Changes in the Quality and Microflora of Yellowtail Seriola quinqueradiata Muscles during Cold Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Viable Bacterial Count
2.3. Biochemical Measurements
2.4. Solid Phase Micro Extraction and Gas Liquid Chromatography-Mass Spectrometry
2.5. Sensory Test
2.6. DNA Extraction and Next-Generation Sequencing
2.7. Sequencing Data
2.8. Statistical Analyses
3. Results and Discussion
3.1. Viable Bacterial Count
3.2. Biochemical Measurements
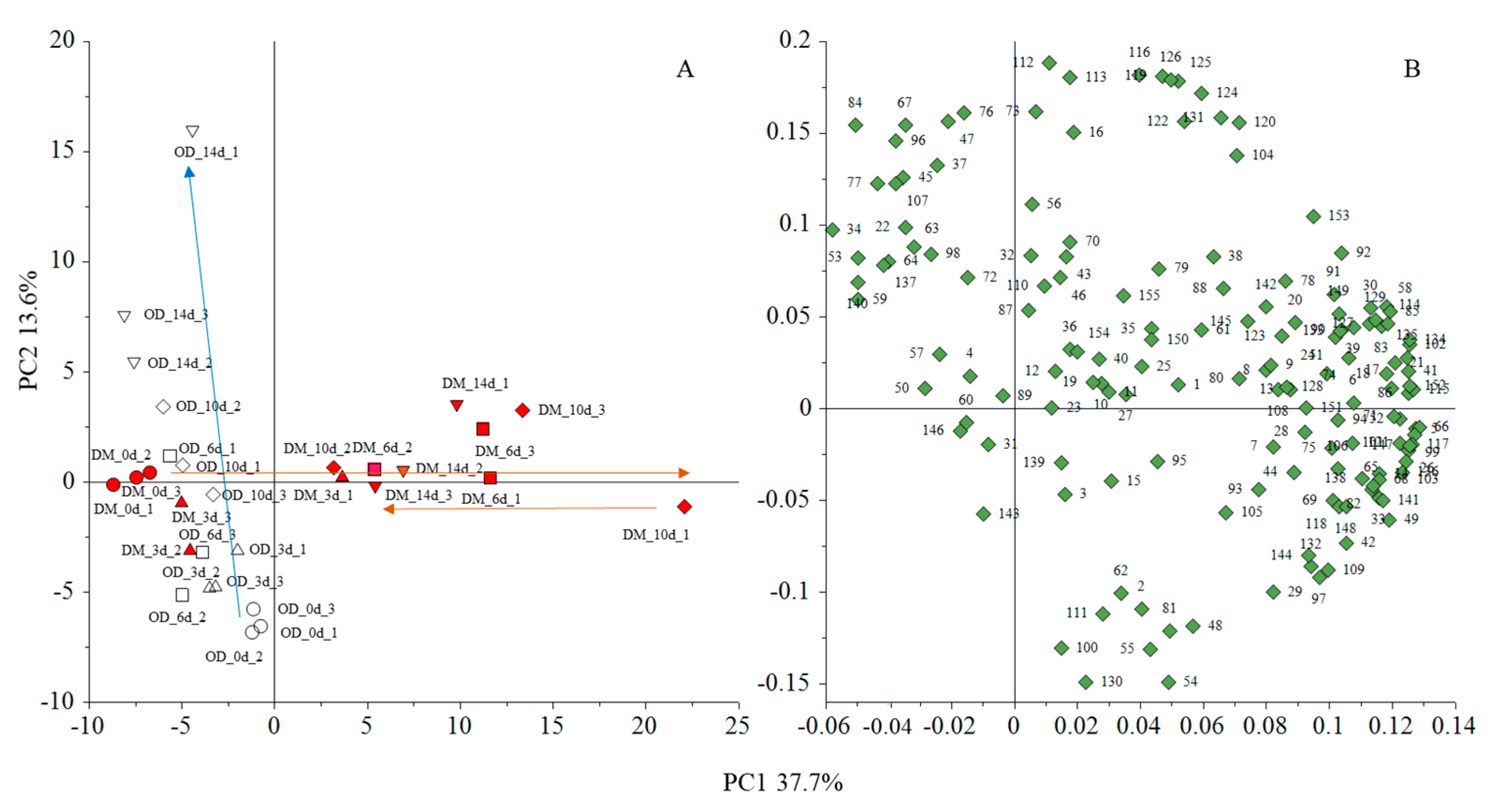
3.3. Volatile Compounds by Solid Phase Micro Extraction and Gas Liquid Chromatography-Mass Spectrometry
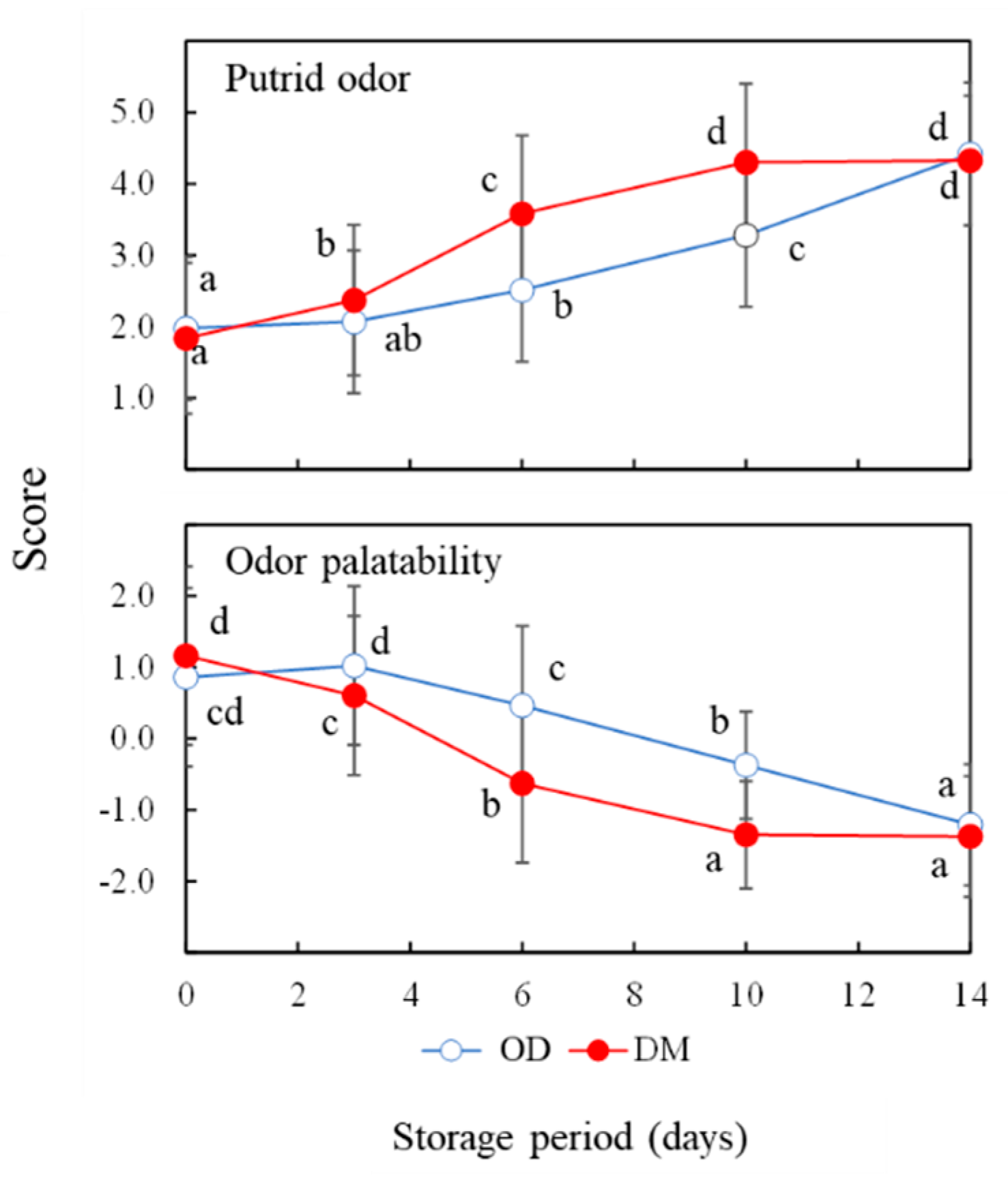
3.4. Sensory Test
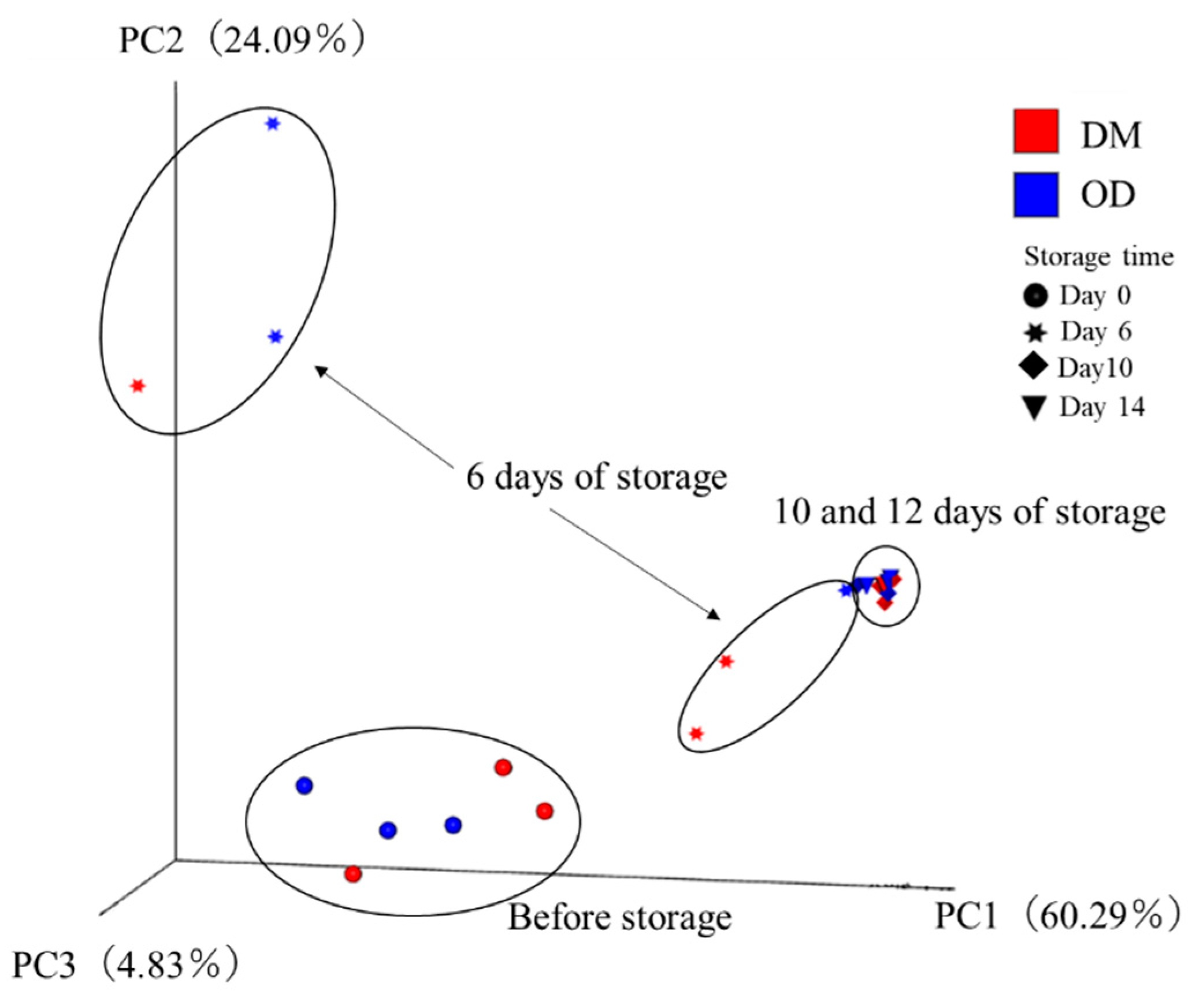
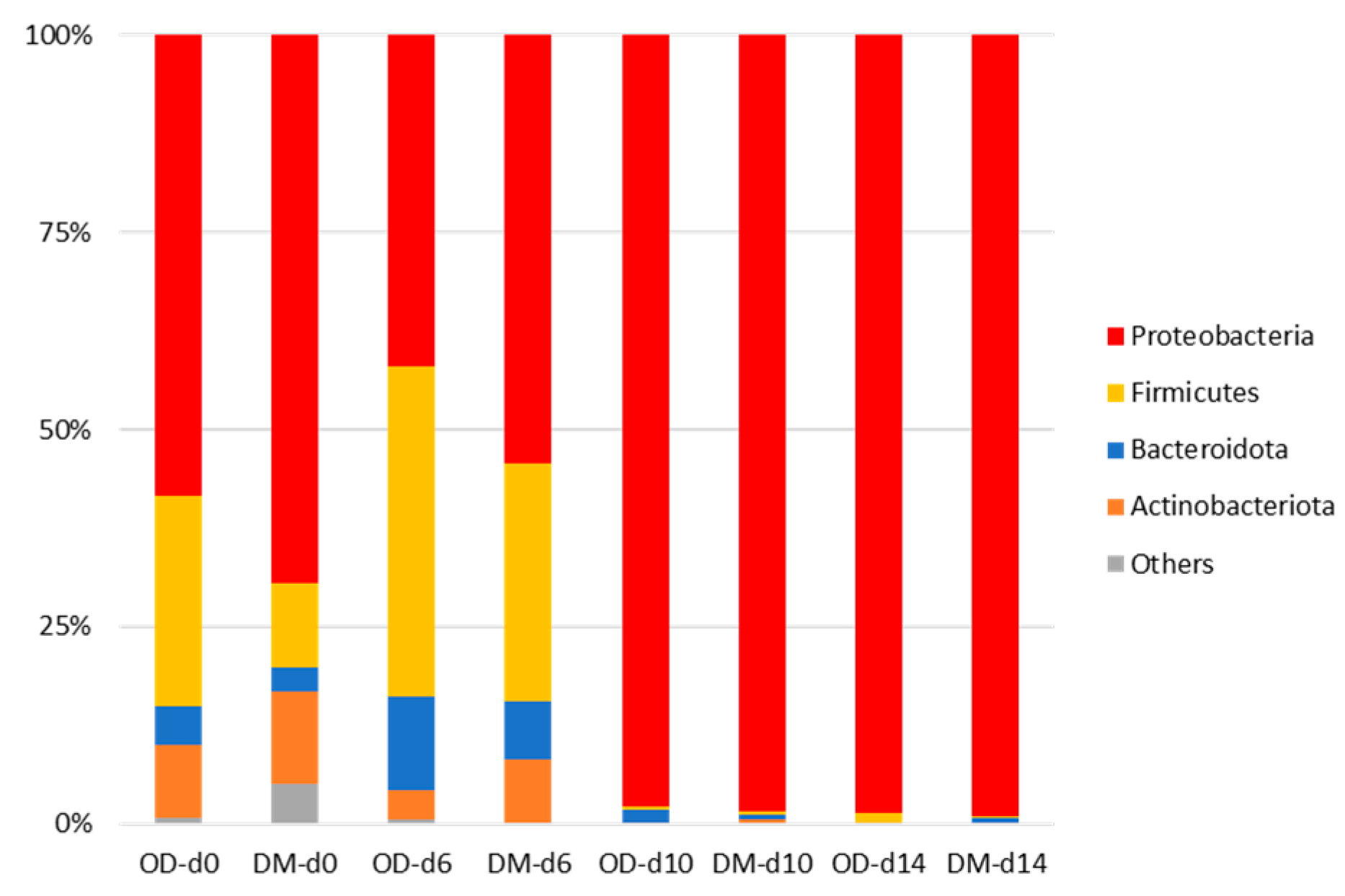
3.5. Next-Generation Sequencing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhuang, S.; Hong, H.; Zhang, L.; Luo, Y. Spoilage-related microbiota in fish and crustaceans during storage: Research progress and future trends. Compr. Rev. Food Sci. Food Saf. 2021, 20, 252–288. [Google Scholar] [CrossRef]
- Yu, D.; Wu, L.; Regenstein, J.M.; Jiang, Q.; Yang, F.; Xu, Y.; Xia, W. Recent advances in quality retention of non-frozen fish and fishery products: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1747–1759. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, L. The microbial safety of fish and fish products: Recent advances in understanding its significance, contamination sources, and control strategies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 738–786. [Google Scholar] [CrossRef]
- Reynisson, E.; Lauzon, H.; Magnusson, H.; Jonsdottir, R.; Olafsdottir, G.; Marteinsson, V.; Hreggvidsson, G. Bacterial composition and succession during storage of North-Atlantic cod (Gadus morhua) at superchilled temperatures. BMC Microbiol. 2009, 9, 250. [Google Scholar] [CrossRef]
- Rudi, K.; Maugesten, T.; Hannevik, S.E.; Nissen, H. Explorative multivariate analyses of 16S rRNA gene data from microbial communities in modified atmosphere-packed salmon and coalfish. Appl. Environ. Microbiol. 2004, 70, 5010–5018. [Google Scholar] [CrossRef]
- Juste, A.; Thomma, B.; Lievens, B. Recent advances in molecular techniques to study microbial communities in food-associated matrices and processes. Food Microbiol. 2008, 25, 745–761. [Google Scholar] [CrossRef]
- Emborg, J.; Laursen, B.G.; Rathjen, T.; Dalgaard, P. Microbial spoilage and formation of biogenic amines in fresh and thawed modified atmosphere packed salmon (Salmo salar) at 2 °C. J. Appl. Microbiol. 2002, 92, 790–799. [Google Scholar] [CrossRef]
- Mayo, B.; Rachid, C.T.C.C.; Alegría, Á.; Leite, A.M.O.; Peixoto, R.S.; Delgado, S. Impact of next generation sequencing techniques in food microbiology. Curr. Genom. 2014, 15, 293–309. [Google Scholar] [CrossRef]
- Dimitrios, A.A.; Parlapani, F.F.; Boziaris, I.S. The evolution of knowledge on seafood spoilage microbiota from the 20th to the 21st century: Have we finished or just begun? Trends Food Sci. Technol. 2022, 120, 236–247. [Google Scholar]
- Parlapani, F.F.; Mallouchos, A.; Haroutounian, S.A.; Boziarisa, I.S. Volatile organic compounds of microbial and non-microbial origin produced on model fish substrate un-inoculated and inoculated with gilt-head sea bream spoilage bacteria. LWT-Food Sci. Technol. 2017, 78, 54–62. [Google Scholar] [CrossRef]
- Parlapani, F.F. Microbial diversity of seafood. Curr. Opin. Food Sci. 2021, 37, 45–51. [Google Scholar] [CrossRef]
- Kawai, T. Fish flavor. Crit. Rev. Food Sci. Nutr. 1996, 36, 257–298. [Google Scholar] [CrossRef]
- Ganeko, N.; Shoda, M.; Hirohara, I.; Bhadra, A.; Ishida, T.; Matsuda, H.; Takamura, H.; Matoba, T. Analysis of volatile flavor compounds of sardine (Sardinops melanostica) by solid phase microextraction. J. Food Sci. 2008, 73, S83–S88. [Google Scholar] [CrossRef]
- Miyasaki, T.; Hamaguchi, M.; Yokoyama, S. Change of volatile compounds in fresh fish meat during ice storage. J. Food Sci. 2011, 76, C1319–C1325. [Google Scholar] [CrossRef]
- François, L.; Pascal, T.; Nathalie, K.; Frédéric, M.; Pierre, M.; Ossarath, K.; Jean, L.B.; Guillaume, D. Evolution of volatile odorous compounds during the storage of European seabass (Dicentrarchus labrax). Food Chem. 2012, 131, 1304–1311. [Google Scholar]
- Moreira, N.; Valente, L.M.P.; Castro-Cunha, M.; Cunha, L.M.; Pinho, P.G. Effect of storage time and heat processing on the volatile profile of Senegalese sole (Solea senegalensis Kaup, 1858) muscle. Food Chem. 2013, 138, 2365–23731. [Google Scholar] [CrossRef]
- Foteini, F.P.; Athanasios, M.; Serkos, A.H.; Ioannis, S.B. Microbiological spoilage and investigation of volatile profile during storage of sea bream fillets under various conditions. Inter. J. Food Microbiol. 2014, 189, 153–163. [Google Scholar]
- Josephson, D.B.; Lindsay, R.C.; Stuiber, D.A. Biogenesis of lipid-derived volatile aroma compounds in the emerald shiner (Notropis atherinoides). J. Agric. Food Chem. 1984, 32, 1347–1352. [Google Scholar] [CrossRef]
- Koizumi, C.; Kieu-Thu Thi, C.; Nonaka, J. Undesirable odor of cooked sardine meat. Nippon. Suisan Gakkaishi 1979, 45, 1307–1312. [Google Scholar] [CrossRef]
- Howgate, P.A. Critical review of total volatile bases and trimethylamine as indices of freshness of fish. Part 2. Formation of the bases, and application in quality assurance. Electronic J. Environ. Agric. Food Chem. 2010, 9, 58–88. [Google Scholar]
- Liu, X.; Huang, Z.; Jia, S.; Zhang, J.; Li, K.; Luo, Y. The roles of bacteria in the biochemical changes of chill-stored bighead carp (Aristichthys nobilis): Proteins degradation, biogenic amines accumulation, volatiles production, and nucleotides catabolism. Food Chem. 2018, 255, 174–181. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Burke, C.M.; Christopher, C.J.; Bolch, C.C.J.; Stanley, R. Seafood spoilage microbiota and associated volatile organic compounds at different storage temperatures and packaging conditions. Inter. J. Food Microbiol. 2018, 280, 87–99. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.A.; Parlapani, F.F.; Mallouchos, A.; Angelidou, A.; Syropoulou, F.; Minos, G.; Boziaris, I.S. Volatile organic compounds and 16s metabarcoding in ice-stored red seabream Pagrus major. Food 2022, 11, 666. [Google Scholar] [CrossRef]
- Hassoun, A.; Jagtap, S.; Garcia-Garcia, G.; Trollman, H.; Pateiro, M.; Lorenzo, J.M.; Trif, M.; Rusu, A.V.; Aadil, R.M.; Šimat, V.; et al. Food quality 4.0: From traditional approaches to digitalized automated analysis. J. Food Eng. 2023, 337, 111216. [Google Scholar] [CrossRef]
- Gardner, G.A. Streptomycin-thallous acetate-actidione (STAA) agar: A medium for the selective enumeration of Brochothrix thermosphacta. Inter. J. Food Microbiol. 1985, 2, 69–70. [Google Scholar] [CrossRef]
- Conway, E.J. Microdiffusion analysis and volumetric error. Nature 1948, 161, 583. [Google Scholar]
- Hamakawa, Y.; Mukojima, K.; Uchiyama, M.; Okada, S.; Mabuchi, R.; Furuta, A.; Tanimoto, S. Effect of different heating conditions on odor of yellowtail Seriola quinqueradiata muscles. Biosci. Biotechnol. Biochem. 2021, 85, 2030–2041. [Google Scholar] [CrossRef]
- Mukojima, K.; Yoshii, M.; Tone, A.; Mabuchi, R.; Furuta, A.; Tanimoto, S. Effect of storage after heating on odor of muscles of yellowtail (Seriola quinqueradiata). Biosci. Biotechnol. Biochem. 2022, 86, 902–915. [Google Scholar] [CrossRef]
- The Ethical Guidelines for Medical and Biological Research Involving Human Subjects. 2023. Available online: https://square.umin.ac.jp/kasuitai/pdf/2_gakkai_rinri_2023.pdf (accessed on 24 January 2024). (In Japanese).
- The Act on the Protection of Personal Information. 2023. Available online: https://www.japaneselawtranslation.go.jp/ja/laws/view/130 (accessed on 24 January 2024).
- Wang, R.; Hirabayashi, M.; Furuta, A.; Okazaki, T.; Tanimoto, S. Changes in extractive components and bacterial flora in live mussels Mytilus galloprovincialis during storage at different temperatures. J. Food Sci. 2023, 88, 1654–1671. [Google Scholar] [CrossRef]
- Metaboanalyst 5.0. 2023. Available online: https://www.metaboanalyst.ca/ (accessed on 24 January 2024).
- Nomura, H.; Miura, Y.; Ozaki, Y. Hygienic study of frozen foods (Part 3) Some microbiological tests on the freshness of frozen fish (1). J. Home Econ. Jpn. 1971, 22, 418–423. [Google Scholar]
- Papadopoulou, O.S.; Iliopoulos, V.; Mallouchos, A.; Panagou, E.Z.; Chorianopoulos, N.; Tassou, C.C.; Nychas, G.E. Spoilage potential of pseudomonas (P. fragi, P. putida) and LAB (Leuconostoc mesenteroides, Lactobacillus sakei) strains and their volatilome profile during storage of sterile pork meat using GC/MS and data analytics. Foods 2020, 9, 633. [Google Scholar] [CrossRef]
- Sallam, K.I. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Cont. 2007, 18, 566–575. [Google Scholar] [CrossRef]
- Endo, K. Methods for determining freshness of fish and shellfish. Sci. Cook. 1973, 6, 14–19. [Google Scholar]
- European Commission Commission Decision of 8 March 1995 fixing the total volatile basic nitrogen (TVB-N) limit values for certain categories of fishery products and specifying the analysis methods to be used. Off. J. Eur. Communities 1995, 84–87.
- Nevigato, T.; Masci, M.; Casini, I.; Caproni, R.; Orban, E. Trimethylamine as a freshness indicator for seafood stored in ice: Analysis by GC-FID of four species caught in the Tyrrhenian sea. J. Food Sci. 2018, 30, 522–534. [Google Scholar]
- Gram, L.; Dalgaard, P. Fish spoilage bacteria—Problems and solutions. Curr. Opin. Biotechnol. 2002, 13, 262–266. [Google Scholar] [CrossRef]
- Rathod, N.B.; Nirmal, N.P.; Pagarkar, A.; Özogul, F.; Rocha, J.M. Antimicrobial Impacts of Microbial metabolites on the preservation of fish and fishery products: A review with current knowledge. Microorganisms 2022, 10, 77. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Murata, M. Distribution of free amino acids, creatine, and trimethylamine oxide in mackerel and yellowtail. Nippon. Suisan Gakkaishi 1986, 52, 685–689. [Google Scholar] [CrossRef]
- Kawabata, T. Studies on the trimethylamine oxide-reductase-I. Reduction of trimethylamine oxide in the dark muscle of pelagic migrating fish under aseptic condition. Nippon. Suisan Gakkaishi 1953, 19, 505–512. [Google Scholar] [CrossRef]
- Tokunaga, T. Trimethylamine oxide and its decomposition in the bloody muscle of fish-II. Formation of DMA and TMA during storage. Nippon. Suisan Gakkaishi 1970, 36, 510–515. [Google Scholar] [CrossRef]
- Tanimoto, S.; Kikutani, H.; Kitabayashi, K.; Ohkita, T.; Arita, R.; Nishimura, S.; Takemoto, R.; Mabuchi, R.; Shimoda, M. Qualitative changes in each part of yellowtail Seriola quinqueradiata flesh during cold storage. Fish Sci. 2018, 84, 135–148. [Google Scholar] [CrossRef]
- Viji, P.; Tanuja, S.; Ninan, G.; Lalitha, K.V.; Zynudheen, A.A.; Binsi, P.K.; Srinivasagopal, T.K. Biochemical, textural, microbiological and sensory attributes of gutted and ungutted sutchi catfish (Pangasianodon hypophthalmus) stored in ice. J. Food Sci. Technol. 2015, 52, 3312–3321. [Google Scholar] [CrossRef]
- Ho, C.T.; Chen, Q. ACS Symposium Series; Lipids in food flavors: Chapter 1. In Lipids in food Flavors an Overview; Ho, C.-T., Hartman, T.G., Eds.; American Chemical Society: Washington, DC, USA, 1994; pp. 2–14. [Google Scholar]
- Shahidi, F.; Hossain, A. Role of lipids in food flavor generation. Molecules 2022, 27, 5014. [Google Scholar] [CrossRef]
- Tanimoto, S.; Song, X.A.; Sakaguchi, M.; Sugawara, T.; Hirata, T. Levels of glutathione and related enzymes in yellowtail muscle subjected to ice storage in a modified atmosphere. J. Food Sci. 2011, 76, C974–C979. [Google Scholar] [CrossRef]
- Matsuura, F.; Hashimoto, K. Chemical studies on the red muscle (“chiai”) of fishes. –III. Determinations of the content of hemoglobin, myoglobin and cytochrome c in the muscles of fishes. Nippon. Suisan Gakkaishi 1954, 20, 308–312. [Google Scholar] [CrossRef]
- Casaburi, A.; Piombino, P.; Nychas, G.J.; Villani, F.; Ercolini, D. Bacterial populations and the volatilome associated to meat spoilage. Food Microbiol. 2015, 45, 83–102. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, X.; Xie, J. Characterization of metabolite, genome and volatile organic compound changes provides insights into the spoilage and cold adaptive markers of Acinetobacter johnsonii XY27. LWT—Food Sci. Technol. 2022, 162, 113453. [Google Scholar] [CrossRef]
- Yi, Z.; Xie, J. Prediction in the dynamics and spoilage of Shewanella putrefaciens in bigeye tuna (Thunnus obesus) by gas sensors stored at different refrigeration temperatures. Foods 2021, 10, 2132. [Google Scholar] [CrossRef]
- Quality and Quality Changes in Fresh Fish (1995) 5. Postmortem Changes in Fish. Available online: https://www.fao.org/3/v7180e/v7180e06.htm (accessed on 24 January 2024).
- NCBI Taxonomy Browser. Available online: https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi?id=1236 (accessed on 24 January 2024).
- Yang, S.P.; Xie, J.; Qian, Y.F. Determination of spoilage microbiota of pacific white shrimp during ambient and cold storage using next generation sequencing and culture-dependent method. J. Food Sci. 2017, 82, 1178–1183. [Google Scholar] [CrossRef]
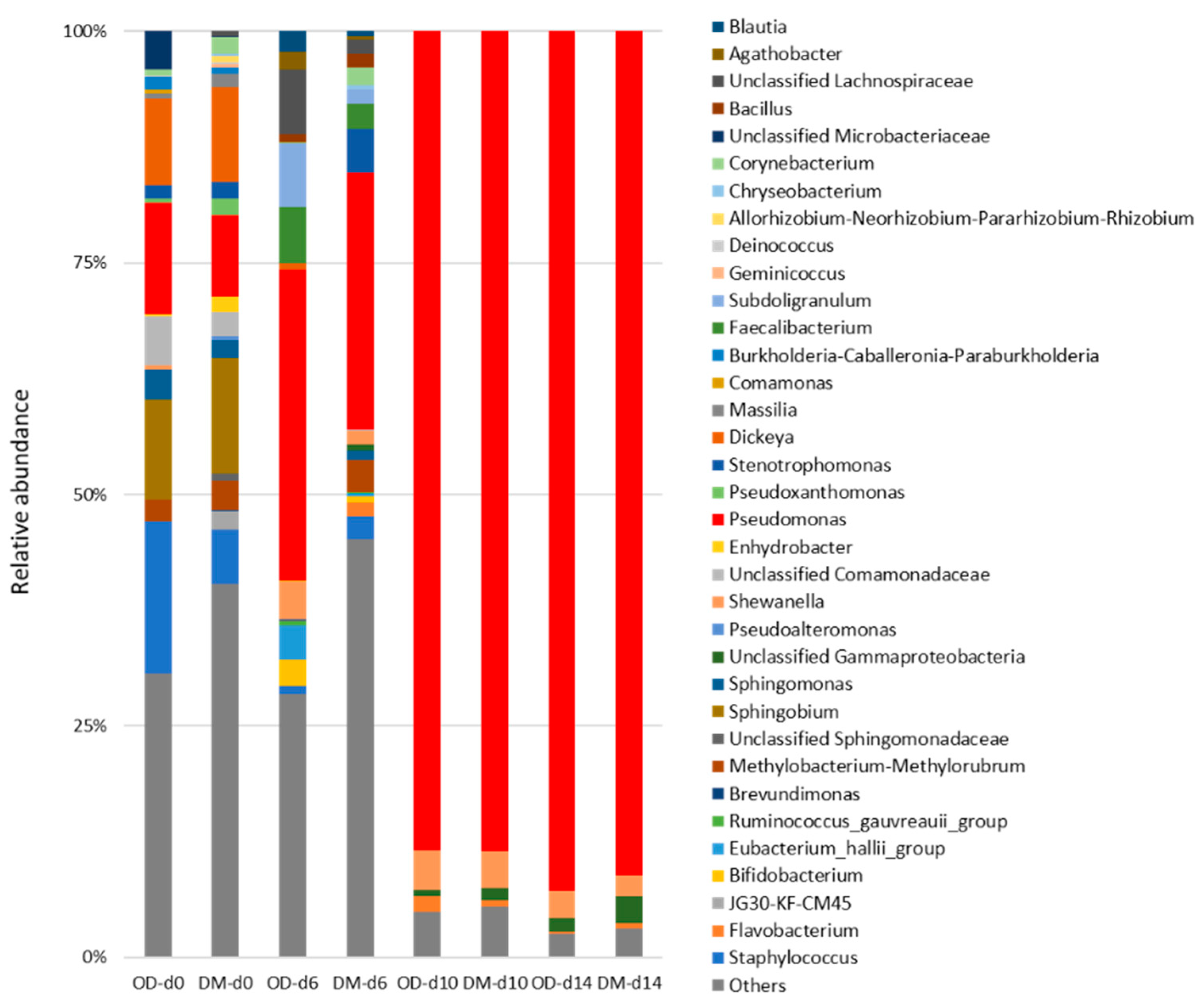
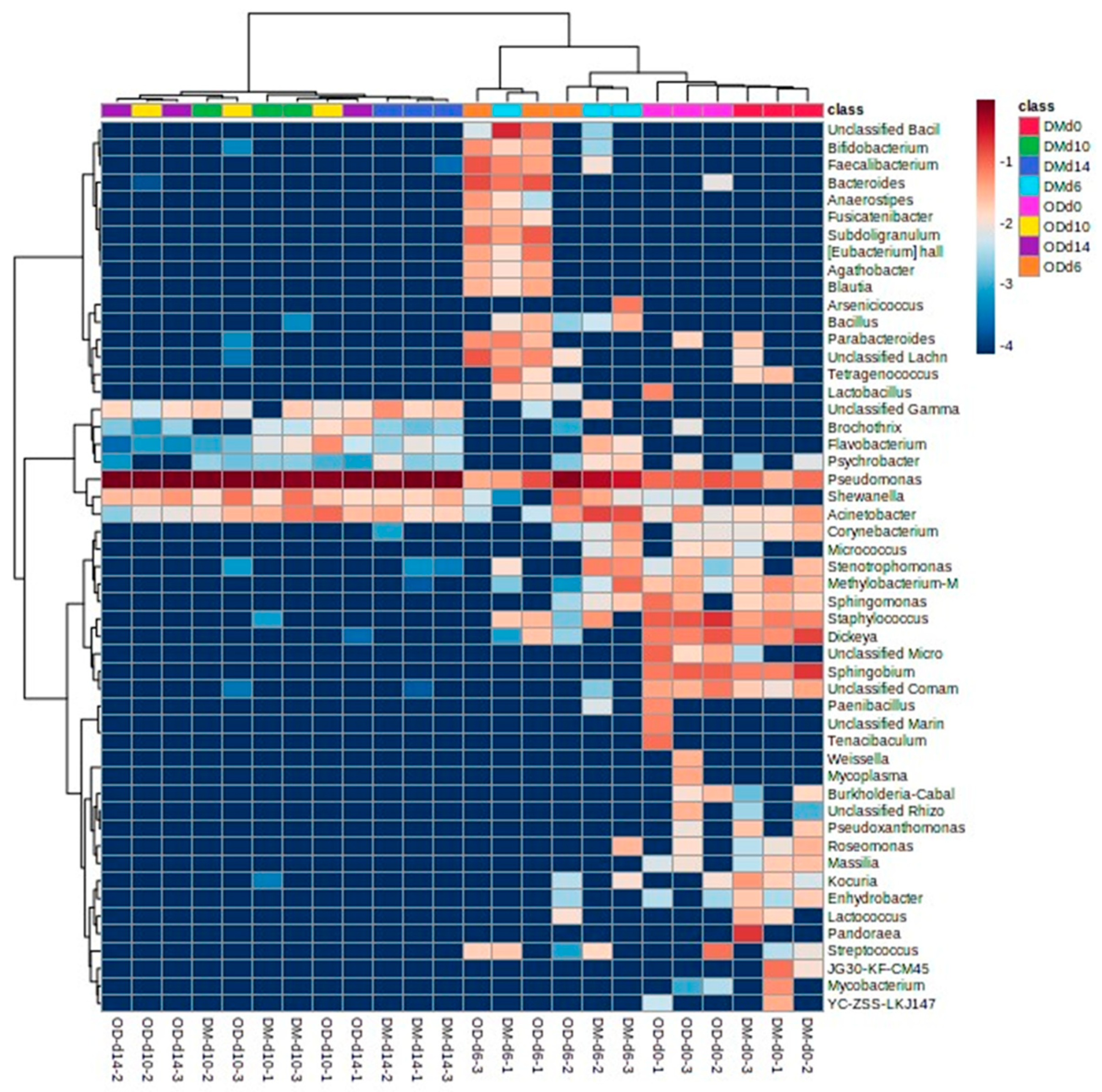
| Muscle Type | Storage Periods (Days) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 10 | 14 | |||||||
| Total viable count | OD | 3.0 | a | 2.5 | a | 4.0 | b | 7.3 | c† | 8.6 | d |
| DM | 3.0 | a | 2.7 | a | 4.0 | b | 6.6 | c | 8.2 | d | |
| Heterotrophic marine bacteria | OD | 1.6 | a | 2.1 | a | 4.2 | b | 7.0 | c | 8.5 | d† |
| DM | 2.3 | a | 2.9 | ab | 3.4 | b | 6.6 | c | 7.9 | d | |
| Lactic Acid Bacteria | OD | 0.6 | a | 0.6 | a | 0.8 | a | 1.2 | a | 1.2 | a |
| DM | 0.3 | a | 0.7 | ab | 1.0 | ab | 1.5 | b | 1.1 | ab | |
| Enterobacteriaceae | OD | 1.3 | a | 0.9 | a | 3.1 | b | 6.4 | c | 7.5 | c |
| DM | 1.7 | b | 0.2 | a | 2.7 | c | 5.9 | d | 7.3 | e | |
| Pseudomonas spp. | OD | 0.7 | a | 2.2 | ab | 3.5 | b† | 6.1 | c | 7.6 | c |
| DM | 1.8 | a | 1.5 | ab | 2.8 | b | 5.9 | c | 7.2 | d | |
| Aeromonas spp. | OD | 1.5 | a | 2.7 | b | 4.2 | c† | 7.2 | d | 8.4 | e† |
| DM | 1.6 | a | 2.3 | a | 3.7 | b | 6.7 | c | 8.2 | d | |
| Brochothrix thermosphacta | OD | 0.3 | a | 0.0 | a | 0.9 | a | 3.6 | b | 5.4 | b† |
| DM | 0.0 | a | 0.0 | a | 0.5 | a | 3.7 | b | 4.8 | b | |
| H2S-producing bacteria | OD | 0.0 | a | 1.2 | b | 3.1 | c | 5.1 | d | 6.5 | e |
| DM | 0.0 | a | 0.6 | a | 2.9 | b | 4.8 | c | 6.1 | d | |
| Muscle Type | Storage Periods (Days) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 6 | 10 | 14 | |||||||
| TVB-N (mg/100 g) | OD | 14.9 | a† | 14.6 | a† | 15.8 | a† | 15.9 | B | 18.3 | b† |
| DM | 10.4 | a | 11.9 | ab | 13.0 | b | 15.9 | b | 16.9 | b | |
| TMA (μg/g) | OD | 0.21 | c† | 1.28 | c† | 1.36 | c† | 3.67 | b† | 12.23 | a† |
| DM | 13.95 | d | 39.70 | cd | 69.05 | bc | 108.68 | ab | 145.31 | a | |
| TBARS (μmol/g) | OD | 0.004 | a† | 0.008 | a† | 0.012 | a | 0.019 | b† | 0.016 | b† |
| DM | 0.326 | a | 0.384 | ab | 0.935 | bc | 1.823 | cd† | 1.943 | d | |
| Muscle Type | Storage Periods (Days) | ||||||||
| 0 | 6 | 10 | 14 | ||||||
| OUTs | OD | 51.0 | 49.7 | 22.7 | 23.0 | † | |||
| DM | 77.0 | a | 56.7 | ab | 23.7 | B | 27.0 | b | |
| Chao1 | OD | 51.0 | 49.7 | 22.7 | 24.0 | † | |||
| DM | 77.3 | a | 57.0 | ab | 23.7 | C | 27.3 | bc | |
| ACE | OD | 51.0 | 49.7 | 22.7 | 24.0 | † | |||
| DM | 77.3 | a | 57.0 | ab | 23.7 | C | 27.3 | bc | |
| Goods_ coverage (%) | OD | 1.000 | 1.000 | 1.000 | 1.000 | ||||
| DM | 1.000 | 1.000 | 1.000 | 1.000 | |||||
| Shannon | OD | 4.73 | a | 4.67 | a | 2.67 | B | 2.71 | b |
| DM | 5.24 | a | 4.59 | a | 2.67 | B | 2.83 | b | |
| Simpson | OD | 0.947 | a | 0.936 | a | 0.748 | B | 0.725 | b |
| DM | 0.944 | a | 0.921 | a | 0.764 | B | 0.740 | b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanimoto, S.; Hirata, Y.; Ishizu, S.; Wang, R.; Furuta, A.; Mabuchi, R.; Okada, G. Changes in the Quality and Microflora of Yellowtail Seriola quinqueradiata Muscles during Cold Storage. Foods 2024, 13, 1086. https://doi.org/10.3390/foods13071086
Tanimoto S, Hirata Y, Ishizu S, Wang R, Furuta A, Mabuchi R, Okada G. Changes in the Quality and Microflora of Yellowtail Seriola quinqueradiata Muscles during Cold Storage. Foods. 2024; 13(7):1086. https://doi.org/10.3390/foods13071086
Chicago/Turabian StyleTanimoto, Shota, Yuka Hirata, Shinta Ishizu, Run Wang, Ayumi Furuta, Ryota Mabuchi, and Genya Okada. 2024. "Changes in the Quality and Microflora of Yellowtail Seriola quinqueradiata Muscles during Cold Storage" Foods 13, no. 7: 1086. https://doi.org/10.3390/foods13071086





