Agronomic and Genetic Strategies to Enhance Selenium Accumulation in Crops and Their Influence on Quality
Abstract
:1. Introduction
2. Agronomic Strategies for Se Biofortification
2.1. Se Fertilization Strategies
2.1.1. Effects of Se Species and Dosages on Crop Se Accumulation
2.1.2. Effect of Crop Fertilization Site and Time on Se Accumulation
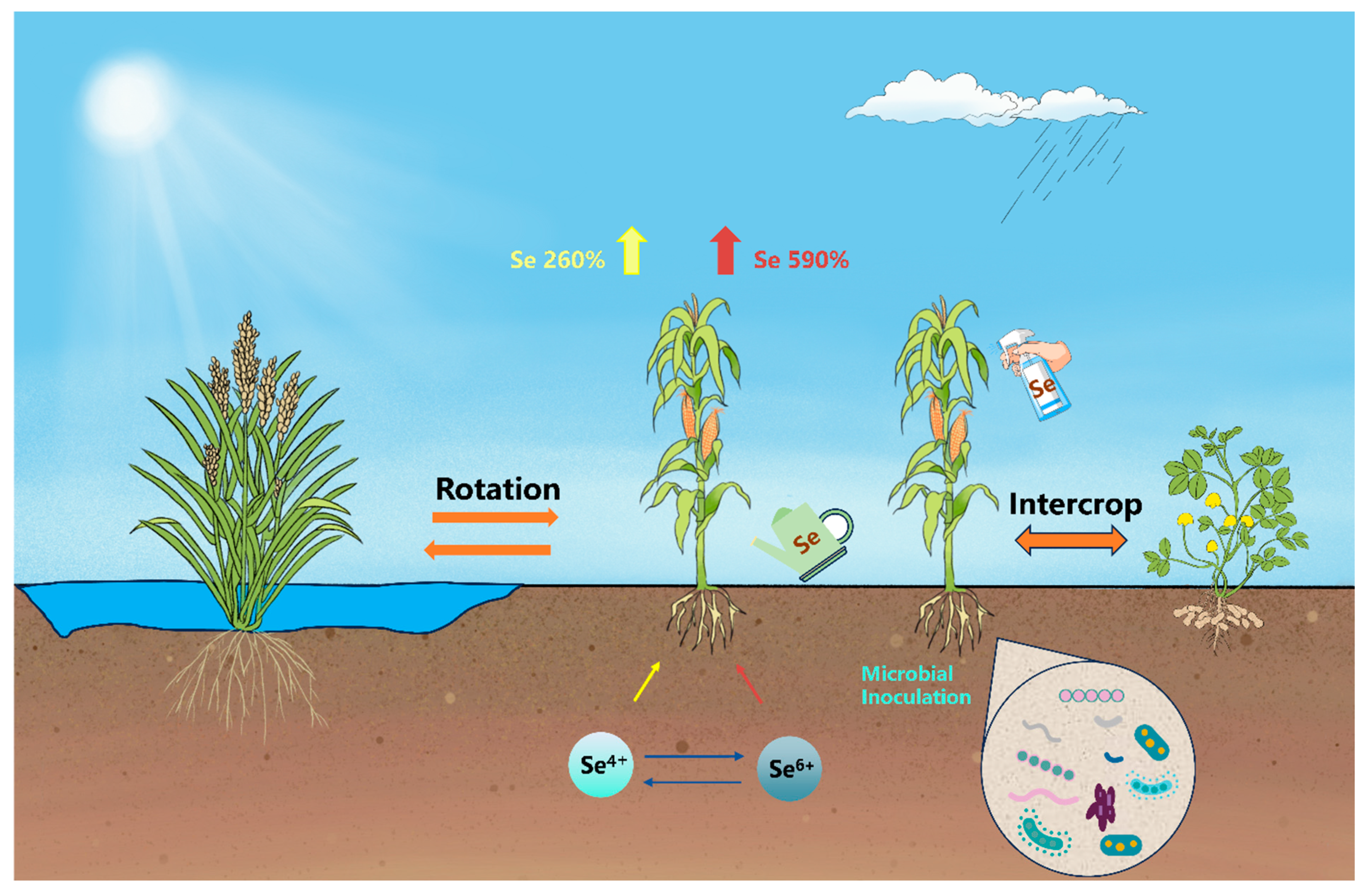
2.2. Agronomic Management Strategies
2.2.1. Crop Rotation and Intercropping
2.2.2. Soil and Water Management
2.2.3. Microbial-Assisted Biofortification
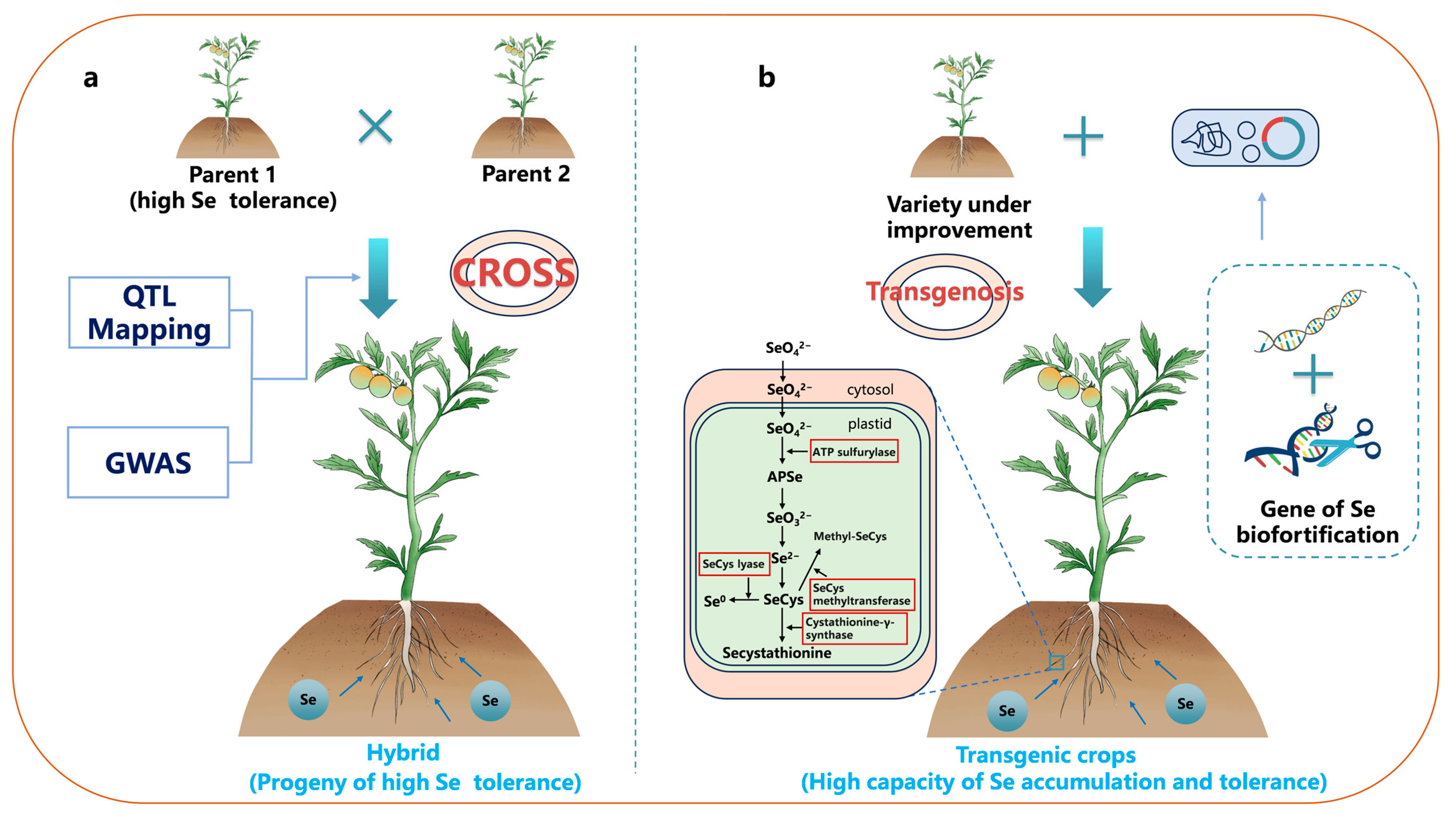
3. Genetic Strategies for Se Biofortification
3.1. Breeding Techniques for Gene Improvement in Crops
3.2. Transgenic Technology Expanding the Traditional Crop Gene Pool
4. Impact of Se Biofortification on Crop Growth and Quality
4.1. Influence of Se Biofortification on Crop Growth
4.1.1. Influence of Se Biofortification on Growth Status and Antioxidant Activity
| Species | Applications | Growth Parameters | Saccharides | Proteins | Fats | Antioxidant Activity | Antioxidant Enzyme | References |
|---|---|---|---|---|---|---|---|---|
| Triticum aestivum, L. cv. Baegjoongmil microgreens | Sodium selenite 0.125–1.0 mg L−1—hydroponic | Yield ↓* Microgreen weight ↓ Microgreen height ↓ | – | – | – | NSA ↑0.25 mg L−1 ABTS NS; DPPH NS | SOD ↑0.125, 1.00 mg L−1 | [169] |
| Oryza sativa L.’Ariete’ grains | Sodium selenate 30–300 g ha−1—foliar | – | Total Sugars ↑60–300 g ha−1 | ↑120–300 g ha−1 | ↑180 g ha−1 | – | – | [25] |
| Ipomoea aquatica Forsk. ‘XGDB’ | Selenite 0.2 mg L−1—Foliar | Biomass NS | – | NS | – | MDA NS | SOD NS; POD NS; CAT NS; | [29] |
| Triticum aestivum L. Shoots ‘BRS 264′ | Sodium selenate 12–120 g ha−1 Se—foliar | Yield ↑ | Total Soluble Sugars ↑ Sucrose ↑21, 120 g ha−1 | NS | – | MDA NS H2O2 NS | APX ↑12–38 g ha−1; CAT NS; SOD NS | [78] |
| Solanum tuberosum L. Tubers | Sodium selenite 0.75–5.0 mg kg−1—soil | Production ↑0.75 mg kg−1 ↓3–5 mg kg−1 | – | – | – | MDA ↑3.0–5.0 mg kg−1 H2O2 ↓ | CAT ↑1.5–3.0 mg kg−1 SOD ↑1.5–5.0 mg kg−1 | [162] |
| Coffea arabica red Itucaí Leaves | Sodium selenate 10–160 mg L−1—foliar | Yield ↑10–40, 120 mg L−1 | – | – | – | MDA ↓; H2O2 ↓20–80 mg L−1, ↑160 mg L−1 | CAT ↑20, 120–160 mg L−1 APX ↑20–160 mg L−1 SOD ↑20–160 mg L−1 | [164] |
4.1.2. Influence of Se Biofortification on Small-Molecule Antioxidant Contents in Crops
4.2. Influence of Se Biofortification in Crops on Element Accumulation
4.2.1. Influence of Se Biofortification on Human Health-Related Mineral Elements
4.2.2. Influence of Se Biofortification on Toxic Heavy Metals
5. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schwarz, K.; Foltz, C.M. Selenium as an integral part of factor 3 against dietary necrotic liver degeneration. J. Am. Chem. Soc. 1957, 79, 200–214. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Zhao, F.-J. Strategies for increasing the selenium content of wheat. J. Cereal Sci. 2007, 41, 282–292. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 371, 1256–1268. [Google Scholar] [CrossRef] [PubMed]
- Badawy, S.A.; Zayed, B.A.; Bassiouni, S.; Mahdi, A.H.; Majrashi, A.; Ali, E.F.; Seleiman, M.F. Influence of nano silicon and nano selenium on root characters, growth, ion selectivity, yield, and yield components of rice (Oryza sativa L.) under salinity conditions. Plants 2021, 11, 1657. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, D.L.; Tsuji, P.A.; Carlson, B.A.; Gladyshev, V.N. Selenium and selenocysteine: Roles in cancer, health, and development. Trends Biochem. Sci. 2014, 31, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Jitaru, P.; Barbante, C. Selenium biochemistry and its role for human health. Metallomics 2014, 1, 25–54. [Google Scholar] [CrossRef]
- Rayman, M.P.; Stranges, S.; Griffin, B.A.; Pastor-Barriuso, R.; Guallar, E. Effect of Supplementation with High-Selenium Yeast on Plasma Lipids: A Randomized Trial. Ann. Intern. Med. 2011, 151, 656–665. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 61, 4075–4097. [Google Scholar] [CrossRef]
- Niedzielski, P.; Rudnicka, M.; Wachelka, M.; Kozak, L.; Rzany, M.; Wozniak, M.; Kaskow, Z. Selenium species in selenium fortified dietary supplements. Food Chem. 2016, 190, 454–459. [Google Scholar] [CrossRef]
- Daniels, L.A. Selenium metabolism and bioavailability. Biol. Trace Elem. Res. 1996, 51, 185–199. [Google Scholar] [CrossRef]
- Haug, A.; Graham, R.D.; Christophersen, O.A.; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 11, 209–228. [Google Scholar]
- Yang, H.; Yang, X.; Ning, Z.; Kwon, S.Y.; Li, M.-L.; Tack, F.M.G.; Kwon, E.E.; Rinklebe, J.; Yin, R. The beneficial and hazardous effects of selenium on the health of the soil-plant-human system: An overview. J. Hazard. Mater. 2022, 422, 126876. [Google Scholar] [CrossRef]
- Taha, R.S.; Seleiman, M.F.; Shami, A.; Alhammad, B.A.; Mahdi, A.H.A. Integrated Application of Selenium and Silicon Enhances Growth and Anatomical Structure, Antioxidant Defense System and Yield of Wheat Grown in Salt-Stressed Soil. Plants 2021, 11, 1040. [Google Scholar] [CrossRef]
- Trippe, R.C., III; Pilon-Smits, E.A.H. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J. Hazard. Mater. 2021, 404, 124178. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- El Kassis, E.; Cathala, N.; Rouached, H.; Fourcroy, P.; Berthomieu, P.; Terry, N.; Davidian, J.-C. Characterization of a selenate-resistant Arabidopsis mutant. Root growth as a potential target for selenate toxicity. Plant Physiol. 2007, 141, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Shibagaki, N.; Rose, A.; McDermott, J.P.; Fujiwara, T.; Hayashi, H.; Yoneyama, T.; Davies, J.P. Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J. 2002, 21, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Terry, N.; Zayed, A.M.; de Souza, M.P.; Tarun, A.S. Selenium in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 401–432. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Mitani, N.; Yamaji, N.; Shen, R.F.; Ma, J.F. Involvement of Silicon Influx Transporter OsNIP2;1 in Selenite Uptake in Rice. Plant Physiol. 2010, 151, 1871–1877. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, B.; Li, W.; Che, R.; Deng, K.; Li, H.; Yu, F.; Ling, H.; Li, Y.; Chu, C. OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol. 2014, 201, 1183–1191. [Google Scholar] [CrossRef]
- Sors, T.G.; Ellis, D.R.; Na, G.N.; Lahner, B.; Lee, S.; Leustek, T.; Pickering, I.J.; Salt, D.E. Analysis of sulfur and selenium assimilation in Astragalus plants with varying capacities to accumulate selenium. Plant J. 2005, 41, 785–797. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 111, 217–235. [Google Scholar] [CrossRef]
- Rios, J.J.; Rosales, M.A.; Blasco, B.; Cervilla, L.M.; Romero, L.; Ruiz, J.M. Biofortification of Se and induction of the antioxidant capacity in lettuce plants. Sci. Hortic. 2008, 111, 248–255. [Google Scholar] [CrossRef]
- Varo, P.; Alfthan, G.; Ekholm, P.; Aro, A.; Koivistoinen, P. Selenium Intake and Serum Selenium in Finland—Effects of Soil Fertilization with Selenium. Am. J. Clin. Nutr. 1988, 41, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Lidon, F.C.; Oliveira, K.; Ribeiro, M.M.; Pelica, J.; Pataco, I.; Ramalho, J.C.; Leitao, A.E.; Almeida, A.S.; Campos, P.S.; Ribeiro-Barros, A.I.; et al. Selenium biofortification of rice grains and implications on macronutrients quality. J. Cereal Sci. 2018, 81, 22–29. [Google Scholar] [CrossRef]
- Hao, S.; Liu, P.; Qin, J.; Song, L.; Yang, W.; Feng, M.; Zhang, M.; Wang, C.; Song, X. Effects of Applying Different Doses of Selenite to Soil and Foliar at Different Growth Stage on Selenium Content and Yield of Different Oat Varieties. Plants 2022, 11, 1810. [Google Scholar] [CrossRef] [PubMed]
- Danso, O.P.; Asante-Badu, B.; Zhang, Z.; Song, J.; Wang, Z.; Yin, X.; Zhu, R. Selenium Biofortification: Strategies, Progress and Challenges. Agriculture 2023, 11, 416. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets—Iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 181, 49–84. [Google Scholar] [CrossRef]
- Tang, L.; Hamid, Y.; Liu, D.; Shohag, M.J.I.; Zehra, A.; He, Z.; Feng, Y.; Yang, X. Foliar application of zinc and selenium alleviates cadmium and lead toxicity of water spinach—Bioavailability/cytotoxicity study with human cell lines. Environ. Int. 2020, 145, 106122. [Google Scholar] [CrossRef]
- Groth, S.; Budke, C.; Neugart, S.; Ackermann, S.; Kappenstein, F.-S.; Daum, D.; Rohn, S. Influence of a Selenium Biofortification on Antioxidant Properties and Phenolic Compounds of Apples (Malus domestica). Antioxidants 2020, 1, 187. [Google Scholar] [CrossRef]
- Hu, T.; Li, L.; Hui, G.; Zhang, J.; Li, H.; Wu, W.; Wei, X.; Guo, Y. Selenium biofortification and its effect on multi-element change in Auricularia auricular. Food Chem. 2019, 295, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Yang, Z.; Shui, Y.; Liu, X.; Chen, J.; Khan, S.; Wang, J.; Gao, Z. Methods of Selenium Application Differentially Modulate Plant Growth, Selenium Accumulation and Speciation, Protein, Anthocyanins and Concentrations of Mineral Elements in Purple-Grained Wheat. Front. Plant Sci. 2020, 11, 1114. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Miao, P.; Li, D.; Wu, Y.; Zhou, C.; Pan, C. Improving red pitaya fruit quality by nano-selenium biofortification to enhance phenylpropanoid and betalain biosynthesis. Ecotoxicol. Environ. Saf. 2023, 267, 115653. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Rayman, M.P. Food-chain selenium and human health: Emphasis on intake. Br. J. Nutr. 2008, 101, 254–268. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Hao, J.; Fan, S.; Dong, R.; Zeng, H.; Liu, C.; Han, Y. Effects of selenate and selenite on selenium accumulation and speciation in lettuce. Plant Physiol. Biochem. 2022, 192, 162–171. [Google Scholar] [CrossRef]
- Di, X.; Qin, X.; Zhao, L.; Liang, X.; Xu, Y.; Sun, Y.; Huang, Q. Selenium distribution, translocation and speciation in wheat (Triticum aestivum L.) after foliar spraying selenite and selenate. Food Chem. 2023, 400, 134077. [Google Scholar] [CrossRef]
- Antoshkina, M.; Golubkina, N.; Poluboyarinov, P.; Skrypnik, L.; Sekara, A.; Tallarita, A.; Caruso, G. Effect of sodium selenate and selenocystine on Savoy cabbage yield, morphological and biochemical characteristics under Chlorella supply. Plants 2023, 11, 1020. [Google Scholar] [CrossRef]
- Luo, L.; Zhang, J.; Zhang, K.; Wen, Q.; Ming, K.; Xiong, H.; Ning, F. Peanut selenium distribution, concentration, speciation, and effects on proteins after exogenous selenium biofortification. Food Chem. 2021, 354, 129515. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, S.; Jiang, Z.; Wang, Y.; Zhang, Z. Selenium Biofortification Modulates Plant Growth, Microelement and Heavy Metal Concentrations, Selenium Uptake, and Accumulation in Black-Grained Wheat. Front. Plant Sci. 2021, 12, 748523. [Google Scholar] [CrossRef]
- De Lima, A.B.; Vilalta, T.d.A.; Lessa, J.H.d.L.; Lopes, G.; Guilherme, L.R.G.; Guerra, M.B.B. Selenium bioaccessibility in rice grains biofortified via soil or foliar application of inorganic Se. J. Food Compos. Anal. 2023, 124, 105652. [Google Scholar] [CrossRef]
- Liu, P.; Song, L.; Hao, S.; Qin, J.; Yang, C.; Yang, W.; Feng, M.; Zhang, M.; Wang, C.; Song, X. Effects of selenium application concentration, period and method on the selenium content and grain yield of Tartary buckwheat of different varieties. J. Sci. Food Agric. 2022, 101, 6868–6876. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, J.; Li, W.; Gong, Z.; Jia, C.; Li, P. Effect of foliar application of the selenium-rich nutrient solution on the selenium accumulation in grains of Foxtail millet (Zhangzagu 10). Environ. Sci. Pollut. Res. 2022, 21, 5569–5576. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yu, Y.; Li, J.; Wang, Y.; Huang, Q.; Guo, Y.; Li, H. Effects of Different Forms of Selenium Fertilizers on Se Accumulation, Distribution, and Residual Effect in Winter Wheat-Summer Maize Rotation System. J. Agric. Food Chem. 2017, 61, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Borowska, K.; Koper, J.; Milanowski, M. Seasonal changes of selenium and selected oxidoreductases in soil under different fertilization and crop rotation. Ecol. Chem. Eng. A-Chem. I Inz. Ekol. A 2012, 11, 719–730. [Google Scholar]
- Borowska, K.; Koper, J. The effect of long-term organic-mineral fertilisation on selenium content and chosen oxidoreductases activity under winter wheat cultivation. Chem. Ecol. 2010, 26, 111–116. [Google Scholar] [CrossRef]
- Tang, W.; Tang, W.; Xie, Y.; Li, X.; Li, H.; Lin, L.; Huang, Z.; Sun, B.; Sun, G.; Tu, L.; et al. Effects of intercropping on Se accumulation and growth of pakchoi, lettuce and radish. Int. J. Phytoremediation 2023, 21, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Lu, R.; Li, H.; Lin, L.; Li, L.; Xiang, J.; Chen, L.; Tang, Y. Mutual intercropping affects selenium uptake of eggplant seedlings. Int. J. Environ. Anal. Chem. 2021, 101, 2866–2875. [Google Scholar] [CrossRef]
- Lin, L.; Xu, X.; Wang, J.; Wang, X.; Lv, X.; Tang, Y.; Deng, H.; Liang, D.; Xia, H. Intercropping of Cyphomandra betacea with Different Ploidies of Solanum Sect. Solanum (Solanaceae) Wild Vegetables Increase Their Selenium Uptakes. Plants 2023, 11, 716. [Google Scholar] [CrossRef]
- Ragalyi, P.; Takacs, T.; Fuzy, A.; Uzinger, N.; Dobosy, P.; Zaray, G.; Szucs-Vasarhelyi, N.; Rekasi, M. Effect of Se-Enriched Irrigation Water and Soil Texture on Biomass Production and Elemental Composition of Green Pea and Carrot and Their Contribution to Human Se Intake. Agriculture 2022, 11, 496. [Google Scholar] [CrossRef]
- Mrstina, T.; Praus, L.; Kaplan, L.; Szakova, J.; Tlustos, P. Efficiency of selenium biofortification of spring wheat: The role of soil properties and organic matter amendment. Plant Soil Environ. 2022, 61, 572–579. [Google Scholar] [CrossRef]
- Ozpinar, S. Nutrient concentration and yield of maize (Zea mays L.) after vetch (Vicia sativa L.) in conventional and reduced tillage systems. J. Plant Nutr. 2016, 31, 1697–1712. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Centofanti, T.; Zambrano, M.C.; Vang, K.; Lone, T.A. Salsola soda as selenium biofortification crop under high saline and boron growing conditions. Front. Plant Sci. 2022, 13, 996502. [Google Scholar] [CrossRef] [PubMed]
- Vega-Ravello, R.; Belen Romero-Poma, M.; de Oliveira, C.; Guimaraes Guilherme, L.R.; Lopes, G. Soil Selenium Addition for Producing Se-Rich Quinoa and Alleviating Water Deficit on the Peruvian Coast. J. Soil Sci. Plant Nutr. 2023, 21, 238–250. [Google Scholar] [CrossRef]
- Zhao, F.-J.; Lopez-Bellido, F.J.; Gray, C.W.; Whalley, W.R.; Clark, L.J.; McGrath, S.P. Effects of soil compaction and irrigation on the concentrations of selenium and arsenic in wheat grains. Sci. Total Environ. 2007, 372, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Marfetan, J.A.; Gallo, A.L.; Farias, M.E.; Velez, M.L.; Pescuma, M.; Ordonez, O.F. Exiguobacterium sp. as a bioinoculant for plant-growth promotion and Selenium biofortification strategies in horticultural plants. World J. Microbiol. Biotechnol. 2023, 31, 134. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Sun, H.; Qin, Y.; Zhou, Y.; Zhu, H.; Yao, Q. A synthetic community of siderophore-producing bacteria increases soil selenium bioavailability and plant uptake through regulation of the soil microbiome. Sci. Total Environ. 2023, 871, 162076. [Google Scholar] [CrossRef]
- Dhiman, K.; Sharma, D.; Kumari, R.; Tomar, P. Biofortification of crops using microbes—A promising sustainable agriculture strategy. J. Plant Nutr. 2022, 46, 2912–2935. [Google Scholar] [CrossRef]
- Ros, G.H.; van Rotterdam, A.M.D.; Bussink, D.W.; Bindraban, P.S. Selenium fertilization strategies for bio-fortification of food: An agro-ecosystem approach. Plant Soil 2016, 404, 99–112. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Hartikainen, H.; Hajiboland, R.; Seppanen, M.M. Uptake and remobilization of selenium in Brassica napus L. plants supplied with selenate or selenium-enriched plant residues. J. Plant Nutr. Soil Sci. 2019, 181, 196–202. [Google Scholar] [CrossRef]
- Hu, T.; Hui, G.; Li, H.; Guo, Y. Selenium biofortification in Hericium erinaceus (Lion’s Mane mushroom) and its in vitro bioaccessibility. Food Chem. 2020, 331, 127287. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ali, F.; Wang, M.; Quang Toan, D.; Zhou, F.; Banuelos, G.S.; Liang, D. Understanding boosting selenium accumulation in Wheat (Triticum aestivum L.) following foliar selenium application at different stages, forms, and doses. Environ. Sci. Pollut. Res. 2020, 21, 717–728. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Bowen, H.C.; Marshall, B.; Broadley, M.R. Extraordinarily high leaf selenium to sulfur ratios define ‘Se-accumulator’ plants. Ann. Bot. 2007, 101, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Izydorczyk, G.; Ligas, B.; Mikula, K.; Witek-Krowiak, A.; Moustakas, K.; Chojnacka, K. Biofortification of edible plants with selenium and iodine—A systematic literature review. Sci. Total Environ. 2021, 754, 141983. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.; Lytle, C.M.; Terry, N. Accumulation and volatilization of different chemical species of selenium by plants. Planta 1998, 201, 284–292. [Google Scholar] [CrossRef]
- De Souza, M.P.; Pilon-Smits, E.A.H.; Lytle, C.M.; Hwang, S.; Tai, J.; Honma, T.S.U.; Yeh, L.; Terry, N. Rate-limiting steps in selenium assimilation and volatilization by Indian mustard. Plant Physiol. 1998, 111, 1487–1494. [Google Scholar] [CrossRef]
- D’Amato, R.; Fontanella, M.C.; Falcinelli, B.; Beone, G.M.; Bravi, E.; Marconi, O.; Benincasa, P.; Businelli, D. Selenium Biofortification in Rice (Oryza sativa L.) Sprouting: Effects on Se Yield and Nutritional Traits with Focus on Phenolic Acid Profile. J. Agric. Food Chem. 2018, 61, 4082–4090. [Google Scholar] [CrossRef]
- Marschall, T.A.; Bornhorst, J.; Kuehnelt, D.; Schwerdtle, T. Differing cytotoxicity and bioavailability of selenite, methylselenocysteine, selenomethionine, selenosugar 1 and trimethylselenonium ion and their underlying metabolic transformations in human cells. Mol. Nutr. Food Res. 2016, 61, 2622–2632. [Google Scholar] [CrossRef]
- Mimmo, T.; Tiziani, R.; Valentinuzzi, F.; Lucini, L.; Nicoletto, C.; Sambo, P.; Scampicchio, M.; Pii, Y.; Cesco, S. Selenium Biofortification in Fragaria × ananassa: Implications on Strawberry Fruits Quality, Content of Bioactive Health Beneficial Compounds and Metabolomic Profile. Front. Plant Sci. 2017, 8, 1887. [Google Scholar] [CrossRef]
- Wu, Z.; Banuelos, G.S.; Lin, Z.-Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef]
- Quang Toan, D.; Wang, M.; Thi Anh Thu, T.; Zhou, F.; Wang, D.; Zhai, H.; Peng, Q.; Xue, M.; Du, Z.; Banuelos, G.S.; et al. Bioavailability of selenium in soil-plant system and a regulatory approach. Crit. Rev. Environ. Sci. Technol. 2019, 41, 443–517. [Google Scholar]
- Wang, P.; Lombi, E.; Zhao, F.-J.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Poblaciones, M.J.; Rodrigo, S.; Santamaria, O.; Chen, Y.; McGrath, S.P. Agronomic selenium biofortification in Triticum durum under Mediterranean conditions: From grain to cooked pasta. Food Chem. 2014, 146, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wen, X.; Yi, N.; Liu, Y.; Wu, J.; Li, H.; Liu, G. Effect of foliar application of silicon, selenium and zinc on heavy metal accumulation in wheat grains in field studies. Environ. Pollut. Bioavailab. 2022, 31, 246–252. [Google Scholar] [CrossRef]
- Yuan, Z.; Long, W.; Liang, T.; Zhu, M.; Zhu, A.; Luo, X.; Fu, L.; Hu, Z.; Zhu, R.; Wu, X. Effect of foliar spraying of organic and inorganic selenium fertilizers during different growth stages on selenium accumulation and speciation in rice. Plant Soil 2023, 486, 87–101. [Google Scholar] [CrossRef]
- Ramos, D.P.; Herrera Chan, G.A.; Ramos Dias, M.A.; Silva, D.V.; Reis Sousa, P.L.; Mascena, N.R., Jr.; Viana Leal, T.H.; de Oliveira, W.T.M.; Dias, D.S.; Cavallini, G.S.; et al. Effect of foliar application with selenium on biofortification and physiological attributes of irrigated rice cultivars. J. Food Compos. Anal. 2023, 123, 105534. [Google Scholar] [CrossRef]
- Sharma, S.; Bansal, A.; Dhillon, S.K.; Dhillon, K.S. Comparative effects of selenate and selenite on growth and biochemical composition of rapeseed (Brassica napus L.). Plant Soil 2010, 329, 339–348. [Google Scholar] [CrossRef]
- Lara, T.S.; de Lima Lessa, J.H.; Dazio de Souza, K.R.; Branco Corguinha, A.P.; Dias Martins, F.A.; Lopes, G.; Guimaraes Guilherme, L.R. Selenium biofortification of wheat grain via foliar application and its effect on plant metabolism. J. Food Compos. Anal. 2019, 81, 10–18. [Google Scholar] [CrossRef]
- Venter, Z.S.; Jacobs, K.; Hawkins, H.-J. The impact of crop rotation on soil microbial diversity: A meta-analysis. Pedobiologia 2016, 51, 215–223. [Google Scholar] [CrossRef]
- Karlen, D.L.; Varvel, G.E.; Bullock, D.G.; Cruse, R.M. Crop Rotations for the 21st-Century. Adv. Agron. 1994, 53, 1–45. [Google Scholar]
- Kumar, A.; Yadav, D.S. Use of organic manure and fertilizer in rice (Oryza sativa) wheat (Triticum aestivum) cropping system for sustainability. Indian J. Agric. Sci. 1995, 61, 703–707. [Google Scholar]
- Seleiman, M.F.; Hafez, E.M. Optimizing inputs management for sustainable agricultural development. In Mitigating Environmental Stresses for Agricultural Sustainability in Egypt; Springer: Cham, Switzerland, 2021; pp. 487–507. [Google Scholar]
- MacLeod, J.A.; Gupta, U.C.; Milburn, P.; Sanderson, J.B. Selenium concentration in plant material, drainage and surface water as influenced by Se applied to barley foliage in a barley red clover potato rotation. Can. J. Soil Sci. 1998, 71, 685–688. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.-F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 201, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Liancourt, P.; Tielboerger, K.; Travis, J.M.J.; Anthelme, F.; et al. Facilitation in plant communities: The past, the present, and the future. J. Ecol. 2008, 91, 18–34. [Google Scholar] [CrossRef]
- He, Q.; Bertness, M.D.; Altieri, A.H. Global shifts towards positive species interactions with increasing environmental stress. Ecol. Lett. 2013, 11, 695–706. [Google Scholar] [CrossRef] [PubMed]
- El-Shamy, M.A.; Seleiman, M.; Rady, T.E.-G.H. Effect of different sowing methods on growth, yield and its components of wheat under intercropping patterns with Egyptian clover var. Fahl. Assiut J. Agric. Sci. 2017, 48, 67–80. [Google Scholar]
- Willey, R.W. Resource Use in Intercropping Systems. Agric. Water Manag. 1990, 17, 215–231. [Google Scholar] [CrossRef]
- Mazurak, A.P. Glossary of Soil Science Terms—Soil Physics; Soil Science Society of America: Madison, WI, USA, 1997. [Google Scholar]
- Nawaz, M.F.; Bourrie, G.; Trolard, F. Soil compaction impact and modelling. A review. Agron. Sustain. Dev. 2013, 31, 291–309. [Google Scholar] [CrossRef]
- Kooistra, M.J.; Schoonderbeek, D.; Boone, F.R.; Veen, B.W.; Vannoordwijk, M. Root-soil contact of maize, as measured by a thin-section technique.2. Effects of soil compaction. Plant Soil 1992, 131, 119–129. [Google Scholar] [CrossRef]
- Arvidsson, J. Nutrient uptake and growth of barley as affected by soil compaction. Plant Soil 1999, 201, 9–19. [Google Scholar] [CrossRef]
- Kausch, M.F.; Pallud, C.E. Modeling the impact of soil aggregate size on selenium immobilization. Biogeosciences 2013, 11, 1323–1336. [Google Scholar] [CrossRef]
- Roy, R.; Nunez-Delgado, A.; Sultana, S.; Wang, J.; Munir, A.; Battaglia, M.L.; Sarker, T.; Seleiman, M.F.; Barmon, M.; Zhang, R. Additions of optimum water, spent mushroom compost and wood biochar to improve the growth performance of Althaea rosea in drought-prone coal-mined spoils. J. Environ. Manag. 2021, 295, 113076. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Steinbach, H.S. A review of the effects of tillage systems on some soil physical properties, water content, nitrate availability and crops yield in the Argentine Pampas. Soil Tillage Res. 2009, 101, 1–15. [Google Scholar] [CrossRef]
- Cooper, J.; Baranski, M.; Stewart, G.; Nobel-de Lange, M.; Barberi, P.; Fliessbach, A.; Peigne, J.; Berner, A.; Brock, C.; Casagrande, M.; et al. Shallow non-inversion tillage in organic farming maintains crop yields and increases soil C stocks: A meta-analysis. Agron. Sustain. Dev. 2016, 31, 22. [Google Scholar] [CrossRef]
- Torabian, S.; Farhangi-Abriz, S.; Denton, M.D. Do tillage systems influence nitrogen fixation in legumes? A review. Soil Tillage Res. 2019, 185, 113–121. [Google Scholar] [CrossRef]
- Lessa, J.H.L.; Araujo, A.M.; Silva, G.N.T.; Guilherme, L.R.G.; Lopes, G. Adsorption-desorption reactions of selenium (VI) in tropical cultivated and uncultivated soils under Cerrado biome. Chemosphere 2016, 164, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Bellido, F.J.; Sanchez, V.; Rivas, I.; Lopez-Bellido, R.J.; Lopez-Bellido, L. Wheat grain selenium content as affected by year and tillage system in a rainfed Mediterranean Vertisol. Field Crops Res. 2019, 233, 41–48. [Google Scholar] [CrossRef]
- Hamza, M.A.; Anderson, W.K. Soil compaction in cropping systems—A review of the nature, causes and possible solutions. Soil Tillage Res. 2005, 81, 121–145. [Google Scholar] [CrossRef]
- Massari, C.; Modanesi, S.; Dari, J.; Gruber, A.; De Lannoy, G.J.M.; Girotto, M.; Quintana-Segui, P.; Le Page, M.; Jarlan, L.; Zribi, M.; et al. A Review of Irrigation Information Retrievals from Space and Their Utility for Users. Remote Sens. 2021, 11, 4112. [Google Scholar] [CrossRef]
- Eiche, E.; Nothstein, A.K.; Goettlicher, J.; Steininger, R.; Dhillon, K.S.; Neumann, T. The behaviour of irrigation induced Se in the groundwater-soil-plant system in Punjab, India. Environ. Sci. Process. Impacts 2019, 21, 957–969. [Google Scholar] [CrossRef]
- Eid, M.A.; Abdel-Salam, A.A.; Salem, H.M.; Mahrous, S.E.; Seleiman, M.F.; Alsadon, A.A.; Solieman, T.H.; Ibrahim, A.A. Interaction effects of nitrogen source and irrigation regime on tuber quality, yield, and water Use efficiency of Solanum tuberosum L. Plants 2020, 1, 110. [Google Scholar] [CrossRef]
- Broadley, M.R.; White, P.J.; Bryson, R.J.; Meacham, M.C.; Bowen, H.C.; Johnson, S.E.; Hawkesford, M.J.; McGrath, S.P.; Zhao, F.J.; Breward, N.; et al. Biofortification of UK food crops with selenium. Proc. Nutr. Soc. 2006, 61, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhou, F.; Yu, D.; Zhang, N.; Qi, M.; Li, Y.; Wu, F.; Liang, D. Irrigation leads to new Se-toxicity paddy fields in and around typical Se-toxicity area. Sci. Total Environ. 2023, 892, 164433. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yang, J.; Fang, Z.; Zhang, S.; Zhou, X. Effects of Water Management on Selenium Accumulation in Rice Grains and Bacterial Community Diversity in Rhizosphere Soil. Acta Pedol. Sin. 2021, 51, 1574–1584. [Google Scholar]
- Duran, P.; Acuna, J.J.; Jorquera, M.A.; Azcon, R.; Borie, F.; Cornejo, P.; Mora, M.L. Enhanced selenium content in wheat grain by co-inoculation of selenobacteria and arbuscular mycorrhizal fungi: A preliminary study as a potential Se biofortification strategy. J. Cereal Sci. 2013, 51, 275–280. [Google Scholar] [CrossRef]
- Acuna, J.J.; Jorquera, M.A.; Barra, P.J.; Crowley, D.E.; de la Luz Mora, M. Selenobacteria selected from the rhizosphere as a potential tool for Se biofortification of wheat crops. Biol. Fertil. Soils 2013, 41, 175–185. [Google Scholar] [CrossRef]
- Sheng, X.-F.; Xia, J.-J. Improvement of rape (Brassica napus) plant growth and cadmium uptake by cadmium-resistant bacteria. Chemosphere 2006, 61, 1036–1042. [Google Scholar] [CrossRef]
- Lee, S.; Doolittle, J.J.; Woodard, H.J. Selenite Adsorption and Desorption in Selected South Dakota Soils as a Function of pH and Other Oxyanions. Soil Sci. 2011, 171, 73–79. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Gu, M.; Li, H.; Shohag, M.J.I.; Shen, F.; Wang, X.; Wei, Y. Combined use of arbuscular mycorrhizal fungus and selenium fertilizer shapes microbial community structure and enhances organic selenium accumulation in rice grain. Sci. Total Environ. 2020, 748, 141166. [Google Scholar] [CrossRef]
- Ye, Y.; Qu, J.; Pu, Y.; Rao, S.; Xu, F.; Wu, C. Selenium Biofortification of Crop Food by Beneficial Microorganisms. J. Fungi 2020, 1, 59. [Google Scholar] [CrossRef] [PubMed]
- Ike, M.; Takahashi, K.; Fujita, T.; Kashiwa, M.; Fujita, M. Selenate reduction by bacteria isolated from aquatic environment free from selenium contamination. Water Res. 2000, 31, 3019–3025. [Google Scholar] [CrossRef]
- Trivedi, G.; Patel, P.; Saraf, M. Synergistic effect of endophytic selenobacteria on biofortification and growth of Glycine max under drought stress. South Afr. J. Bot. 2020, 134, 27–35. [Google Scholar] [CrossRef]
- De Souza, M.P.; Chu, D.; Zhao, M.; Zayed, A.M.; Ruzin, S.E.; Schichnes, D.; Terry, N. Rhizosphere bacteria enhance selenium accumulation and volatilization by Indian mustard. Plant Physiol. 1999, 111, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.J.; Trivedi, G.R.; Shah, R.K.; Saraf, M. Selenorhizobacteria: As biofortification tool in sustainable agriculture. Biocatal. Agric. Biotechnol. 2018, 14, 198–203. [Google Scholar] [CrossRef]
- Yasin, M.; El-Mehdawi, A.F.; Anwar, A.; Pilon-Smits, E.A.H.; Faisal, M. Microbial-enhanced Selenium and Iron Biofortification of Wheat (Triticum aestivum L.)—Applications in Phytoremediation and Biofortification. Int. J. Phytoremediation 2015, 11, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Duran, P.; Acuna, J.J.; Jorquera, M.A.; Azcon, R.; Paredes, C.; Rengel, Z.; de la Luz Mora, M. Endophytic bacteria from selenium-supplemented wheat plants could be useful for plant-growth promotion, biofortification and Gaeumannomyces graminis biocontrol in wheat production. Biol. Fertil. Soils 2014, 51, 983–990. [Google Scholar] [CrossRef]
- Cartes, P.; Jara, A.A.; Pinilla, L.; Rosas, A.; Mora, M.L. Selenium improves the antioxidant ability against aluminium-induced oxidative stress in ryegrass roots. Ann. Appl. Biol. 2010, 151, 297–307. [Google Scholar] [CrossRef]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are improving Lives of Millions of People around the world. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 21, 65–134. [Google Scholar] [CrossRef]
- Pena, C.; Restrepo-Sanchez, L.-P.; Kushalappa, A.; Rodriguez-Molano, L.-E.; Mosquera, T.; Narvaez-Cuenca, C.-E. Nutritional contents of advanced breeding clones of Solanum tuberosum group Phureja. LWT Food Sci. Technol. 2015, 61, 76–82. [Google Scholar] [CrossRef]
- Kumar, J.; Sen Gupta, D.; Kumar, S.; Gupta, S.; Singh, N.P. Current Knowledge on Genetic Biofortification in Lentil. J. Agric. Food Chem. 2016, 61, 6383–6396. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, P.; Yamaji, N.; Ma, J.F. Plant Nutrition for Human Nutrition: Hints from Rice Research and Future Perspectives. Mol. Plant 2020, 11, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Souza, G.A.; Hart, J.J.; Carvalho, J.G.; Rutzke, M.A.; Albrecht, J.C.; Guilherme, L.R.G.; Kochian, L.V.; Li, L. Genotypic variation of zinc and selenium concentration in grains of Brazilian wheat lines. Plant Sci. 2014, 224, 27–35. [Google Scholar] [CrossRef]
- White, P.J. The Genetics of Selenium Accumulation by Plants. In Selenium in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.Q., Eds.; Springer: Cham, Switzerland, 2017; Volume 11, pp. 143–163. [Google Scholar]
- Chen, J.; Huang, X.-Y.; Salt, D.E.; Zhao, F.-J. Mutation in OsCADT1 enhances cadmium tolerance and enriches selenium in rice grain. New Phytol. 2020, 221, 838–850. [Google Scholar] [CrossRef] [PubMed]
- Thavarajah, D.; Ruszkowski, J.; Vandenberg, A. High potential for selenium biofortification of lentils (Lens culinaris L.). J. Agric. Food Chem. 2008, 51, 10747–10753. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Erskine, W.; Zaman, M.; Thavarajah, P.; Thavarajah, D.; Siddique, K. Selenium biofortification in lentil (Lens culinaris Medikus subsp. culinaris): Farmers’ field survey and genotype× environment effect. Food Res. Int. 2013, 51, 1596–1604. [Google Scholar]
- Wang, P.; Wang, H.; Liu, Q.; Tian, X.; Shi, Y.; Zhang, X. QTL mapping of selenium content using a RIL population in wheat. PLoS ONE 2017, 11, e0184351. [Google Scholar] [CrossRef]
- Huang, Y.; Sun, C.; Min, J.; Chen, Y.; Tong, C.; Bao, J. Association Mapping of Quantitative Trait Loci for Mineral Element Contents in Whole Grain Rice (Oryza sativa L.). J. Agric. Food Chem. 2015, 61, 10885–10892. [Google Scholar] [CrossRef]
- Liu, C.; Ding, S.; Zhang, A.; Hong, K.; Jiang, H.; Yang, S.; Ruan, B.; Zhang, B.; Dong, G.; Guo, L.; et al. Development of nutritious rice with high zinc/selenium and low cadmium in grains through QTL pyramiding. J. Integr. Plant Biol. 2020, 61, 349–359. [Google Scholar] [CrossRef]
- Singh, K.; Batra, R.; Sharma, S.; Saripalli, G.; Gautam, T.; Singh, R.; Pal, S.; Malik, P.; Kumar, M.; Jan, I.; et al. WheatQTLdb: A QTL database for wheat. Mol. Genet. Genom. 2021, 291, 1051–1056. [Google Scholar] [CrossRef] [PubMed]
- Ates, D.; Sever, T.; Aldemir, S.; Yagmur, B.; Temel, H.Y.; Kaya, H.B.; Alsaleh, A.; Kahraman, A.; Ozkan, H.; Vandenberg, A.; et al. Identification QTLs Controlling Genes for Se Uptake in Lentil Seeds. PLoS ONE 2016, 11, e0149210. [Google Scholar]
- Zhang, L.-H.; Abdel-Ghany, S.E.; Freeman, J.L.; Ackley, A.R.; Schiavon, M.; Pilon-Smits, E.A.H. Investigation of selenium tolerance mechanisms in Arabidopsis thaliana. Physiol. Plant. 2006, 121, 212–223. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, F.; Liu, D.; Chen, K.; Du, B.; Qiu, X.; Xu, J.; Xing, D. Distribution characteristics of selenium, cadmium and arsenic in rice grains and their genetic dissection by genome-wide association study. Front. Genet. 2022, 13, 1007896. [Google Scholar] [CrossRef] [PubMed]
- Marcotuli, I.; Miguel Soriano, J.; Gadaleta, A. A consensus map for quality traits in durum wheat based on genome-wide association studies and detection of ortho-meta QTL across cereal species. Front. Genet. 2022, 13, 982418. [Google Scholar] [CrossRef] [PubMed]
- Ozkuru, E.; Ates, D.; Nemli, S.; Erdogmus, S.; Karaca, N.; Yilmaz, H.; Yagmur, B.; Kartal, C.; Tosun, M.; Ocak, O.O.; et al. Genome-wide association studies of molybdenum and selenium concentrations in C. arietinum and C. reticulatum seeds. Mol. Breed. 2019, 31, 46. [Google Scholar] [CrossRef]
- Myles, S.; Peiffer, J.; Brown, P.J.; Ersoz, E.S.; Zhang, Z.; Costich, D.E.; Buckler, E.S. Association Mapping: Critical Considerations Shift from Genotyping to Experimental Design. Plant Cell 2009, 21, 2194–2202. [Google Scholar] [CrossRef]
- Zhang, G.-M.; Zheng, T.-Q.; Chen, Z.; Wang, Y.-L.; Wang, Y.; Shi, Y.-M.; Wang, C.-C.; Zhang, L.-Y.; Ma, J.-T.; Deng, L.-W.; et al. Joint Exploration of Favorable Haplotypes for Mineral Concentrations in Milled Grains of Rice (Oryza sativa L.). Front. Plant Sci. 2018, 9, 447. [Google Scholar] [CrossRef]
- Yan, J.; Xue, W.-T.; Yang, R.-Z.; Qin, H.-B.; Zhao, G.; Tzion, F.; Cheng, J.-P. Quantitative Trait Loci Conferring Grain Selenium Nutrient in Durum Wheat × Wild Emmer Wheat RIL Population. Czech J. Genet. Plant Breed. 2018, 51, 52–58. [Google Scholar] [CrossRef]
- Pu, Z.-E.; Yu, M.; He, Q.-Y.; Chen, G.-Y.; Wang, J.-R.; Liu, Y.-X.; Jiang, Q.-T.; Li, W.; Dai, S.-F.; Wei, Y.-M.; et al. Quantitative Trait Loci Associated with Micronutrient Concentrations in Two Recombinant Inbred Wheat Lines. J. Integr. Agric. 2014, 11, 2322–2329. [Google Scholar] [CrossRef]
- Norton, G.J.; Deacon, C.M.; Xiong, L.; Huang, S.; Meharg, A.A.; Price, A.H. Genetic mapping of the rice ionome in leaves and grain: Identification of QTLs for 17 elements including arsenic, cadmium, iron and selenium. Plant Soil 2010, 329, 139–153. [Google Scholar] [CrossRef]
- Zhang, L.; Byrne, P.F.; Pilon-Smits, E.A. Mapping quantitative trait loci associated with selenate tolerance in Arabidopsis thaliana. New Phytol. 2006, 171, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Welch, R.M.; Graham, R.D. Agriculture: The real nexus for enhancing bioavailable micronutrients in food crops. J. Trace Elem. Med. Biol. 2005, 11, 299–307. [Google Scholar] [CrossRef]
- Zhu, C.; Naqvi, S.; Gomez-Galera, S.; Pelacho, A.M.; Capell, T.; Christou, P. Transgenic strategies for the nutritional enhancement of plants. Trends Plant Sci. 2007, 11, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Pilon-Smits, E.A.H.; LeDuc, D.L. Phytoremediation of selenium using transgenic plants. Curr. Opin. Biotechnol. 2009, 21, 207–212. [Google Scholar] [CrossRef]
- Li, Z.; Tian, Y.; Wang, B.; Peng, R.; Xu, J.; Fu, X.; Han, H.; Wang, L.; Zhang, W.; Deng, Y.; et al. Enhanced phytoremediation of selenium using genetically engineered rice plants. J. Plant Physiol. 2022, 271, 153665. [Google Scholar] [CrossRef]
- Ellis, D.R.; Sors, T.G.; Brunk, D.G.; Albrecht, C.; Orser, C.; Lahner, B.; Wood, K.V.; Harris, H.H.; Pickering, I.J.; Salt, D.E. Production of Se-methylselenocysteine in transgenic plants expressing selenocysteine methyltransferase. BMC Plant Biol. 2004, 4, 1. [Google Scholar] [CrossRef]
- McKenzie, M.J.; Hunter, D.A.; Pathirana, R.; Watson, L.M.; Joyce, N.I.; Matich, A.J.; Rowan, D.D.; Brummell, D.A. Accumulation of an organic anticancer selenium compound in a transgenic Solanaceous species shows wider applicability of the selenocysteine methyltransferase transgene from selenium hyperaccumulators. Transgenic Res. 2009, 11, 407–424. [Google Scholar] [CrossRef]
- Brummell, D.A.; Watson, L.M.; Pathirana, R.; Joyce, N.I.; West, P.J.; Hunter, D.A.; McKenzie, M.J. Biofortification of Tomato (Solanum lycopersicum) Fruit with the Anticancer Compound Methylselenocysteine Using a Selenocysteine Methyltransferase from a Selenium Hyperaccumulator. J. Agric. Food Chem. 2011, 51, 10987–10994. [Google Scholar] [CrossRef]
- Hung, C.-Y.; Holliday, B.M.; Kaur, H.; Yadav, R.; Kittur, F.S.; Xie, J. Identification and characterization of selenate- and selenite-responsive genes in a Se-hyperaccumulator Astragalus racemosus. Mol. Biol. Rep. 2012, 31, 7635–7646. [Google Scholar] [CrossRef]
- Hefferon, K.L. Can biofortified crops help attain food security? Curr. Mol. Biol. Rep. 2016, 2, 180–185. [Google Scholar] [CrossRef]
- Watanabe, K.N.; Sassa, Y.; Suda, E.; Chen, C.-H.; Inaba, M.; Kikuchi, A. Global political, economic, social and technological issues on trasngenic crops. Plant Biotechnol. 2005, 21, 515–522. [Google Scholar] [CrossRef]
- Newman, R.; Waterland, N.; Moon, Y.; Tou, J.C. Selenium Biofortification of Agricultural Crops and Effects on Plant Nutrients and Bioactive Compounds Important for Human Health and Disease Prevention—A Review. Plant Foods Hum. Nutr. 2019, 71, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Saidi, I.; Chtourou, Y.; Djebali, W. Selenium alleviates cadmium toxicity by preventing oxidative stress in sunflower (Helianthus annuus) seedlings. J. Plant Physiol. 2014, 171, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium in cancer prevention: A review of the evidence and mechanism of action. Proc. Nutr. Soc. 2005, 61, 527–542. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.G.; Moon, Y.; Sams, C.E.; Tou, J.C.; Waterland, N.L. Biofortification of Sodium Selenate Improves Dietary Mineral Contents and Antioxidant Capacity of Culinary Herb Microgreens. Front. Plant Sci. 2021, 12, 716437. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Xiao, Y.; Wu, H. Selenium accumulation, speciation, and its effect on nutritive value of Flammulina velutipes (Golden needle mushroom). Food Chem. 2021, 350, 128667. [Google Scholar] [CrossRef]
- De Oliveira, V.C.; Faquin, V.; Andrade, F.R.; Carneiro, J.P.; da Silva Junior, E.C.; Dazio de Souza, K.R.; Pereira, J.; Guimaraes Guilherme, L.R. Physiological and Physicochemical Responses of Potato to Selenium Biofortification in Tropical Soil. Potato Res. 2019, 61, 315–331. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, S.; Li, Y.; Xu, S.; Shi, G.; Ding, Z. Effect of selenium on mushroom growth and metabolism: A review. Trends Food Sci. Technol. 2021, 118, 328–340. [Google Scholar] [CrossRef]
- De Brito Mateus, M.P.; Rimoldi Tavanti, R.F.; Tavanti, T.R.; Santos, E.F.; Jalal, A.; dos Reis, A.R. Selenium biofortification enhances ROS scavenge system increasing yield of coffee plants. Ecotoxicol. Environ. Saf. 2021, 209, 111772. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.; Malorgio, F.; Pezzarossa, B. Selenium Enrichment of Horticultural Crops. Molecules 2017, 21, 933. [Google Scholar] [CrossRef] [PubMed]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.-T. An Overview of Hazardous Impacts of Soil Salinity in Crops, Tolerance Mechanisms, and Amelioration through Selenium Supplementation. Int. J. Mol. Sci. 2020, 21, 148. [Google Scholar] [CrossRef] [PubMed]
- Tamaoki, M.; Freeman, J.L.; Pilon-Smits, E.A.H. Cooperative ethylene and jasmonic acid signaling regulates selenite resistance in Arabidopsis. Plant Physiol. 2008, 141, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- White, P.J. Selenium metabolism in plants. Biochim. Biophys. Acta-Gen. Subj. 2018, 1861, 2333–2342. [Google Scholar] [CrossRef]
- Islam, M.Z.; Park, B.-J.; Kang, H.-M.; Lee, Y.-T. Influence of selenium biofortification on the bioactive compounds and antioxidant activity of wheat microgreen extract. Food Chem. 2020, 309, 125763. [Google Scholar] [CrossRef]
- Yu, L.L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Qian, M. Free radical scavenging properties of wheat extracts. J. Agric. Food Chem. 2002, 51, 1619–1624. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 21, 2452. [Google Scholar] [CrossRef]
- Huang, J.; Qian, J.; Wang, S.; Li, Y.; Zhai, X.; Olajide, T.M.; Shen, G.X.; Liao, X. Effect of selenium biofortification on bioactive compounds and antioxidant activity in germinated black soybean. J. Food Sci. 2022, 81, 1009–1019. [Google Scholar] [CrossRef]
- Golubkina, N.; Kekina, H.; Caruso, G. Yield, Quality and Antioxidant Properties of Indian Mustard (Brassica juncea L.) in Response to Foliar Biofortification with Selenium and Iodine. Plants 2018, 1, 80. [Google Scholar] [CrossRef]
- Golubkina, N.A.; Kosheleva, O.V.; Krivenkov, L.V.; Dobrutskaya, H.G.; Nadezhkin, S.; Caruso, G. Intersexual differences in plant growth, yield, mineral composition and antioxidants of spinach (Spinacia oleracea L.) as affected by selenium form. Sci. Hortic. 2017, 225, 350–358. [Google Scholar] [CrossRef]
- Mohammad Azmin, S.N.H.; Sulaiman, N.S.; Mat Nor, M.S.; Abdullah, P.S.; Abdul Kari, Z.; Pati, S. A Review on Recent Advances on Natural Plant Pigments in Foods: Functions, Extraction, Importance and Challenges. Appl. Biochem. Biotechnol. 2022, 191, 4655–4672. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Goel, S.; Kaur, N.; Sharma, S.; Singh, I.; Nayyar, H. Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ. Exp. Bot. 2012, 77, 242–248. [Google Scholar] [CrossRef]
- Pannico, A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y. Selenium Biofortification Impacts the Nutritive Value, Polyphenolic Content, and Bioactive Constitution of Variable Microgreens Genotypes. Antioxidants 2020, 1, 272. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Antoshkina, M.; Bondareva, L.; Sekara, A.; Campagna, E.; Caruso, G. Effect of Foliar Application of Sodium Selenate on Mineral Relationships in Brassicaceae Crops. Horticulturae 2023, 1, 535. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Lannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005, 11, 21–34. [Google Scholar] [CrossRef]
- Lai, X.; Yang, X.; Rao, S.; Zhu, Z.; Cong, X.; Ye, J.; Zhang, W.; Liao, Y.; Cheng, S.; Xu, F. Advances in physiological mechanisms of selenium to improve heavy metal stress tolerance in plants. Plant Biol. 2022, 21, 913–919. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Garcia-Caparros, P.; Parvin, K.; Zulfiqar, F.; Ahmed, N.; Fujita, M. Selenium Supplementation and Crop Plant Tolerance to Metal/Metalloid Toxicity. Front. Plant Sci. 2022, 12, 792770. [Google Scholar] [CrossRef]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium Biofortification and Interaction with Other Elements in Plants: A Review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef]
- Lanza, M.G.D.B.; dos Reis, A.R. Roles of selenium in mineral plant nutrition: ROS scavenging responses against abiotic stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lin, W.; Jiao, H.; Liu, J.; Chan, L.; Liu, X.; Wang, R.; Chen, T. Uptake, transport, and metabolism of selenium and its protective effects against toxic metals in plants: A review. Metallomics 2021, 11, mfab040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, X.; Chan, H.M.; Larssen, T. New Insights into Traditional Health Risk Assessments of Mercury Exposure: Implications of Selenium. Environ. Sci. Technol. 2014, 41, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhou, J.; Liu, H.; Zhang, W.; Hu, Y.; Liang, J.; Zhou, J. Foliar spraying with silicon and selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. Sci. Total Environ. 2018, 631–632, 1100–1108. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, X.; Zhu, J.; Sapkota, A.; Meng, B.; Yao, H.; Qin, H.; Larssen, T. Selenium in Soil Inhibits Mercury Uptake and Trans location in Rice (Oryza sativa L.). Environ. Sci. Technol. 2012, 41, 10040–10046. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2018, 251, 459–469. [Google Scholar] [CrossRef]
| Crop Types | Mapping Population | No. of QTL | References | |
|---|---|---|---|---|
| Cross | Type (Number) | Se | ||
| Lens culinaris Medik. | PI 320937 × Eston | RIL (96) | 36 | [135] |
| T. aestivum, L. | TN18 × LM6 | RIL (184) | 16 | [131] |
| T. aestivum, L. | Triticum dicoccoides × Langdon | F6 RIL (152) | 15 | [142] |
| T. aestivum, L. | SHW-L1 × Chuanmai 32 | RIL (-) | 4 | [143] |
| Oryza sativa L. | Oryza sativa ssp. indica inbred variety 93–11 × Oryza sativa ssp. indica photo-thermo-sensitive male sterile line PA64s | RIL (132) | 2 | [133] |
| Oryza sativa L. | Bala (an indica) × Azucena (a japonica) | F6 RIL (105) | 6 | [144] |
| Arabidopsis thaliana | Ler-0 × Col-4 | F8 RIL (96) | 3 | [145] |
| Crop | Applications | Bioactive Compounds | Crop Pigments | References |
|---|---|---|---|---|
| Oryza sativa L. | Sodium selenite | Soluble Free Phenolic Acids (PA) ↑* 45–405 mg L−1 | Total chlorophyll content (TChlC)↑15–45 mg L−1, ↓135–405 mg L−1 | [67] |
| (Shoots of 10-Day Old Rice Sprouts) | 15–405 mg L−1—plastic trays | Bound PA ↑45 mg L−1, ↓15, 135–405 mg L−1 Soluble Conjugated PA ↑ | Total Carotenoid content (TCC) ↓45–405 mg L−1 | |
| Oryza sativa L. | Sodium selenate | Soluble Free PA ↑ | TChlC ↓45–135 mg L−1 | |
| (Shoots of 10-Day Old Rice Sprouts) | 15–135 mg L−1—plastic trays | Bound PA ↓ | TCC ↓45–135 mg L−1 | |
| Fragaria × ananassa cv. Fruits | Sodium selenate | Total phenolic content (TPC) NS; Total Flavonoids ↓ | – | [69] |
| 10, 100 μM—hydroponic | Total Flavonols ↓ | |||
| Brassica juncea L. Leaves | Sodium selenate | Vitamin C ↑; Carotene NS | Chlorophyll a NS; Chlorophyll b ↑ | [173] |
| 50 mg L−1 | Flavonoids ↑ | |||
| Coriandrum sativum L. | Sodium selenate | Total polyphenols ↑16 μM | β-carotene ↑8 μM, ↓16 μM | [177] |
| 8, 16 μM—capillary mat | Lutein ↓ | |||
| Ocimum basilicum L. ‘green basil’ | Sodium selenate | Total polyphenols ↑8 μM | β-carotene ↓ | |
| 8, 16 μM—capillary mat | Lutein ↑8 μM, ↓16 μM | |||
| Spinacia oleracea L. | Sodium selenate | Vitamin C NS | Chlorophyll a ↓; Chlorophyll b ↓ | [174] |
| male crop Leaves | 0.28 mM—foliar | TChlC ↓; Carotenes ↓ | ||
| Spinacia oleracea L. | Sodium selenite | Vitamin C ↑ | Chlorophyll a ↑; Chlorophyll b ↑ | |
| male crop Leaves | 0.28 mM—foliar | TChlC ↑; Carotenes ↑ | ||
| Spinacia oleracea L. | Sodium selenate | Vitamin C ↑ | Chlorophyll a NS; Chlorophyll b ↑ | |
| female crop Leaves | 0.28 mM—foliar | TChlC NS; Carotenes ↑ | ||
| Spinacia oleracea L. | Sodium selenite | Vitamin C ↑ | Chlorophyll a NS; Chlorophyll b ↑ | |
| female crop Leaves | 0.28 mM—foliar | TChlC ↑; Carotenes ↑ |
| Crop | Application | Cd | Pb | Cr | Hg | As | References |
|---|---|---|---|---|---|---|---|
| Triticum aestivum L. | Se ore powder | – * | NS | NS | NS | NS | [40] |
| ‘Xihei 88′ Grain (black-grained wheat) | 1080–4320 g ha−1—soil | ||||||
| Triticum aestivum L. | Se ore powder | – | NS | NS | NS | NS | |
| Heidali’ Grain (black-grained wheat) | 1080–4320 g ha−1—soil | ||||||
| Triticum aestivum L. | Se ore powder | – | NS | NS | NS | NS | |
| H. ericium erinaceus fruiting bodies | Selenate | NS 40 μg g−1 | – | NS 40 μg g−1 | ↑40 μg g−1 | NS 40 μg g−1 | [61] |
| 0.5–200 μg g−1—substrate | |||||||
| H. ericium erinaceus fruiting bodies | Selenite | ↓40 μg g−1 | – | ↑40 μg g−1 | NS 40 μg g−1 | ↓40 μg g−1 | |
| 0.5–200 μg g−1—substrate | |||||||
| H. ericium erinaceus fruiting bodies | SeMet | NS 40 μg g−1 | – | ↑40 μg g−1 | ↓40 μg g−1 | ↓40 μg g−1 | |
| 0.5–200 μg g−1—substrate | |||||||
| Brassica juncea L. Leaves | Sodium selenate | ↓ | NS | NS | – | NS | [173] |
| 50 mg L−1 | |||||||
| Triticum aestivum L. | Sodium selenite | ↓ | ↓ | ↓ | – | – | [32] |
| 202w17 (purple-grain)’ Grains | 10 mg ml−1—foliar | ||||||
| Triticum aestivum L. | Sodium selenite | ↓ | ↓ | NS | – | – | |
| ‘202w17 (purple-grain)’ Grains | 50 mg kg−1—soil |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, B.; Cao, H.; Wu, Q.; Mao, K.; Yang, X.; Su, J.; Zhang, H. Agronomic and Genetic Strategies to Enhance Selenium Accumulation in Crops and Their Influence on Quality. Foods 2023, 12, 4442. https://doi.org/10.3390/foods12244442
Zhou B, Cao H, Wu Q, Mao K, Yang X, Su J, Zhang H. Agronomic and Genetic Strategies to Enhance Selenium Accumulation in Crops and Their Influence on Quality. Foods. 2023; 12(24):4442. https://doi.org/10.3390/foods12244442
Chicago/Turabian StyleZhou, Bingqi, Haorui Cao, Qingqing Wu, Kang Mao, Xuefeng Yang, Junxia Su, and Hua Zhang. 2023. "Agronomic and Genetic Strategies to Enhance Selenium Accumulation in Crops and Their Influence on Quality" Foods 12, no. 24: 4442. https://doi.org/10.3390/foods12244442








