Characterization of Volatile Flavor Compounds and Aroma Active Components in Button Mushroom (Agaricus bisporus) across Various Cooking Methods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Button Mushroom Samples and Cooking Methods
- Steamed: Room temperature water was added at a 1:20 (g/mL) ratio in a steamer. The water was heated to boiling, and the mushroom slices were placed on the steamer rack in the steamer and steamed for 7 min.
- Boiled: Room temperature water was added at a ratio of 1:20 (g/mL) in a pan and heated on an induction cooker (Guangdong Midea Life Electric Appliance Manu-facturing Co., Foshan, China) until the water boiled. Then, the sliced mushrooms were poured into the pan and were cooked for 7 min.
- Baked: The oven (Guangdong Midea Life Electric Appliance Manufacturing Co., Foshan, China) was preheated to 163 °C, and the mushroom slices were baked in the 163 °C oven for about 4 min on each side, totaling 8 min.
2.3. E-Nose Measurement
2.4. Headspace Solid-Phase Micro-Extraction (HS-SPME) of Aroma Compounds
2.5. GC-MS Analysis
2.6. Calculation of Odor Activity Values (OAVs)
2.7. Aroma Recombination and Omission Experiments
2.8. Statistical Analysis
3. Results and Discussion
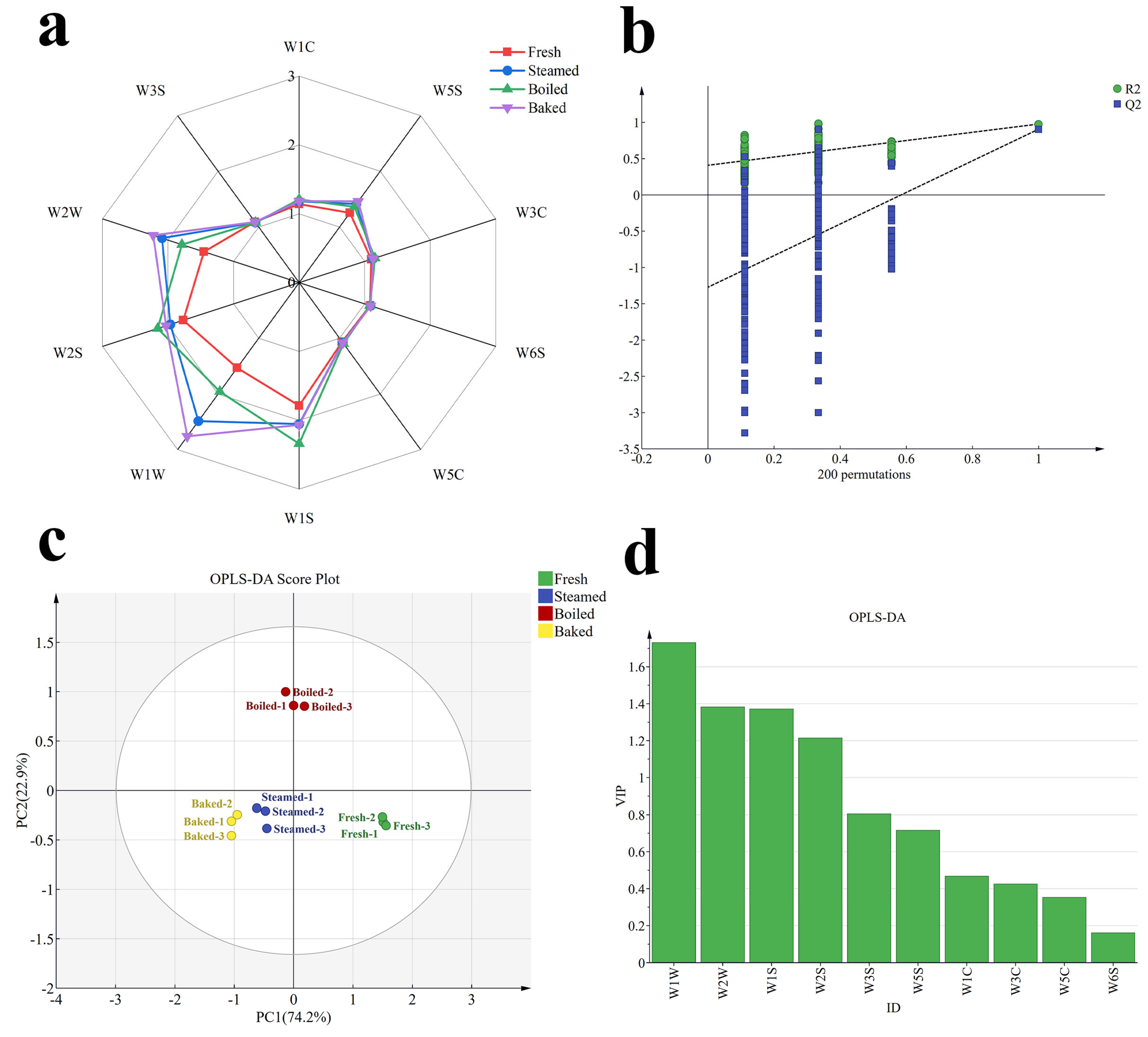
3.1. E-Nose Analysis
3.2. Characterization of Volatile Compound Species
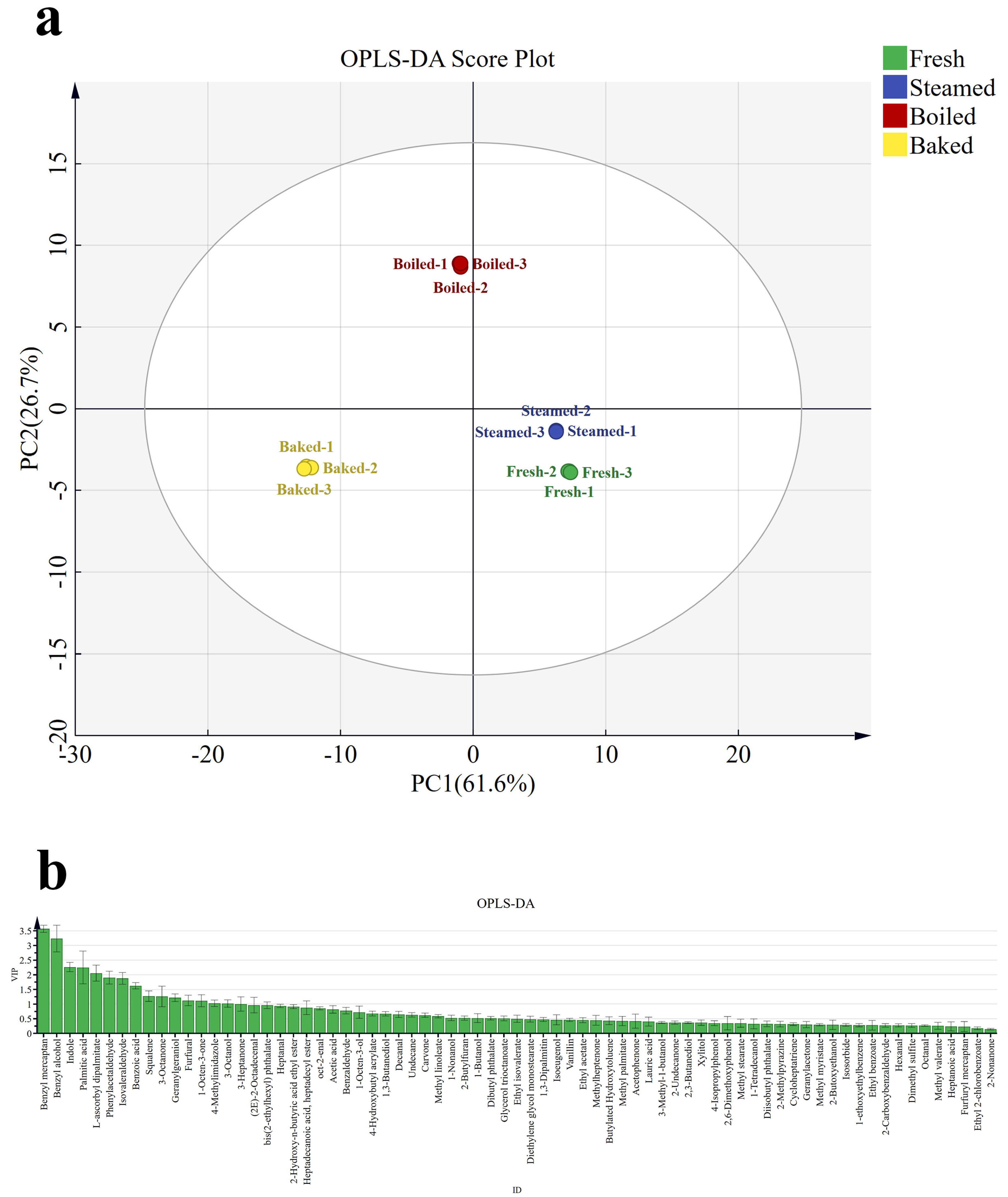
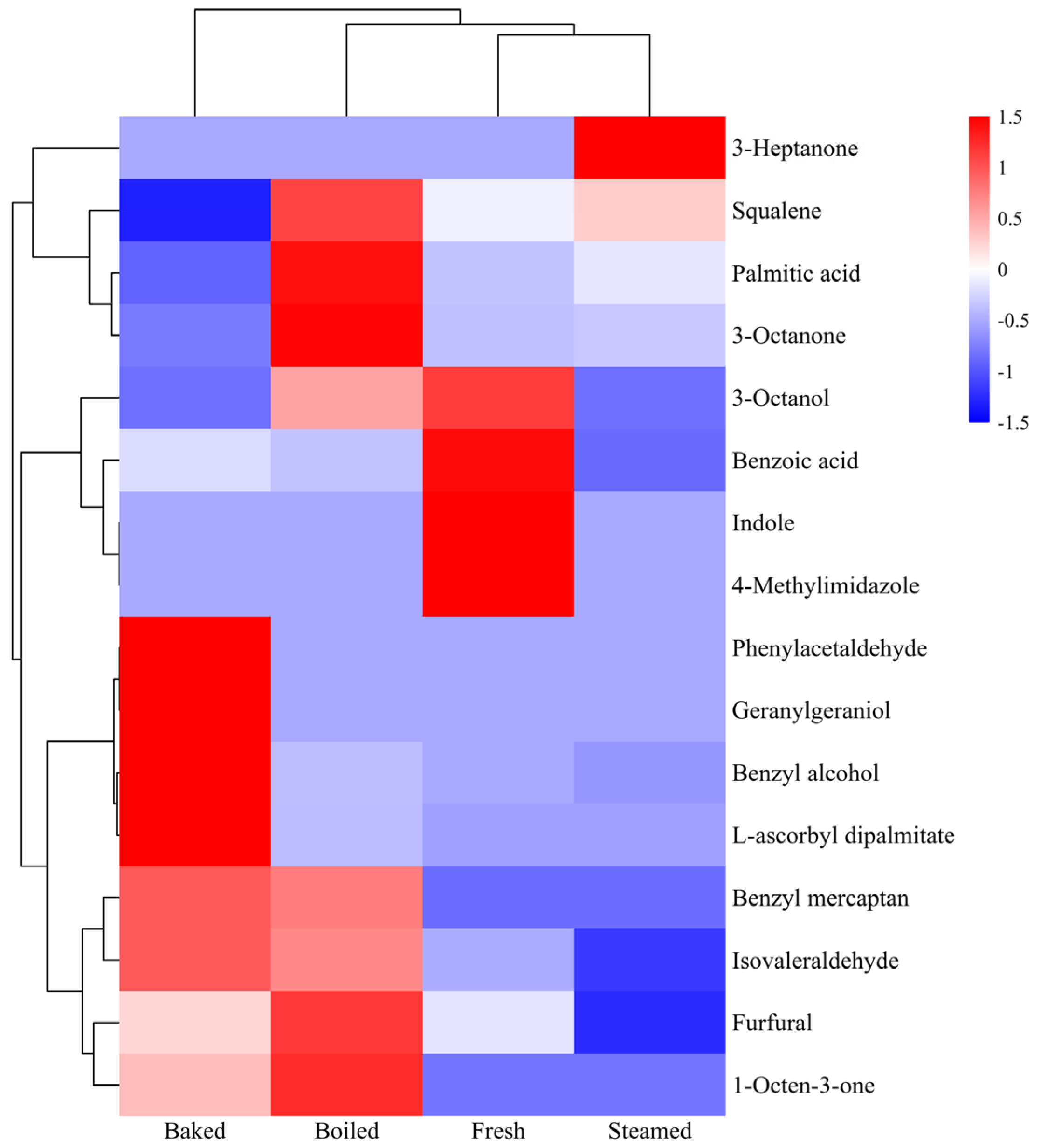
3.3. Differential Aroma Analysis
3.4. OAV Analysis
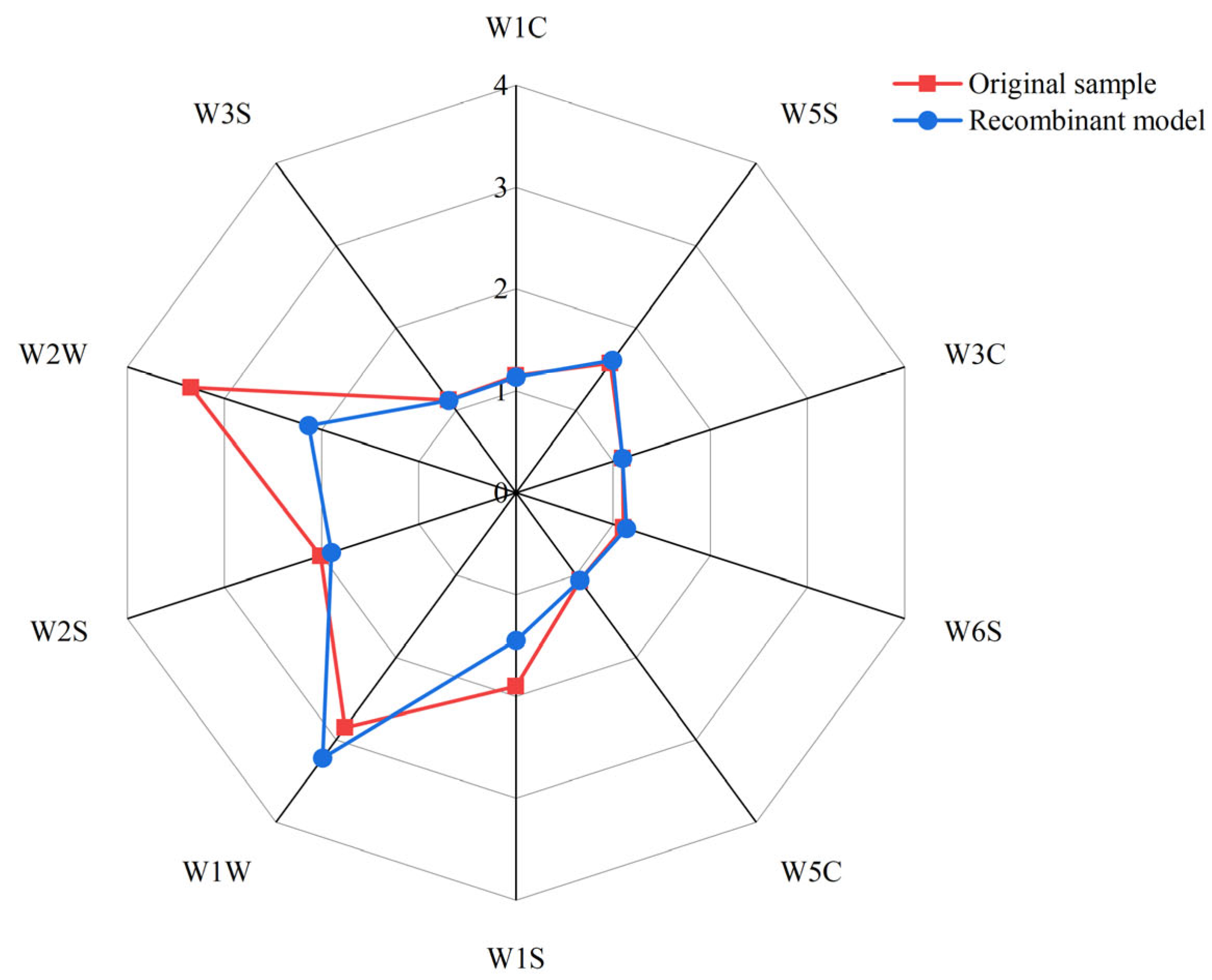
3.5. Aroma Recombination and Omission Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feng, T.; Yang, M.; Ma, B.; Zhao, Y.; Zhuang, H.; Zhang, J.; Chen, D. Volatile profiles of two genotype Agaricus bisporus species at different growth stages. Food Res. Int. 2021, 140, 109761. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthi, R.; Srinivash, M.; Mahalingam, P.U.; Malaikozhundan, B. Dietary nutrients in edible mushroom, Agaricus bisporus and their radical scavenging, antibacterial, and antifungal effects. Process Biochem. 2022, 121, 10–17. [Google Scholar] [CrossRef]
- Rauf, A.; Joshi, P.B.; Ahmad, Z.; Hemeg, H.A.; Olatunde, A.; Naz, S.; Hafeez, N.; Simal-Gandara, J. Edible mushrooms as potential functional foods in amelioration of hypertension. Phytother. Res. 2023, 37, 2644–2660. [Google Scholar] [CrossRef] [PubMed]
- Bassam, S.M.; Noleto-Dias, C.; Farag, M.A. Dissecting grilled red and white meat flavor: Its characteristics, production mechanisms, influencing factors and chemical hazards. Food Chem. 2022, 371, 131139. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Guan, Q.; Wang, Y.; Lin, D.; Du, B. Effect of Different Cooking Methods on Nutrients, Antioxidant Activities and Flavors of Three Varieties of Lentinus edodes. Foods 2022, 11, 2713. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, X.; Tian, Y.; Wang, Q.; Li, X.; An, F.; Luo, Z.; Shang, P.; Liu, Z.; Huang, Q. Mechanisms of cooking methods on flavor formation of Tibetan pork. Food Chem. X 2023, 19, 100873. [Google Scholar] [CrossRef]
- Shakoor, A.; Zhang, C.; Xie, J.; Yang, X. Maillard reaction chemistry in formation of critical intermediates and flavour compounds and their antioxidant properties. Food Chem. 2022, 393, 133416. [Google Scholar] [CrossRef]
- Flaskerud, K.; Bukowski, M.; Golovko, M.; Johnson, L.; Brose, S.; Ali, A.; Cleveland, B.; Picklo, M.; Raatz, S. Effects of cooking techniques on fatty acid and oxylipin content of farmed rainbow trout (Oncorhynchus mykiss). Food Sci. Nutr. 2017, 5, 1195–1204. [Google Scholar] [CrossRef]
- Li, W.; Li, R.; Chen, W.; Feng, J.; Wu, D.; Zhang, Z.; Zhang, J.; Yang, Y. The anabolism of sulphur aroma volatiles responds to enzymatic and non-enzymatic reactions during the drying process of shiitake mushrooms. Food Chem. 2022, 371, 131123. [Google Scholar] [CrossRef]
- Hou, Z.; Xia, R.; Li, Y.; Xu, H.; Wang, Y.; Feng, Y.; Pan, S.; Wang, Z.; Ren, H.; Qian, G.; et al. Key components, formation pathways, affecting factors, and emerging analytical strategies for edible mushrooms aroma: A review. Food Chem. 2024, 438, 137993. [Google Scholar] [CrossRef]
- Davila, M.; Muniz, A.; Du, X. The impact of roasting and steaming on savory flavors contributed by amino acids, 5′-nucleotides, and volatiles in Agaricus bisporus mushrooms. Int. J. Gastron. Food Sci. 2022, 30, 100590. [Google Scholar] [CrossRef]
- Selli, S.; Guclu, G.; Sevindik, O.; Kelebek, H. Variations in the key aroma and phenolic compounds of champignon (Agaricus bisporus) and oyster (Pleurotus ostreatus) mushrooms after two cooking treatments as elucidated by GC–MS-O and LC-DAD-ESI-MS/MS. Food Chem. 2021, 354, 129576. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Hu, Z.; Liang, M.; Song, L.; Wu, W.; Li, R.; Li, Z.; Zhang, J. Evaluation of the flavor compounds of Pleurotus eryngii as affected by baking temperatures using HS-SPME-GC–MS and electronic nose. J. Food Process. Preserv. 2022, 46, e17056. [Google Scholar] [CrossRef]
- Chi, X.; Shao, Y.; Pan, M.; Yang, Q.; Yang, Y.; Zhang, X.; Ai, N.; Sun, B. Distinction of volatile flavor profiles in various skim milk products via HS-SPME–GC–MS and E-nose. Eur. Food Res. Technol. 2021, 247, 1539–1551. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, P.; Xia, W.; Jiang, Q.; Liu, S.; Xu, Y. Characterization of key aroma compounds in low-salt fermented sour fish by gas chromatography-mass spectrometry, odor activity values, aroma recombination and omission experiments. Food Chem. 2022, 397, 133773. [Google Scholar] [CrossRef] [PubMed]
- Capone, S.; Tufariello, M.; Francioso, L.; Montagna, G.; Casino, F.; Leone, A.; Siciliano, P. Aroma analysis by GC/MS and electronic nose dedicated to Negroamaro and Primitivo typical Italian Apulian wines. Sens. Actuators B 2013, 179, 259–269. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Lv, Z.; Zeng, Q.; Fu, X.; Chen, Q.; Luo, Z.; Luo, C.; Wang, D.; Zhang, W. Analysis of the volatile profiles of kiwifruits experiencing soft rot using E-nose and HS-SPME/GC–MS. LWT 2023, 173, 114405. [Google Scholar] [CrossRef]
- Jin, G.; Zhu, Z.; Wu, Z.; Wang, F.; Li, J.; Raghavan, V.; Li, B.; Song, C. Characterization of volatile components of microwave dried perilla leaves using GC–MS and E-nose. Food Biosci. 2023, 56, 103083. [Google Scholar] [CrossRef]
- Zhou, J.; Feng, T.; Ye, R. Differentiation of Eight Commercial Mushrooms by Electronic Nose and Gas Chromatography-Mass Spectrometry. J. Sens. 2015, 2015, 374013. [Google Scholar] [CrossRef]
- Fujioka, K.; Shimizu, N.; Manome, Y.; Ikeda, K.; Yamamoto, K.; Tomizawa, Y. Discrimination Method of the Volatiles from Fresh Mushrooms by an Electronic Nose Using a Trapping System and Statistical Standardization to Reduce Sensor Value Variation. Sensors 2013, 13, 15532–15548. [Google Scholar] [CrossRef]
- Gomez, I.; Lavega Gonzalez, R.; Tejedor-Calvo, E.; Perez Clavijo, M.; Carrasco, J. Odor Profile of Four Cultivated and Freeze-Dried Edible Mushrooms by Using Sensory Panel, Electronic Nose and GC-MS. J. Fungi 2022, 8, 953. [Google Scholar] [CrossRef]
- Sun, Y.; Lv, F.; Tian, J.; Ye, X.Q.; Chen, J.; Sun, P. Domestic cooking methods affect nutrient, phytochemicals, and flavor content in mushroom soup. Food Sci. Nutr. 2019, 7, 1969–1975. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.; Choi, Y.; Kim, Y.; Jeong, H.S.; Lee, J. Effect of Different Cooking Methods on the True Retention of Vitamins, Minerals, and Bioactive Compounds in Shiitake Mushrooms (Lentinula edodes). Food Sci. Technol. Res. 2019, 25, 115–122. [Google Scholar] [CrossRef]
- Roncero-Ramos, I.; Mendiola-Lanao, M.; Perez-Clavijo, M.; Delgado-Andrade, C. Effect of different cooking methods on nutritional value and antioxidant activity of cultivated mushrooms. Int. J. Food Sci. Nutr. 2017, 68, 287–297. [Google Scholar] [CrossRef]
- Guo, Q.; Adelina, N.M.; Hu, J.; Zhang, L.; Zhao, Y. Comparative analysis of volatile profiles in four pine-mushrooms using HS-SPME/GC-MS and E-nose. Food Control 2022, 134, 108711. [Google Scholar] [CrossRef]
- Adelina, N.M.; Wang, H.; Zhang, L.; Zhao, Y. Comparative analysis of volatile profiles in two grafted pine nuts by headspace-SPME/GC–MS and electronic nose as responses to different roasting conditions. Food Res. Int. 2021, 140, 110026. [Google Scholar] [CrossRef]
- Lu, B.; Perez-Moreno, J.; Zhang, F.; Rinaldi, A.C.; Yu, F. Aroma profile of two commercial truffle species from Yunnan and Sichuan, China: Inter- and intraspecific variability and shared key compounds. Food Sci. Hum. Wellness 2021, 10, 163–173. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Zhang, D.; Shen, Q.; Pan, T.; Hui, T.; Ma, J. Characterization of Key Aroma Compounds in Beijing Roasted Duck by Gas Chromatography–Olfactometry–Mass Spectrometry, Odor-Activity Values, and Aroma-Recombination Experiments. J. Agric. Food Chem. 2019, 67, 5847–5856. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Xiao, Q.; Xie, J.; Cheng, J.; Sun, B.; Du, W.; Wang, Y.; Wang, T. Aroma Compounds in Chicken Broths of Beijing Youji and Commercial Broilers. J. Agric. Food Chem. 2018, 66, 10242–10251. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Li, J.; Liu, H.; Wang, Y. Volatile compounds and aroma characteristics of mushrooms: A review. Crit. Rev. Food Sci. Nutr. 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Cui, C.; Zhang, S.; Zhu, J.; Peng, C.; Cai, H.; Yang, X.; Hou, R. Use of headspace GC/MS combined with chemometric analysis to identify the geographic origins of black tea. Food Chem. 2021, 360, 130033. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Zhang, Y.; Qin, Y.; Li, Y.; Su, L.; Li, L.; Bian, Z.; Lu, T. E-eye, flash GC E-nose and HS-GC-MS combined with chemometrics to identify the adulterants and geographical origins of Ziziphi Spinosae Semen. Food Chem. 2023, 424, 136270. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.; Dan, M.; Zhao, G.; Wang, D. Recent advances in chromatography-mass spectrometry and electronic nose technology in food flavor analysis and detection. Food Chem. 2023, 405, 134814. [Google Scholar] [CrossRef] [PubMed]
- Grosshauser, S.; Schieberle, P. Characterization of the Key Odorants in Pan-Fried White Mushrooms (Agaricus bisporus L.) by Means of Molecular Sensory Science: Comparison with the Raw Mushroom Tissue. J. Agric. Food Chem. 2013, 61, 3804–3813. [Google Scholar] [CrossRef]
- Maga, J.A. Influence of Maturity, Storage and Heating on the Flavor of Mushroom (Agaricus bisporus) Caps and Stems. J. Food Process Preserv. 1981, 5, 95–101. [Google Scholar] [CrossRef]
- Çaǧlarırmak, N. Determination of nutrients and volatile constituents of Agaricus bisporus (brown) at different stages. J. Sci. Food Agric. 2009, 89, 634–638. [Google Scholar] [CrossRef]
- Pei, F.; Yang, W.; Ma, N.; Fang, Y.; Zhao, L.; An, X.; Xin, Z.; Hu, Q. Effect of the two drying approaches on the volatile profiles of button mushroom (Agaricus bisporus) by headspace GC–MS and electronic nose. LWT-Food Sci. Technol. 2016, 72, 343–350. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Y.; Wang, Y.; Bu, H.; Dong, T. Changes in cell wall metabolism and flavor qualities of mushrooms (Agaricus bernardii) under EMAP treatments during storage. Food Packag. Shelf Life 2021, 29, 100732. [Google Scholar] [CrossRef]
- Gao, F.; Xie, W.; Zhang, H.; Li, S.; Li, T. Variations of quality and volatile components of morels (Morchella sextelata) during storage. J. Plant Physiol. 2023, 290, 154094. [Google Scholar] [CrossRef]
- Feng, Y.; Xu, H.; Sun, Y.; Xia, R.; Hou, Z.; Li, Y.; Wang, Y.; Pan, S.; Li, L.; Zhao, C.; et al. Effect of light on quality of preharvest and postharvest edible mushrooms and its action mechanism: A review. Trends Food Sci. Technol. 2023, 139, 104119. [Google Scholar] [CrossRef]
- Zhang, K.; Pu, Y.; Sun, D. Recent advances in quality preservation of postharvest mushrooms (Agaricus bisporus): A review. Trends Food Sci. Technol. 2018, 78, 72–82. [Google Scholar] [CrossRef]
- Du, X.; Sissons, J.; Shanks, M.; Plotto, A. Aroma and flavor profile of raw and roasted Agaricus bisporus mushrooms using a panel trained with aroma chemicals. LWT-Food Sci. Technol. 2021, 138, 110596. [Google Scholar] [CrossRef]
- Ashmore, L.; Craske, J.D.; Srzednicki, G. Volatile compounds in fresh, cooked fresh, dried and cooked dried Agaricus bisporus using ambient temperature vacuum distillation. Int. Food Res. J. 2014, 21, 263–268. [Google Scholar]
- Huang, J.; Xiao, L.; Yi, Y.; Li, B.; Sun, R.; Deng, H. Preservation mechanism and flavor variation of postharvest button mushroom (Agaricus bisporus) coated compounds of protocatechuic acid-CaCl2-NaCl-pullulan. LWT-Food Sci. Technol. 2022, 169, 114020. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, X.; Song, Z.; Kong, W.; Kang, Y.; Kong, W.; Ng, T.B. Coating shiitake mushrooms (Lentinus edodes) with a polysaccharide from Oudemansiella radicata improves product quality and flavor during postharvest storage. Food Chem. 2021, 352, 129357. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xu, R.; Jia, Q.; Feng, T.; Huang, Q.; Ho, C.; Song, S. Identification of dihydro-β-ionone as a key aroma compound in addition to C8 ketones and alcohols in Volvariella volvacea mushroom. Food Chem. 2019, 293, 333–339. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Hengchao, E.; He, X.; Li, J.; Zhao, X.; Zhou, C. Characteristic fingerprints and comparison of volatile flavor compounds in Morchella sextelata under different drying methods. Food Res. Int. 2023, 172, 113103. [Google Scholar] [CrossRef]
- Cheng, Y.; Wei, Y.; Zhang, M.; Wang, H. Effect of micro-perforated film packing on physicochemical quality and volatile profile of button mushroom (Agaricus bisporus) during postharvest shelf-life. J. Food Process. Preserv. 2022, 46, e16648. [Google Scholar] [CrossRef]
- Zhu, R.; Wen, Y.; Wu, W.; Zhang, L.; Salman Farid, M.; Shan, S.; Wen, J.; Farag, M.A.; Zhang, Y.; Zhao, C. The flavors of edible mushrooms: A comprehensive review of volatile organic compounds and their analytical methods. Crit. Rev. Food Sci. Nutr. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Combet, E.; Henderson, J.; Eastwood, D.C.; Burton, K.S. Influence of Sporophore Development, Damage, Storage, and Tissue Specificity on the Enzymic Formation of Volatiles in Mushrooms (Agaricus bisporus). J. Agric. Food Chem. 2009, 57, 3709–3717. [Google Scholar] [CrossRef]
- Schmidberger, P.C.; Schieberle, P. Characterization of the Key Aroma Compounds in White Alba Truffle (Tuber. magnatum pico) and Burgundy Truffle (Tuber uncinatum) by Means of the Sensomics Approach. J. Agric. Food Chem. 2017, 65, 9287–9296. [Google Scholar] [CrossRef] [PubMed]
- Vahdatzadeh, M.; Splivallo, R. Improving truffle mycelium flavour through strain selection targeting volatiles of the Ehrlich pathway. Sci. Rep. 2018, 8, 9304. [Google Scholar] [CrossRef] [PubMed]
- Pueschel, V.A.; Schieberle, P. Changes in the key aroma compounds of matsutake mushroom (Tricholoma matsutake Sing.) from Canada during pan-frying elucidated by application of the sensomics approach. Eur. Food Res. Technol. 2020, 247, 51–65. [Google Scholar] [CrossRef]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS-GC–IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Schmidberger, P.C.; Schieberle, P. Changes in the Key Aroma Compounds of Raw Shiitake Mushrooms (Lentinula edodes) Induced by Pan-Frying as Well as by Rehydration of Dry Mushrooms. J. Agric. Food Chem. 2020, 68, 4493–4506. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.F.; Wickramasinghe, P.C.K.; Munafo, J.P. Key Odorants from the Fragrant Bolete, Suillus punctipes. J. Agric. Food Chem. 2020, 68, 8621–8628. [Google Scholar] [CrossRef]
- Zhang, H.; Pu, D.; Sun, B.; Ren, F.; Zhang, Y.; Chen, H. Characterization and comparison of key aroma compounds in raw and dry porcini mushroom (Boletus edulis) by aroma extract dilution analysis, quantitation and aroma recombination experiments. Food Chem. 2018, 258, 260–268. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. A paradigm of volatile aroma compounds in rice and their product with extraction and identification methods: A comprehensive review. Food Res. Int. 2020, 130, 108924. [Google Scholar] [CrossRef]
| No. | Volatile Compounds | CAS | RT | Odorant | Concentration (μg/g) | p-Value | VIP | |||
|---|---|---|---|---|---|---|---|---|---|---|
| (Min) | Description | Fresh | Steamed | Boiled | Baked | |||||
| Alcohols | ||||||||||
| 1 | Benzyl alcohol | 100-51-6 | 24.097 | floral | 4.55 ± 0.12 c | 2.32 ± 0.20 d | 7.26 ± 0.20 b | 47.56 ± 1.50 a | 4.01 × 10−12 | 3.24 |
| 2 | 2,3-Butanediol | 513-85-9 | 18.286 | - | 0.30 ± 0.02 | ND | ND | ND | 2.33 × 10−10 | 0.37 |
| 3 | 1,3-Butanediol | 107-88-0 | 18.527 | - | 1.01 ± 0.09 | ND | ND | ND | 4.38 × 10−9 | 0.67 |
| 4 | 3-Methyl-1-butanol | 123-51-3 | 24.711 | alcoholic, fruity, fatty, banana-like | 0.31 ± 0.02 | ND | ND | ND | 5.31 × 10−10 | 0.37 |
| 5 | Isosorbide | 652-67-5 | 22.972 | - | ND | ND | ND | 0.33 ± 0.03 | 2.26 × 10−9 | 0.29 |
| 6 | Xylitol | 87-99-0 | 20.564 | - | ND | ND | ND | 0.54 ± 0.09 | 7.25 × 10−7 | 0.37 |
| 7 | 1-Octen-3-ol | 3391-86-4 | 21.946 | mushroom-like | ND | ND | 1.66 ± 0.17 a | 0.42 ± 0.03 b | 5.03 × 10−8 | 0.72 |
| 8 | Geranylgeraniol | 24034-73-9 | 33.572 | - | ND | ND | ND | 5.96 ± 0.13 | 8.98 × 10−14 | 1.22 |
| 9 | 3-Octanol | 589-98-0 | 22.481 | earthy, mushroom-like | 2.07 ± 0.12 a | ND | 1.45 ± 0.20 b | ND | 3.17 × 10−8 | 1.02 |
| 10 | 1-Butanol | 71-36-3 | 20.236 | floral, fragrant, fruity | ND | ND | 0.79 ± 0.04 | ND | 4.75 × 10−9 | 0.52 |
| 11 | 1-Nonanol | 143-08-8 | 22.671 | green, fatty, sweet | 0.63 ± 0.06 | ND | ND | ND | 8.74 × 10-9 | 0.53 |
| 12 | 1-Tetradecanol | 112-72-1 | 30.917 | waxy | 0.25 ± 0.09 | ND | ND | ND | 4.19 × 10−4 | 0.32 |
| Esters | ||||||||||
| 13 | Methyl palmitate | 112-39-0 | 29.961 | - | 0.31 ± 0.10 b | ND | 0.49 ± 0.12 a | 0.19 ± 0.07 b | 1.00 × 10−3 | 0.42 |
| 14 | Dibutyl phthalate | 84-74-2 | 29.412 | - | 0.60 ± 0.06 | ND | ND | ND | 8.65 × 10−9 | 0.52 |
| 15 | 4-Hydroxybutyl acrylate | 2478-10-6 | 28.818 | - | ND | ND | ND | 1.85 ± 0.07 | 6.62 × 10−12 | 0.68 |
| 16 | Ethyl acetate | 141-78-6 | 19.33 | fruity, sweet | 0.93 ± 0.07 a | 0.46 ± 0.11 b | 0.44 ± 0.08 b | 0.31 ± 0.05 b | 6.10 × 10−5 | 0.45 |
| 17 | Methyl linoleate | 112-63-0 | 37.345 | - | 0.75 ± 0.05 a | ND | ND | 0.40 ± 0.05 b | 1.26 × 10−8 | 0.59 |
| 18 | Methyl valerate | 624-24-8 | 22.158 | - | ND | ND | ND | 0.28 ± 0.08 | 5.60 × 10−5 | 0.26 |
| 19 | Heptadecanoic acid, heptadecyl ester | 36617-50-2 | 27.682 | - | ND | ND | 2.44 ± 0.08 a | 0.62 ± 0.12 b | 3.07 × 10−10 | 0.88 |
| 20 | 1,3-Dipalmitin | 502-52-3 | 25.549 | - | ND | ND | ND | 0.91 ± 0.05 | 2.47 × 10−10 | 0.48 |
| 21 | L-ascorbyl dipalmitate | 28474-90-0 | 26.106 | - | ND | ND | 1.47 ± 0.15 b | 17.82 ± 0.51 a | 1.21 × 10−12 | 2.06 |
| 22 | Glycerol trioctanoate | 538-23-8 | 25.363 | - | 0.69 ± 0.08 b | 0.42 ± 0.09 c | 0.93 ± 0.03 a | ND | 4.77 × 10−7 | 0.51 |
| 23 | Ethyl 2-chlorobenzoate | 7335-25-3 | 23.582 | - | ND | 0.06 ± 0.01 | ND | ND | 8.01 × 10−8 | 0.17 |
| 24 | Ethyl isovalerate | 108-64-5 | 22.814 | fruity, sweet, grape-like | ND | 0.49 ± 0.07 | ND | ND | 3.89 × 10−7 | 0.50 |
| 25 | bis(2-ethylhexyl) phthalate | 117-81-7 | 25.117 | - | ND | 1.98 ± 0.10 b | 2.82 ± 0.12 a | 2.74 ± 0.07 a | 5.58 × 10−10 | 0.96 |
| 26 | Methyl stearate | 112-61-8 | 24.522 | - | ND | ND | 0.36 ± 0.08 | ND | 7.00 × 10−6 | 0.35 |
| 27 | Diethylene glycol monostearate | 106-11-6 | 24.346 | - | 0.53 ± 0.09 | ND | ND | ND | 9.45 × 10−7 | 0.49 |
| 28 | 2-Hydroxy-n-butyric acid ethyl ester | 52089-54-0 | 21.094 | - | 1.87 ± 0.05 | ND | ND | ND | 3.17 × 10−13 | 0.91 |
| 29 | Diisobutyl phthalate | 84-69-5 | 31.856 | - | 0.24 ± 0.05 | ND | ND | ND | 9.00 × 10−6 | 0.32 |
| 30 | Dimethyl sulfite | 616-42-2 | 14.059 | - | 0.17 ± 0.02 | ND | ND | ND | 2.67 × 10−7 | 0.27 |
| 31 | Ethyl benzoate | 93-89-0 | 20.911 | - | 0.19 ± 0.07 | ND | ND | ND | 4.31 × 10−4 | 0.28 |
| 32 | Methyl myristate | 124-10-7 | 28.569 | - | 0.20 ± 0.02 | ND | ND | ND | 2.32 × 10−8 | 0.30 |
| 33 | Benzaldehyde | 100-52-7 | 17.227 | almond-like | 0.29 ± 0.09 c | 0.22 ± 0.02 c | 0.45 ± 0.09 b | 2.80 ± 0.05 a | 1.50 × 10−10 | 0.78 |
| 34 | Phenylacetaldehyde | 122-78-1 | 17.785 | honey-like | ND | ND | ND | 14.53 ± 0.31 | 7.42 × 10−14 | 1.90 |
| 35 | Hexanal | 66-25-1 | 23.865 | green | ND | ND | ND | 0.30 ± 0.04 | 5.87 × 10−8 | 0.27 |
| 36 | (2E)-2-Octadecenal | 51534-37-3 | 33.088 | - | ND | ND | 2.71 ± 0.03 | ND | 1.11 × 10−15 | 0.97 |
| 37 | 2-Carboxybenzaldehyde | 119-67-5 | 31.285 | - | 0.17 ± 0.03 | ND | ND | ND | 1.00 × 10−6 | 0.27 |
| 38 | Furfural | 98-01-1 | 21.348 | bread-like, sweet, fatty, almond-like | 1.98 ± 0.12 c | 0.26 ± 0.07 d | 4.06 ± 0.20 a | 2.59 ± 0.11 b | 4.55 × 10−9 | 1.12 |
| 39 | Isovaleraldehyde | 590-86-3 | 23.368 | apple-like, malty | 7.34 ± 0.23 c | 3.27 ± 0.12 d | 14.33 ± 0.26 b | 15.92 ± 0.14 a | 1.93 × 10−12 | 1.88 |
| 40 | Decanal | 112-31-2 | 32.153 | floral, green, fruity, waxy, orange-like, | ND | ND | 0.45 ± 0.10 b | 1.89 ± 0.06 a | 3.61 × 10−10 | 0.65 |
| 41 | oct-2-enal | 2363-89-5 | 29.133 | fruity, green, nutty | ND | ND | 2.17 ± 0.09 b | 2.87 ± 0.06 a | 5.93 × 10−12 | 0.85 |
| 42 | Heptanal | 111-71-7 | 27.4 | green, fatty | 0.29 ± 0.06 d | 1.15 ± 0.13 c | 3.42 ± 0.07 b | 3.85 ± 0.07 a | 4.54 × 10−11 | 0.94 |
| 43 | Vanillin | 121-33-5 | 28.166 | - | 0.41 ± 0.02 a | ND | 0.33 ± 0.02 b | ND | 3.11 × 10−10 | 0.46 |
| 44 | Octanal | 124-13-0 | 29.744 | citrus-like, fatty | 0.25 ± 0.04 b | 0.22 ± 0.03 b | 0.30 ± 0.02 a | ND | 2.00 × 10−6 | 0.27 |
| Acids | ||||||||||
| 45 | Acetic acid | 64-19-7 | 16.007 | sour | ND | 1.42 ± 0.04 a | 0.72 ± 0.11 b | 0.69 ± 0.07 b | 5.57 × 10−8 | 0.82 |
| 46 | Benzoic acid | 65-85-0 | 20.728 | bitter gourd-like | 6.66 ± 0.21 a | 0.89 ± 0.07 d | 2.18 ± 0.14 c | 2.56 ± 0.13 b | 1.92 × 10−10 | 1.63 |
| 47 | Lauric acid | 143-07-7 | 30.203 | tallow-like | 0.79 ± 0.06 b | 0.55 ± 0.08 c | 1.09 ± 0.16 a | 0.99 ± 0.16 a b | 3.00 × 10−3 | 0.40 |
| 48 | Palmitic acid | 57-10-3 | 34.048 | - | 4.64 ± 0.12 c | 6.26 ± 0.09 b | 18.12 ± 0.16 a | 0.36 ± 0.11 d | 7.22 × 10−15 | 2.25 |
| 49 | Heptanoic acid | 111-14-8 | 26.343 | - | ND | ND | ND | 0.25 ± 0.11 | 1.00 × 10−3 | 0.24 |
| Ketones | ||||||||||
| 50 | Methylheptenone | 110-93-0 | 23.159 | sweet, fruity | ND | ND | 0.58 ± 0.11 | ND | 2.00 × 10−6 | 0.45 |
| 51 | 3-Octanone | 106-68-3 | 11.184 | earthy, mushroom-like, resinous | 0.96 ± 0.17 b | 1.08 ± 0.14 b | 5.25 ± 0.22 a | ND | 6.32 × 10−10 | 1.26 |
| 52 | 3-Heptanone | 106-35-4 | 11.013 | fruity | ND | 1.97 ± 0.17 | ND | ND | 5.26 × 10−9 | 1.00 |
| 53 | Geranylacetone | 3796-70-1 | 12.536 | green, fruity | 0.20 ± 0.06 | ND | ND | ND | 3.70 × 10−5 | 0.30 |
| 54 | 2-Undecanone | 112-12-9 | 12.267 | floral | 0.31 ± 0.04 | ND | ND | ND | 4.51 × 10−8 | 0.37 |
| 55 | Carvone | 99-49-0 | 12.043 | - | 1.34 ± 0.13 a | 1.23 ± 0.14 a | ND | ND | 1.07 × 10−7 | 0.62 |
| 56 | 1-Octen-3-one | 4312-99-6 | 11.641 | metallic, mushroom-like | ND | ND | 4.21 ± 0.14 a | 2.48 ± 0.24 b | 5.60 × 10−10 | 1.12 |
| 57 | 2-Nonanone | 821-55-6 | 11.835 | fruity, green, cheese-like | ND | ND | ND | 0.08 ± 0.01 | 1.30 × 10−8 | 0.14 |
| 58 | Acetophenone | 98-86-2 | 11.329 | hawthorn-like | ND | ND | 0.53 ± 0.20 | ND | 3.26 × 10−4 | 0.42 |
| Others | ||||||||||
| 59 | Undecane | 1120-21-4 | 15.804 | - | 0.90 ± 0.09 | ND | ND | ND | 9.88 × 10−9 | 0.63 |
| 60 | Squalene | 111-02-4 | 36.81 | - | 3.29 ± 0.26 c | 4.32 ± 0.15 b | 6.49 ± 0.22 a | ND | 7.04 × 10−10 | 1.27 |
| 61 | 2,6-Dimethoxyphenol | 91-10-1 | 33.76 | clove-like, smoky | ND | 0.26 ± 0.11 | ND | ND | 1.00 × 10−3 | 0.35 |
| 62 | Isoeugenol | 97-54-1 | 34.525 | clove-like | ND | 0.43 ± 0.12 | ND | ND | 4.70 × 10−5 | 0.46 |
| 63 | 4-Methylimidazole | 822-36-6 | 16.783 | - | 2.37 ± 0.20 | ND | ND | ND | 4.45 × 10−9 | 1.03 |
| 64 | Indole | 120-72-9 | 33.252 | musty | 11.51 ± 0.26 | ND | ND | ND | 1.00 × 10−13 | 2.26 |
| 65 | 2-Butoxyethanol | 111-76-2 | 18.824 | - | 0.20 ± 0.08 | ND | ND | ND | 1.00 × 10−3 | 0.29 |
| 66 | 2-Methylpyrazine | 109-08-0 | 31.076 | nutty, sweet | 0.21 ± 0.12 a | ND | ND | 0.27 ± 0.07 a | 2.00 × 10−3 | 0.32 |
| 67 | 4-Isopropylphenol | 99-89-8 | 31.623 | - | ND | 0.24 ± 0.07 b | 0.31 ± 0.03 a | ND | 9.00 × 10−6 | 0.35 |
| 68 | Butylated Hydroxytoluene | 128-37-0 | 34.209 | - | ND | ND | 0.55 ± 0.07 | ND | 7.17 × 10−8 | 0.43 |
| 69 | 1-ethoxyethylbenzene | 3299-05-6 | 35.403 | - | ND | ND | ND | 0.32 ± 0.04 | 8.24 × 10−8 | 0.28 |
| 70 | Cycloheptatriene | 544-25-2 | 13.493 | - | ND | ND | ND | 0.40 ± 0.02 | 3.56 × 10−10 | 0.31 |
| 71 | Benzyl mercaptan | 100-53-8 | 37.082 | - | ND | ND | 40.51 ± 1.19 b | 45.38 ± 0.46 a | 2.88 × 10−13 | 3.57 |
| 72 | 2-Butylfuran | 4466-24-4 | 33.938 | noncharacteristic | ND | 0.60 ± 0.06 a | 0.39 ± 0.02 b | 0.44 ± 0.02 b | 2.23 × 10−7 | 0.52 |
| 73 | Furfuryl mercaptan | 98-02-2 | 25.795 | - | ND | ND | ND | 0.24 ± 0.12 | 3.00 × 10−3 | 0.23 |
| Type of Compounds | Fresh | Contents (µg/g) | Steamed | Contents (µg/g) | Boiled | Contents (µg/g) | Baked | Contents (µg/g) |
|---|---|---|---|---|---|---|---|---|
| Alcohols | 7 | 9.11 | 1 | 2.32 | 4 | 11.16 | 5 | 54.81 |
| Esters | 11 | 6.48 | 5 | 3.41 | 7 | 8.94 | 9 | 25.13 |
| Aldehydes | 7 | 10.73 | 5 | 5.12 | 9 | 28.22 | 8 | 44.76 |
| Acids | 3 | 12.09 | 4 | 9.12 | 4 | 22.12 | 5 | 4.86 |
| Ketones | 4 | 2.82 | 3 | 4.28 | 4 | 10.58 | 2 | 2.56 |
| Others | 5 | 18.49 | 5 | 5.84 | 5 | 48.24 | 6 | 47.04 |
| Total | 37 | 59.72 | 23 | 30.09 | 33 | 129.26 | 35 | 179.16 |
| No. | Compounds | Odor Threshold µg/g | OAV | |||
|---|---|---|---|---|---|---|
| Fresh | Steamed | Boiled | Baked | |||
| 1 | Benzyl alcohol | 2.55 | 1.78 | 0.91 | 2.85 | 18.65 |
| 2 | 2,3-Butanediol | 0.0951 | 3.18 | ND | ND | ND |
| 3 | 1,3-Butanediol | 10 | 0.10 | ND | ND | ND |
| 4 | 3-Methyl-1-butanol | 0.25 | 1.24 | ND | ND | ND |
| 5 | 1-Octen-3-ol | 0.1 | ND | ND | 16.60 | 4.17 |
| 6 | 3-Octanol | 0.042 | 49.26 | ND | 34.55 | ND |
| 7 | 1-Butanol | 0.5 | ND | ND | 1.58 | ND |
| 8 | 1-Nonanol | 0.09 | 7.04 | ND | ND | ND |
| 9 | 1-Tetradecanol | 559 | 0.00 | ND | ND | ND |
| 10 | Methyl palmitate | 0.003 | 103.67 | ND | 163.33 | 62.67 |
| 11 | Ethyl acetate | 5 | 0.19 | 0.09 | 0.09 | 0.06 |
| 12 | Methyl valerate | 0.02 | ND | ND | ND | 14.00 |
| 13 | 2-Hydroxy-n-butyric acid ethyl ester | 0.8 | 2.34 | ND | ND | ND |
| 14 | Ethyl benzoate | 0.05 | 3.80 | ND | ND | ND |
| 15 | Benzaldehyde | 0.35 | 0.83 | 0.63 | 1.29 | 8.00 |
| 16 | Phenylacetaldehyde | 0.004 | ND | ND | ND | 3632.50 |
| 17 | Hexanal | 0.02 | ND | ND | ND | 15.00 |
| 18 | Furfural | 9.56 | 0.21 | 0.03 | 0.42 | 0.27 |
| 19 | Isovaleraldehyde | 13 | 0.56 | 0.25 | 1.10 | 1.22 |
| 20 | Decanal | 33 | ND | ND | 0.01 | 0.06 |
| 21 | oct-2-enal | 394 | ND | ND | 0.01 | 0.01 |
| 22 | Heptanal | 0.06 | 4.83 | 19.17 | 57.00 | 64.17 |
| 23 | Octanal | 0.008 | 31.25 | 27.50 | 37.50 | ND |
| 24 | Acetic acid | 22 | ND | 0.06 | 0.03 | 0.03 |
| 25 | Benzoic acid | 1 | 6.66 | 0.89 | 2.18 | 2.56 |
| 26 | Lauric acid | 59 | 0.01 | 0.01 | 0.02 | 0.02 |
| 27 | Palmitic acid | 503 | 0.01 | 0.01 | 0.04 | 0.00 |
| 28 | Heptanoic acid | 10.4 | ND | ND | ND | 0.02 |
| 29 | Methylheptenone | 0.85 | ND | ND | 0.68 | ND |
| 30 | 3-Octanone | 0.07 | 13.71 | 15.43 | 75.00 | ND |
| 31 | 3-Heptanone | 0.08 | ND | 24.63 | ND | ND |
| 32 | Geranylacetone | 0.06 | 3.33 | ND | ND | ND |
| 33 | 2-Undecanone | 0.182 | 1.70 | ND | ND | ND |
| 34 | Carvone | 0.1 | 13.40 | 12.30 | ND | ND |
| 35 | 1-Octen-3-one | 0.00005 | ND | ND | 84,200.00 | 49,600.00 |
| 36 | 2-Nonanone | 0.2 | ND | ND | ND | 0.40 |
| 37 | Acetophenone | 0.065 | ND | ND | 8.15 | ND |
| 38 | Undecane | 10 | 0.09 | ND | ND | ND |
| 39 | 2,6-Dimethoxyphenol | 1.85 | ND | 0.14 | ND | ND |
| 40 | Isoeugenol | 0.1 | ND | 4.30 | ND | ND |
| 41 | Indole | 25 | 0.46 | ND | ND | ND |
| 42 | Butylated Hydroxytoluene | 0.161 | ND | ND | 3.42 | ND |
| 43 | 2-Butylfuran | 0.005 | ND | 120.00 | 78.00 | 88.00 |
| 44 | Furfuryl mercaptan | 0.01 | ND | ND | ND | 23.80 |
| Compound(s) Omitted | Degree of Odor Difference |
|---|---|
| 1-Octen-3-one | W2S↓ * |
| Benzyl alcohol | W2S↓ ** |
| Methyl valerate | W2S↓ ***, W2W↓ *** |
| Phenylacetaldehyde | ns |
| 1-Octen-3-ol | W2S↓ ***, W2W↓ *** |
| Benzoic acid | ns |
| Isovaleraldehyde | W2S↓ *** |
| Hexanal | ns |
| Methyl palmitate | ns |
| Furfuryl mercaptan | W2S↓ ***, W2W↓ *** |
| 2-Butylfuran | ns |
| Heptanal | W2S↓ *** |
| Benzaldehyde | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Guo, S.; Rao, H.; Lan, B.; Zheng, B.; Zhang, N. Characterization of Volatile Flavor Compounds and Aroma Active Components in Button Mushroom (Agaricus bisporus) across Various Cooking Methods. Foods 2024, 13, 685. https://doi.org/10.3390/foods13050685
Xie L, Guo S, Rao H, Lan B, Zheng B, Zhang N. Characterization of Volatile Flavor Compounds and Aroma Active Components in Button Mushroom (Agaricus bisporus) across Various Cooking Methods. Foods. 2024; 13(5):685. https://doi.org/10.3390/foods13050685
Chicago/Turabian StyleXie, Limei, Shaoli Guo, Hongting Rao, Bingying Lan, Baodong Zheng, and Ningning Zhang. 2024. "Characterization of Volatile Flavor Compounds and Aroma Active Components in Button Mushroom (Agaricus bisporus) across Various Cooking Methods" Foods 13, no. 5: 685. https://doi.org/10.3390/foods13050685
APA StyleXie, L., Guo, S., Rao, H., Lan, B., Zheng, B., & Zhang, N. (2024). Characterization of Volatile Flavor Compounds and Aroma Active Components in Button Mushroom (Agaricus bisporus) across Various Cooking Methods. Foods, 13(5), 685. https://doi.org/10.3390/foods13050685






