A.G. Synthesis, Characterization, and Biological Evaluation of New Chitosan Derivative Bearing Diphenyl Pyrazole Moiety. Int. J. Biol. Macromol. 2023, 243, 125180. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Elgammal, W.E.; Dawaba, A.M.; Ibrahim, A.G.; Fouda, A.; Hassan, S.M. A Novel 1,3,4-Thiadiazole Modified Chitosan: Synthesis, Characterization, Antimicrobial Activity, and Release Study from Film Dressings. Appl. Biol. Chem. 2022, 65, 54. [Google Scholar] [CrossRef]
- Chabbi, J.; Aqil, A.; Katir, N.; Vertruyen, B.; Jerôme, C.; Lahcini, M.; El Kadib, A. Aldehyde-Conjugated Chitosan-Graphene Oxide Glucodynamers: Ternary Cooperative Assembly and Controlled Chemical Release. Carbohydr. Polym. 2020, 230, 115634. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Seoane, I.; Manfredi, L.; Cyras, V.; Torre, L.; Fortunati, E.; Puglia, D. Effect of Cellulose Nanocrystals and Bacterial Cellulose on Disintegrability in Composting Conditions of Plasticized PHB Nanocomposites. Polymers 2017, 9, 561. [Google Scholar] [CrossRef]
- Kaya, M.; Khadem, S.; Cakmak, Y.S.; Mujtaba, M.; Ilk, S.; Akyuz, L.; Salaberria, A.M.; Labidi, J.; Abdulqadir, A.H.; Deligöz, E. Antioxidative and Antimicrobial Edible Chitosan Films Blended with Stem, Leaf and Seed Extracts of Pistacia terebinthus for Active Food Packaging. RSC Adv. 2018, 8, 3941–3950. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and Chitosan: Production and Application of Versatile Biomedical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Thadathil, N.; Velappan, S.P. Recent Developments in Chitosanase Research and Its Biotechnological Applications: A Review. Food Chem. 2014, 150, 392–399. [Google Scholar] [CrossRef]
- Lucas, N.; Bienaime, C.; Belloy, C.; Queneudec, M.; Silvestre, F.; Nava-Saucedo, J.-E. Polymer Biodegradation: Mechanisms and Estimation Techniques—A Review. Chemosphere 2008, 73, 429–442. [Google Scholar] [CrossRef]
- Ratte, H.T. Bioaccumulation and Toxicity of Silver Compounds: A Review. Environ. Toxicol. Chem. 1999, 18, 89–108. [Google Scholar] [CrossRef]
- Feng, Y.; Lu, K.; Mao, L.; Guo, X.; Gao, S.; Petersen, E.J. Degradation of 14C-Labeled Few Layer Graphene via Fenton Reaction: Reaction Rates, Characterization of Reaction Products, and Potential Ecological Effects. Water Res. 2015, 84, 49–57. [Google Scholar] [CrossRef]
- Navarro, D.A.; Kah, M.; Losic, D.; Kookana, R.S.; McLaughlin, M.J. Mineralisation and Release of 14C-Graphene Oxide (GO) in Soils. Chemosphere 2020, 238, 124558. [Google Scholar] [CrossRef]
- Huang, C.; Xia, T.; Niu, J.; Yang, Y.; Lin, S.; Wang, X.; Yang, G.; Mao, L.; Xing, B. Transformation of 14C-Labeled Graphene to 14CO2 in the Shoots of a Rice Plant. Angew. Chem. Int. Ed. 2018, 57, 9759–9763. [Google Scholar] [CrossRef]
- Kuo, P.-C.; Sahu, D.; Yu, H.H. Properties and Biodegradability of Chitosan/Nylon 11 Blending Films. Polym. Degrad. Stab. 2006, 91, 3097–3102. [Google Scholar] [CrossRef]
- Akyuz, L.; Kaya, M.; Ilk, S.; Cakmak, Y.S.; Salaberria, A.M.; Labidi, J.; Yılmaz, B.A.; Sargin, I. Effect of Different Animal Fat and Plant Oil Additives on Physicochemical, Mechanical, Antimicrobial and Antioxidant Properties of Chitosan Films. Int. J. Biol. Macromol. 2018, 111, 475–484. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of Chitosan-Based Biodegradable Active Films Using Bio-Waste Enriched with Polyphenol Propolis Extract Envisaging Food Packaging Applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef]
- Chang, X.; Hou, Y.; Liu, Q.; Hu, Z.; Xie, Q.; Shan, Y.; Li, G.; Ding, S. Physicochemical and Antimicrobial Properties of Chitosan Composite Films Incorporated with Glycerol Monolaurate and Nano-TiO2. Food Hydrocoll. 2021, 119, 106846. [Google Scholar] [CrossRef]
- Maron, P.-A.; Mougel, C.; Ranjard, L. Soil Microbial Diversity: Methodological Strategy, Spatial Overview and Functional Interest. Comptes Rendus Biol. 2011, 334, 403–411. [Google Scholar] [CrossRef]
- Sinha, S.; Tripathi, P.; Chand, S. A New Bifunctional Chitosanase Enzyme from Streptomyces Sp. and Its Application in Production of Antioxidant Chitooligosaccharides. Appl. Biochem. Biotechnol. 2012, 167, 1029–1039. [Google Scholar] [CrossRef]
- Swiontek Brzezinska, M.; Jankiewicz, U.; Burkowska, A.; Walczak, M. Chitinolytic Microorganisms and Their Possible Application in Environmental Protection. Curr. Microbiol. 2014, 68, 71–81. [Google Scholar] [CrossRef]
- Bagheri-Khoulenjani, S.; Taghizadeh, S.M.; Mirzadeh, H. An Investigation on the Short-Term Biodegradability of Chitosan with Various Molecular Weights and Degrees of Deacetylation. Carbohydr. Polym. 2009, 78, 773–778. [Google Scholar] [CrossRef]
- Zhang, H.; Neau, S.H. In Vitro Degradation of Chitosan by a Commercial Enzyme Preparation: Effect of Molecular Weight and Degree of Deacetylation. Biomaterials 2001, 22, 1653–1658. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-Based Biomaterials for Tissue Engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Nakashima, T.; Matsuo, M.; Bin, Y.; Nakano, Y.; Kobayashi, T.; Komemushi, S.; Sakagami, Y. Mechanical Properties and Antibacterial Efficacy of Chitosan Films. Biocontrol Sci. 2006, 11, 27–36. [Google Scholar] [CrossRef]
- Makarios-Laham, I.; Lee, T.-C. Biodegradability of Chitin- and Chitosan-Containing Films in Soil Environment. J. Environ. Polym. Degr. 1995, 3, 31–36. [Google Scholar] [CrossRef]
- Pavoni, J.M.F.; Luchese, C.L.; Tessaro, I.C. Impact of Acid Type for Chitosan Dissolution on the Characteristics and Biodegradability of Cornstarch/Chitosan Based Films. Int. J. Biol. Macromol. 2019, 138, 693–703. [Google Scholar] [CrossRef]
- Giannakas, A.; Vlacha, M.; Salmas, C.; Leontiou, A.; Katapodis, P.; Stamatis, H.; Barkoula, N.-M.; Ladavos, A. Preparation, Characterization, Mechanical, Barrier and Antimicrobial Properties of Chitosan/PVOH/Clay Nanocomposites. Carbohydr. Polym. 2016, 140, 408–415. [Google Scholar] [CrossRef]


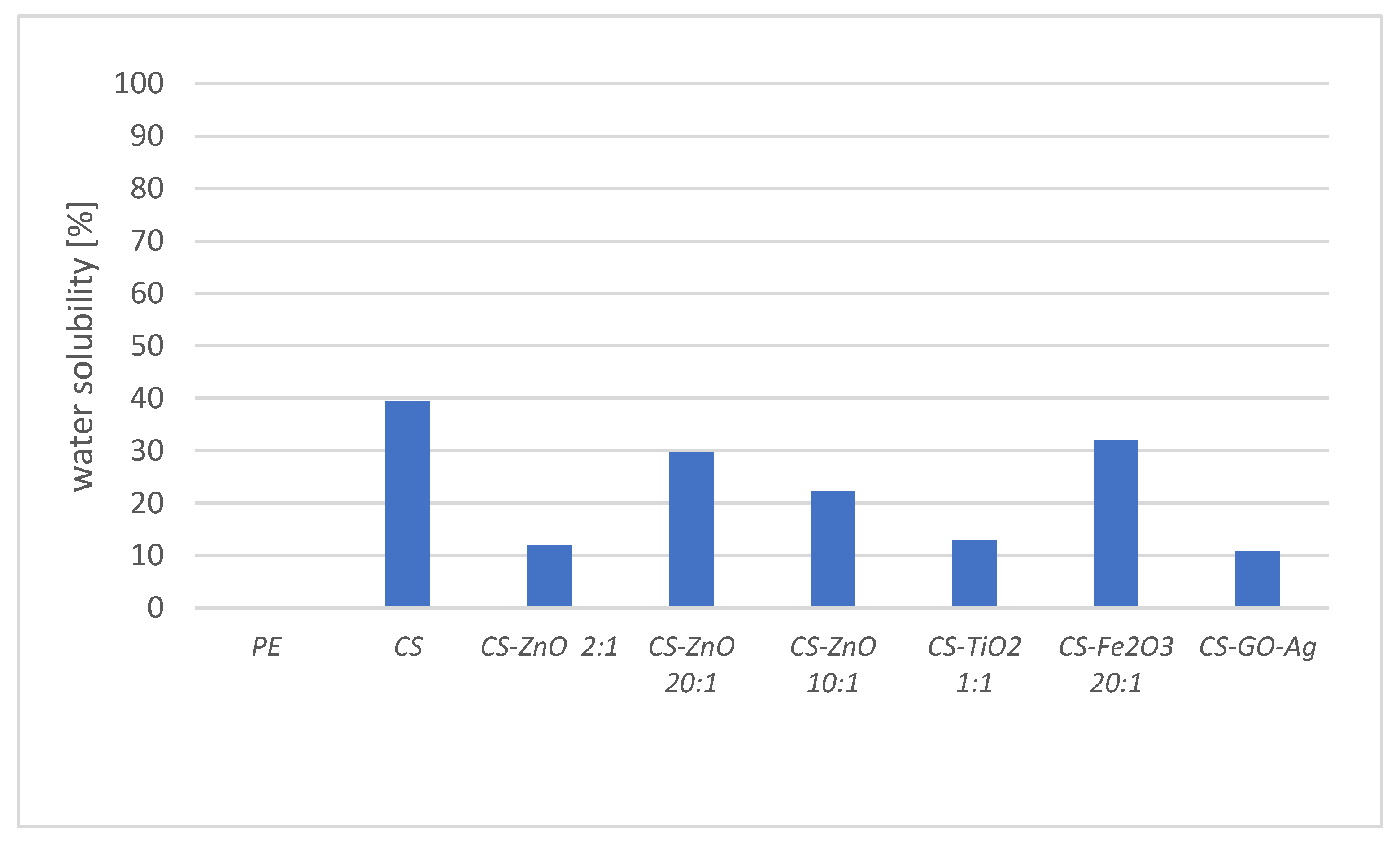
| Type of Chitosan Film | Properties | References |
|---|---|---|
| CS/ZnO | improvement of the quality of pork meat during cold storage | [11] |
| (CS)/Fe3O4, (Gr)/CS/Fe3O4 | antimicrobial activity | [12] |
| CS/GEL/AgNPs | antimicrobial activity | [13] |
| CS/TiO2 NPs | exposure to UV light, triggered higher antimicrobial activity | [14] |
| CS/TiO2 NPs | antimicrobial, antioxidant, and ethylene scavenging properties | [15] |
| CS/TiO2 NPs | good antimicrobial activity under dark and light conditions | [16] |
| RS/CMCh | enhanced antioxidant and antimicrobial properties | [17] |
| CS/garlic essential oil | antioxidant and antimicrobial activity | [18] |
| CS | antioxidant system of figs during storage | [19] |
| CS/propolis coatings | antioxidant system of strawberries during storage | [20] |
| CS/GO films | antimicrobial activity | [21] |
| CS/metal oxide films CS/GO films | antimicrobial activity | [22,23] |
| DPPS-CH (chitosan bearing pyrazole derivative) | antimicrobial activity | [24] |
| Cs-EATT Cs-BATT (chitosan bearing 1,3,4-thiadiazole derivative) | antimicrobial activity | [25] |
| Sample Code | Metal Precursors | Molar Ratio NH2: Metal Precursor |
|---|---|---|
| PE | - | |
| CS | - | |
| CS-ZnO 2:1 | Zinc acetate | 2:1 |
| CS-ZnO 10:1 | Zinc acetate | 10:1 |
| CS-ZnO 20:1 | Zinc acetate | 20:1 |
| CS-TiO2 1:1 | Titanium diisopropoxide bis(acac) | 1:1 |
| CS-Fe2O3 20:1 | Iron(III) acetylacetonate | 20:1 |
| Sample Code | Functionalized Graphene Fillers | Metal Precursors |
|---|---|---|
| CS-GO-Ag | GO (3 wt%) | Silver nitrate (3%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrońska, N.; Katir, N.; Nowak-Lange, M.; El Kadib, A.; Lisowska, K. Biodegradable Chitosan-Based Films as an Alternative to Plastic Packaging. Foods 2023, 12, 3519. https://doi.org/10.3390/foods12183519
Wrońska N, Katir N, Nowak-Lange M, El Kadib A, Lisowska K. Biodegradable Chitosan-Based Films as an Alternative to Plastic Packaging. Foods. 2023; 12(18):3519. https://doi.org/10.3390/foods12183519
Chicago/Turabian StyleWrońska, Natalia, Nadia Katir, Marta Nowak-Lange, Abdelkrim El Kadib, and Katarzyna Lisowska. 2023. "Biodegradable Chitosan-Based Films as an Alternative to Plastic Packaging" Foods 12, no. 18: 3519. https://doi.org/10.3390/foods12183519
APA StyleWrońska, N., Katir, N., Nowak-Lange, M., El Kadib, A., & Lisowska, K. (2023). Biodegradable Chitosan-Based Films as an Alternative to Plastic Packaging. Foods, 12(18), 3519. https://doi.org/10.3390/foods12183519







