Fermentation of Orange Peels by Lactic Acid Bacteria: Impact on Phenolic Composition and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Samples
2.2. Lactic Acid Bacteria Strains and Culture Media
2.3. Fermentation of Orange Peels
2.4. Extraction of Phenolic Compounds
2.5. Determination of Phenolic Compounds by HPLC-ESI-TOF-MS
2.6. Antioxidant Assays: DPPH and ABTS
3. Results and Discussion
3.1. Growth of LAB Strains in Orange Peels
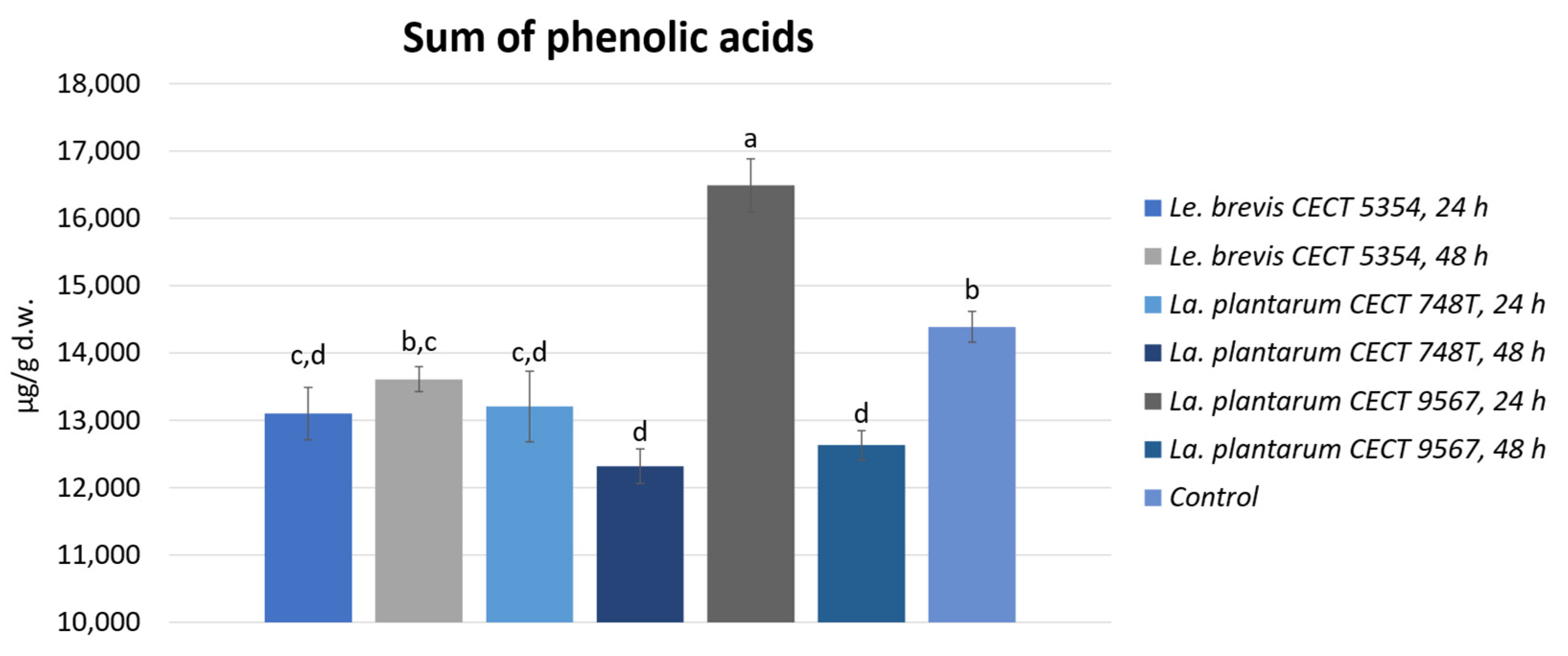
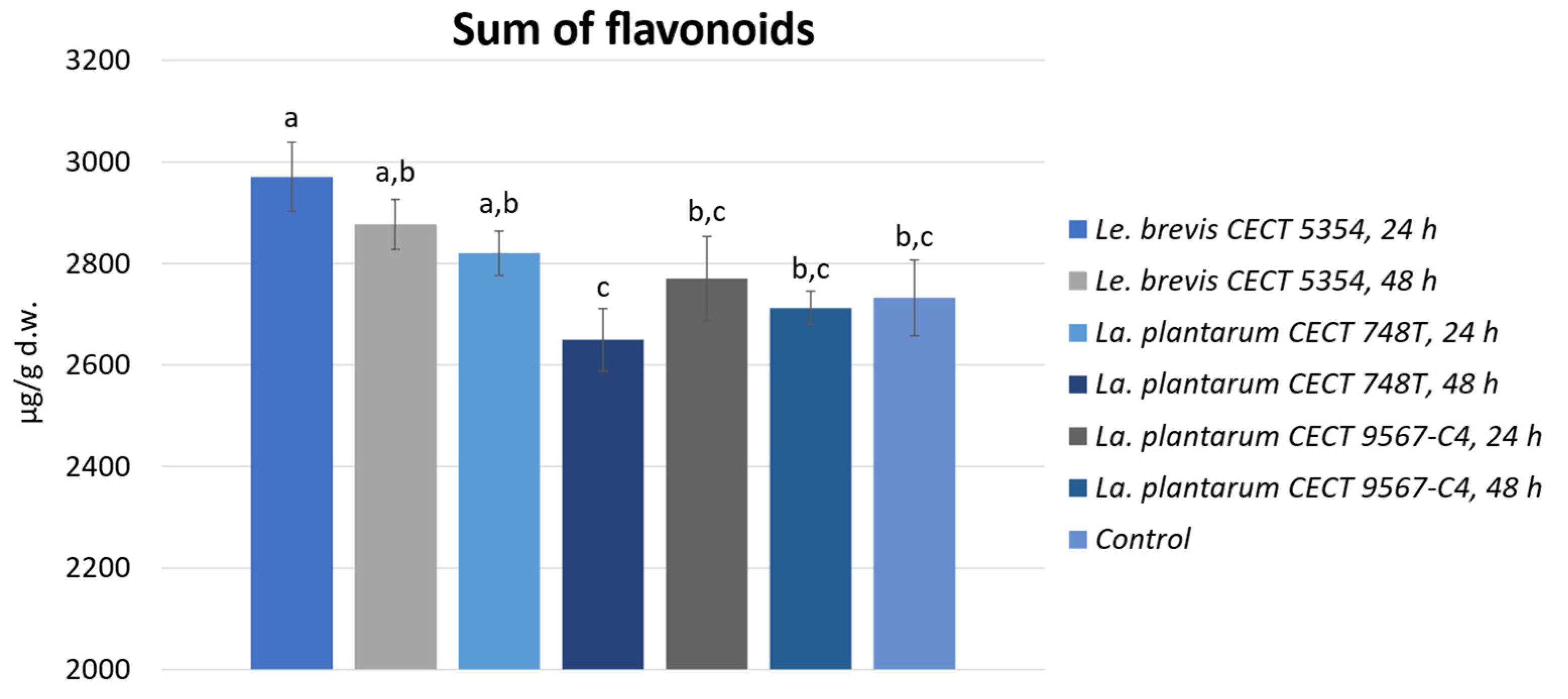
3.2. Quantification of Phenolic Compounds by HPLC-ESI-TOF-MS and Biotransformation during Fermentation in Orange Peels
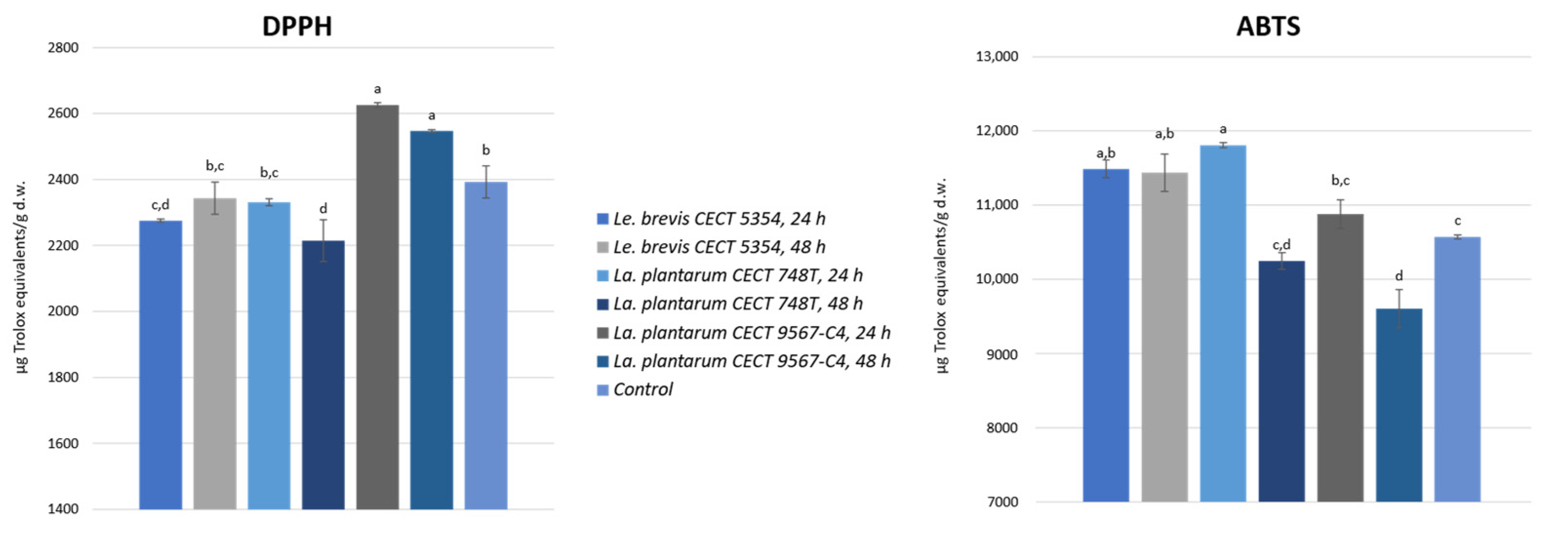
3.3. Antioxidant Activity in Fermented Orange Peel
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista. Available online: https://www.statista.com/ (accessed on 4 May 2022).
- Razola-Díaz, M.d.C.; Guerra-Hernández, E.J.; Rodríguez-Pérez, C.; Gómez-Caravaca, A.M.; García-Villanova, B.; Verardo, V. Optimization of Ultrasound-Assisted Extraction via Sonotrode of Phenolic Compounds from Orange By-Products. Foods 2021, 10, 1120. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic composition, antioxidant potential and health benefits of citrus peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, Y.R.; Thakur, R.; Thakur, P.; Mittal, A.; Chakrabarti, S.; Siwal, S.S.; Thakur, V.K.; Saini, R.V.; Saini, A.K. Food fermentation—Significance to public health and sustainability challenges of modern diet and food systems. Int. J. Food Microbiol. 2022, 371, 109666. [Google Scholar] [CrossRef] [PubMed]
- Aravantinos-Zafiris, G.; Tzia, C.; Oreopoulou, V.; Thomopoulos, C.D. Fermentation of orange processing wastes for citric acid production. J. Sci. Food Agric. 1994, 65, 117–120. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Torre, P.; Converti, A.; Domínguez, J.M. Submerged citric acid fermentation on orange peel autohydrolysate. J. Agric. Food Chem. 2008, 56, 2380–2387. [Google Scholar] [CrossRef] [PubMed]
- Leh, D.S.; Biz, A.; de Paula, D.H.F.; Richard, P.; Gonçalves, A.G.; Noseda, M.D.; Mitchell, D.A.; Krieger, N. Conversion of citric pectin into D-galacturonic acid with high substrate loading using a fermented solid with pectinolytic activity. Biocatal. Agric. Biotechnol. 2017, 11, 214–219. [Google Scholar] [CrossRef]
- Kuivanen, J.; Dantas, H.; Mojzita, D.; Mallmann, E.; Biz, A.; Krieger, N.; Mitchell, D.; Richard, P. Conversion of orange peel to L-galactonic acid in a consolidated process using engineered strains of Aspergillus niger. AMB Express 2014, 4, 33. [Google Scholar] [CrossRef]
- Li, Q.; Siles, J.A.; Thompson, I.P. Succinic acid production from orange peel and wheat straw by batch fermentations of Fibrobacter succinogenes S85. Appl. Microbiol. Biotechnol. 2010, 88, 671–678. [Google Scholar] [CrossRef]
- Gaind, S. Exploitation of Orange Peel for Fungal Solubilization of Rock Phosphate by Solid State Fermentation. Waste Biomass Valorization 2017, 8, 1351–1360. [Google Scholar] [CrossRef]
- Yalemtesfa, B.; Alemu, T.; Santhanam, A. Solid substrate fermentation and conversion of orange waste in to fungal biomass using Aspergillus niger KA-06 and Chaetomium Spp KC-06. Afr. J. Microbiol. Res. 2010, 4, 1275–1281. [Google Scholar]
- Ahmadi, F.; Zamiri, M.J.; Khorvash, M.; Banihashemi, Z.; Bayat, A.R. Chemical composition and protein enrichment of orange peels and sugar beet pulp after fermentation by two Trichoderma species. Iran. J. Vet. Res. 2015, 16, 25–30. [Google Scholar] [PubMed]
- Mantzouridou, F.T.; Paraskevopoulou, A.; Lalou, S. Yeast flavour production by solid state fermentation of orange peel waste. Biochem. Eng. J. 2015, 101, 1–8. [Google Scholar] [CrossRef]
- Kantifedaki, A.; Kachrimanidou, V.; Mallouchos, A.; Papanikolaou, S.; Koutinas, A.A. Orange processing waste valorisation for the production of bio-based pigments using the fungal strains Monascus purpureus and Penicillium purpurogenum. J. Clean. Prod. 2018, 185, 882–890. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Laredo-Alcalá, E.; Buenrostro-Figueroa, J.J.; Ascacio-Valdés, J.A.; Genisheva, Z.; Aguilar, C.; Teixeira, J. Ellagic acid production using polyphenols from orange peel waste by submerged fermentation. Electron. J. Biotechnol. 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Ahmed, N.E.; Awad, H.M. Optimizing the production of pectinase of orange peel waste by penicillium chrysogenum mf318506 using response surface methodology in submerged fermentation. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e3931. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, I.; Ladero, M.; Santos, V.E. d-lactic acid production from orange waste enzymatic hydrolysates with L. delbrueckii cells in growing and resting state. Ind. Crops Prod. 2020, 146, 112176. [Google Scholar] [CrossRef]
- Drouault, S.; Corthier, G. Health effects of lactic acid bacteria ingested in fermented milk. Vet. Res. 2001, 32, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Signorini, M.; Salazar, J.A.; Ponce-Alquicira, E.; Guerrero-Legarreta, I. Effect of lactic acid and lactic acid bacteria treatment on myofibrillar protein degradation and dynamic rheology of beef. J. Texture Stud. 2007, 38, 373–392. [Google Scholar] [CrossRef]
- Verni, M.; De Mastro, G.; De Cillis, F.; Gobbetti, M.; Rizzello, C.G. Lactic acid bacteria fermentation to exploit the nutritional potential of Mediterranean faba bean local biotypes. Food Res. Int. 2019, 125, 108571. [Google Scholar] [CrossRef]
- Bergillos-Meca, T.; Cabrera-Vique, C.; Artacho, R.; Moreno-Montoro, M.; Navarro-Alarcón, M.; Olalla, M.; Giménez, R.; Seiquer, I.; Ruiz-López, M.D. Does Lactobacillus plantarum or ultrafiltration process improve Ca, Mg, Zn and P bioavailability from fermented goats’ milk? Food Chem. 2015, 187, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Curiel, J.A.; Pinto, D.; Marzani, B.; Filannino, P.; Farris, G.A.; Gobbetti, M.; Rizzello, C.G. Lactic acid fermentation as a tool to enhance the antioxidant properties of Myrtus communis berries. Microb. Cell Fact. 2015, 14, 67. [Google Scholar] [CrossRef]
- Pontonio, E.; Montemurro, M.; Pinto, D.; Marzani, B.; Trani, A.; Ferrara, G.; Mazzeo, A.; Gobbetti, M.; Rizzello, C.G. Lactic acid fermentation of pomegranate juice as a tool to improve antioxidant activity. Front. Microbiol. 2019, 10, 460471. [Google Scholar] [CrossRef]
- Muñoz, R.; de las Rivas, B.; López de Felipe, F.; Reverón, I.; Santamaría, L.; Esteban-Torres, M.; Curiel, J.A.; Rodríguez, H.; Landete, J.M. Chapter 4—Biotransformation of Phenolics by Lactobacillus plantarum in Fermented Foods. In Fermented Foods in Health and Disease Prevention; Frias, J., Martinez-Villaluenga, C., Peñas, E., Eds.; Academic Press: Boston, MA, USA, 2017; pp. 63–83. ISBN 978-0-12-802309-9. [Google Scholar]
- Mathur, H.; Beresford, T.P.; Cotter, P.D. Health Benefits of Lactic Acid Bacteria (LAB) Fermentates. Nutrients 2020, 12, 1679. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cai, Y. Lactic Acid Bacteria: Fundamentals and Practice; Springer Dordrecht: Berlin, Germany, 2014; ISBN 9789401788410. [Google Scholar]
- Huang, J.Y.; Huang, M.L.; Kao, C.Y.; Fang, T.J. Orange peel fiber and Tremella flava fermented powder effectively induce exopolysaccharide production by Lactobacillus plantarum SLC 13. Int. J. Agric. Biol. 2017, 19, 437–444. [Google Scholar] [CrossRef]
- Ricci, A.; Díaz, A.B.; Lazzi, C.; Blandino Garrido, A.M. Valorization of orange peels exploiting fungal solid-state and lacto-fermentation. J. Sci. Food Agric. 2023, 103, 4614–4624. [Google Scholar] [CrossRef]
- Razola-Díaz, M.d.C.; Verardo, V.; Gómez-Caravaca, A.M.; García-Villanova, B.; Guerra-Hernández, E.J. Mathematical Modelling of Convective Drying of Orange By-Product and Its Influence on Phenolic Compounds and Ascorbic Acid Content, and Its Antioxidant Activity. Foods 2023, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- De Montijo-Prieto, S.; Castro, D.J.; Reina, J.C.; Jimenez-Valera, M.; Ruiz-Bravo, A. Draft genome sequence of Lactobacillus plantarum C4 (CECT 9567), a potential probiotic strain isolated from kefir. Arch. Microbiol. 2019, 201, 409–414. [Google Scholar] [CrossRef]
- De Montijo-Prieto, S.; Razola-Díaz, M.d.C.; Barbieri, F.; Tabanelli, G.; Gardini, F.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Impact of Lactic Acid Bacteria Fermentation on Phenolic Compounds and Antioxidant Activity of Avocado Leaf Extracts. Antioxidants 2023, 12, 298. [Google Scholar] [CrossRef]
- Razola-Díaz, M.d.C.; De Montijo-Prieto, S.; Aznar-Ramos, M.J.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Verardo, V.; Gómez-Caravaca, A.M. Effect of Lactic Acid Bacteria Fermentation on the Polar Compounds Content with Antioxidant and Antidiabetic Activity of Avocado Seed Extracts. Fermentation 2023, 9, 420. [Google Scholar] [CrossRef]
- Verni, M.; Pontonio, E.; Krona, A.; Jacob, S.; Pinto, D.; Rinaldi, F.; Verardo, V.; Díaz-de-Cerio, E.; Coda, R.; Rizzello, C.G. Bioprocessing of Brewers’ Spent Grain Enhances Its Antioxidant Activity: Characterization of Phenolic Compounds and Bioactive Peptides. Front. Microbiol. 2020, 11, 1831. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A.; Diaz, A.B.; Caro, I.; Bernini, V.; Galaverna, G.; Lazzi, C.; Blandino, A. Orange peels: From by-product to resource through lactic acid fermentation. J. Sci. Food Agric. 2019, 99, 6761–6767. [Google Scholar] [CrossRef]
- Fritsch, C.; Jänsch, A.; Ehrmann, M.A.; Toelstede, S.; Vogel, R.F. Characterization of Cinnamoyl Esterases from Different Lactobacilli and Bifidobacteria. Curr. Microbiol. 2017, 74, 247–256. [Google Scholar] [CrossRef]
- Muñoz, R.; de Las Rivas, B.; Curiel, J.A.; Rodríguez, H.; Esteban-Torres, M.; Reverón, I.; Santamaría, L.; Landete, J.M.; Plaza-Vinuesa, L.; Sánchez-Arroyo, A.; et al. Food phenolics and Lactiplantibacillus plantarum. Int. J. Food Microbiol. 2024, 412, 110555. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Maldonado, A.F.; Schieber, A.; Gänzle, M.G. Structure-function relationships of the antibacterial activity of phenolic acids and their metabolism by lactic acid bacteria. J. Appl. Microbiol. 2011, 111, 1176–1184. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Gänzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef]
- Rozès, N.; Peres, C. Effects of phenolic compounds on the growth and the fatty acid composition of Lactobacillus plantarum. Appl. Microbiol. Biotechnol. 1998, 49, 108–111. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef]
- Esteban-Torres, M.; Landete, J.M.; Reverón, I.; Santamaría, L.; de las Rivas, B.; Muñoz, R. A Lactobacillus plantarum esterase active on a broad range of phenolic esters. Appl. Environ. Microbiol. 2015, 81, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Soumya, M.P.; Nampoothiri, K.M. An overview of functional genomics and relevance of glycosyltransferases in exopolysaccharide production by lactic acid bacteria. Int. J. Biol. Macromol. 2021, 184, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Qi, T.; Xu, L.; Lu, L.; Xiao, M. Recent progress in the enzymatic glycosylation of phenolic compounds. J. Carbohydr. Chem. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, P.; Cui, Y.; Li, K.; Qiao, X.; Zhang, Y.T.; Li, S.M.; Cox, R.J.; Wu, B.; Ye, M.; et al. Regio- and Stereospecific O-Glycosylation of Phenolic Compounds Catalyzed by a Fungal Glycosyltransferase from Mucor hiemalis. Adv. Synth. Catal. 2017, 359, 995–1006. [Google Scholar] [CrossRef]
- Xie, K.; Dou, X.; Chen, R.; Chen, D.; Fang, C.; Xiao, Z.; Dai, J. Two novel fungal phenolic UDP glycosyltransferases from Absidia coerulea and Rhizopus japonicus. Appl. Environ. Microbiol. 2017, 83, e03103-16. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, L.; Bai, J.; Yue, Q.; Zhang, M.; Li, J.; Wang, C.; Xu, Y. Methylglucosylation of Phenolic Compounds by Fungal Glycosyltransferase-Methyltransferase Functional Modules. J. Agric. Food Chem. 2019, 67, 8573–8580. [Google Scholar] [CrossRef] [PubMed]
- Kralj, S.; Van Geel-Schutten, G.H.; Rahaoui, H.; Leer, R.J.; Faber, E.J.; Van der Maarel, M.J.E.C.; Dijkhuizen, L. Molecular Characterization of a Novel Glucosyltransferase from Lactobacillus reuteri Strain 121 Synthesizing a Unique, Highly Branched Glucan with α-(1→4) and α-(1→6) Glucosidic Bonds. Appl. Environ. Microbiol. 2002, 68, 4283. [Google Scholar] [CrossRef]
- Lim, E.K.; Higgins, G.S.; Li, Y.; Bowles, D.J. Regioselectivity of glucosylation of caffeic acid by a UDP-glucose:glucosyltransferase is maintained in planta. Biochem. J. 2003, 373, 987–992. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Kometani, T.; Takii, H.; Terada, Y.; Okada, S. Glucosylation of caffeic acid with Bacillus subtilis X-23 α-amylase and a description of the glucosides. J. Ferment. Bioeng. 1995, 80, 18–23. [Google Scholar] [CrossRef]
- Kang, J.; Kim, Y.M.; Kim, N.; Kim, D.W.; Nam, S.H.; Kim, D. Synthesis and characterization of hydroquinone fructoside using Leuconostoc mesenteroides levansucrase. Appl. Microbiol. Biotechnol. 2009, 83, 1009–1016. [Google Scholar] [CrossRef]
- Lu, L.; Guo, Y.; Xu, L.; Qi, T.; Jin, L.; Xu, L.; Xiao, M. Galactosylation of caffeic acid by an engineered β-galactosidase. Drug Discov. Ther. 2015, 9, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.B.; Mani, J.S.; Broszczak, D.; Prasad, S.S.; Ekanayake, C.P.; Strappe, P.; Valeris, P.; Naiker, M. Hitting the sweet spot: A systematic review of the bioactivity and health benefits of phenolic glycosides from medicinally used plants. Phytother. Res. 2021, 35, 3484–3508. [Google Scholar] [CrossRef] [PubMed]
- Mallampalli, V.; Owens, D.K.; Kumar, D. Expression and Biochemical Function of Putative Flavonoid GT Clones from Grapefruit and Identification of New Clones using the harvEST Database. Master’s Thesis, East Tennessee State University, Johnson City, TN, USA, 2009. [Google Scholar] [CrossRef]
- Guo, X.; Guo, A.; Li, E. Biotransformation of two citrus flavanones by lactic acid bacteria in chemical defined medium. Bioprocess Biosyst. Eng. 2021, 44, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Deba-Rementeria, S.; Paz, A.; Estrada, O.; Vázquez-Araújo, L. Consumer perception and physicochemical characterization of a new product made from lactic acid fermented orange peels. Int. J. Gastron. Food Sci. 2023, 31, 100647. [Google Scholar] [CrossRef]
- Razola-Díaz, M.d.C.; Sevenich, R.; Rossi Ribeiro, L.; Guerra-Hernández, E.-J.; Schlüter, O.; Verardo, V. Combined effect of pulse electric field and probe ultrasound technologies for obtaining phenolic compounds from orange by-product. LWT 2024, 198, 115950. [Google Scholar] [CrossRef]
- Rozan, M.; Alamri, E.; Bayomy, H. Fermented Hass avocado kernel: Nutritional properties and use in the manufacture of biscuits. Saudi J. Biol. Sci. 2022, 29, 103295. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Teng, J.; Lyu, Y.; Hu, X.; Zhao, Y.; Wang, M. Enhanced Antioxidant Activity for Apple Juice Fermented with Lactobacillus plantarum ATCC14917. Molecules 2019, 24, 51. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, J.; Hu, D.; Li, J.; Zhu, W.; Yuan, L.; Chen, X.; Yao, J. Gamma-Aminobutyric Acid-Producing Levilactobacillus brevis Strains as Probiotics in Litchi Juice Fermentation. Foods 2023, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Li, T.; Feng, J.; Yin, J.; Zou, X.; Wang, J.; Wang, B. Enhanced DPPH Radical Scavenging Activity and Enriched γ-Aminobutyric Acid in Mulberry Juice Fermented by the Probiotic Lactobacillus brevis S3. Fermentation 2023, 9, 829. [Google Scholar] [CrossRef]
- Piazzon, A.; Vrhovsek, U.; Masuero, D.; Mattivi, F.; Mandoj, F.; Nardini, M. Antioxidant activity of phenolic acids and their metabolites: Synthesis and antioxidant properties of the sulfate derivatives of ferulic and caffeic acids and of the acyl glucuronide of ferulic acid. J. Agric. Food Chem. 2012, 60, 12312–12323. [Google Scholar] [CrossRef]
- Ben Abdallah, M.; Chadni, M.; M’hiri, N.; Brunissen, F.; Rokbeni, N.; Allaf, K.; Besombes, C.; Ioannou, I.; Boudhrioua, N. Intensifying Effect of Instant Controlled Pressure Drop (DIC) Pre-Treatment on Hesperidin Recovery from Orange Byproducts: In Vitro Antioxidant and Antidiabetic Activities of the Extracts. Molecules 2023, 28, 1858. [Google Scholar] [CrossRef] [PubMed]

| 0 h | 24 h | 48 h | ||||
|---|---|---|---|---|---|---|
| Log10 (CFU/mL) | pH | Log10 (CFU/mL) | pH | Log10 (CFU/mL) | pH | |
| Le. brevis CECT 5354 | 7.92 ± 0.03 | 5.8 | 8.09 ± 0.09 | 3.7 | 8.21 ± 0.03 | 3.5 |
| La. plantarum CECT 748T | 7.94 ± 0.02 | 5.8 | 8.18 ± 0.13 | 3.7 | 8.01 ± 0.02 | 3.5 |
| La. plantarum CECT 9567 | 7.90 ± 0.03 | 5.8 | 8.60 ± 0.03 | 3.4 | 8.51 ± 0.01 | 3.2 |
| Compound | Le. brevis CECT 5354 | La. plantarum CECT 748T | La. plantarum CECT 9567 | Control | |||
|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | ||
| Caffeic acid 3-O-glucuronide isomer I | 247.31 ± 6.16 b | 292.08 ± 4.35 c | 299.02 ± 8.16 b | 291.53 ± 7.42 b | 344.24 ± 10.58 a | 331.17 ± 10.82 a | 324.25 ± 2.11 a |
| Caffeic acid 3-O-glucuronide isomer II | 1013.41 ± 9.26 b | 1086.94 ± 2.52 a | 962.02 ± 35.99 c | 926.84 ± 9.88 c | 1136.69 ± 9.70 a | 835.58 ± 1.85 d | 1031.70 ± 26.31 b |
| Caffeoylglycolic acid methyl ester | 566.91 ± 4.51 d | 586.10 ± 0.43 d | 699.64 ± 32.07 a | 609.64 ± 6.84 c,d | 658.41 ± 20.12 a,b | 672.53 ± 11.32 a,b | 643.56 ± 4.80 b,c |
| Caffeoylmalic acid isomer I | 784.93 ± 16.56 c | 822.56 ± 21.66 b,c | 913.38 ± 32.92 a | 816.35 ± 12.40 b,c | 925.70 ± 33.03 a | 939.33 ± 12.71 a | 878.32 ± 20.35 a,b |
| Caffeoylmalic acid isomer II | 358.13 ± 10.94 c | 365.61 ± 11.82 c | 432.31 ± 20.23 a,b | 362.31 ± 4.25 c | 460.45 ± 9.25 a | 440.02 ± 10.83 a,b | 426.22 ± 4.97 b |
| 2-(E)-O-Feruloyl-D-galactaric acid isomer I | 535.18 ± 25.98 e | 618.73 ± 3.04 c | 602.65 ± 25.53 c,d | 561.19 ± 7.69 d,e | 785.52 ± 19.31 a | 582.45 ± 27.15 c–e | 682.90 ± 15.40 b |
| 2-(E)-O-Feruloyl-D-galactaric acid isomer II | 959.38 ± 30.16 c,d | 1013.35 ± 9.56 b,c | 923.94 ± 45.24 d | 907.83 ± 17.36 d | 1217.10 ± 37.75 a | 890.69 ± 8.30 d | 1081.95 ± 11.02 b |
| 2-(E)-O-Feruloyl-D-galactaric acid isomer III | 1114.06 ± 12.43 c,d | 1176.40 ± 48.97 b,c | 1040.48 ± 50.14 d,e | 993.00 ± 42.88 e,f | 1578.57 ± 47.40 a | 913.48 ± 24.26 f | 1253.86 ± 9.81 b |
| 2-(E)-O-Feruloyl-D-galactaric acid isomer IV | 2660.23 ± 110.54 c | 2834.30 ± 2.67 b | 2447.51 ± 88.72 d | 2370.97 ± 22.84 d,e | 3395.69 ± 25.11 a | 2261.85 ± 46.95 e | 2882.53 ± 33.35 b |
| 2-(E)-O-Feruloyl-D-galactaric acid isomer V | 1435.76 ± 67.27 c,d | 1503.91 ± 32.86 b,c | 1388.00 ± 49.60 c,d | 1307.67 ± 31.65 d | 1984.38 ± 90.00 a | 1288.17 ± 11.13 d | 1603.01 ± 65.47 b |
| Feruloyl isocitric acid isomer I | 322.02 ± 15.04 c,d | 309.86 ± 12.00 d | 342.57 ± 13.62 b,c | 313.79 ± 8.48 c,d | 403.49 ± 5.95 a | 391.67 ± 8.09 a | 354.75 ± 4.66 b |
| Feruloyl isocitric acid isomer II | 1122.62 ± 24.61 c,d | 1112.85 ± 8.54 c,d | 1187.72 ± 53.48 b,c | 1102.26 ± 32.63 d | 1383.98 ± 34.79 a | 1361.08 ± 1.97 a | 1232.35 ± 9.59 b |
| Feruloyl isocitric acid isomer III | 485.96 ± 9.92 b,c | 450.20 ± 2.54 c | 535.49 ± 19.92 a | 466.59 ± 15.53 c | 536.72 ± 16.04 a | 512.50 ± 10.84 a,b | 507.51 ± 6.34 a,b |
| Ferulic acid O-glucoside | 302.87 ± 5.65 a | 277.48 ± 1.75 b | 259.58 ± 2.74 b,c | 253.43 ± 6.73 c,d | 240.32 ± 9.06 d | 194.82 ± 6.28 e | 252.46 ± 9.04 c,d |
| Sinapic acid O-glucoside | 241.00 ± 6.57 a | 224.12 ± 0.18 b | 215.36 ± 2.51 b,c | 203.23 ± 0.98 c | 211.73 ± 6.59 b,c | 142.59 ± 5.61 d | 211.80 ± 4.18 b,c |
| Sinapinic acid-O-glucuronide isomer I | 811.12 ± 27.33 c | 805.08 ± 18.41 c | 795.88 ± 34.20 c | 703.32 ± 23.89 d | 1070.21 ± 14.86 a | 726.92 ± 17.21 d | 873.06 ± 0.10 b |
| Sinapinic acid-O-glucuronide isomer II | 138.55 ± 5.43 b,c | 130.91 ± 5.17 c | 158.04 ± 7.84 a | 129.10 ± 3.20 c | 154.65 ± 6.99 a | 145.25 ± 0.07 a,b | 147.95 ± 1.46 a,b |
| Compound | Le. brevis CECT 5354 | La. plantarum CECT 748T | La. plantarum CECT 9567 | Control | |||
|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | ||
| Quercetin-3-O-rutinoside-7-O-Glucoside | 27.64 ± 1.17 a,b | 25.46 ± 0.88 c,d | 25.44 ± 0.77 c,d | 23.84 ± 0.76 d | 28.64 ± 0.72 a | 23.97 ± 0.05 d | 26.23 ± 0.20 b,c |
| Luteolin 7-O-glucoside | 62.27 ± 0.95 a,b | 61.33 ± 2.26 a,b | 57.68 ± 1.99 a,b | 52.04 ± 1.93 c | 62.68 ± 1.65 a | 57.29 ± 2.35 b | 59.21 ± 0.77 a,b |
| Prunin | 149.44 ± 1.35 a | 141.97 ± 2.65 b | 138.38 ± 2.75 b | 120.88 ± 1.30 c | 124.48 ± 1.71 c | 137.31 ± 0.14 b | 123.74 ± 2.72 c |
| Isorhamnetin-3-O-rutinoside isomer I | 95.29 ± 3.24 a | 91.70 ± 0.63 a,b | 85.68 ± 2.65 c | 88.01 ± 0.35 b,c | 95.80 ± 1.46 a | 84.86 ± 1.59 c | 92.54 ± 1.41 a,b |
| Isorhamnetin-3-O-rutinoside isomer II | 45.12 ± 2.14 a | 43.67 ± 1.40 a,b | 40.22 ± 1.72 b,c | 38.48 ± 0.79 c | 43.74 ± 1.51 a,b | 38.71 ± 1.74 c | 42.46 ± 1.14 a–c |
| Vitexin-O-pentoside | 128.65 ± 0.81 a | 125.94 ± 0.01 a,b | 121.50 ± 3.51 b,c | 116.85 ± 0.21 c | 125.34 ± 4.05 a,b | 121.79 ± 0.52 b,c | 122.45 ± 0.45 b,c |
| Naringin hydrate | 18.83 ± 0.48 b,c | 19.03 ± 0.90 a-c | 17.68 ± 0.68 c,d | 16.71 ± 0.81 d | 20.67 ± 0.72 a | 16.97 ± 0.41 d | 19.49 ± 0.06 a,b |
| Apigenin-di-C-hexoside (Vicenin-2) isomer I | 490.70 ± 6.40 a | 481.67 ± 0.88 a | 473.24 ± 9.59 a,b | 431.92 ± 3.21 c | 448.12 ± 15.82 b,c | 470.90 ± 1.79 a,b | 444.48 ± 19.28 b,c |
| Apigenin-di-C-hexoside (Vicenin-2) isomer II | 28.47 ± 0.89 b | 27.58 ± 0.02 b | 27.71 ± 1.17 b | 28.48 ± 0.84 b | 32.04 ± 0.79 a | 27.40 ± 0.91 b | 30.84 ± 0.35 a |
| Apigenin-di-C-hexoside (Vicenin-2) isomer III | 17.27 ± 0.72 d | 17.88 ± 0.49 c,d | 17.30 ± 0.02 d | 18.77 ± 0.48 b,c | 21.41 ± 0.30 a | 17.44 ± 0.75 c,d | 20.03 ± 0.49 a,b |
| Apigenin 7-O-neohesperidoside | 28.46 ± 0.58 a | 27.64 ± 0.78 a | 26.48 ± 0.96 a–c | 23.68 ± 0.67 d | 27.31 ± 0.99 a,b | 24.98 ± 0.57 c,d | 25.38 ± 0.10 b–d |
| Narirutin | 621.47 ± 8.42 a | 610.59 ± 5.64 a,b | 585.25 ± 2.80 b,c | 558.30 ± 15.65 c,d | 537.37 ± 18.90 d | 588.76 ± 12.61 a–c | 560.05 ± 16.29 c,d |
| Rutin isomer I | 97.43 ± 2.67 a,b | 92.69 ± 2.28 b,c | 86.39 ± 2.88 c,d | 84.59 ± 3.54 d | 100.77 ± 0.51 a | 89.37 ± 0.85 c,d | 91.25 ± 3.82 b–d |
| Rutin isomer II | 63.38 ± 2.13 a,b | 63.58 ± 2.20 a,b | 60.96 ± 2.48 b | 57.69 ± 1.75 b | 68.30 ± 3.12 a | 58.31 ± 1.47 b | 63.48 ± 1.21 a,b |
| Hesperidin | 960.16 ± 31.76 a | 907.31 ± 23.27 a,b | 924.52 ± 5.57 a,b | 863.24 ± 25.80 b,c | 873.10 ± 26.05 b,c | 833.32 ± 4.76 c | 868.09 ± 23.69 b,c |
| Neohesperidin | 17.48 ± 0.12 b,c | 18.85 ± 0.59 a | 17.79 ± 0.36 a,b | 15.84 ± 0.03 d | 16.24 ± 0.12 d | 16.51 ± 0.48 c,d | 16.09 ± 0.79 d |
| Alpha-Glucosyl Hesperidin isomer I | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Alpha-Glucosyl Hesperidin isomer II | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Kaempferol 3-O-[3″,6″-di-O-(E)-cinnamoyl]-b-D-glucopyranoside isomer I | 14.98 ± 0.60 b,c | 16.14 ± 0.66 a,b | 15.62 ± 0.72 b,c | 14.80 ± 0.56 b,c | 17.41 ± 0.87 a | 14.41 ± 0.15 c | 16.00 ± 0.06 a–c |
| Kaempferol 3-O-[3″,6″-di-O-(E)-cinnamoyl]-b-D glucopyranoside isomer II | 19.89 ± 0.57 a,b | 20.46 ± 0.52 a | 19.45 ± 0.83 a–c | 17.98 ± 0.70 c | 20.54 ± 0.90 a | 18.36 ± 0.52 b,c | 19.82 ± 0.05 a,b |
| Kaempferol 3-[2″-glucosyl-6″-acetyl-galactoside]7-glucoside isomer I | 28.58 ± 1.10 c,d | 29.77 ± 0.75 b,c | 28.32 ± 0.89 c,d | 27.95 ± 0.69 c,d | 33.89 ± 1.22 a | 26.92 ± 0.20 d | 31.60 ± 0.10 b |
| Kaempferol 3-[2″-glucosyl-6″-acetyl-galactoside]7-glucoside isomer II | 31.98 ± 1.25 b | 30.58 ± 1.48 b,c | 29.27 ± 0.56 c | 28.92 ± 0.73 c | 37.23 ± 0.96 a | 25.95 ± 0.08 d | 32.48 ± 0.98 b |
| Kaempferol-dihexosyl acetate | 10.52 ± 0.50 c,d | 11.33 ± 0.05 c | 11.04 ± 0.30 c,d | 10.41 ± 0.19 d | 15.19 ± 0.38 a | 8.99 ± 0.22 e | 12.74 ± 0.17 b |
| Kaempferol 3-O-(6″-O-acetyl) glucoside-7-O-rhamnoside | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Kaempferol 3-apiosyl-(1->4)-rhamnoside-7-rhamnoside | 2.50 ± 0.08 d,e | 2.81 ± 0.11 c | 2.67 ± 0.13 c,d | 2.13 ± 0.10 f | 4.79 ± 0.15 a | 2.24 ± 0.08 e,f | 3.15 ± 0.12 b |
| Kaempferol 3-O-sinapoyl-caffeoyl-sophoroside 7-O-glucoside isomer I | 5.80 ± 0.06 b | 5.72 ± 0.21 b | 4.61 ± 0.20 c | 4.63 ± 0.18 c | 8.97 ± 0.23 a | 4.88 ± 0.12 c | 6.25 ± 0.28 b |
| Kaempferol 3-O-sinapoyl-caffeoyl-sophoroside 7-O-glucoside isomer II | 4.17 ± 0.07 c | 3.59 ± 0.15 d | 3.11 ± 0.13 f | 3.47 ± 0.10 d,e | 6.45 ± 0.09 a | 3.13 ± 0.10 e,f | 4.71 ± 0.22 b |
| 3′,4′-Didemethylnobiletin | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razola-Díaz, M.d.C.; De Montijo-Prieto, S.; Guerra-Hernández, E.J.; Jiménez-Valera, M.; Ruiz-Bravo, A.; Gómez-Caravaca, A.M.; Verardo, V. Fermentation of Orange Peels by Lactic Acid Bacteria: Impact on Phenolic Composition and Antioxidant Activity. Foods 2024, 13, 1212. https://doi.org/10.3390/foods13081212
Razola-Díaz MdC, De Montijo-Prieto S, Guerra-Hernández EJ, Jiménez-Valera M, Ruiz-Bravo A, Gómez-Caravaca AM, Verardo V. Fermentation of Orange Peels by Lactic Acid Bacteria: Impact on Phenolic Composition and Antioxidant Activity. Foods. 2024; 13(8):1212. https://doi.org/10.3390/foods13081212
Chicago/Turabian StyleRazola-Díaz, María del Carmen, Soumi De Montijo-Prieto, Eduardo Jesús Guerra-Hernández, María Jiménez-Valera, Alfonso Ruiz-Bravo, Ana María Gómez-Caravaca, and Vito Verardo. 2024. "Fermentation of Orange Peels by Lactic Acid Bacteria: Impact on Phenolic Composition and Antioxidant Activity" Foods 13, no. 8: 1212. https://doi.org/10.3390/foods13081212
APA StyleRazola-Díaz, M. d. C., De Montijo-Prieto, S., Guerra-Hernández, E. J., Jiménez-Valera, M., Ruiz-Bravo, A., Gómez-Caravaca, A. M., & Verardo, V. (2024). Fermentation of Orange Peels by Lactic Acid Bacteria: Impact on Phenolic Composition and Antioxidant Activity. Foods, 13(8), 1212. https://doi.org/10.3390/foods13081212









