Inhibition of Amyloid β Accumulation by Protease-Digested Whitebait (Shirasu) in a Murine Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Protease Digestion of Fish
2.2. Fluorescent BACE1 Assay
2.3. Evaluation of BACE1 Activity by Fluorescence HPLC
2.4. Preparation of Feed Administration
2.5. Animal Test
2.6. Quantification of Insoluble Aβ
2.7. Western Blotting
2.8. Statistical Analyses
3. Results
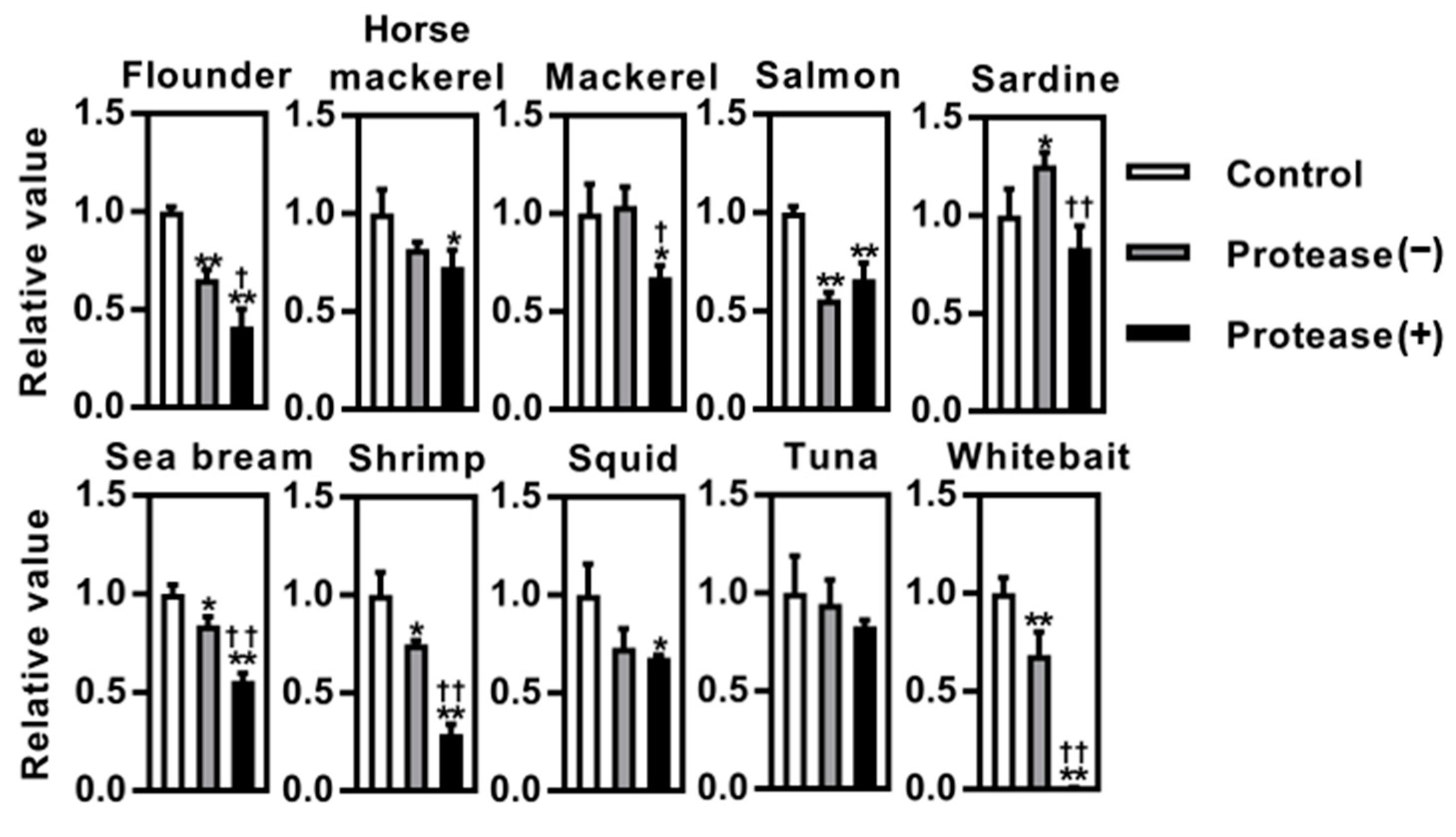
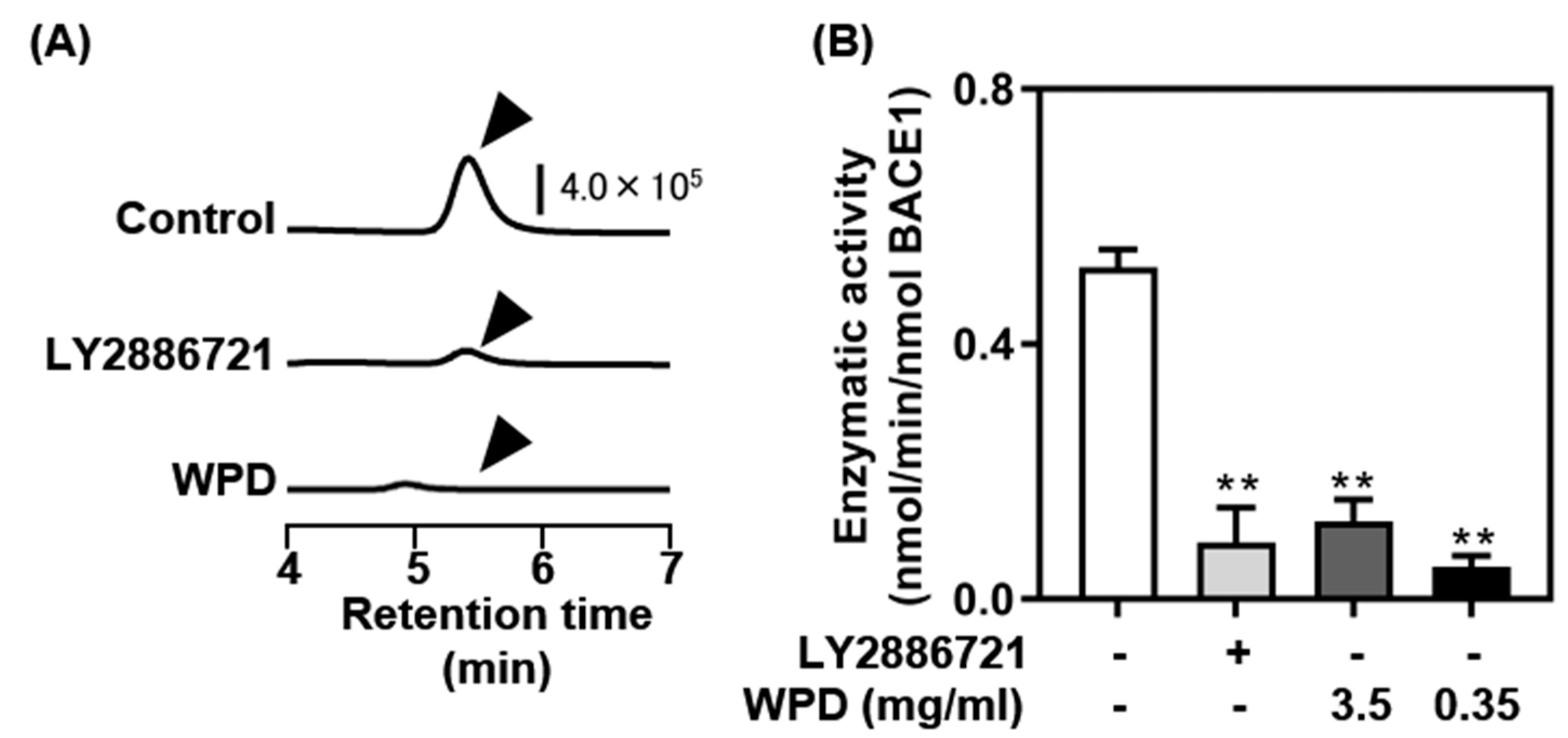
3.1. Screening of BACE1 Inhibitory Activity in Vitro
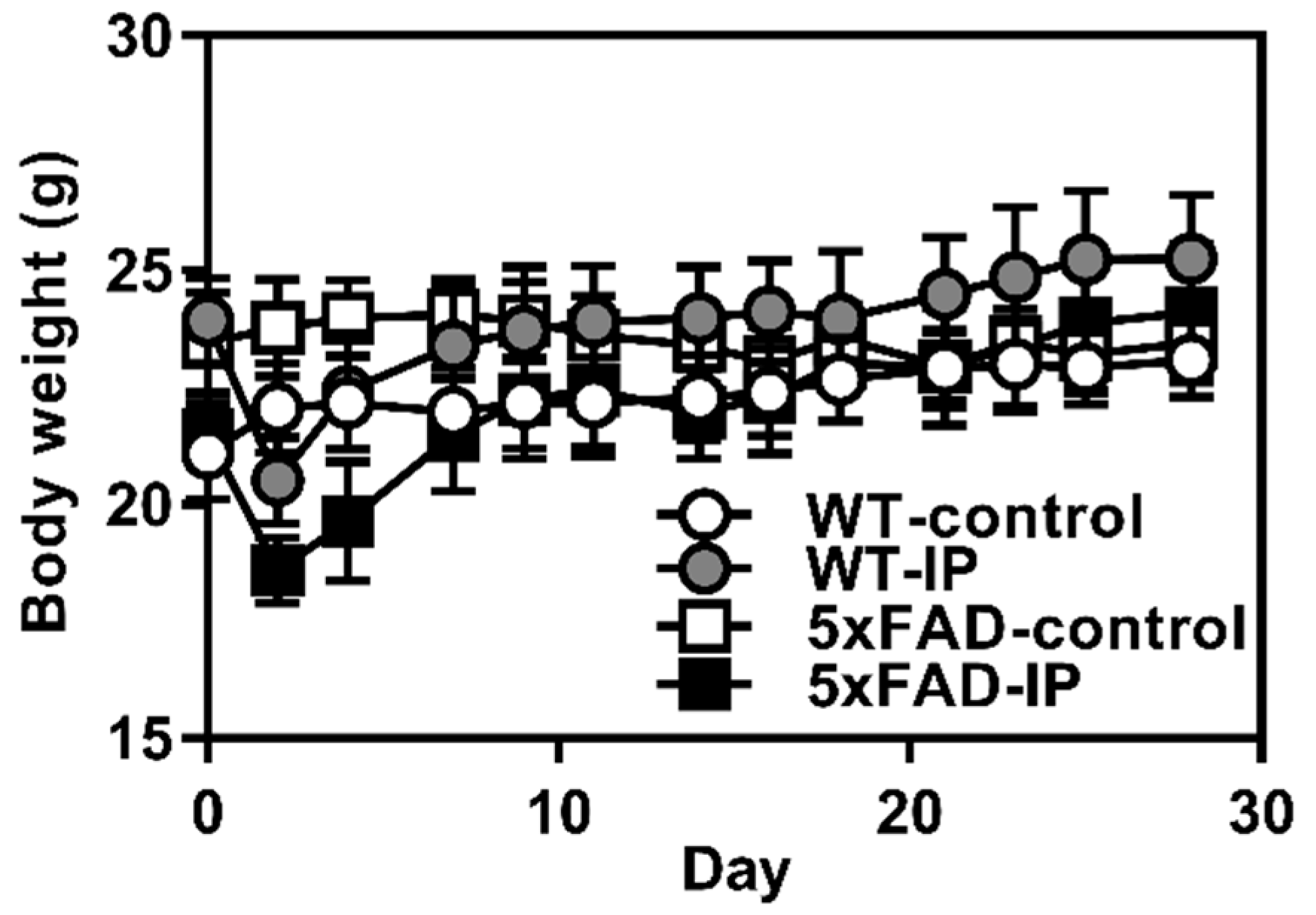
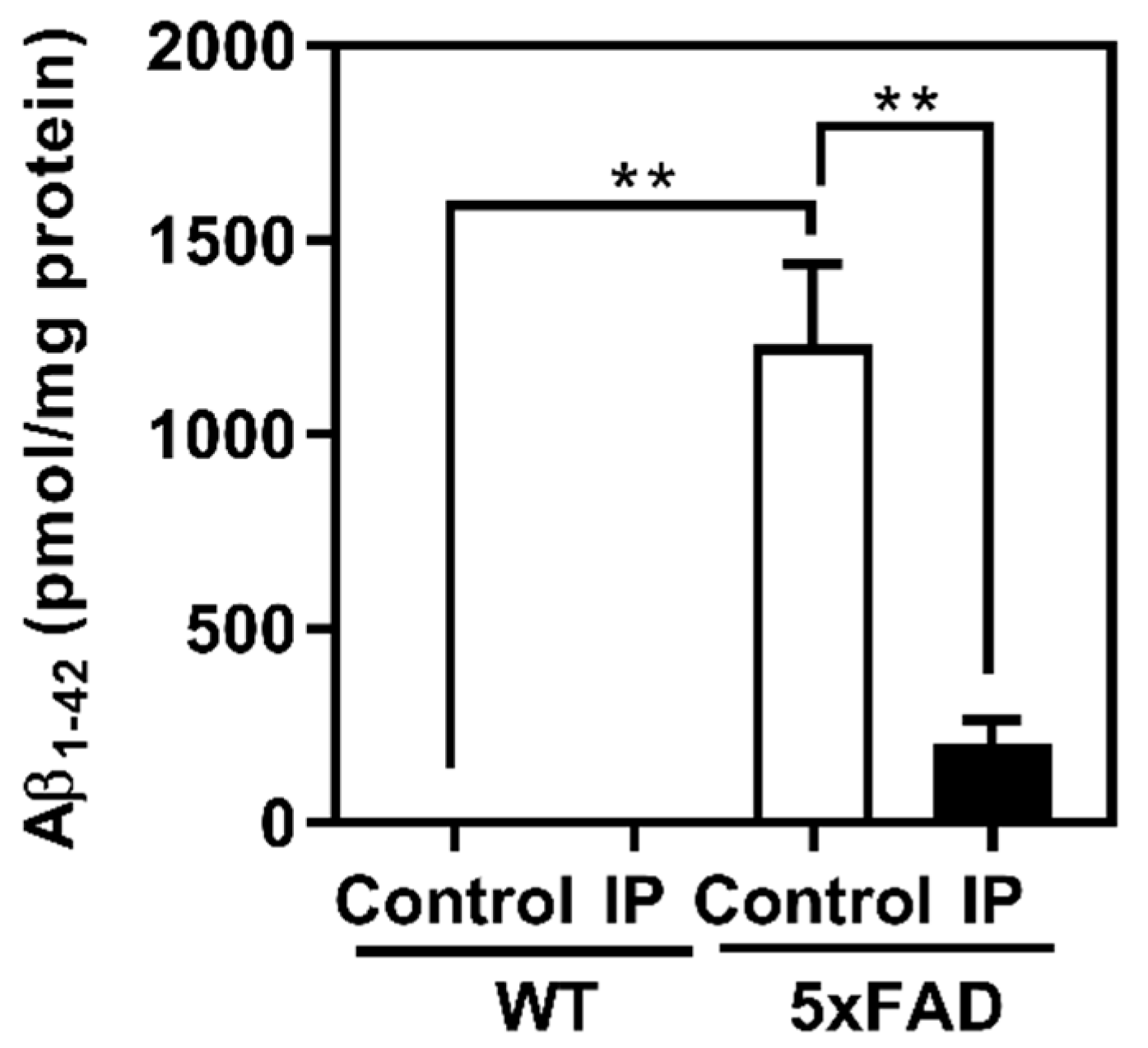
3.2. Evaluation of Effects of WPD Administration on 5xFAD Mice
3.3. Analysis of Astrogliosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Crous-Bou, M.; Minguillon, C.; Gramunt, N.; Molinuevo, J.L. Alzheimer’s disease prevention: From risk factors to early intervention. Alzheimers Res. Ther. 2017, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Passeri, E.; Elkhoury, K.; Morsink, M.; Broersen, K.; Linder, M.; Tamayol, A.; Malaplate, C.; Yen, F.T.; Arab-Tehrany, E. Alzheimer’s disease: Treatment strategies and their limitations. Int. J. Mol. Sci. 2022, 23, 13954. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Wolfe, M.S. When loss is gain: Reduced presenilin proteolytic function leads to increased Abeta42/Abeta40. Talking Point on the role of presenilin mutations in Alzheimer disease. EMBO. Rep. 2007, 8, 136–140. [Google Scholar] [CrossRef]
- Hampel, H.; Vassar, R.; De Strooper, B.; Hardy, J.; Willem, M.; Singh, N.; Zhou, J.; Yan, R.; Vanmechelen, E.; De Vos, A.; et al. The beta-secretase BACE1 in Alzheimer’s disease. Biol. Psychiatry 2021, 89, 745–756. [Google Scholar] [CrossRef]
- Devi, L.; Tang, J.; Ohno, M. Beneficial effects of the β-secretase inhibitor GRL-8234 in 5XFAD Alzheimer’s transgenic mice lessen during disease progression. Curr. Alzheimer Res. 2015, 12, 13–21. [Google Scholar] [CrossRef]
- Egan, M.F.; Kost, J.; Tariot, P.N.; Aisen, P.S.; Cummings, J.L.; Vellas, B.; Sur, C.; Mukai, Y.; Voss, T.; Furtek, C.; et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2018, 378, 1691–1703. [Google Scholar] [CrossRef]
- Sperling, R.; Henley, D.; Aisen, P.S.; Raman, R.; Donohue, M.C.; Ernstrom, K.; Rafii, M.S.; Streffer, J.; Shi, Y.; Karcher, K.; et al. Findings of efficacy, safety, and biomarker outcomes of atabecestat in preclinical Alzheimer disease: A truncated randomized phase 2b/3 clinical trial. JAMA Neurol. 2021, 78, 293–301. [Google Scholar] [CrossRef]
- Lo, A.C.; Evans, C.D.; Mancini, M.; Wang, H.; Shcherbinin, S.; Lu, M.; Natanegara, F.; Willis, B.A. Phase II (NAVIGATE-AD study) results of LY3202626 effects on patients with mild Alzheimer’s disease dementia. J. Alzheimers Dis. Rep. 2021, 5, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Wessels, A.M.; Tariot, P.N.; Zimmer, J.A.; Selzler, K.J.; Bragg, S.M.; Andersen, S.W.; Landry, J.; Krull, J.H.; Downing, A.M.; Willis, B.A.; et al. Efficacy and safety of lanabecestat for treatment of early and mild Alzheimer disease: The AMARANTH and DAYBREAK-ALZ randomized clinical trials. JAMA Neurol. 2020, 77, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Youn, K.; Park, J.H.; Lee, S.; Lee, S.; Lee, J.; Yun, E.Y.; Jeong, W.S.; Jun, M. BACE1 inhibition by genistein: Biological evaluation, kinetic analysis, and molecular docking simulation. J. Med. Food 2018, 21, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.Y.; Bae, K.; Seong, Y.H.; Song, K.S. Green tea catechins as a BACE1 (beta-secretase) inhibitor. Bioorg. Med. Chem. Lett. 2003, 13, 3905–3908. [Google Scholar] [CrossRef]
- Das, S.; Sengupta, S.; Chakraborty, S. Scope of β-secretase (BACE1)-targeted therapy in Alzheimer’s disease: Emphasizing the flavonoid based natural scaffold for BACE1 inhibition. ACS Chem. Neurosci. 2020, 11, 3510–3522. [Google Scholar] [CrossRef]
- Mycroft-West, C.J.; Devlin, A.J.; Cooper, L.C.; Guimond, S.E.; Procter, P.; Guerrini, M.; Miller, G.J.; Fernig, D.G.; Yates, E.A.; Lima, M.A.; et al. Glycosaminoglycans from Litopenaeus vannamei inhibit the Alzheimer’s disease beta secretase, BACE1. Mar. Drugs 2021, 19, 203. [Google Scholar] [CrossRef]
- Mycroft-West, C.J.; Devlin, A.J.; Cooper, L.C.; Procter, P.; Miller, G.J.; Fernig, D.G.; Guerrini, M.; Guimond, S.E.; Lima, M.A.; Yates, E.A.; et al. Inhibition of BACE1, the beta-secretase implicated in Alzheimer’s disease, by a chondroitin sulfate extract from Sardina pilchardus. Neural Regen. Res. 2020, 15, 1546–1553. [Google Scholar] [CrossRef]
- Mycroft-West, C.J.; Cooper, L.C.; Devlin, A.J.; Procter, P.; Guimond, S.E.; Guerrini, M.; Fernig, D.G.; Lima, M.A.; Yates, E.A.; Skidmore, M.A. A Glycosaminoglycan extract from Portunus pelagicus inhibits BACE1, the beta secretase implicated in Alzheimer’s disease. Mar. Drugs 2019, 17, 293. [Google Scholar] [CrossRef]
- Lee, J.K.; Li-Chan, E.C.Y.; Cheung, I.W.Y.; Jeon, Y.J.; Ko, J.Y.; Byun, H.G. Neuroprotective effect of β-secretase inhibitory peptide from Pacific hake (Merluccius productus) fish protein hydrolysate. Curr. Alzheimer Res. 2019, 16, 1028–1038. [Google Scholar] [CrossRef]
- Lee, J.K.; Li-Chan, E.C.Y.; Byun, H.-G. Characterization of β-secretase inhibitory peptide purified from skate skin protein hydrolysate. Eur. Food Res. Technol. 2015, 240, 129–136. [Google Scholar] [CrossRef]
- Li-Chan, E.C.Y.; Cheung, I.W.Y.; Byun, H.-G. Shrimp (Pandalopsis dispar) waste hydrolysate as a source of novel β–secretase inhibitors. Fish. Aquat. Sci. 2016, 19, 11. [Google Scholar] [CrossRef]
- Youn, K.; Yoon, J.H.; Lee, N.; Lim, G.; Lee, J.; Sang, S.; Ho, C.T.; Jun, M. Discovery of sulforaphane as a potent BACE1 inhibitor based on kinetics and computational studies. Nutrients 2020, 12, 3026. [Google Scholar] [CrossRef] [PubMed]
- Hafez Ghoran, S.; Kijjoa, A. Marine-derived compounds with anti-Alzheimer’s disease activities. Mar. Drugs 2021, 19, 410. [Google Scholar] [CrossRef] [PubMed]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Kristinsson, H.G.; Rasco, B.A. Fish protein hydrolysates: Production, biochemical, and functional properties. Crit. Rev. Food Sci. Nutr. 2000, 40, 43–81. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef]
- Kinno, A.; Kasamatsu, S.; Akaike, T.; Ihara, H. Reactive sulfur species omics analysis in the brain tissue of the 5xFAD mouse model of Alzheimer’s disease. Antioxidants 2023, 12, 1105. [Google Scholar] [CrossRef]
- Itagaki, S.; McGeer, P.L.; Akiyama, H.; Zhu, S.; Selkoe, D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J. Neuroimmunol. 1989, 24, 173–182. [Google Scholar] [CrossRef]
- Rogers, J.; Webster, S.; Lue, L.F.; Brachova, L.; Civin, W.H.; Emmerling, M.; Shivers, B.; Walker, D.; McGeer, P. Inflammation and Alzheimer’s disease pathogenesis. Neurobiol. Aging 1996, 17, 681–686. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, S.R.; Byun, H.-G. Purification and characterization of β-secretase inhibitory peptide from sea hare (Aplysia kurodai) by enzymatic hydrolysis. Fish. Aquat. Sci. 2018, 21, 13. [Google Scholar] [CrossRef]
- Ano, Y.; Ohya, R.; Takaichi, Y.; Washinuma, T.; Uchida, K.; Takashima, A.; Nakayama, H. Beta-lactolin, a whey-derived lacto-tetrapeptide, prevents Alzheimer’s disease pathologies and cognitive decline. J. Alzheimers Dis. 2020, 73, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Tanaka, M.; Yoshino, A.; Nagasato, Y.; Takata, F.; Dohgu, S.; Matsui, T. A memory-improving dipeptide, Tyr-Pro, can reach the mouse brain after oral administration. Sci. Rep. 2023, 13, 16908. [Google Scholar] [CrossRef]
- Urbi, Z.; Azmi, N.S.; Ming, L.C.; Hossain, M.S. A Concise review of extraction and characterization of chondroitin sulphate from fish and fish wastes for pharmacological application. Curr. Issues Mol. Biol. 2022, 44, 3905–3922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, J.; Liu, C.; Song, C.; Li, P.; Yin, F.; Xiao, Y.; Li, J.; Jiang, W.; Zong, A.; et al. Protective effects of low molecular weight chondroitin sulfate on amyloid beta (Aβ)-induced damage in vitro and in vivo. Neuroscience 2015, 305, 169–182. [Google Scholar] [CrossRef]
- Zhao, N.; Wu, L.; Zhang, X.; Jiang, W.; Wang, F. Low molecular weight chondroitin sulfate ameliorates pathological changes in 5XFAD mice by improving various functions in the brain. Neuropharmacology 2021, 199, 108796. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Dohata, A.; Taniguchi, Y.; Hoshi, A.; Uchida, K.; Takashima, A.; Nakayama, H. Iso-α-acids, bitter components of beer, prevent inflammation and cognitive decline induced in a mouse model of Alzheimer’s disease. J. Biol. Chem. 2017, 292, 3720–3728. [Google Scholar] [CrossRef]
- Tang, J.J.; Huang, L.F.; Deng, J.L.; Wang, Y.M.; Guo, C.; Peng, X.N.; Liu, Z.; Gao, J.M. Cognitive enhancement and neuroprotective effects of OABL, a sesquiterpene lactone in 5xFAD Alzheimer’s disease mice model. Redox Biol. 2022, 50, 102229. [Google Scholar] [CrossRef]
- Yang, X.; Ye, T.; He, Y.; Wen, L.; Cheng, X. Qi-fu-yin attenuated cognitive disorders in 5xFAD mice of Alzheimer’s disease animal model by regulating immunity. Front. Neurol. 2023, 14, 1183764. [Google Scholar] [CrossRef]
- Devi, L.; Ohno, M. Phospho-eIF2α level is important for determining abilities of BACE1 reduction to rescue cholinergic neurodegeneration and memory defects in 5XFAD mice. PLoS ONE 2010, 5, e12974. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsuki, T.; Ogi, K.; Kinno, A.; Kasamatsu, S.; Ihara, H.; Sumitani, H. Inhibition of Amyloid β Accumulation by Protease-Digested Whitebait (Shirasu) in a Murine Model of Alzheimer’s Disease. Foods 2024, 13, 2858. https://doi.org/10.3390/foods13182858
Katsuki T, Ogi K, Kinno A, Kasamatsu S, Ihara H, Sumitani H. Inhibition of Amyloid β Accumulation by Protease-Digested Whitebait (Shirasu) in a Murine Model of Alzheimer’s Disease. Foods. 2024; 13(18):2858. https://doi.org/10.3390/foods13182858
Chicago/Turabian StyleKatsuki, Takahiro, Kayako Ogi, Ayaka Kinno, Shingo Kasamatsu, Hideshi Ihara, and Hidenobu Sumitani. 2024. "Inhibition of Amyloid β Accumulation by Protease-Digested Whitebait (Shirasu) in a Murine Model of Alzheimer’s Disease" Foods 13, no. 18: 2858. https://doi.org/10.3390/foods13182858
APA StyleKatsuki, T., Ogi, K., Kinno, A., Kasamatsu, S., Ihara, H., & Sumitani, H. (2024). Inhibition of Amyloid β Accumulation by Protease-Digested Whitebait (Shirasu) in a Murine Model of Alzheimer’s Disease. Foods, 13(18), 2858. https://doi.org/10.3390/foods13182858






