Tracking the Mandorla di Avola Almond Variety by Means of ICP Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Collection
2.3. Sample Treatment
2.3.1. Soil
2.3.2. Almonds and Other Vegetal Matrices
- Acid digestion was applied to 0.5 g of almonds in PTFE vessels, then 1 mL of 30% hydrogen peroxide, 3 mL of nitric acid 69%, and 4 mL of HPW were added, and the vessels were put inside the microwave oven system. During the heating treatment, the temperature was increased from 25 °C to 180 °C over a 10 min period and the temperature was kept constant for 15 min. The resulting solution was taken to 50 mL with HPW in a polypropylene tube. Solutions were then diluted 1:10 with HPW prior to ICP analysis.
- Dry ashing was applied by weighting ca. 10 g of almonds into a porcelain capsule and put inside a Milestone (Sorisole, Italy) Pyro 260 microwave ashing system. The heating cycle was as follows: room temperature to 150 °C in 10 min; hold at 150 °C for 20 min; raise temperature up to 500 °C in 20 min; hold at 500 °C for 30 min; raise temperature up to 750 °C in 10 min; hold at 750 °C for 30 min; raise temperature up to 1000 °C in 10 min; hold at 1000 °C for 30 min. The different heating steps were chosen in order to remove specific groups of molecules. The resulting ash was completely dissolved in 2.0 mL of ultrapure concentrated nitric acid and taken up to 50 mL with HPW in a polypropylene tube. Solutions were then diluted 1:10 with HPW prior to ICP analysis.
2.4. ICP-MS Analysis
2.5. ICP-OES Analysis
2.6. Analysis of Certified Samples
2.7. Data Analysis
3. Results
- the soil on which the almond trees grow;
- the leaves of the almond tree;
- almonds;
- almond husks.
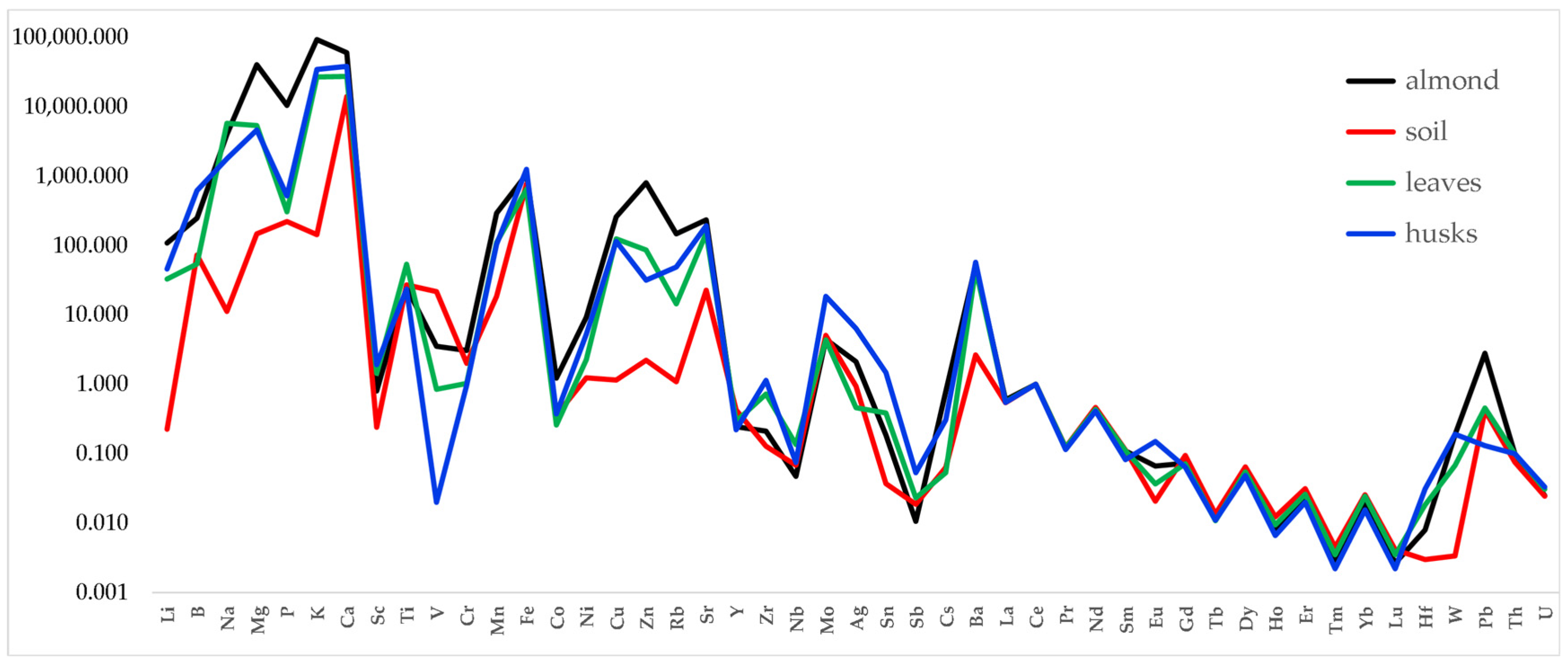
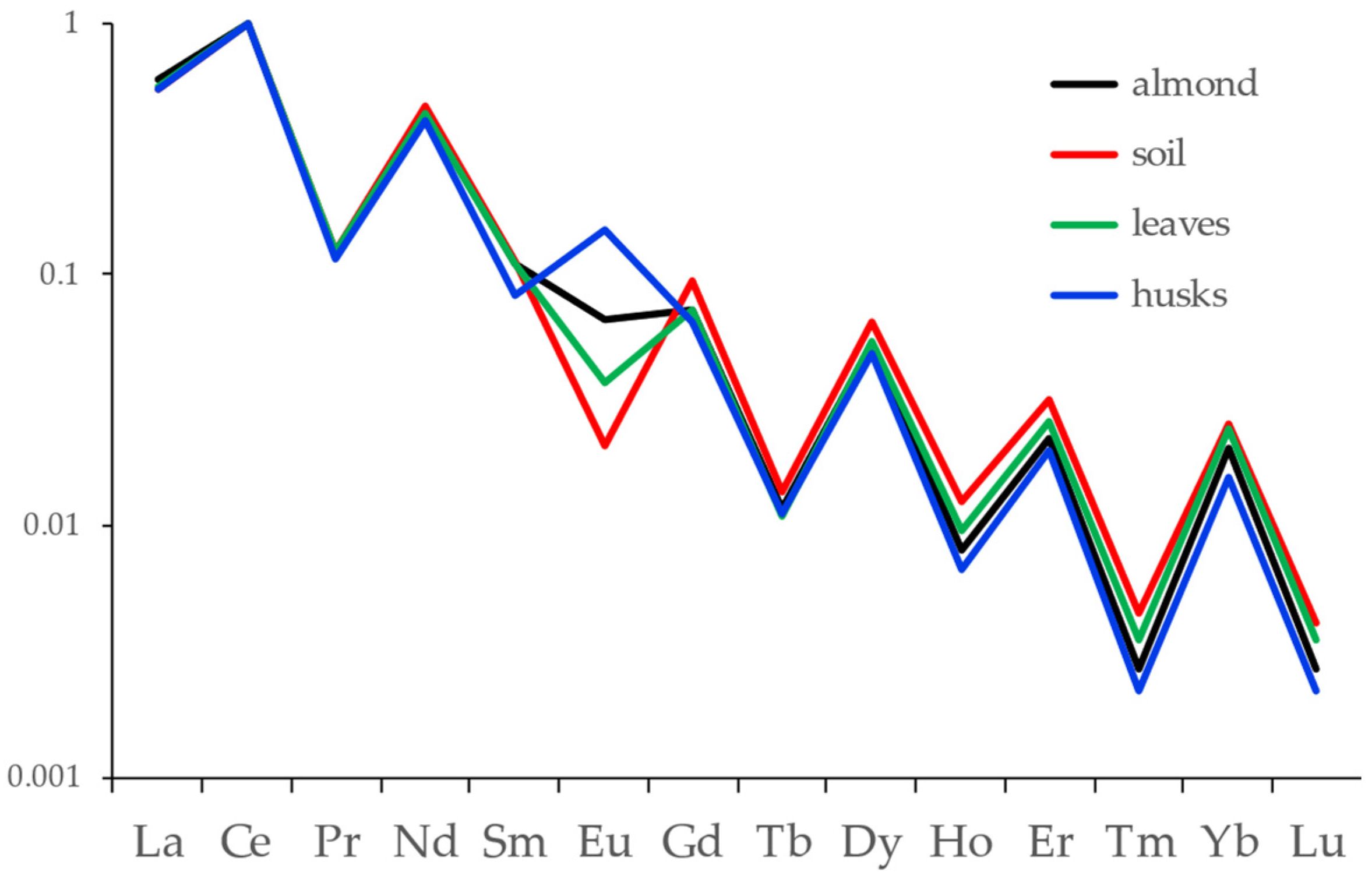
3.1. Traceability of the Almond Chain
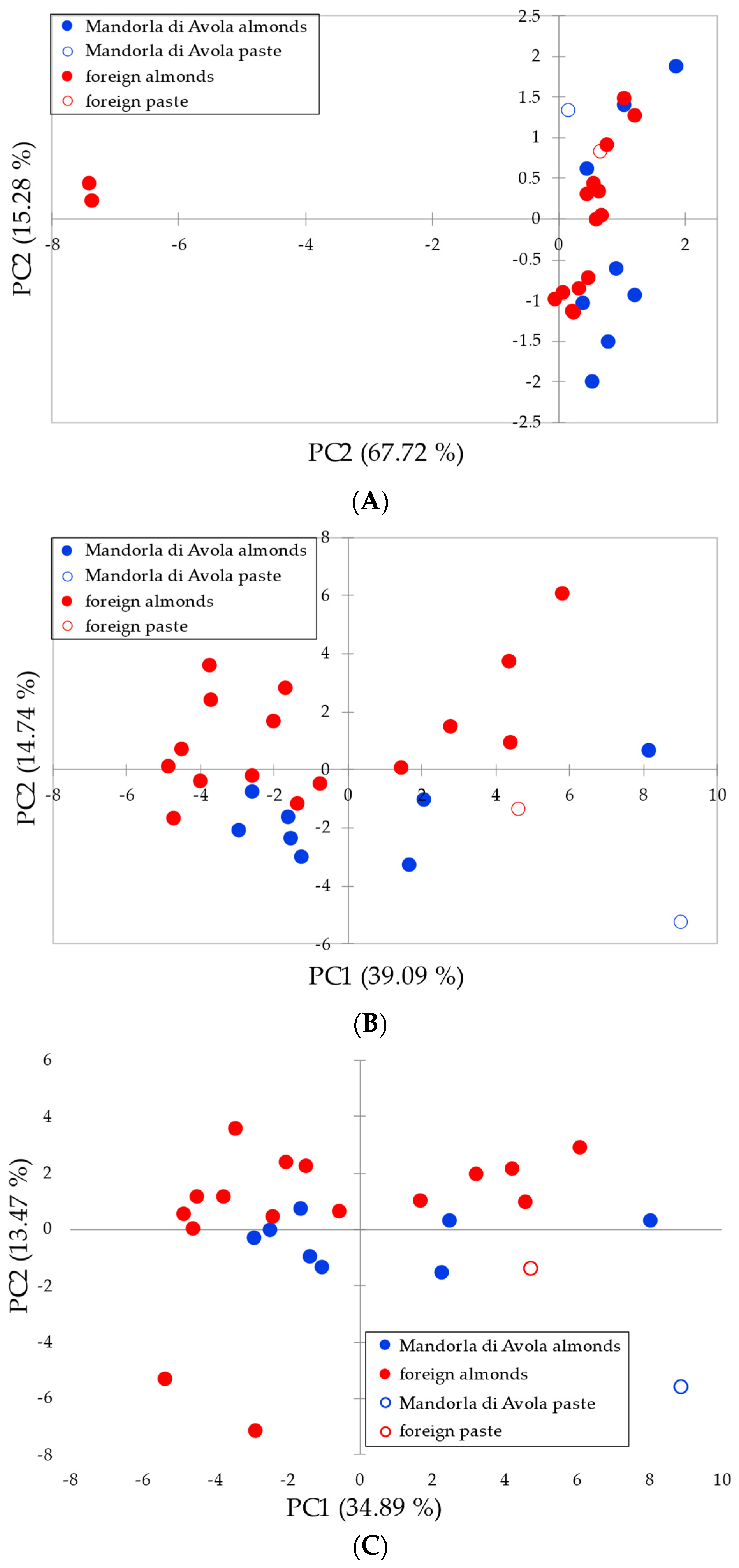
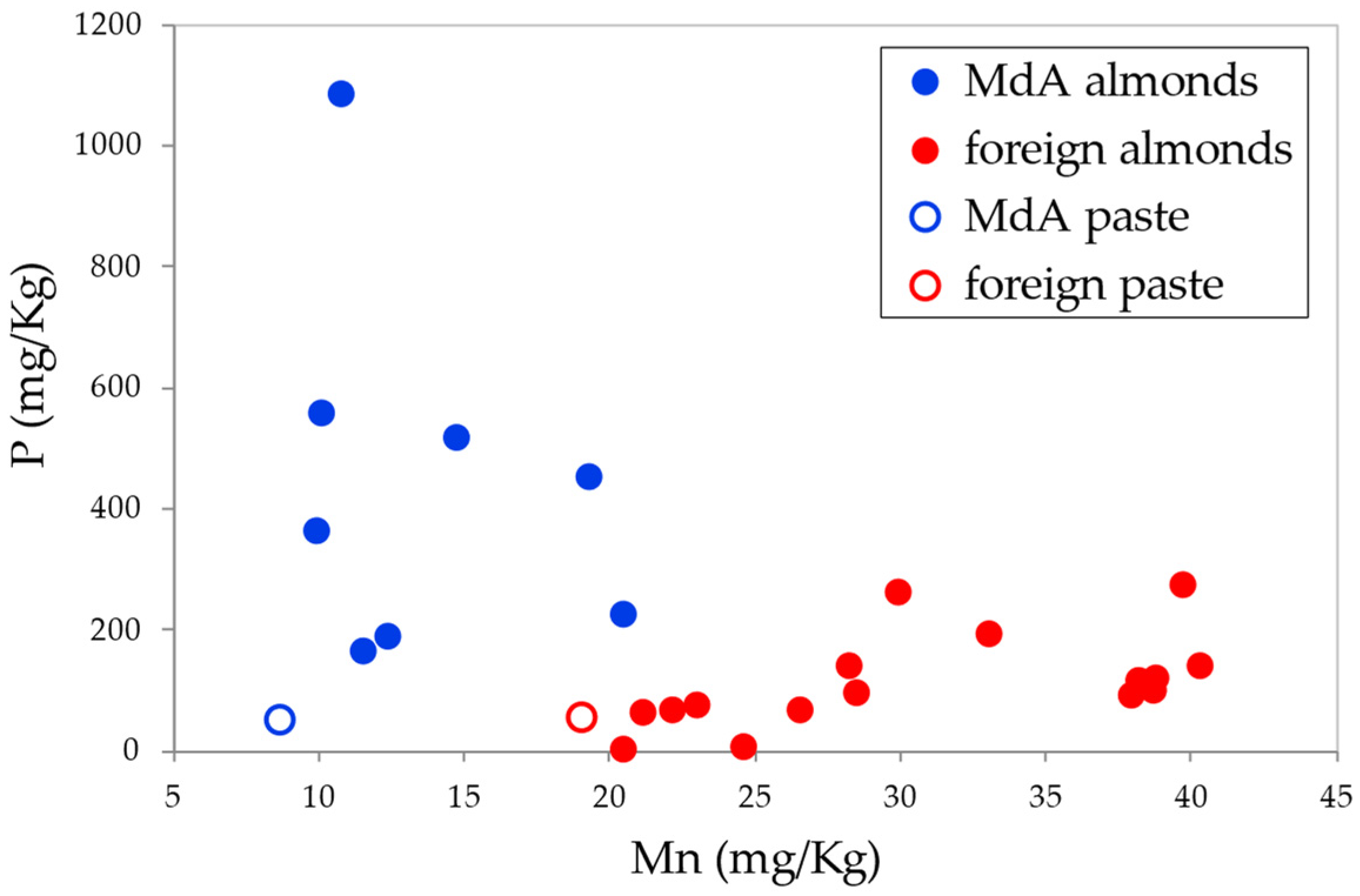
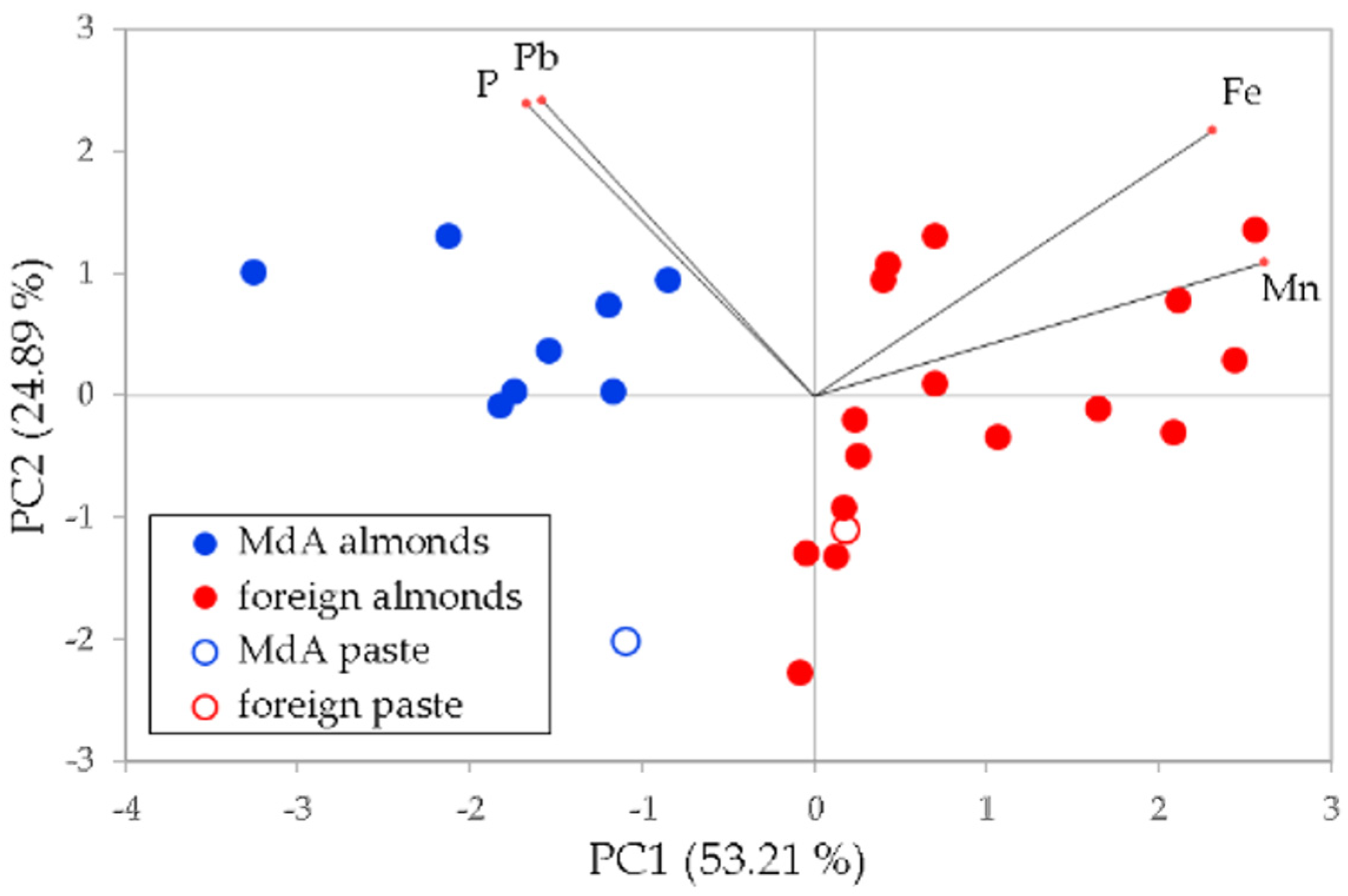
3.2. Authentication on the Avola Almond
- eight samples of Mandorla di Avola variety;
- six samples from California;
- two samples from Spain;
- six samples from Sicily;
- two samples from Italy (unspecified region);
- two samples of almond paste, one made from Avola almonds and one from foreign almonds.
4. Discussion
4.1. Traceability of the Almond Chain
4.2. Authentication on the Avola Almond
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Singar, S.; Kadyan, S.; Patoine, C.; Park, G.; Arjmandi, B.; Nagpal, R. The Effects of Almond Consumption on Cardiovascular Health and Gut Microbiome: A Comprehensive Review. Nutrients 2024, 16, 1964. [Google Scholar] [CrossRef] [PubMed]
- Ouzir, M. Nutritional and Health-Beneficial Values of Almond Nuts Consumption. Nutrire 2023, 48, 53. [Google Scholar] [CrossRef]
- Özcan, M.M. A Review on Some Properties of Almond: Impact of Processing, Fatty Acids, Polyphenols, Nutrients, Bioactive Properties, and Health Aspects. J. Food Sci. Technol. 2023, 60, 1493–1504. [Google Scholar] [CrossRef]
- Lacivita, V.; Derossi, A.; Caporizzi, R.; Lamacchia, C.; Speranza, B.; Guerrieri, A.; Racioppo, A.; Corbo, M.R.; Sinigaglia, M.; Severini, C. Discover Hidden Value of Almond By-Products: Nutritional, Sensory, Technological and Microbiological Aspects. Future Foods 2024, 10, 100398. [Google Scholar] [CrossRef]
- Barral-Martinez, M.; Fraga-Corral, M.; Garcia-Perez, P.; Simal-Gandara, J.; Prieto, M.A. Almond By-Products: Valorization for Sustainability and Competitiveness of the Industry. Foods 2021, 10, 1793. [Google Scholar] [CrossRef]
- Consorzio Mandorla Di Avola. Available online: https://consorziomandorlaavola.it/ (accessed on 19 June 2024).
- Bottone, A.; Montoro, P.; Masullo, M.; Pizza, C.; Piacente, S. Metabolite Profiling and Antioxidant Activity of the Polar Fraction of Italian Almonds (Toritto and Avola): Analysis of Seeds, Skins, and Blanching Water. J. Pharm. Biomed. Anal. 2020, 190, 113518. [Google Scholar] [CrossRef]
- Smeriglio, A.; Mandalari, G.; Bisignano, C.; Filocamo, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Polyphenolic Content and Biological Properties of Avola Almond (Prunus dulcis Mill. D.A. Webb) Skin and Its Industrial Byproducts. Ind. Crops Prod. 2016, 83, 283–293. [Google Scholar] [CrossRef]
- Bottone, A.; Masullo, M.; Montoro, P.; Pizza, C.; Piacente, S. HR-LC-ESI-Orbitrap-MS Based Metabolite Profiling of Prunus dulcis Mill. (Italian Cultivars Toritto and Avola) Husks and Evaluation of Antioxidant Activity. Phytochem. Anal. 2019, 30, 415–423. [Google Scholar] [CrossRef]
- Kahlaoui, M.; Borotto Dalla Vecchia, S.; Giovine, F.; Ben Haj Kbaier, H.; Bouzouita, N.; Barbosa Pereira, L.; Zeppa, G. Characterization of Polyphenolic Compounds Extracted from Different Varieties of Almond Hulls (Prunus dulcis L.). Antioxidants 2019, 8, 647. [Google Scholar] [CrossRef]
- Bottone, A.; Montoro, P.; Masullo, M.; Pizza, C.; Piacente, S. Metabolomics and Antioxidant Activity of the Leaves of Prunus dulcis Mill. (Italian Cvs. Toritto and Avola). J. Pharm. Biomed. Anal. 2018, 158, 54–65. [Google Scholar] [CrossRef]
- De Giorgio, D.; Leo, L.; Zacheo, G.; Lamascese, N. Evaluation of 52 Almond (Prunus amygdalus Batsch) Cultivars from the Apulia Region in Southern Italy. J. Hortic. Sci. Biotechnol. 2007, 82, 541–546. [Google Scholar] [CrossRef]
- Ingegneri, M.; Smeriglio, A.; Rando, R.; Gervasi, T.; Tamburello, M.P.; Ginestra, G.; La Camera, E.; Pennisi, R.; Sciortino, M.T.; Mandalari, G.; et al. Composition and Biological Properties of Blanched Skin and Blanch Water Belonging to Three Sicilian Almond Cultivars. Nutrients 2023, 15, 1545. [Google Scholar] [CrossRef] [PubMed]
- Almond of Avola, DNA Analysis Reveals Two Maxi-Frauds on Sugared Almonds. Available online: https://www.greatitalianfoodtrade.it/en/mercati/mandorla-di-avola-lanalisi-del-dna-svela-due-maxi-frodi-sui-confetti/ (accessed on 19 June 2024).
- Beltrán Sanahuja, A.; Maestre Pérez, S.E.; Grané Teruel, N.; Valdés García, A.; Prats Moya, M.S. Variability of Chemical Profile in Almonds (Prunus dulcis) of Different Cultivars and Origins. Foods 2021, 10, 153. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Casal, S.; Ferreira, I.C.F.R.; Peres, A.M.; Pereira, J.A.; Oliveira, M.B.P.P. Supervised Chemical Pattern Recognition in Almond (Prunus dulcis) Portuguese PDO Cultivars: PCA- and LDA-Based Triennial Study. J. Agric. Food Chem. 2012, 60, 9697–9704. [Google Scholar] [CrossRef] [PubMed]
- Kalogiouri, N.P.; Mitsikaris, P.D.; Klaoudatos, D.; Papadopoulos, A.N.; Samanidou, V.F. A Rapid HPLC-UV Protocol Coupled to Chemometric Analysis for the Determination of the Major Phenolic Constituents and Tocopherol Content in Almonds and the Discrimination of the Geographical Origin. Molecules 2021, 26, 5433. [Google Scholar] [CrossRef] [PubMed]
- Arndt, M.; Rurik, M.; Drees, A.; Ahlers, C.; Feldmann, S.; Kohlbacher, O.; Fischer, M. Food Authentication: Determination of the Geographical Origin of Almonds (Prunus dulcis Mill.) via near-Infrared Spectroscopy. Microchem. J. 2021, 160, 105702. [Google Scholar] [CrossRef]
- von Wuthenau, K.; Segelke, T.; Müller, M.-S.; Behlok, H.; Fischer, M. Food Authentication of Almonds (Prunus dulcis Mill.). Origin Analysis with Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Chemometrics. Food Control 2022, 134, 108689. [Google Scholar] [CrossRef]
- von Wuthenau, K.; Müller, M.-S.; Cvancar, L.; Oest, M.; Fischer, M. Food Authentication of Almonds (Prunus dulcis Mill.). Fast Origin Analysis with Laser Ablation Inductively Coupled Plasma Mass Spectrometry and Chemometrics. J. Agric. Food Chem. 2022, 70, 5237–5244. [Google Scholar] [CrossRef]
- Amorello, D.; Orecchio, S.; Pace, A.; Barreca, S. Discrimination of Almonds (Prunus dulcis) Geographical Origin by Minerals and Fatty Acids Profiling. Nat. Prod. Res. 2016, 30, 2107–2110. [Google Scholar] [CrossRef]
- Firmani, P.; Bucci, R.; Marini, F.; Biancolillo, A. Authentication of “Avola Almonds” by near Infrared (NIR) Spectroscopy and Chemometrics. J. Food Compos. Anal. 2019, 82, 103235. [Google Scholar] [CrossRef]
- Scappaticci, C.; Foschi, M.; Plaku, A.; Biancolillo, A.; D’Archivio, A.A. Enhancing Traceability of Italian Almonds through IR Spectroscopy and Chemometric Classifiers. Appl. Sci. 2023, 13, 12765. [Google Scholar] [CrossRef]
- Salvo, A.; Rotondo, A.; Mangano, V.; Grimaldi, M.; Stillitano, I.; D’Ursi, A.M.; Dugo, G.; Rastrelli, L. High-Resolution Magic Angle Spinning Nuclear Magnetic Resonance (HR-MAS-NMR) as Quick and Direct Insight of Almonds. Nat. Prod. Res. 2020, 34, 71–77. [Google Scholar] [CrossRef]
- Di Guardo, M.; Farneti, B.; Khomenko, I.; Luca, L.; Modica, G.; Mosca, A.; Distefano, G.; Bianco, L.; Troggio, M.; Sottile, F.; et al. Direct-Injection Spectrometry and Whole-Genome Genotyping Unravel the Genetic Regulation of Fresh and Roasted Kernels of Almond. Acta Hortic. 2023, 1362, 367–372. [Google Scholar] [CrossRef]
- Di Guardo, M.; Farneti, B.; Khomenko, I.; Modica, G.; Mosca, A.; Distefano, G.; Bianco, L.; Troggio, M.; Sottile, F.; La Malfa, S.; et al. Genetic Characterization of an Almond Germplasm Collection and Volatilome Profiling of Raw and Roasted Kernels. Hortic. Res. 2021, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Ibourki, M.; Hallouch, O.; Devkota, K.; Guillaume, D.; Hirich, A.; Gharby, S. Elemental Analysis in Food: An Overview. J. Food Compos. Anal. 2023, 120, 105330. [Google Scholar] [CrossRef]
- Mazarakioti, E.C.; Zotos, A.; Thomatou, A.-A.; Kontogeorgos, A.; Patakas, A.; Ladavos, A. Inductively Coupled Plasma-Mass Spectrometry (ICP-MS), a Useful Tool in Authenticity of Agricultural Products’ and Foods’ Origin. Foods 2022, 11, 3705. [Google Scholar] [CrossRef]
- Karabagias, I.K. Advances of Spectrometric Techniques in Food Analysis and Food Authentication Implemented with Chemometrics. Foods 2020, 9, 1550. [Google Scholar] [CrossRef]
- Hu, B.; He, M.; Chen, B.; Xu, C.; Zhang, Q.; Ma, J.; Feng, Y.; Cui, Z. Elemental Mass Spectrometry in Food and Environmental Chemistry. In Mass Spectrometry in Food and Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2022; pp. 53–97. [Google Scholar]
- Calà, E.; Fracchia, A.; Robotti, E.; Gulino, F.; Gullo, F.; Oddone, M.; Massacane, M.; Cordone, G.; Aceto, M. On the Traceability of the Hazelnut Production Chain by Means of Trace Elements. Molecules 2022, 27, 3854. [Google Scholar] [CrossRef] [PubMed]
- Gulino, F.; Calà, E.; Cozzani, C.; Vaccari, L.; Oddone, M.; Aceto, M. On the Traceability of Honey by Means of Lanthanide Distribution. Foods 2023, 12, 1803. [Google Scholar] [CrossRef] [PubMed]
- Aceto, M.; Calà, E.; Musso, D.; Regalli, N.; Oddone, M. A Preliminary Study on the Authentication and Traceability of Extra Virgin Olive Oil Made from Taggiasca Olives by Means of Trace and Ultra-Trace Elements Distribution. Food Chem. 2019, 298, 125047. [Google Scholar] [CrossRef]
- Aceto, M.; Musso, D.; Calà, E.; Arieri, F.; Oddone, M. Role of Lanthanides in the Traceability of the Milk Production Chain. J. Agric. Food Chem. 2017, 65, 4200–4208. [Google Scholar] [CrossRef] [PubMed]
- Aceto, M.; Gulino, F.; Calà, E.; Robotti, E.; Petrozziello, M.; Tsolakis, C.; Cassino, C. Authentication and Traceability Study on Barbera d’asti and Nizza Docg Wines: The Role of Trace-and Ultra-Trace Elements. Beverages 2020, 6, 63. [Google Scholar] [CrossRef]
- Nguyen, Q.; Nguyen, T.; Le, V.; Nguyen, N.; Truong, N.; Hoang, M.; Pham, T.; Bui, Q. Towards a Standardized Approach for the Geographical Traceability of Plant Foods Using Inductively Coupled Plasma Mass Spectrometry (ICP-MS) and Principal Component Analysis (PCA). Foods 2023, 12, 1848. [Google Scholar] [CrossRef] [PubMed]
| Parameters | Value |
|---|---|
| Forward power | 1550 W |
| Plasma gas flow | 14.0 L/min |
| Nebulizer gas flow | 0.9 L/min |
| Auxiliary gas flow | 0.8 L/min |
| Isotopes used | 45Sc, 49Ti, 51V, 52Cr, 55Mn, 57Fe, 59Co, 60Ni, 63Cu, 66Zn, 85Rb, 88Sr, 89Y, 90Zr, 93Nb, 95Mo, 107Ag, 118Sb, 121Sb, 133Cs, 137Ba, 139La, 140Ce, 141Pr, 146Nd, 147Sm, 153Eu, 157Gd, 159Tb, 163Dy, 165Ho, 166Er, 169Tm, 172Yb, 175Lu, 179Hf, 182W, 208Pb, 232Th and 238U |
| Internal standards | 103Rh, 115In and 193Ir at 10 μg/L |
| CeO+/Ce+ | <0.5% in KED mode |
| Parameters | Value |
|---|---|
| Plasma observation | axial |
| Forward power | 1400 W |
| Plasma gas flow | 12.0 L/min |
| Nebulizer gas flow | 0.96 L/min |
| Auxiliary gas flow | 0.90 L/min |
| Wavelengths used | B (249.773 nm) Ca (317.993 nm) K (766.491 nm) Li (670.780 nm) Mg (285.213 nm) Na (589.592 nm) P (177.495 nm) |
| RF generator | 40 MHz |
| RF power | 1300 W |
| Elements | F Value |
|---|---|
| Mn | 36.72 |
| P | 17.91 |
| Pb | 12.14 |
| Fe | 5.98 |
| Variables | PC1 | PC2 |
|---|---|---|
| Fe | 0.806 | 0.518 |
| Mn | 0.911 | 0.260 |
| P | −0.586 | 0.571 |
| Pb | −0.552 | 0.579 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulino, F.; Siragusa, C.; Calà, E.; Gullo, F.; Aceto, M. Tracking the Mandorla di Avola Almond Variety by Means of ICP Analysis. Foods 2024, 13, 2634. https://doi.org/10.3390/foods13162634
Gulino F, Siragusa C, Calà E, Gullo F, Aceto M. Tracking the Mandorla di Avola Almond Variety by Means of ICP Analysis. Foods. 2024; 13(16):2634. https://doi.org/10.3390/foods13162634
Chicago/Turabian StyleGulino, Federica, Cassandra Siragusa, Elisa Calà, Francesca Gullo, and Maurizio Aceto. 2024. "Tracking the Mandorla di Avola Almond Variety by Means of ICP Analysis" Foods 13, no. 16: 2634. https://doi.org/10.3390/foods13162634
APA StyleGulino, F., Siragusa, C., Calà, E., Gullo, F., & Aceto, M. (2024). Tracking the Mandorla di Avola Almond Variety by Means of ICP Analysis. Foods, 13(16), 2634. https://doi.org/10.3390/foods13162634







