Mass Transfer Resistance and Reaction Rate Kinetics for Carbohydrate Digestion with Cell Wall Degradation by Cellulase
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. A Beaker and Stirrer System
2.2.2. Starch Cell Wall Degradation by Cellulase
2.2.3. Starch Hydrolysis by Amylase
2.2.4. SEM Analysis
2.2.5. Particle Size Measurement
2.3. Data Analysis
3. Results and Discussion
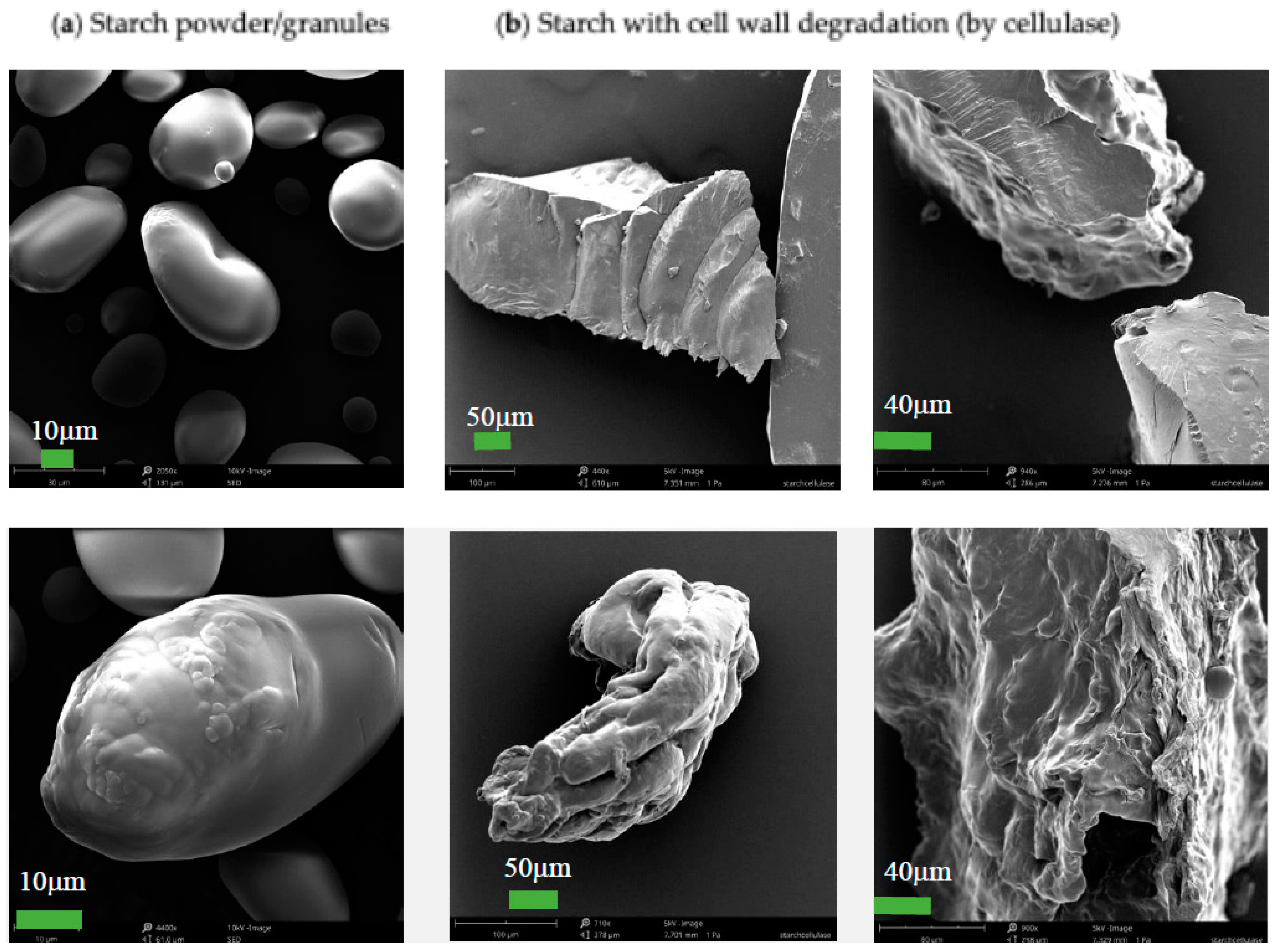
3.1. Degradation of Starch Cell Walls
3.2. Starch Hydrolysis with Degraded Cell Walls
3.3. Particle Size Analysis
3.4. Estimation of the Damköhler Number for Starch Hydrolysis with Degraded Cell Walls
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Ritchie, H.; Rosado, P.; Roser, M. Environmental Impacts of Food Production. 2022. Available online: https://ourworldindata.org/environmental-impacts-of-food (accessed on 1 August 2024).
- Poore, J.; Nemecek, T. Reducing Food’s Environmental Impacts through Producers and Consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef] [PubMed]
- WHO European Office for the Prevention and Control of Noncommunicable Diseases. Plant-Based Diets and Their Impact on Health, Sustainability and the Environment a Review of the Evidence, WHO European Office for the Prevention and Control of Noncommunicable Diseases; WHO European Office for the Prevention and Control of Noncommunicable Diseases: Geneva, Switzerland, 2021. [Google Scholar]
- Langrish, T.A.G. Multifilm Mass Transfer and Time Constants for Mass Transfer in Food Digestion: Application to Gut-on-Chip Models. Appl. Biosci. 2022, 1, 101–112. [Google Scholar] [CrossRef]
- Cussler, E.L. Diffusion: Mass Transfer in Fluid Systems, 3rd ed.; Cambridge University Press: New York, NY, USA, 2009; ISBN 9780521871211. [Google Scholar]
- Amos, R.A.; Mohnen, D. Critical Review of Plant Cell Wall Matrix Polysaccharide Glycosyltransferase Activities Verified by Heterologous Protein Expression. Front. Plant Sci. 2019, 10, 915. [Google Scholar] [CrossRef] [PubMed]
- Tingley, J.P.; Low, K.E.; Xing, X.; Abbott, D.W. Combined Whole Cell Wall Analysis and Streamlined in Silico Carbohydrate-Active Enzyme Discovery to Improve Biocatalytic Conversion of Agricultural Crop Residues. Biotechnol. Biofuels 2021, 14, 16. [Google Scholar] [CrossRef]
- Fry, S.C. Plant Cell Walls. From Chemistry to Biology. Ann. Bot. 2011, 108, viii–ix. [Google Scholar] [CrossRef]
- Zwolinski, B.J.; Eyring, H.; Reese, C.E. Diffusion and Membrane Permeability. J. Phys. Chem. 1949, 53, 1426–1453. [Google Scholar] [CrossRef]
- Morrod, R.S. A New Method for Measuring the Permeability of Plant Cell Membranes Using Epidermis-Free Leaf Discs. J. Exp. Bot. 1974, 25, 521–533. [Google Scholar] [CrossRef]
- Chaffey, N.; Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell. 4th edn. Ann. Bot. 2003, 91, 401. [Google Scholar] [CrossRef]
- Abramson, M.; Shoseyov, O.; Shani, Z. Plant Cell Wall Reconstruction toward Improved Lignocellulosic Production and Processability. Plant Sci. 2010, 178, 61–72. [Google Scholar] [CrossRef]
- Giovannoni, M.; Gramegna, G.; Benedetti, M.; Mattei, B. Industrial Use of Cell Wall Degrading Enzymes: The Fine Line Between Production Strategy and Economic Feasibility. Front. Bioeng. Biotechnol. 2020, 8, 356. [Google Scholar] [CrossRef]
- Derntl, C.; Mach-Aigner, A.R.; Mach, R.L. Trichoderma Proteins with Disruption Activity on Cellulosic Substrates. In Biotechnology and Biology of Trichoderma; Elsevier B.V.: Amsterdam, The Netherlands, 2014; pp. 309–317. ISBN 9780444595768. [Google Scholar]
- Santamaria, M.; Garzon, R.; Moreira, R.; Rosell, C.M. Estimation of Viscosity and Hydrolysis Kinetics of Corn Starch Gels Based on Microstructural Features Using a Simplified Model. Carbohydr. Polym. 2021, 273, 118549. [Google Scholar] [CrossRef] [PubMed]
- Aleixandre, A.; Benavent-Gil, Y.; Rosell, C.M. Effect of Bread Structure and in Vitro Oral Processing Methods in Bolus Disintegration and Glycemic Index. Nutrients 2019, 11, 2105. [Google Scholar] [CrossRef] [PubMed]
- Levenspiel, O. Fluid-Particle Reactions: Kinetics. In Chemical Reaction Engineering; John Wiley & Sons: New York, NY, USA, 1999. [Google Scholar]
- Catchpole, J.P.; Fulford, G. Dimensionless Groups. Ind. Eng. Chem. 1966, 58, 46–60. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Li, H.; Dhital, S.; Gidley, M.J.; Gilbert, R.G. A More General Approach to Fitting Digestion Kinetics of Starch in Food. Carbohydr. Polym. 2019, 225, 115244. [Google Scholar] [CrossRef]
- Carroll, A.; Somerville, C. Cellulosic Biofuels. Annu. Rev. Plant Biol. 2009, 60, 165–182. [Google Scholar] [CrossRef]
- Attia, M.S.; Youssef, A.O. Enhancement of the Efficiency of a Salivary Alpha Amylase Biomarker for the Sympathetic Nervous System by a Nano-Optical Sensor Tb–Acetyl Acetone Complex. New J. Chem. 2016, 40, 7529–7535. [Google Scholar] [CrossRef]
- Goñi, I.; Garcia-Alonso, A.; Saura-Calixto, F. A Starch Hydrolysis Procedure to Estimate Glycemic Index. Nutr. Res. 1997, 17, 427–437. [Google Scholar] [CrossRef]
- Dhital, S.; Shrestha, A.K.; Gidley, M.J. Relationship between Granule Size and in Vitro Digestibility of Maize and Potato Starches. Carbohydr. Polym. 2010, 82, 480–488. [Google Scholar] [CrossRef]
- Langrish, T.A.G.; Zhong, C.; Sun, L. Probing Differences in Mass-Transfer Coefficients in Beaker and Stirrer Digestion Systems and the Usp Dissolution Apparatus 2 Using Benzoic Acid Tablets. Processes 2021, 9, 2168. [Google Scholar] [CrossRef]
- McMillin, J.M. Blood Glucose. In Clinical Methods: The History, Physical, and Laboratory Examinations; Butterworths: Boston, MA, USA, 1990; pp. 662–665. [Google Scholar]
- Schugmann, M.; Foerst, P. Systematic Investigation on the Glass Transition Temperature of Binary and Ternary Sugar Mixtures and the Applicability of Gordon–Taylor and Couchman–Karasz Equation. Foods 2022, 11, 1679. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.; Zaccaron, S.; Zwirchmayr, N.S.; Hettegger, H.; Hofinger, A.; Bacher, M.; Henniges, U.; Hosoya, T.; Potthast, A.; Rosenau, T. Yellowing and Brightness Reversion of Celluloses: CO or COOH, Who Is the Culprit? Cellulose 2019, 26, 429–444. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Z.; Zhong, C.; Lei, Z.; Langrish, T.A.G. Comparing Mass Transfer and Reaction Rate Kinetics in Starch Hydrolysis during Food Digestion. Appl. Res. 2024, e202400023. [Google Scholar] [CrossRef]
- Özbek, B.; Yüceer, S. α-Amylase Inactivation during Wheat Starch Hydrolysis Process. Process Biochem. 2001, 37, 87–95. [Google Scholar] [CrossRef]
- Roldan-Cruz, C.; Garcia-Hernandez, A.; Alvarez-Ramirez, J.; Vernon-Carter, E.J. Effect of the Stirring Speed in the in Vitro Activity of α-Amylase. Food Hydrocoll. 2021, 110, 106127. [Google Scholar] [CrossRef]
- Sanders, W.T.M.; Geerink, M.; Zeeman, G.; Lettinga, G. Anaerobic Hydrolysis Kinetics of Particulate Substrates. Water Sci. Technol. 2000, 41, 17–24. [Google Scholar] [CrossRef]






| Samples | Stirrer Speed (rpm) | Estimated Maximum Glucose Concentration (mg/mL) | Estimated Time Constant (tau) for the Reaction (h) | Slope of the Glucose Concentration–Time Curve (dC/dt) (mg/mL/s) |
|---|---|---|---|---|
| Starch powder | 100 | 3.1 | 9.8 | 7.2 × 10−5 |
| 200 | 2.5 | 24.1 | 2.8 × 10−5 | |
| 1 h degradation—starch and cellulase | 100 | 3.2 | 3.1 | 2.8 × 10−4 |
| 200 | 2.9 | 3.1 | 2.7 × 10−4 | |
| 4 h degradation—starch and cellulase | 100 | 3.3 | 3.5 | 2.8 × 10−4 |
| 200 | 3.0 | 3.5 | 2.6 × 10−4 |
| Starch Samples | Specific Surface Area (m2/kg) |
|---|---|
| 1 h, degradation | 131 ± 0.4 |
| 1 h, soaking | 96 ± 5 |
| 4 h, degradation | 127 ± 3 |
| 4 h, soaking | 118 ± 9 |
| Samples | Stirrer Speed (rpm) | Estimated Overall Mass-Transfer Coefficient (m/s) | Estimated Overall Mass-Transfer Resistance (s/m) | Estimated Internal Mass-Transfer Resistance (s/m) |
|---|---|---|---|---|
| Starch powder | 100 | 3.6 × 10−8 | 2.8 × 107 | 2.2 × 107 |
| 200 | 1.4 × 10−8 | 7.2 × 107 | 6.6 × 107 | |
| 1 h degradation—starch and cellulase | 100 | 1.8 × 10−7 | 5.5 × 106 | Negligible |
| 200 | 1.7 × 10−7 | 5.8 × 106 | Negligible | |
| 4 h degradation—starch and cellulase | 100 | 1.8 × 10−7 | 5.5 × 106 | Negligible |
| 200 | 1.7 × 10−7 | 6.0 × 106 | Negligible |
| Time Constant for Overall Mass Transfer (h) | Time Constant for Reaction (h) | Da (Time Constant for Overall Mass Transfer/Time Constant for Reaction) | |||||
|---|---|---|---|---|---|---|---|
| Stirrer speed (rpm) | 100 | 200 | 100 | 200 | 100 | 200 | |
| System | |||||||
| Starch powder | 4.6 | 12.0 | 9.8 | 24.1 | 0.47 | 0.50 | |
| 1 h degradation (starch and cellulase) | 1.2 | 1.2 | 3.1 | 3.1 | 0.38 | 0.40 | |
| 4 h degradation (starch and cellulase) | 1.2 | 1.3 | 3.5 | 3.5 | 0.34 | 0.36 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Cheng, S.; Cheng, J.; Langrish, T.A.G. Mass Transfer Resistance and Reaction Rate Kinetics for Carbohydrate Digestion with Cell Wall Degradation by Cellulase. Foods 2024, 13, 2881. https://doi.org/10.3390/foods13182881
Sun Y, Cheng S, Cheng J, Langrish TAG. Mass Transfer Resistance and Reaction Rate Kinetics for Carbohydrate Digestion with Cell Wall Degradation by Cellulase. Foods. 2024; 13(18):2881. https://doi.org/10.3390/foods13182881
Chicago/Turabian StyleSun, Yongmei, Shu Cheng, Jingying Cheng, and Timothy A. G. Langrish. 2024. "Mass Transfer Resistance and Reaction Rate Kinetics for Carbohydrate Digestion with Cell Wall Degradation by Cellulase" Foods 13, no. 18: 2881. https://doi.org/10.3390/foods13182881
APA StyleSun, Y., Cheng, S., Cheng, J., & Langrish, T. A. G. (2024). Mass Transfer Resistance and Reaction Rate Kinetics for Carbohydrate Digestion with Cell Wall Degradation by Cellulase. Foods, 13(18), 2881. https://doi.org/10.3390/foods13182881







