Spatial Distribution and Enrichment Dynamics of Foodborne Norovirus in Oyster Tissues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Artificial Contamination Oysters with NoV
2.2. Sample Collection and Processing Procedures
2.3. Quantitative Real-Time PCR (RT-qPCR) for NoV
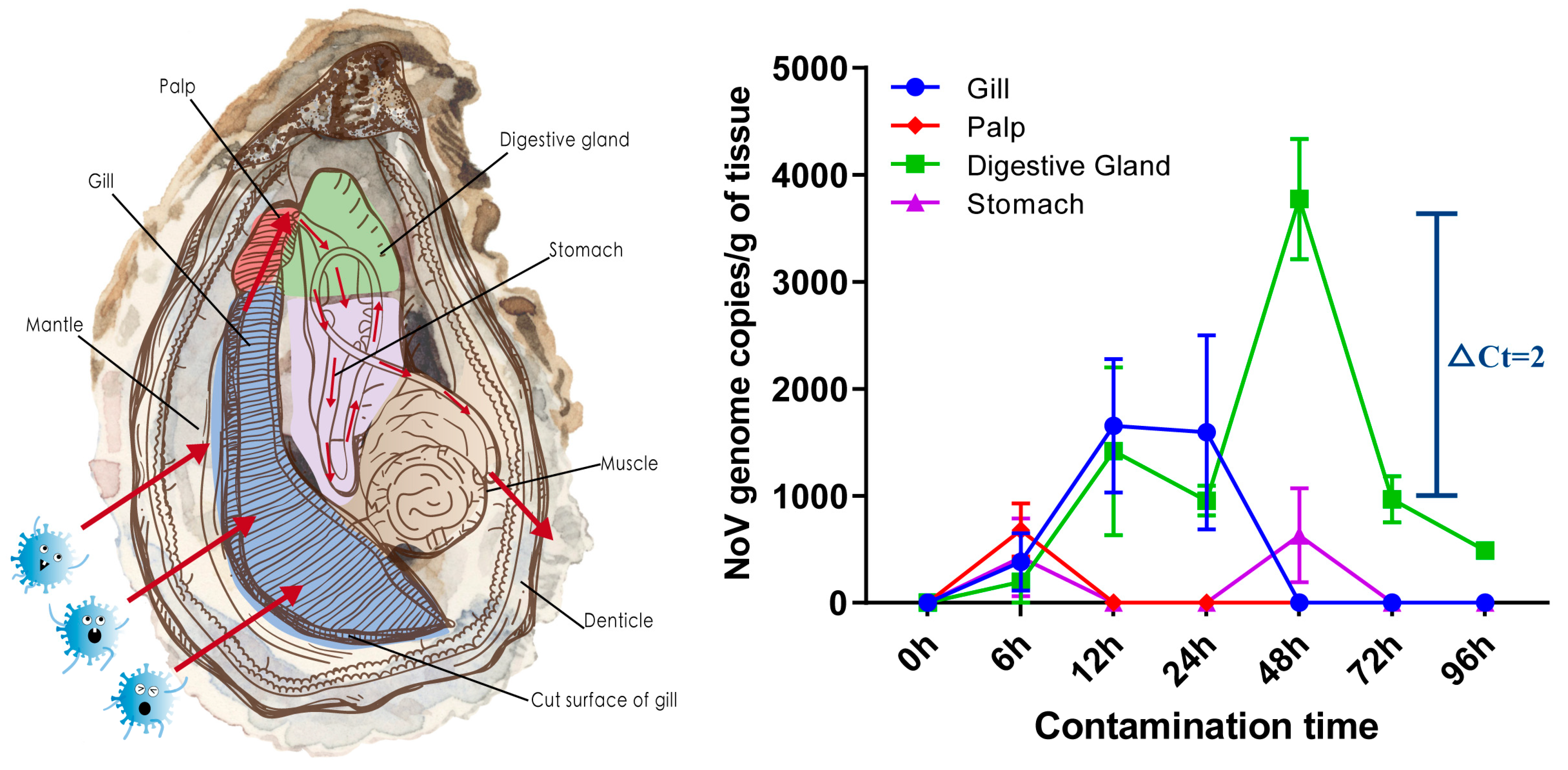
3. Results
3.1. Dynamics of NoV Contamination in Oyster Tissues
3.2. Relationship between Sampling Sites and the Detection Rate
3.3. Relationship between Contamination Time and Detection Rate
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rupnik, A.; Keaveney, S.; Devilly, L.; Butler, F.; Doré, W. The Impact of Winter Relocation and Depuration on Norovirus Concentrations in Pacific Oysters Harvested from a Commercial Production Site. Food Environ. Virol. 2018, 10, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World health organization global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Atmar, R.L.; Opekun, A.R.; Gilger, M.A.; Estes, M.K.; Crawford, S.E.; Neill, F.H.; Graham, D.Y. Norwalk Virus Shedding after Experimental Human Infection. Emerg. Infect. Dis. 2008, 14, 1553–1557. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, P.; de Graaf, M.; Parra, G.I.; Chan, M.C.; Green, K.; Martella, V.; Wang, Q.; White, P.A.; Katayama, K.; Vennema, H.; et al. Updated classification of norovirus genogroups and genotypes. J. Gen. Virol. 2019, 100, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Wu, S.; Kong, X.; Xie, H.; Fu, J.; He, Y.; Feng, W.; Liu, N.; Li, J.; Rainey, J.J.; et al. Norovirus Outbreak Surveillance, China, 2016–2018. Emerg. Infect. Dis. 2020, 26, 437–445. [Google Scholar] [CrossRef]
- Cannon, J.L.; Barclay, L.; Collins, N.R.; Wikswo, M.E.; Castro, C.J.; Magaña, L.C.; Gregoricus, N.; Marine, R.L.; Chhabra, P.; Vinjé, J. Genetic and Epidemiologic Trends of Norovirus Outbreaks in the United States from 2013 to 2016 Demonstrated Emergence of Novel GII.4 Recombinant Viruses. J. Clin. Microbiol. 2017, 55, 2208–2221. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Begall, L.F.; Mauroy, A.; Thiry, E. Noroviruses—The state of the art, nearly fifty years after their initial discovery. Viruses 2021, 13, 1541. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.J.A.; Lees, D.N. Environmental transmission of human noroviruses in shellfish waters. Appl. Environ. Microb. 2014, 80, 3552–3561. [Google Scholar] [CrossRef]
- Desdouits, M.; Wacrenier, C.; Ollivier, J.; Schaeffer, J.; Le Guyader, F.S. A targeted metagenomics approach to study the diversity of norovirus GII in shellfish implicated in outbreaks. Viruses 2020, 12, 978. [Google Scholar] [CrossRef]
- Scientific Opinion on an update on the present knowledge on the occurrence and control of foodborne viruses. EFSA J. 2011, 9, 2190.
- Lowther, J.; Gustar, N.; Powell, A.; Brien, S.O.; Lees, D.N. A one-year survey of norovirus in UK oysters collected at the point of sale. Food Environ. Virol. 2018, 10, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Chassaing, M.; Boudaud, N.; Belliot, G.; Estienney, M.; Majou, D.; de Rougemont, A.; Gantzer, C. Interaction between norovirus and Histo-Blood Group Antigens: A key to understanding virus transmission and inactivation through treatments? Food Microbiol. 2020, 92, 103594. [Google Scholar] [CrossRef] [PubMed]
- Green, T.J.; Yin Walker, C.; Leduc, S.; Michalchuk, T.; Mcallister, J.; Roth, M.; Janes, J.K.; Krogh, E.T. Spatial and temporal pattern of norovirus dispersal in an oyster growing region in the northeast pacific. Viruses 2022, 14, 762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, D.; Zhang, Z.; Hewitt, J.; Li, X.; Hou, P.; Wang, D.; Wu, Q. Surveillance of human norovirus in oysters collected from production area in Shandong Province, China during 2017–2018. Food Control 2021, 121, 107649. [Google Scholar] [CrossRef]
- Li, Y.; Xue, L.; Gao, J.; Cai, W.; Zhang, Z.; Meng, L.; Miao, S.; Hong, X.; Xu, M.; Wu, Q.; et al. A systematic review and meta-analysis indicates a substantial burden of human noroviruses in shellfish worldwide, with GII.4 and GII.2 being the predominant genotypes. Food Microbiol. 2023, 109, 104140. [Google Scholar] [CrossRef] [PubMed]
- Raymond, P.; Paul, S.; Guy, R.A. Impact of capsid and genomic integrity tests on norovirus extraction recovery rates. Foods 2023, 12, 826. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Huang, J.; Wang, T.; Fan, C.; Kang, J.; Zhang, Q.; Li, Y.; Chen, S. Ultrasensitive and visual detection of human norovirus genotype GII.4 or GII.17 using CRISPR-Cas12a assay. Virol. J. 2022, 19, 150. [Google Scholar] [CrossRef]
- Rupprom, K.; Chavalitshewinkoon-Petmitr, P.; Diraphat, P.; Kittigul, L. Evaluation of real-time RT-PCR assays for detection and quantification of norovirus genogroups I and II. Virol. Sin. 2017, 32, 139–146. [Google Scholar] [CrossRef]
- ISO 15216-1:2017/Amd 1:2021; Microbiology of the Food Chain—Horizontal Method for Determination of Hepatitis a Virus and Norovirus Using Real-Time RT-PCR—Part 1: Method for Quantification. International Organization for Standardization (ISO): Geneva, Switzerland, 2021.
- Teunis, P.F.M.; Moe, C.L.; Liu, P.E.; Miller, S.; Lindesmith, L.; Baric, R.S.; Le Pendu, J.; Calderon, R.L. Norwalk virus: How infectious is it? J. Med. Virol. 2008, 80, 1468–1476. [Google Scholar] [CrossRef]
- Tunyakittaveeward, T.; Rupprom, K.; Pombubpa, K.; Howteerakul, N.; Kittigul, L. Norovirus monitoring in oysters using two different extraction methods. Food Environ. Virol. 2019, 11, 374–382. [Google Scholar] [CrossRef]
- Quang Le, H.; Suffredini, E.; Tien Pham, D.; Kim To, A.; De Medici, D. Development of a method for direct extraction of viral RNA from bivalve molluscs. Lett. Appl. Microbiol. 2018, 67, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Plante, D.; Bran Barrera, J.A.; Lord, M.; Iugovaz, I.; Nasheri, N. Development of an RNA extraction protocol for norovirus from raw oysters and detection by qRT-PCR and Droplet-Digital RT-PCR. Foods 2021, 10, 1804. [Google Scholar] [CrossRef] [PubMed]
- Lowmoung, T.; Pombubpa, K.; Duangdee, T.; Tipayamongkholgul, M.; Kittigul, L. Distribution of naturally occurring norovirus genogroups i, II, and IV in oyster tissues. Food Environ. Virol. 2017, 9, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Pilotto, M.R.; Souza, D.S.M.; Barardi, C.R.M. Viral uptake and stability in Crassostrea gigas oysters during depuration, storage and steaming. Mar. Pollut. Bull. 2019, 149, 110524. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, Z.; Wu, Q.; Tian, P.; Geng, H.; Xu, T.; Wang, D. Redesigned duplex RT-qPCR for the detection of GI and GII human noroviruses. Engineering 2020, 6, 442–448. [Google Scholar] [CrossRef]
- Kibenge, F.S.B.; Strange, R.J. Chapter 1—Introduction to the anatomy and physiology of the major aquatic animal species in aquaculture. In Aquaculture Pharmacology; Kibenge, F.S.B., Baldisserotto, B., Chong, R.S., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1–111. [Google Scholar]
- Maalouf, H.; Zakhour, M.; Le Pendu, J.; Le Saux, J.; Atmar, R.L.; Le Guyader, F.S. Distribution in tissue and seasonal variation of norovirus genogroup i and II ligands in oysters. Appl. Environ. Microb. 2010, 76, 5621–5630. [Google Scholar] [CrossRef] [PubMed]
- Le Guyader, F.S.; Loisy, F.; Atmar, R.L.; Hutson, A.M.; Estes, M.K.; Ruvoën-Clouet, N.; Pommepuy, M.; Le Pendu, J. Norwalk Virus–specific Binding to Oyster Digestive Tissues. Emerg. Infect. Dis. 2006, 12, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.; Engelbrektson, A.L.; Jiang, X.; Zhong, W.; Mandrell, R.E. Norovirus recognizes histo-blood group antigens on gastrointestinal cells of clams, mussels, and oysters: A possible mechanism of bioaccumulation. J. Food Prot. 2007, 70, 2140–2147. [Google Scholar] [CrossRef]
- Tan, M.; Xia, M.; Cao, S.; Huang, P.; Farkas, T.; Meller, J.; Hegde, R.S.; Li, X.; Rao, Z.; Jiang, X. Elucidation of strain-specific interaction of a GII-4 norovirus with HBGA receptors by site-directed mutagenesis study. Virology 2008, 379, 324–334. [Google Scholar] [CrossRef]
- Morozov, V.; Hanisch, F.; Wegner, K.M.; Schroten, H. Pandemic GII.4 sydney and epidemic GII.17 kawasaki308 noroviruses display distinct specificities for Histo-Blood group antigens leading to different transmission vector dynamics in pacific oysters. Front. Microbiol. 2018, 9, 2826. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Wu, Q.; Wang, D.; Johnson, K.N.; Johnson, K.N. Oyster heat shock protein 70 plays a role in binding of human noroviruses. Appl. Environ. Microb. 2021, 87, e79021. [Google Scholar] [CrossRef] [PubMed]
- Torok, V.; Hodgson, K.; Mcleod, C.; Tan, J.; Malhi, N.; Turnbull, A. National survey of foodborne viruses in Australian oysters at production. Food Microbiol. 2018, 69, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Manuel, C.S.; Moore, M.D.; Jaykus, L. Predicting human norovirus infectivity—Recent advances and continued challenges. Food Microbiol. 2018, 76, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Shen, J.; Reece, K.S. A deterministic model for understanding nonlinear viral dynamics in oysters. Appl. Environ. Microb. 2022, 88, e236021. [Google Scholar] [CrossRef] [PubMed]
- Hartard, C.; Leclerc, M.; Rivet, R.; Maul, A.; Loutreul, J.; Banas, S.; Boudaud, N.; Gantzer, C. F-Specific RNA bacteriophages, especially members of subgroup II, should be reconsidered as good indicators of viral pollution of oysters. Appl. Environ. Microb. 2018, 84, e01866-17. [Google Scholar] [CrossRef]
- Lowther, J.A.; Gustar, N.E.; Hartnell, R.E.; Lees, D.N. Comparison of norovirus RNA levels in Outbreak-Related oysters with background environmental levels. J. Food Protect. 2012, 75, 389–393. [Google Scholar] [CrossRef]
- Dore, B.; Keaveney, S.; Flannery, J.; Rajko-Nenow, P. Management of health risks associated with oysters harvested from a norovirus contaminated area, Ireland, February–March 2010. Euro Surveill 2010, 15, 19567. [Google Scholar] [CrossRef]
- Campos, C.J.A.; Kershaw, S.; Morgan, O.C.; Lees, D.N. Risk factors for norovirus contamination of shellfish water catchments in England and Wales. Int. J. Food Microbiol. 2017, 241, 318–324. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, M.; Zhang, Z.; Zhao, X.; Geng, H.; Xue, L.; Liu, D. Spatial Distribution and Enrichment Dynamics of Foodborne Norovirus in Oyster Tissues. Foods 2024, 13, 128. https://doi.org/10.3390/foods13010128
Mao M, Zhang Z, Zhao X, Geng H, Xue L, Liu D. Spatial Distribution and Enrichment Dynamics of Foodborne Norovirus in Oyster Tissues. Foods. 2024; 13(1):128. https://doi.org/10.3390/foods13010128
Chicago/Turabian StyleMao, Mao, Zilei Zhang, Xuchong Zhao, Haoran Geng, Liang Xue, and Danlei Liu. 2024. "Spatial Distribution and Enrichment Dynamics of Foodborne Norovirus in Oyster Tissues" Foods 13, no. 1: 128. https://doi.org/10.3390/foods13010128
APA StyleMao, M., Zhang, Z., Zhao, X., Geng, H., Xue, L., & Liu, D. (2024). Spatial Distribution and Enrichment Dynamics of Foodborne Norovirus in Oyster Tissues. Foods, 13(1), 128. https://doi.org/10.3390/foods13010128





