Computer-Aided Design to Improve the Thermal Stability of Rhizomucor miehei Lipase
Abstract
1. Introduction
2. Materials and Methods
2.1. Genes, Plasmids, and Strains
2.2. Main Materials
2.3. Construction of RML Mutants
2.4. Expression and Purification of Wild-Type and Mutant Proteins
2.5. Enzymatic Property Assays of the Wild Type and Mutants
2.6. Determination of Enzymatic Activity of Wild-Type and Mutant RML on Camphor Seed Oil
2.7. Computational Analysis
2.7.1. Homology Modeling of RML
2.7.2. Computational Design of RML Mutants
2.7.3. Evolutionary Conservation Analysis
2.7.4. Residue Interaction Network Analysis
2.7.5. Molecular Docking
2.7.6. Molecular Dynamics Simulations
3. Results and Discussion
3.1. Computational Design of RML
3.2. Expression and Purification of the Wild-Type RML and Its Mutants in E. coli
3.3. Enzymatic Characteristics of Single-Point Mutants
3.4. Characteristics of Combinatorial Mutants
3.5. Enzymatic Activities of the Wild Type and Mutants to Camphor Tree Seed Oil
3.6. Analysis of Residue Interaction Networks
3.7. Analysis of Molecular Docking
3.8. Analysis of Molecular Dynamics Simulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palomo, J.M. Synthetic complexity created by lipases. Nat. Catal. 2020, 3, 335–336. [Google Scholar] [CrossRef]
- Rafiee, F.; Rezaee, M. Different strategies for the lipase immobilization on the chitosan based supports and their applications. Int. J. Biol. Macromol. 2021, 179, 170–195. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Nian, B. Computer-aided lipase engineering for improving their stability and activity in the food industry: State of the Art. Molecules 2023, 28, 5848. [Google Scholar] [CrossRef] [PubMed]
- Poppe, J.K.; Matte, C.R.; Fernandez-Lafuente, R.; Rodrigues, R.C.; Ayub, M.A.Z. Transesterification of waste frying oil and soybean oil by combi-lipases under ultrasound-assisted reactions. Appl. Biochem. Biotechnol. 2018, 186, 576–589. [Google Scholar] [CrossRef] [PubMed]
- Alikhani, N.; Shahedi, M.; Habibi, Z.; Yousefi, M.; Ghasemi, S.; Mohammadi, M. A multi-component approach for co-immobilization of lipases on silica-coated magnetic nanoparticles: Improving biodiesel production from waste cooking oil. Bioprocess. Biosyst. Eng. 2022, 45, 2043–2060. [Google Scholar] [CrossRef]
- Stemler, C.D.; Scherf, K.A. Improvement of cake baking properties by lipases compared to a traditional emulsifier. Food Chem. X 2022, 15, 100442. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, R.; Xu, W.; Cocolin, L.; Liang, H.; Ji, C.; Zhang, S.; Chen, Y.; Lin, X. Combined effects of lipase and Lactiplantibacillus plantarum 1-24-LJ on physicochemical property, microbial succession and volatile compounds formation in fermented fish product. J. Sci. Food Agric. 2023, 103, 2304–2312. [Google Scholar] [CrossRef]
- Sutar, V.P.; Mali, G.; Upadhye, V.; Singh, V.K.; Sinha, R.P. Purification of lipase from Pseudomonas aeruginosa VSJK R-9 and its application in combination with the lipolytic consortium for bioremediation of restaurant wastewater. Appl. Biochem. Biotechnol. 2023, 195, 1888–1903. [Google Scholar] [CrossRef]
- Tian, M.; Huang, S.; Wang, Z.; Fu, J.; Lv, P.; Miao, C.; Liu, T.; Yang, L.; Luo, W. Enhanced activity of Rhizomucor miehei lipase by directed saturation mutation of the propeptide. Enzym. Microb. Technol. 2021, 150, 109870. [Google Scholar] [CrossRef]
- Bommarius, A.S.; Blum, J.K.; Abrahamson, M.J. Status of protein engineering for biocatalysts: How to design an industrially useful biocatalyst. Curr. Opin. Chem. Biol. 2011, 15, 194–200. [Google Scholar] [CrossRef]
- Yan, X.; Gong, X.; Zeng, Z.; Xia, J.; Ma, M.; Zhao, J.; Zhang, G.; Wang, P.; Wan, D.; Yu, P.; et al. Geographic pattern of variations in chemical composition and nutritional value of Cinnamomum camphora seed kernels from China. Foods 2023, 12, 2630. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yan, X.; Wu, S.; Ma, M.; Yu, P.; Gong, D.; Deng, S.; Zeng, Z. Ethanol extracts from Cinnamomum camphora seed kernel: Potential bioactivities as affected by alkaline hydrolysis and simulated gastrointestinal digestion. Food Res. Int. 2020, 137, 109363. [Google Scholar] [CrossRef]
- Ma, X.; Hu, Z.; Mao, J.; Xu, Y.; Zhu, X.; Xiong, H. Synthesis of cocoa butter substitutes from Cinnamomum camphora seed oil and fully hydrogenated palm oil by enzymatic interesterification. J. Food Sci. Technol.-Mysore 2019, 56, 835–845. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, Y.; Zeng, Z.; Peng, T.; Yan, X.; Zhao, J.; Xia, J.; Yu, P.; Wen, X.; Gong, D. Effect of processing method on chemical composition, physicochemical property, antioxidant activity and volatile compound of Cinnamomum camphora seed kernel oil. Ind. Crop. Prod. 2023, 201, 116907. [Google Scholar] [CrossRef]
- Zhao, M.; Tang, L.; Zhu, X.; Hu, J.; Li, H.; Luo, L.; Lei, L.; Deng, Z. Enzymatic production of zero-trans plastic fat rich in α-linolenic acid and medium-chain fatty acids from highly hydrogenated soybean oil, Cinnamomum camphora seed oil, and perilla oil by lipozyme TL IM. J. Agric. Food Chem. 2013, 61, 1189–1195. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Cen, Y.K.; Zou, S.P.; Xue, Y.P.; Zheng, Y.G. Recent advances in the improvement of enzyme thermostability by structure modification. Crit. Rev. Biotechnol. 2020, 40, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Li, W.F.; Zhou, X.X.; Lu, P. Structural features of thermozymes. Biotechnol. Adv. 2005, 23, 271–281. [Google Scholar] [CrossRef]
- Li, G.; Fang, X.; Su, F.; Chen, Y.; Xu, L.; Yan, Y. Enhancing the thermostability of Rhizomucor miehei Lipase with a limited screening library by rational-design point mutations and disulfide bonds. Appl. Environ. Microbiol. 2018, 84, e2117–e2129. [Google Scholar] [CrossRef]
- Yin, X.; Yao, Y.; Wu, M.C.; Zhu, T.D.; Zeng, Y.; Pang, Q.F. A unique disulfide bridge of the thermophilic xylanase syxyn11 plays a key role in its thermostability. Biochem.-Mosc. 2014, 79, 531–537. [Google Scholar] [CrossRef]
- Zhao, Z.; Hou, S.; Lan, D.; Wang, X.; Liu, J.; Khan, F.I.; Wang, Y. Crystal structure of a lipase from Streptomyces sp strain W007-implications for thermostability and regiospecificity. Febs J. 2017, 284, 3506–3519. [Google Scholar] [CrossRef]
- Tan, Z.; Li, J.; Wu, M.; Wang, J. Enhancing the thermostability of a cold-active lipase from Penicillium cyclopium by in silico design of a disulfide bridge. Appl. Biochem. Biotechnol. 2014, 173, 1752–1764. [Google Scholar] [CrossRef] [PubMed]
- Gurpilhares, D.B.; Cinelli, L.P.; Simas, N.K.; Pessoa, A.J.; Sette, L.D. Marine prebiotics: Polysaccharides and oligosaccharides obtained by using microbial enzymes. Food Chem. 2019, 280, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wang, H.; Hwang, J.; Tseng, C. Protein thermal stability enhancement by designing salt bridges: A combined computational and experimental study. Public Libr. Sci. ONE 2014, 9, e112751. [Google Scholar] [CrossRef]
- Robinson-Rechavi, M.; Alibes, A.; Godzik, A. Contribution of electrostatic interactions, compactness and quaternary structure to protein thermostability: Lessons from structural genomics of Thermotoga maritima. J. Mol. Biol. 2006, 356, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhou, X.; Wang, Y.; Gao, Y.; Nakanishi, H.; Fujita, M.; Li, Z. Enhancement of thermostability and expression level of Rasamsonia emersonii lipase in Pichia pastoris and its application in biodiesel production in a continuous flow reactor. Int. J. Biol. Macromol. 2024, 278, 134481. [Google Scholar] [CrossRef]
- Ashkan, Z.; Hemmati, R.; Homaei, A.; Dinari, A.; Jamlidoost, M.; Tashakor, A. Immobilization of enzymes on nanoinorganic support materials: An update. Int. J. Biol. Macromol. 2021, 168, 708–721. [Google Scholar] [CrossRef]
- Lu, M.; Gao, Z.; Xing, S.; Long, J.; Li, C.; He, L.; Wang, X. Purification, characterization, and chemical modification of Bacillus velezensis SN-14 fibrinolytic enzyme. Int. J. Biol. Macromol. 2021, 177, 601–609. [Google Scholar] [CrossRef]
- Bloom, J.D.; Labthavikul, S.T.; Otey, C.R.; Arnold, F.H. Protein stability promotes evolvability. Proc. Natl. Acad. Sci. USA 2006, 103, 5869–5874. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhu, W.; Zhang, Q.; Zheng, R.; Liu, L.; Cao, H. Enhanced thermal stability of polyphosphate-dependent glucomannokinase by directed evolution. Catalysts 2022, 12, 1112. [Google Scholar] [CrossRef]
- Zhang, H.; Sang, J.; Zhang, Y.; Sun, T.; Liu, H.; Yue, R.; Zhang, J.; Wang, H.; Dai, Y.; Lu, F.; et al. Rational design of a Yarrowia lipolytica derived lipase for improved thermostability. Int. J. Biol. Macromol. 2019, 137, 1190–1198. [Google Scholar] [CrossRef]
- Kepp, K.P. Towards a “Golden Standard” for computing globin stability: Stability and structure sensitivity of myoglobin mutants. Biochim. Biophys. Acta 2015, 1854, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Bednar, D.; Beerens, K.; Sebestova, E.; Bendl, J.; Khare, S.; Chaloupkova, R.; Prokop, Z.; Brezovsky, J.; Baker, D.; Damborsky, J. FireProt: Energy- and evolution-based computational design of thermostable multiple-point mutants. PLoS Comput. Biol. 2015, 11, e1004556. [Google Scholar] [CrossRef] [PubMed]
- Bryksin, A.V.; Matsumura, I. Overlap extension PCR cloning: A simple and reliable way to create recombinant plasmids. Biotechniques 2010, 48, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Hiol, A.; Jonzo, M.D.; Rugani, N.; Druet, D.; Sarda, L.; Comeau, L.C. Purification and characterization of an extracellular lipase from a thermophilic Rhizopus oryzae strain isolated from palm fruit. Enzym. Microb. Technol. 2000, 26, 421–430. [Google Scholar] [CrossRef]
- Sha, C.; Yu, X.; Lin, N.; Zhang, M.; Xu, Y. Enhancement of lipase r27RCL production in Pichia pastoris by regulating gene dosage and co-expression with chaperone protein disulfide isomerase. Enzym. Microb. Technol. 2013, 53, 438–443. [Google Scholar] [CrossRef]
- Wang, P.; Wan, D.; Peng, T.; Yang, Y.; Wen, X.; Yan, X.; Xia, J.; Zhu, Q.; Yu, P.; Gong, D.; et al. Acute oral toxicity and genotoxicity test and evaluation of Cinnamomum camphora seed kernel oil. Foods 2023, 12, 293. [Google Scholar] [CrossRef]
- Delgado, J.; Radusky, L.G.; Cianferoni, D.; Serrano, L. FoldX 5.0: Working with RNA, small molecules and a new graphical interface. Bioinformatics 2019, 35, 4168–4169. [Google Scholar] [CrossRef]
- Bon, L.; Lucchetti, C.; Portolan, F.; Pagan, M. MUPRO: A multipurpose robot. Int. J. Neurosci. 2002, 112, 855–868. [Google Scholar] [CrossRef]
- Myung, Y.; Rodrigues, C.; Ascher, D.B.; Pires, D. MCSM-AB2: Guiding rational antibody design using graph-based signatures. Bioinformatics 2020, 36, 1453–1459. [Google Scholar] [CrossRef]
- Pandurangan, A.P.; Ochoa-Montano, B.; Ascher, D.B.; Blundell, T.L. SDM: A server for predicting effects of mutations on protein stability. Nucleic Acids Res. 2017, 45, 229–235. [Google Scholar] [CrossRef]
- Pires, D.E.V.; Ascher, D.B.; Blundell, T.L. DUET: A server for predicting effects of mutations on protein stability using an integrated computational approach. Nucleic Acids Res. 2014, 42, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Ding, F.; Dokholyan, N.V. Eris: An automated estimator of protein stability. Nat. Methods 2007, 4, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, O.; Erez, E.; Nimrod, G.; Ben-Tal, N. The conSurf-DB: Pre-calculated evolutionary conservation profiles of protein structures. Nucleic Acids Res. 2009, 37, D323–D327. [Google Scholar] [CrossRef] [PubMed]
- Lasowskii, R.A.; Macarthur, M.W.; Moss, D.S.; Thornton, J.S. PROCHECK- a Program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Fang, X.; Huang, J.; Zhang, R.; Wang, F.; Zhang, Q.; Li, G.; Yan, J.; Zhang, H.; Yan, Y.; Xu, L. Convolution neural network-based prediction of protein thermostability. J. Chem. Inf. Model. 2019, 59, 4833–4843. [Google Scholar] [CrossRef]
- Xu, S.Y.; Chu, R.L.; Liu, H.T.; Weng, C.Y.; Wang, Y.J.; Zheng, Y.G. Computer-directed rational design enhanced the thermostability of carbonyl reductase LsCR for the synthesis of ticagrelor precursor. Biotechnol. Bioeng. 2024, 121, 1532–1542. [Google Scholar] [CrossRef]
- Chorin, A.B.; Masrati, G.; Kessel, A.; Narunsky, A.; Sprinzak, J.; Lahav, S.; Ashkenazy, H.; Ben-Tal, N. ConSurf-DB: An accessible repository for the evolutionary conservation patterns of the majority of PDB proteins. Protein Sci. 2020, 29, 258–267. [Google Scholar] [CrossRef]
- Gopal, G.J.; Kumar, A. Strategies for the production of recombinant protein in Escherichia coli. Protein J. 2013, 32, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, D.; Xiong, Z.; Yang, Y.; Tian, G.; Wu, X.; Wang, Y.; Zhuang, Y.; Chu, J.; Tian, X. Optimization of the fermentative production of Rhizomucor miehei Lipase in Aspergillus oryzae by Controlling Morphology. Bioengineering 2022, 9, 610. [Google Scholar] [CrossRef]
- Li, Z.; Miao, Y.; Yang, J.; Zhao, F.; Lin, Y.; Han, S. Efficient improvement of surface displayed lipase from Rhizomucor miehei in PichiaPink™ protease-deficient system. Protein Expr. Purif. 2021, 180, 105804. [Google Scholar] [CrossRef]
- Pace, C.N.; Fu, H.; Fryar, K.L.; Landua, J.; Trevino, S.R.; Shirley, B.A.; Hendricks, M.M.; Iimura, S.; Gajiwala, K.; Scholtz, J.M.; et al. Contribution of hydrophobic interactions to protein stability. J. Mol. Biol. 2011, 408, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Noel, M.; Combes, D. Effects of temperature and pressure on Rhizomucor miehei lipase stability. J. Biotechnol. 2003, 102, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Davari, M.D.; Schwaneberg, U. Recombination of single beneficial substitutions obtained from protein engineering by computer-assisted recombination (CompassR). Methods Mol. Biol. 2022, 2461, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Gihaz, S.; Kanteev, M.; Pazy, Y.; Fishman, A. Filling the void: Introducing aromatic interactions into solvent tunnels to enhance lipase stability in methanol. Appl. Environ. Microbiol. 2018, 84, e02143-18. [Google Scholar] [CrossRef]
- Chahinian, H.; Sarda, L. Distinction between esterases and lipases: Comparative biochemical properties of sequence-related carboxylesterases. Protein Pept. Lett. 2009, 16, 1149–1161. [Google Scholar] [CrossRef]
- Sena, R.O.; Carneiro, C.; Moura, M.V.H.; Breda, G.C.; Pinto, M.C.C.; Fe, L.X.S.G.; Fernandez-Lafuente, R.; Manoel, E.A.; Almeida, R.V.; Freire, D.M.G.; et al. Application of Rhizomucor miehei lipase-displaying Pichia pastoris whole cell for biodiesel production using agro-industrial residuals as substrate. Int. J. Biol. Macromol. 2021, 189, 734–743. [Google Scholar] [CrossRef]
- Zhang, X.; Ai, Y.; Xu, Y.; Yu, X. High-level expression of Aspergillus niger lipase in Pichia pastoris: Characterization and gastric digestion in vitro. Food Chem. 2019, 274, 305–313. [Google Scholar] [CrossRef]
- Mohammadi, M.; Sepehrizadeh, Z.; Ebrahim-Habibi, A.; Shahverdi, A.R.; Faramarzi, M.A.; Setayesh, N. Enhancing activity and thermostability of lipase A from Serratia marcescens by site-directed mutagenesis. Enzym. Microb. Technol. 2016, 93–94, 18–28. [Google Scholar] [CrossRef]
- Fu, J.; Zeng, C.; Zeng, Z.; Wang, B.; Gong, D. Cinnamomum camphora seed kernel oil ameliorates oxidative stress and iInflammation in diet-induced obese rats. J. Food Sci. 2016, 81, H1295–H1300. [Google Scholar] [CrossRef]
- Lee, Y.; Tang, T.; Lai, O. Health Benefits, enzymatic production, and application of medium- and long-chain Triacylglycerol (MLCT) in food industries: A review. J. Food Sci. 2012, 77, R137–R144. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, M.; Yan, X.; Zhang, G.; Xia, J.; Zeng, Z.; Yu, P.; Deng, Q.; Gong, D. Green synthesis of polydopamine functionalized magnetic mesoporous biochar for lipase immobilization and its application in interesterification for novel structured lipids production. Food Chem. 2022, 379, 132148. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Yan, X.; Zeng, Z.; Xia, J.; Zhao, J.; Zeng, G.; Yu, P.; Wen, X.; Gong, D. Enzymatic interesterification improves the lipid composition, physicochemical properties and rheological behavior of Cinnamomum camphora seed kernel oil, Pangasius bocourti stearin and perilla seed oil blends. Food Chem. 2024, 430, 137026. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Xu, F.; Zhang, N.; Wu, Y.; Ju, X.; Wang, L. Dietary a novel structured lipid synthesized by soybean oil and coconut oil alter fatty acid metabolism in C57BL/6J mice. Food Biosci. 2021, 44, 101396. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, J.; Lee, W.J.; Akoh, C.C.; Li, A.; Wang, Y. Modification of palm-based oil blend via interesterification: Physicochemical properties, crystallization behaviors and oxidative stabilities. Food Chem. 2021, 347, 129070. [Google Scholar] [CrossRef]
- Xiao, Q.; Liang, J.; Luo, H.; Li, H.; Yang, J.; Huang, S. Investigations of conformational structures and activities of trypsin and pepsin affected by food colourant allura red. J. Mol. Liq. 2020, 319, 114359. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Xu, Y.; Yu, X. Engineering of a thermo-alkali-stable lipase from Rhizopus chinensis by rational design of a buried disulfide bond and combinatorial mutagenesis. J. Ind. Microbiol. Biotechnol. 2020, 47, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Hibi, T.; Kume, A.; Kawamura, A.; Itoh, T.; Fukada, H.; Nishiya, Y. Hyperstabilization of tetrameric Bacillus sp. TB-90 urate oxidase by introducing disulfide bonds through structural plasticity. Biochemistry 2016, 55, 724–732. [Google Scholar] [CrossRef]
- Wu, J.; Li, M.; Zhou, Y.; Yang, L.; Xu, G. Introducing a salt bridge into the lipase of Stenotrophomonas maltophilia results in a very large increase in thermal stability. Biotechnol. Lett. 2015, 37, 403–407. [Google Scholar] [CrossRef]
- Pleiss, J.; Fischer, M.; Schmid, R.D. Anatomy of lipase binding sites: The scissile fatty acid binding site. Chem. Phys. Lipids 1998, 93, 67–80. [Google Scholar] [CrossRef]
- Anuar, N.F.S.K.; Wahab, R.A.; Huyop, F.; Amran, S.I.; Hamid, A.A.A.; Halim, K.B.A.; Hood, M.H.M. Molecular docking and molecular dynamics simulations of a mutant Acinetobacter haemolyticus alkaline-stable lipase against tributyrin. J. Biomol. Struct. Dyn. 2021, 39, 2079–2091. [Google Scholar] [CrossRef]
- Derewenda, Z.S.; Lee, L.; Derewenda, U. The occurrence of C-H…O hydrogen bonds in proteins. J. Mol. Biol. 1995, 252, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.X.; Wang, Y.B.; Pan, Y.J.; Li, W.F. Differences in amino acids composition and coupling patterns between mesophilic and thermophilic proteins. Amino Acids 2008, 34, 25–33. [Google Scholar] [CrossRef]
- Badieyan, S.; Bevan, D.R.; Zhang, C. Study and design of stability in GH5 cellulases. Biotechnol. Bioeng. 2012, 109, 31–44. [Google Scholar] [CrossRef]
- Wang, R.; Wang, S.; Xu, Y.; Yu, X. Enhancing the thermostability of Rhizopus chinensis lipase by rational design and MD simulations. Int. J. Biol. Macromol. 2020, 160, 1189–1200. [Google Scholar] [CrossRef]
- Albayati, S.H.; Masomian, M.; Ishak, S.N.H.; Ali, M.S.B.M.; Thean, A.L.; Shariff, F.B.M.; Noor, N.D.B.M.; Rahman, R.N.Z.R. Main structural targets for engineering lipase substrate specificity. Catalysts 2020, 10, 747. [Google Scholar] [CrossRef]
- Moroz, O.V.; Blagova, E.; Reiser, V.; Saikia, R.; Dalal, S.; Jorgensen, C.I.; Bhatia, V.K.; Baunsgaard, L.; Andersen, B.; Svendsen, A.; et al. Novel inhibitory function of the Rhizomucor miehei lipase propeptide and three-dimensional structures of its Complexes with the Enzyme. Acs Omega 2019, 4, 9964–9975. [Google Scholar] [CrossRef]
- Zhang, J.H.; Jiang, Y.Y.; Lin, Y.; Sun, Y.F.; Zheng, S.P.; Han, S.Y. Structure-guided modification of Rhizomucor miehei lipase for production of structured lipids. PLoS ONE 2013, 8, e67892. [Google Scholar] [CrossRef]


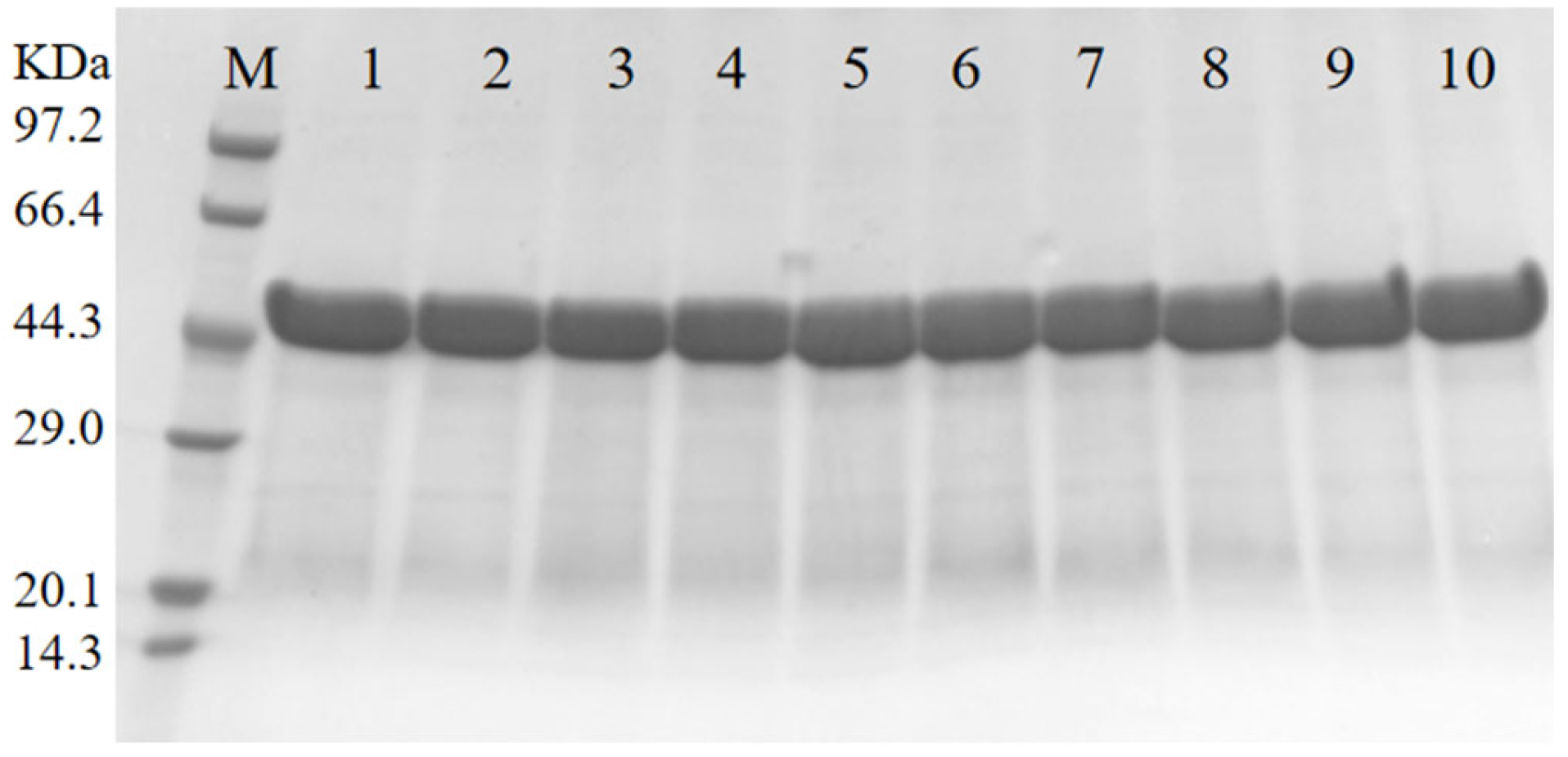



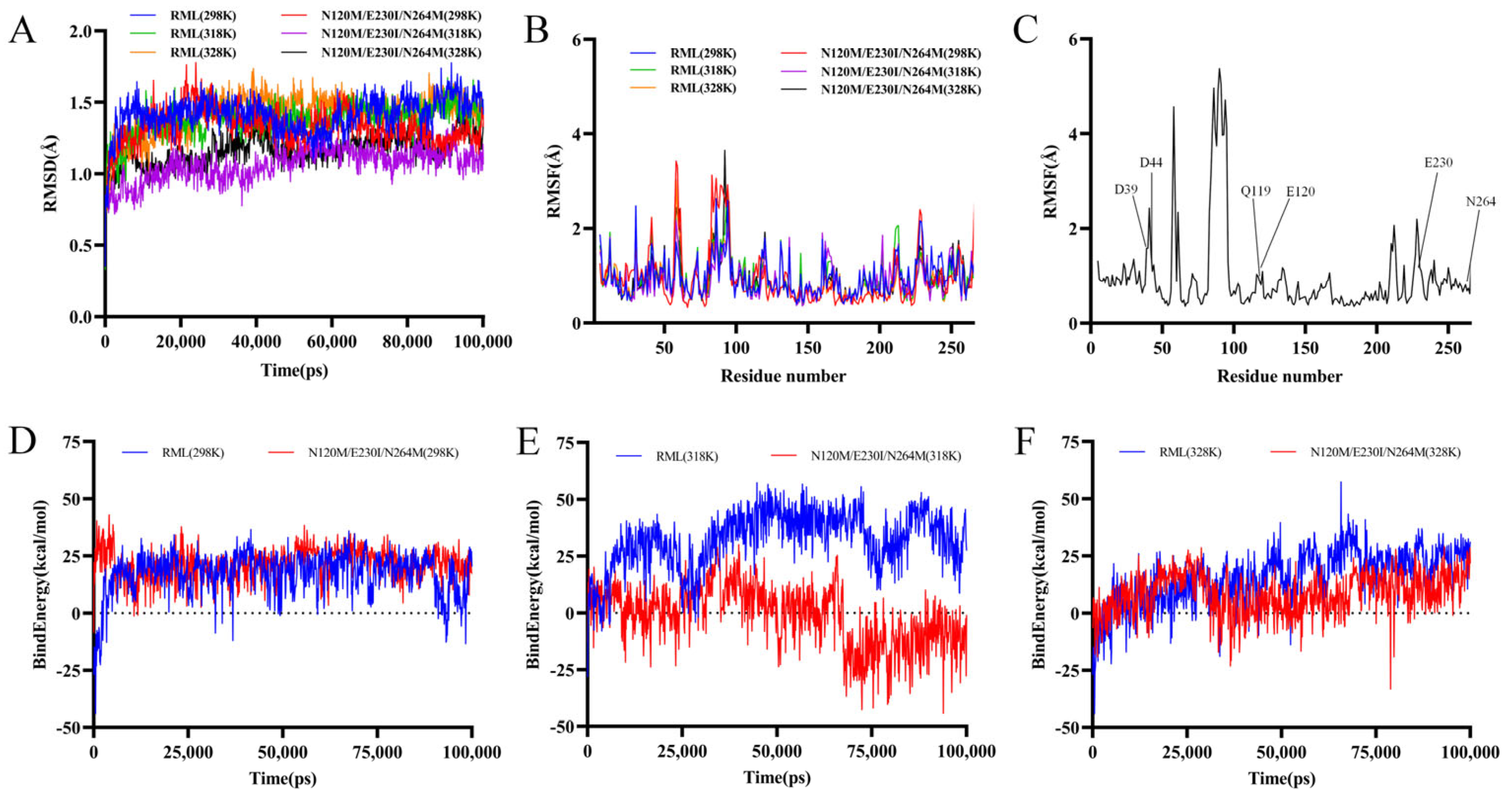
| Mutation Site | Mcsm ΔΔG(kca/mol) | SDM ΔΔG(kca/mol) | DUET ΔΔG(kca/mo) | Epri ΔΔG(kca/mol) |
|---|---|---|---|---|
| D39M | 0.343 | 0.29 | 0.456 | −4.82 |
| D44L | 0.201 | 1.23 | 0.469 | −0.85 |
| Q119L | 0.32 | 0.29 | 0.819 | −2.06 |
| N120L | 0.066 | 1.52 | 0.463 | −2.84 |
| N120M | 0.491 | 0.85 | 0.684 | −4.86 |
| E230L | 0.324 | 0.69 | 0.755 | −2.47 |
| E230I | 0.324 | 0.54 | 0.751 | −2.83 |
| N264M | 0.531 | 0.55 | 0.745 | −3.10 |
| Mutants | t1/2 (min, 50 °C) | Folds |
|---|---|---|
| WT | 45 | 1 |
| D39M | 46.21 | 1.03 |
| D44L | 33.65 | 0.75 |
| Q119L | 23.5 | 0.52 |
| N120M | 53.73 | 1.19 |
| E230I | 115.52 | 2.57 |
| N264M | 78.77 | 1.75 |
| N120M/E230I | 173.29 | 3.85 |
| E230I/N264M | 182.41 | 4.05 |
| N120M/E230I/N264M | 462 | 10.27 |
| HB a | SB a | π–π a | DB a | VW a | π-C a | |
|---|---|---|---|---|---|---|
| WT | 171 | 6 | 12 | 3 | 161 | 2 |
| N120M/E230I/N264M | 172 | 7 | 11 | 3 | 170 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teng, R.; Zhang, J.; Tu, Z.; He, Q.; Li, Y. Computer-Aided Design to Improve the Thermal Stability of Rhizomucor miehei Lipase. Foods 2024, 13, 4023. https://doi.org/10.3390/foods13244023
Teng R, Zhang J, Tu Z, He Q, Li Y. Computer-Aided Design to Improve the Thermal Stability of Rhizomucor miehei Lipase. Foods. 2024; 13(24):4023. https://doi.org/10.3390/foods13244023
Chicago/Turabian StyleTeng, Rong, Jin Zhang, Zhui Tu, Qinghua He, and Yanping Li. 2024. "Computer-Aided Design to Improve the Thermal Stability of Rhizomucor miehei Lipase" Foods 13, no. 24: 4023. https://doi.org/10.3390/foods13244023
APA StyleTeng, R., Zhang, J., Tu, Z., He, Q., & Li, Y. (2024). Computer-Aided Design to Improve the Thermal Stability of Rhizomucor miehei Lipase. Foods, 13(24), 4023. https://doi.org/10.3390/foods13244023





