Fatty Acid Composition, Phytochemistry, Antioxidant Activity on Seed Coat and Kernel of Paeonia ostii from Main Geographic Production Areas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Samples
2.2. Chemicals and Reagents
2.3. Seed Proximate Composition Analysis
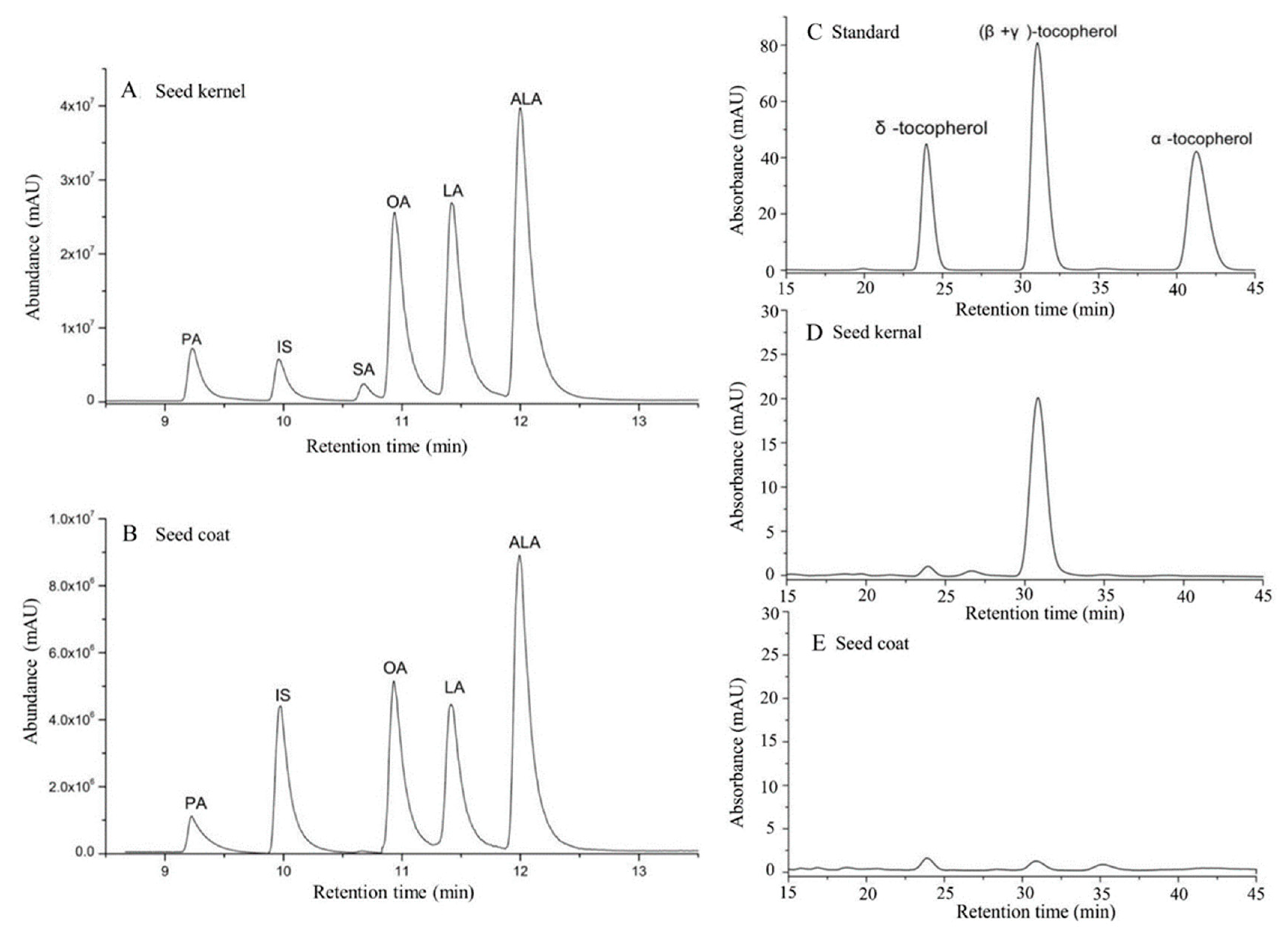
2.4. Fatty Acid Composition and Content Analysis
2.5. Determination of the Components and Content of Tocopherol
2.6. Extraction and Assay of Phytochemical Compounds and HPLC-MS Analysis
2.7. Total Phenolic Content and Antioxidant Activity Analysis
2.8. Antioxidant Potency Composite (APC) Index Analysis
2.9. Statistical Analysis
3. Results
3.1. Proximate Composition Analysis of PSK and PSC
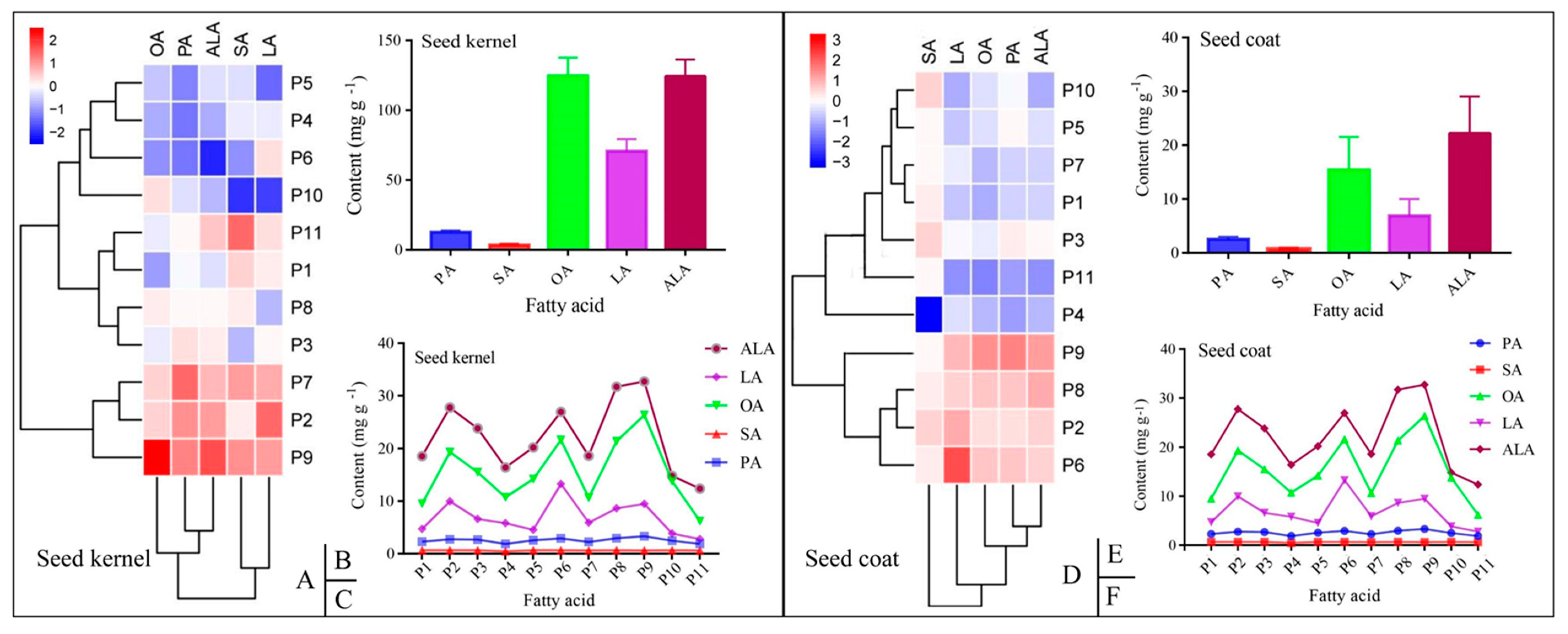
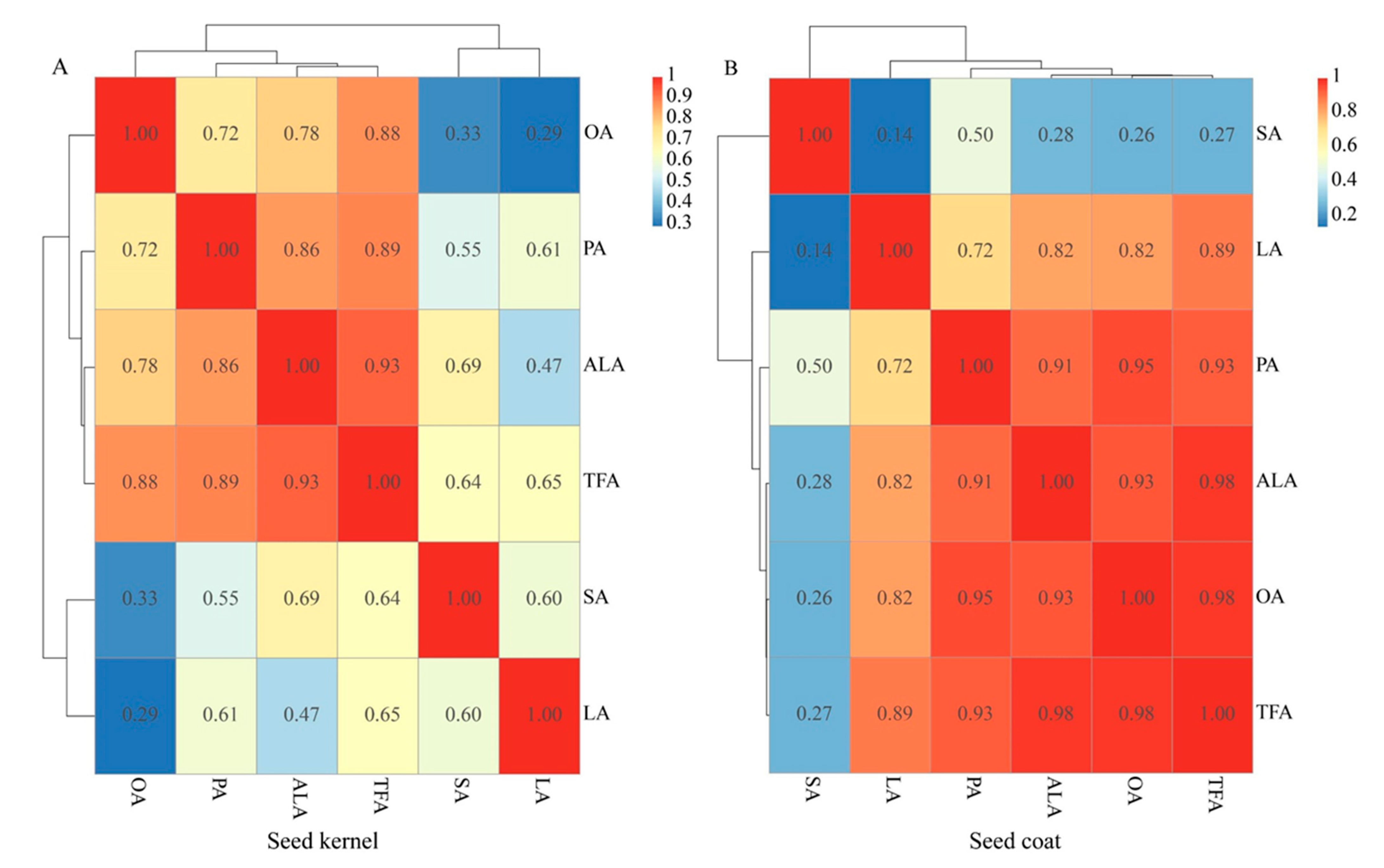
3.2. Composition of the Fatty Acids in the PSK and PSC
3.3. Content of the Five Mainfas in PSK and PSC
3.4. Compounds and Content Analysis of Phytochemical in PSK and PSC
3.5. Antioxidant Activity Analysis of PSK and PSC
3.6. Tocopherols Content Analysis in PSK and PSC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Van Etten, C.H.; Kwolek, W.F.; Peters, J.E.; Barclay, A.S. Plant seeds as protein sources of food or feed. Evaluation based on amino acid composition of 379 species. J. Agric. Food Chem. 1967, 15, 1077–1089. [Google Scholar] [CrossRef]
- Azimova, S.S.; Glushenkova, A.I.; Vinogradova, V.I. Lipids, Lipophilic Components and Essential Oils from Plant Sources; Springer: London, UK, 2012; Volume 1, pp. 1–32. [Google Scholar]
- Kennedy, D.O.; Wightman, E.L. Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2011, 2, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Wang, L.S.; Shu, Q.Y.; Wu, J.; Chen, L.G.; Shao, S.; Yin, D.D. Fatty acid composition of developing tree peony (Paeonia section Moutan DC.) seeds and transcriptome analysis during seed development. BMC Genom. 2015, 16, 194–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minikeeva, A.S.; Freiman, R.É.; Umarov, A.U. A study of the oils of Ocimum basilicum and Cardaria repens. Chem. Nat. Compd. 1971, 7, 5–7. [Google Scholar] [CrossRef]
- Amini, Z.; Ong, H.C.; Harrison, M.D.; Kusumo, F.; Mazaheri, H.; Llham, Z. Biodiesel production by lipase-catalyzed transesterification of Ocimum basilicum L. (sweet basil) seed oil. Energy Conv. Manag. 2017, 132, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Kamila, P.K.; Ray, A.; Sahoo, A.; Nayak, S.; Mohapatra, P.K.; Panda, P.C. Physicochemical characteristics of the lasiococca comberi haines seeds. Nat. Prod. Lett. 2018, 32, 2352–2355. [Google Scholar] [CrossRef]
- Kanmaz, E.Ö.; Ova, G. Genotypic variation on oil content, fatty acid composition and phenolic compounds in linseed (Linum usitatissimum L.). Ege Üniversitesi Ziraat Fakültesi Dergisi 2015, 52, 249. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.X.; Ji, X.Y.; Tan, B.B.; Liang, Y.Z.; Liang, N.N.; Wang, L.X.; Dai, H. Identification of the composition of fatty acids in Eucommia ulmoides seed oil by fraction chain length and mass spectrometry. Food Chem. 2010, 121, 815–819. [Google Scholar] [CrossRef]
- Li, S.S.; Yuan, R.Y.; Chen, L.G.; Wang, L.S.; Hao, X.H.; Wang, L.J.; Zheng, X.C.; Du, H. Systematic qualitative and quantitative assessment of fatty acids in the seeds of 60 tree peony (Paeonia, section Moutan, DC.) cultivars by GC-MS. Food Chem. 2015, 173, 133–140. [Google Scholar] [CrossRef]
- Lee, D.S.; Noh, B.S.; Bae, S.Y.; Kim, K. Characterization of fatty acids composition in vegetable oils by gas chromatography and chemometrics. Anal. Chim. Acta 1998, 358, 163–175. [Google Scholar] [CrossRef]
- Li, J.J. Tree Peony of China; Encyclopedia of China Publishing House: Beijing, China, 2011; pp. 1–20. [Google Scholar]
- Li, Y.C. The strategy on the oil tree peony industry in China. Chin. Eng. Sci. 2014, 10, 58–63. [Google Scholar]
- Lin, P.; Yao, X.H.; Cao, Y.Q.; Xue, L.; Wang, K.L. Variation analysis of fruit characteristics and fatty acid components in Paeonia ostii ‘Phoenix White’. Nonwood For. Res. 2015, 33, 67–72. [Google Scholar]
- NHFPCC (National Health and Family Planning Commission of China). Notice on the Approval of Acer Truncatum Seed Oil and Peony Seed Oil as New Resource Food. Available online: http://www.nhfpc.gov.cn/sps/s7891/201103/cffd9def6007444ea271189c18063b54.shtml (accessed on 22 March 2011).
- Zheng, L.H.; Huang, X.; Wang, L.; Chen, Z.X. Physicochemical properties, chemical composition and antioxidant activity of Dalbergia odorifera T. Chen seed oil. J. Am. Oil Chem. Soc. 2012, 89, 883–890. [Google Scholar]
- Embaby, H.E.; Rayan, A.M. Chemical composition and nutritional evaluation of the seeds of Acacia tortilis (Forssk.) Hayne ssp. raddiana. Food Chem. 2016, 200, 62–68. [Google Scholar] [CrossRef] [PubMed]
- da Silva, B.P.; Anunciaç ão, P.C.; da Silva, J.C.; Della Lucia, C.M.; Martino, H.S.; Pinheiro-Sant’Ana, H.M. Chemical composition of Brazilian chia seeds grown in different places. Food Chem. 2017, 221, 1709–1716. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Q.; Verardo, V.; Contreras, M.M. Fatty acid and sterol composition of tea seed oils: Their comparison by the “fancytiles” approach. Food Chem. 2017, 233, 302–310. [Google Scholar] [CrossRef]
- Nehdi, I.A.; Sbihi, H.M.; Tan, C.P.; Rashid, U.; Al-Resayes, S.I. Chemical composition of date palm (Phoenix dactylifera L.) seed oil from six Saudi Arabian cultivars. J. Food Sci. 2018, 83, 624–630. [Google Scholar] [CrossRef]
- Yu, S.Y.; Du, S.B.; Yuan, J.H.; Hu, Y.H. Fatty acid profile in the seeds and seed tissues of Paeonia L. species as new oil plant resources. Sci. Rep. 2016, 6, 26944. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Li, C.H.; Du, H.; Wang, L.S.; Shu, Q.Y.; Zheng, Y.R.; Xu, Y.J.; Zhang, J.J.; Zhang, J.; Yang, R.Z.; Ge, Y.X. Flavonoid composition and antioxidant activity of tree peony (Paeonia Section, Moutan) yellow flowers. J. Agric. Food Chem. 2009, 57, 8496–8503. [Google Scholar] [CrossRef]
- Feng, C.Y.; Li, S.S.; Yin, D.D.; Zhang, H.J.; Tian, D.K.; Wu, Q.; Wang, L.J.; Su, S.; Wang, L.S. Rapid determination of flavonoids in plumules of sacred lotus cultivars and assessment of their antioxidant activities. Ind. Crops Prod. 2016, 87, 96–104. [Google Scholar] [CrossRef]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]
- He, C.N.; Peng, B.; Dan, Y.; Peng, Y.; Xiao, P.G. Chemical taxonomy of tree peony species from china based on root cortex metabolic fingerprinting. Phytochemistry 2014, 107, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Whiting, P.; Dinan, L. Identification and ecdysteroid antagonist activity of three resveratrol trimers (Suffruticosols A, B and C) from Paeonia suffruticosa. Tetrahedron 1999, 55, 513–524. [Google Scholar] [CrossRef]
- Kim, H.J.; Chang, E.J.; Bae, S.J.; Shim, S.M.; Park, H.D.; Rhee, C.H.; Park, J.H.; Chol, S.W. Cytotoxic and anumutagenic stilbenes from seeds of Paeonia lactiflora. Arch. Pharm. Res. 2002, 25, 293–299. [Google Scholar] [CrossRef]
- Xiao, J.H.; Meng, Q.C.; Cao, K.Q.; Liu, J.F. A study on the relationship between 1000-grain weight and yield in hybrid rice. Hunan Agric. Sci. 2009, 3, 7–9. [Google Scholar]
- Clements, J.; Galek, R.; Kozak, B.; Michalczyk, D.J.; Piotrowicz-Cies’lak, A.L.; Sawicka-Sienkiewicz, E.; Stawin´ski, S.; Zalewski, D. Diversity of selected Lupinus angustifolius L. genotypes at the phenotypic and DNA level with respect to microscopic seed coat structure and thickness. PLoS ONE 2014, 9, e102874. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Li, Y.; Hussain, N.; Li, Z.; Wu, D.; Jiang, L. Allelic variation of BnaC.TT2.a and its association with seed coat color and fatty acids in rapeseed (Brassica napus L.). PLoS ONE 2016, 11, e0146661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.J.; Sun, L.J.; Li, S.; Wang, W.D.; Ding, Y.H.; Swarm, S.A.; Li, L.H.; Wang, X.T.; Tang, X.M.; Zhang, Z.F.; et al. Elevation of soybean seed oil content through selection for seed coat shininess. Nat. Plants 2018, 4, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Orsavova, J.; Misurcova, L.; Ambrozova, J.V.; Vicha, R.; Mlcek, J. Fatty acids composition of vegetable oils and its contribution to dietary energy intake and dependence of cardiovascular mortality on dietary intake of fatty acids. Int. J. Mol. Sci. 2015, 16, 12871–12890. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Mann, J.D.; Faurot, K.R.; Lynch, C.; Imam, S.T.; MacIntosh, B.A.; Hibbeln, J.R.; Loewke, J.; Smith, S.; Coble, R.; et al. Low omega-6 vs. low omega-6 plus high omega-3 dietary intervention for chronic daily headache: Protocol for a randomized clinical trial. Trials 2011, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Salem., J.; Vandal, M.N.; Calon, F. The benefit of docosahexaenoic acid for the adult brain in aging and dementia. Prostaglandins Leukot. Essent. Fatty Acids 2015, 92, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Mišurcová, L.; Vávra Ambrožová, J.; Samek, D. Seaweed lipids as nutraceuticals. Adv. Food Nutr. Res. 2011, 64, 339–355. [Google Scholar]
- Malekbala, M.R.; Soltani, S.M.; Hosseini, S.; Babadi, F.E.; Darajeh, N.; Malekbala, R. Current technologies in the extraction, enrichment and analytical detection of tocopherols and tocotrienols: A review. Crit. Rev. Food Sci. Nutr. 2015, 57, 2935–2942. [Google Scholar] [CrossRef]
- Colombo, M.L. An update on vitamin E, tocopherol and tocotrienol-perspectives. Molecules 2010, 15, 2103–2113. [Google Scholar] [CrossRef] [Green Version]
- Mao, C.X.; Li, G.H.; Li, P.X.; Zhu, P.; Kang, X.M. Analysis of triglycerides composition structure and physicochemical properties of peony seed oil. Mod. Food Sci. Technol. 2014, 30, 142–146. [Google Scholar]
- Yang, X.; Zhang, D.; Song, L.M.; Xu, Q.; Xu, H. Chemical profile and antioxidant activity of the oil from peony seeds (Paeonia suffruticosa Andr.). Oxidative Med. Cell. Longev. 2017, 2017, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Britz, S.J.; Kremer, D.F.; Kenworthy, W.J. Tocopherols in soybean seeds: Genetic variation and environmental effects in field-grown crops. J. Am. Oil Chem. Soc. 2008, 85, 931–936. [Google Scholar] [CrossRef]
- Fritsche, S. A candidate gene-based association study of tocopherol content and composition in rapeseed (Brassica napus). Front. Plant Sci. 2012, 3, 129. [Google Scholar] [CrossRef] [Green Version]
- del Moral, L.; Fernández-Martínez, J.M.; Pérez-Vich, B.; Velasco, L. Accumulation dynamics of seed tocopherols in sunflower lines with modified tocopherol levels. Acta Physiol. Plant. 2013, 35, 3157–3165. [Google Scholar] [CrossRef]
- Brandolini, A.; Hidalgo, A.; Gabriele, S.; Heun, M. Chemical composition of wild and feral diploid wheats and their bearing on domesticated wheats. J. Cereal Sci. 2015, 63, 122–127. [Google Scholar] [CrossRef]
- Hidalgo, A.; Brandolini, A.; Pompei, C.; Piscozzi, R. Carotenoids and tocols of einkorn wheat (Triticum monococcum ssp. monococcum L.). J. Cereal Sci. 2006, 44, 182–193. [Google Scholar] [CrossRef]
- Nagy, K.; Kerrihard, A.L.; Beggio, M.; Craft, B.D.; Pegg, R.B. Modeling the impact of residual fat-soluble vitamin (FSV) contents on the oxidative stability of commercially refined vegetable oils. Food Res. Int. 2016, 84, 26–32. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Anderson, R. A multivariate study of the correlation between tocopherol content and fatty acid composition in vegetable oils. J. Am. Oil Chem. Soc. 1997, 74, 375–380. [Google Scholar] [CrossRef]
- Van Niekerk, P.J.; Burger, A.E.C. The estimation of the composition of edible oil mixtures. J. Am. Oil Chem. Soc. 1985, 62, 531–538. [Google Scholar] [CrossRef]
- Yoshida, H.; Hirooka, N.; Kajimoto, G. Microwave energy effects on quality of some seedoils. J. Food. Sci. 2010, 55, 1412–1416. [Google Scholar] [CrossRef]
- El-Agaimy, M.A.; Neff, W.E.; El-Sayed, M.; Awatif, I.I. Effect of saline irrigation water on olive oil composition. J. Am. Oil Chem. Soc. 1994, 71, 1287–1289. [Google Scholar] [CrossRef]
- Shin, H.S.; Kim, S.W. Lipid composition of Perilla Seed. J. Am. Oil Chem. Soc. 1994, 71, 619–622. [Google Scholar] [CrossRef]
- Hudafaujan, N.; Noriham, A.; Norrakiah, A.S.; Babji, A.S. Antioxidant activity of plants methanolic extracts containing phenolic compounds. Afr. J. Biotechnol. 2009, 8, 484–489. [Google Scholar]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Li, S.S.; Wu, Q.; Yin, D.D.; Feng, C.Y.; Liu, Z.A.; Wang, L.S. Phytochemical variation among the traditional Chinese medicine Mu Dan Pi from Paeonia suffruticosa (tree peony). Phytochemistry 2018, 146, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Chen, Y.W.; Yang, L.Y.; Li, S.L.; Li, Z.Y. Monoterpene glycosides from Paeonia delavayi. Fitoterapia 2007, 78, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Fang, Z.H. New monoterpene glycosides from the root cortex of Paeonia suffruticosa and their potential anti-inflammatory activity. Nat. Prod. Res. 2014, 28, 301–305. [Google Scholar] [CrossRef]
- Zhang, Y.Q.; Li, H.; Huang, M.Q.; Huang, M.; Chu, K.D.; Xu, W.; Zhang, S.N.; Que, J.H.; Chen, L.D. Paeoniflorin, a monoterpene glycoside, protects the brain from cerebral ischemic injury via inhibition of apoptosis. Am. J. Chin. Med. 2015, 43, 543–557. [Google Scholar] [CrossRef]
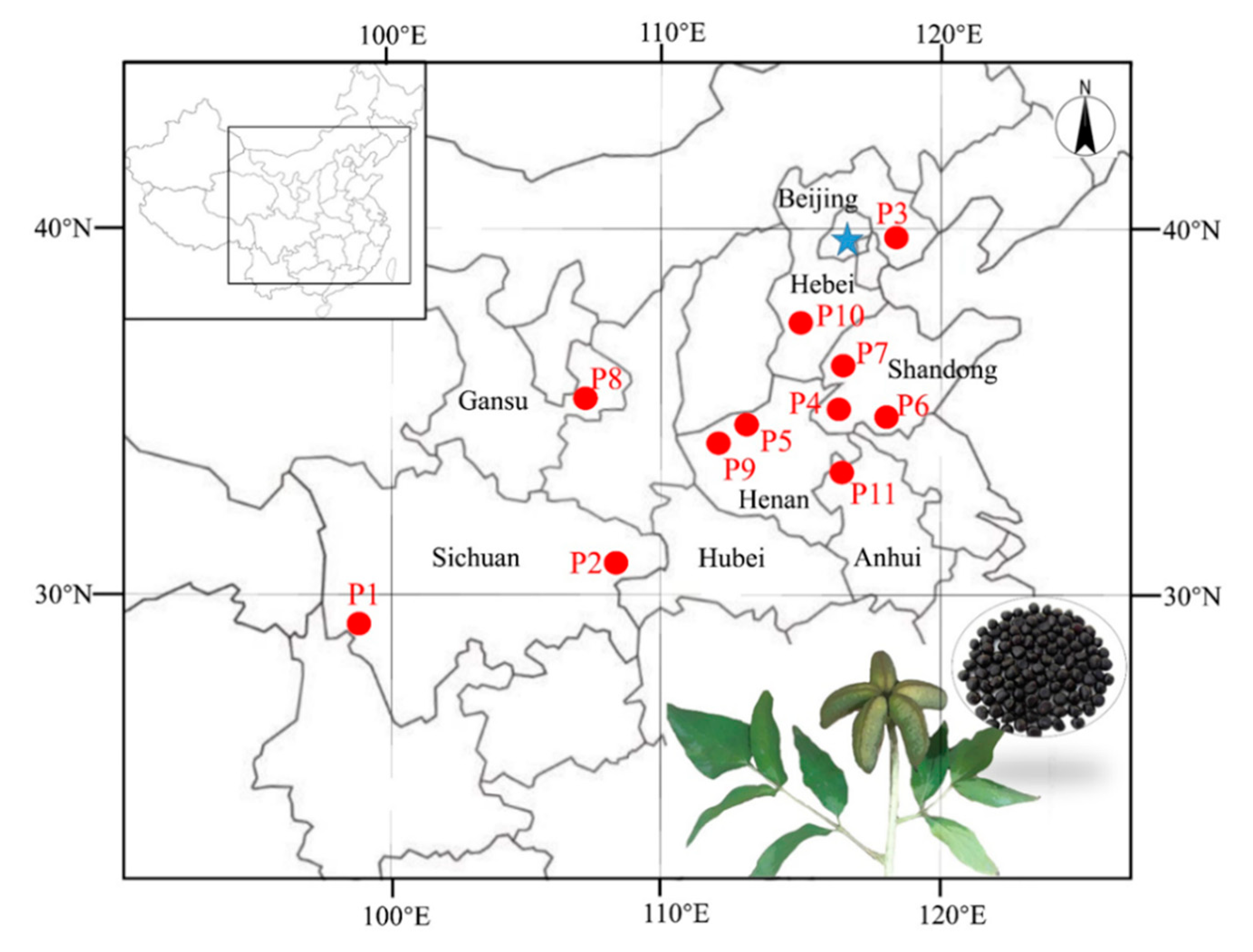


| Code | Neatness (%) | 100-Seed Weight (g) | Seed Coat Rate (%) | Moisture Content (%) | Crude Protein Content (%) | ||
|---|---|---|---|---|---|---|---|
| Seed Coat | Seed Kernel | Seed Coat | Seed Kernel | ||||
| P1 | 98.67 ± 0.23 d | 30.25 ± 0.48 h | 34.15 ± 0.05 bc | 7.41 ± 0.28 bcd | 4.25 ± 0.02 cd | 3.47 ± 0.08 c | 18.65 ± 0.13 b |
| P2 | 97.29 ± 0.30 b | 28.15 ± 0.34 g | 31.13 ± 0.60 a | 6.40 ± 0.28 ab | 3.57 ± 0.05 a | 4.09 ± 0.04 f | 21.33 ± 0.14 e |
| P3 | 99.62 ± 0.08 f | 22.26 ± 0.48 d | 31.62 ± 0.79 a | 6.14 ± 0.39 a | 3.49 ± 0.14 a | 3.60 ± 0.08 cd | 19.78 ± 0.21 c |
| P4 | 97.64 ± 0.21 bc | 24.02 ± 0.41 f | 35.52 ± 1.78 cd | 8.69 ± 0.15 e | 4.36 ± 0.03 cd | 3.68 ± 0.04 d | 20.15 ± 0.11 d |
| P5 | 99.59 ± 0.07 f | 22.41 ± 0.26 d | 32.82 ± 0.39 ab | 7.73 ± 0.60 cde | 5.00 ± 0.11 e | 3.80 ± 0.03 e | 21.39 ± 0.18 e |
| P6 | 93.08 ± 1.30 a | 21.71 ± 0.48 c | 36.38 ± 1.75 de | 7.92 ± 0.48 cde | 3.91 ± 0.29 abc | 3.51 ± 0.02 c | 16.91 ± 0.15 a |
| P7 | 98.80 ± 0.24 d | 23.15 ± 0.34 e | 35.15 ± 0.53 cd | 7.44 ± 0.77 bcd | 4.07 ± 0.10 bc | 3.05 ± 0.06 a | 20.04 ± 0.14 cd |
| P8 | 98.70 ± 0.21 d | 24.13 ± 0.32 f | 35.26 ± 1.06 cd | 8.63 ± 1.77 de | 3.53 ± 0.21 a | 3.66 ± 0.04 d | 18.82 ± 0.08 b |
| P9 | 98.37 ± 0.08 cd | 19.22 ± 0.56 b | 37.24 ± 0.70 e | 6.43 ± 0.23 ab | 3.72 ± 0.32 ab | 4.46 ± 0.09 g | 20.23 ± 0.27 d |
| P10 | 97.59 ± 0.42 bc | 16.08 ± 0.51 a | 38.95 ± 0.07 f | 7.17 ± 0.15 abc | 4.60 ± 0.11 de | 4.62 ± 0.03 h | 22.73 ± 0.15 f |
| P11 | 99.84 ± 0.12 f | 32.88 ± 0.15 i | 38.64 ± 0.81 ef | 6.86 ± 0.25 abc | 3.95 ± 0.66 abc | 3.20 ± 0.17 b | 22.27 ± 0.14 g |
| Range | 93.08–99.84 | 16.08–32.88 | 31.13–38.95 | 6.14–8.69 | 3.49–5.00 | 3.05–4.62 | 16.91–22.73 |
| Average | 98.11 ± 1.85 | 24.02 ± 4.64 | 35.17 ± 2.64 | 7.35 ± 0.10 | 4.04 ± 0.51 | 3.70 ± 0.55 | 20.21 ± 1.65 |
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PA | PSC | 2.27 ± 0.26 ab | 2.75 ± 0.15 abc | 2.69 ± 0.24 abc | 1.86 ± 1.41 a | 2.55 ± 0.15 abc | 2.90 ± 0.42 bc | 2.25 ± 0.39 ab | 2.94 ± 0.10 bc | 3.32 ± 0.43 c | 2.47 ± 0.13 abc | 1.88 ± 0.20 a |
| PSK | 12.71 ± 0.67 abc | 13.84 ± 0.54 abc | 13.11 ± 0.67 abc | 11.31 ± 1.19 a | 11.43 ± 0.36 ab | 11.32 ± 0.72 a | 14.27 ± 1.87 c | 12.82 ± 1.78 abc | 14.04 ± 2.53 bc | 12.32 ± 0.64 abc | 12.76 ± 1.85 abc | |
| SA | PSC | 0.63 ± 0.02 a | 0.65 ± 0.01 a | 0.64 ± 0.02 a | 0.46 ± 0.34 a | 0.63 ± 0.03 a | 0.64 ± 0.02 a | 0.63 ± 0.02 a | 0.64 ± 0.01 a | 0.62 ± 0.01 a | 0.65 ± 0.01 a | 0.63 ± 0.00 a |
| PSK | 3.72 ± 0.31 bcd | 3.53 ± 1.02 bcd | 3.07 ± 0.56 abc | 3.34 ± 0.34 abcd | 3.32 ± 0.22 abcd | 2.85 ± 0.57 ab | 4.00 ± 0.36 cd | 3.57 ± 0.60 bcd | 4.02 ± 0.65 cd | 2.42 ± 0.08 a | 4.24 ± 0.50 d | |
| OA | PSC | 9.51 ± 1.14 ab | 19.34 ± 3.76 cde | 15.51 ± 1.49 bcd | 10.76 ± 8.35 ab | 14.21 ± 0.79 bcd | 21.63 ± 5.31 de | 10.64 ± 4.05 ab | 21.41 ± 3.34 de | 26.40 ± 5.44 e | 13.81 ± 1.39 bc | 6.20 ± 1.59 a |
| PSK | 112.56 ± 5.23 a | 129.67 ± 10.57 a | 122.85 ± 10.97 a | 113.24 ± 15.25 a | 116.46 ± 8.78 a | 111.25 ± 10.66 a | 129.75 ± 18.07 a | 126.63 ± 19.93 a | 157.63 ± 27.49 b | 129.29 ± 6.04 a | 121.97 ± 16.90 a | |
| LA | PSC | 4.71 ± 0.29 ab | 9.98 ± 1.54 cd | 6.60 ± 1.18 abc | 5.80 ± 4.57 abc | 4.54 ± 1.28 ab | 13.26 ± 3.27 d | 5.90 ± 2.52 abc | 8.64 ± 1.30 bc | 9.48 ± 2.77 cd | 3.86 ± 0.66 a | 2.76 ± 0.59 a |
| PSK | 72.41 ± 3.98 bcd | 83.59 ± 7.05 d | 70.95 ± 5.96 abcd | 69.05 ± 10.49 abcd | 58.20 ± 7.00 ab | 73.61 ± 6.29 bcd | 77.41 ± 11.81 cd | 64.50 ± 11.02 abc | 78.92 ± 15.10 cd | 54.39 ± 0.71 a | 73.88 ± 10.61 bcd | |
| ALA | PSC | 18.55 ± 2.33 abc | 27.77 ± 3.93 cd | 23.85 ± 4.42 bcd | 16.40 ± 12.76 ab | 20.20 ± 3.01 abc | 26.98 ± 5.11 cd | 18.61 ± 5.37 abc | 31.72 ± 3.89 d | 32.73 ± 6.45 d | 14.80 ± 1.53 ab | 12.38 ± 2.06 a |
| PSK | 121.15 ± 4.12 abc | 135.06 ± 6.46 bc | 126.67 ± 5.13 abc | 114.27 ± 12.93 ab | 120.52 ± 10.17 abc | 99.85 ± 8.71 a | 132.69 ± 18.07 bc | 124.77 ± 18.88 abc | 144.71 ± 27.45 c | 116.60 ± 2.66 abc | 130.98 ± 20.75 bc | |
| TFA | PSC | 35.69 ± 3.94 ab | 60.50 ± 9.37 cde | 49.32 ± 6.50 bcd | 35.30 ± 27.41 ab | 42.14 ± 4.92 abc | 65.43 ± 14.08 de | 38.05 ± 12.30 ab | 65.37 ± 8.60 de | 72.56 ± 15.10 e | 35.60 ± 3.67 ab | 23.87 ± 4.42 a |
| PSK | 322.58 ± 11.56 a | 365.72 ± 23.34 ab | 336.67 ± 23.03 ab | 311.23 ± 40.09 a | 309.94 ± 26.33 a | 298.89 ± 26.64 a | 358.13 ± 49.87 ab | 332.32 ± 51.55 ab | 399.34 ± 73.16 b | 315.05 ± 9.37 a | 343.85 ± 49.73 ab | |
| UFA | PSC | 32.78 ± 3.69 ab | 57.10 ± 9.23 cde | 45.97 ± 6.64 bcd | 32.97 ± 25.67 ab | 38.95 ± 5.05 abc | 61.89 ± 13.68 de | 35.17 ± 11.94 ab | 61.79 ± 8.50 de | 68.61 ± 14.66 e | 32.48 ± 3.58 ab | 21.35 ± 4.22 a |
| PSK | 306.13 ± 11.35 a | 348.33 ± 21.89 ab | 320.48 ± 21.83 ab | 296.56 ± 38.59 a | 295.19 ± 25.93 a | 284.71 ± 25.59 a | 339.85 ± 47.91 ab | 315.92 ± 49.42 ab | 381.27 ± 69.99 b | 300.30 ± 8.80 a | 326.84 ± 47.43 ab | |
| PUFA | PSC | 23.26 ± 2.61 ab | 37.76 ± 5.47 cd | 30.46 ± 5.59 bcd | 22.21 ± 17.34 ab | 24.74 ± 4.29 abc | 40.25 ± 8.38 d | 24.52 ± 7.89 abc | 40.37 ± 5.16 d | 42.21 ± 9.23 d | 18.66 ± 2.19 ab | 15.15 ± 2.64 a |
| PSK | 193.57 ± 6.60 abc | 218.65 ± 13.15 bc | 197.62 ± 10.90 abc | 183.32 ± 23.34 abc | 178.72 ± 17.15 abc | 173.46 ± 14.99 ab | 210.10 ± 29.88 abc | 189.28 ± 29.86 abc | 223.64 ± 42.54 c | 171.00 ± 2.78 a | 204.86 ± 31.29 abc | |
| n-6/n-3 | PSC | 0.25 | 0.36 | 0.28 | 0.35 | 0.22 | 0.49 | 0.32 | 0.27 | 0.29 | 0.26 | 0.22 |
| PSK | 0.60 | 0.62 | 0.56 | 0.60 | 0.48 | 0.74 | 0.58 | 0.52 | 0.55 | 0.47 | 0.56 | |
| No. | Peak | Retention Time (min) | λmax (nm) | Molecular Formula | [M + H]+ (m/z) | [M−H]− (m/z) | [M + HCOO]− (m/z) | [M + Na]+ (m/z) | Identification | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3-k | 22.4 | 258 | C23H27O12 | 497 | 495 | 519 | Oxypaeoniflora | [26] | |
| 2 | 6-k | 25.8 | 238, 320 | C29H37O16 | 643 | 641 | 687 | 665 | 6′-O-β-Glucopyranosylalbiflorin | [26] |
| 3 | 7-k | 27.9 | 238, 275 | C29H37O16 | 643 | 687 | 611 | 665 | β-Gentiobiosylpaeoniflorin | [26] |
| 4 | 8-k | 27.8 | 238, 275 | C23H27O11 | 481 | 479 | 525 | 503 | Albiflorin | [26] |
| 5 | 9-k | 30.5 | 238, 275 | C23H27O11 | 481 | 479 | 525 | 503 | Paeoniflorin | [26] |
| 6 | 13-k, 1-c | 46.0 | 243, 281 | C42H33O9 | 681 | 679 | 725 | SuffruticosolA | [27] | |
| 7 | 14-k, 2-c | 49.5 | 243, 281 | C42H33O9 | 681 | 679 | 725 | SuffruticosolB | [27] | |
| 8 | 15-k, 4-c | 54.2 | 246, 325 | C28H22O6 | 455 | 453 | 477 | trans-ε-Viniferin | [28] | |
| 9 | 16-k, 5-c | 56.4 | 245, 327 | C42H33O9 | 681 | 679 | 725 | Suffruticosol C | [27] |
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Peak 1 | 72.28 ± 0.14 h | 36.19 ± 1.32 a | 46.02 ± 0.33 c | 46.70 ± 0.64 c | 44.05 ± 0.30 b | 61.33 ± 0.21 f | 55.61 ± 0.99 e | 50.18 ± 0.96 d | 75.14 ± 1.42 i | 43.02 ± 0.34 b | 70.58 ± 1.13 g |
| Peak 2 | 222.25 ± 0.35 i | 105.46 ± 0.37 a | 140.61 ± 1.29 d | 134.02 ± 0.80 c | 121.95 ± 0.22 b | 185.26 ± 1.52 f | 160.10 ± 1.17 e | 159.55 ± 1.13 e | 197.28 ± 2.29 g | 106.74 ± 0.74 a | 204.05 ± 1.24 h |
| Peak 3 | 21.78 ± 0.35 f | 11.24 ± 0.04 a | 14.09 ± 0.24 cd | 13.83 ± 0.07 cd | 13.39 ± 0.08 bc | 17.04 ± 0.40 e | 16.57 ± 0.43 e | 14.67 ± 0.84 d | 22.69 ± 1.18 g | 12.73 ± 0.29 b | 21.50 ± 0.57 f |
| Peak 4 | 81.86 ± 1.78 a | 164.83 ± 0.55 d | 254.93 ± 1.37 h | 159.80 ± 0.51 c | 171.93 ± 1.50 e | 151.96 ± 1.08 b | 212.17 ± 0.93 g | 174.17 ± 0.98 f | 318.39 ± 1.66 i | 383.00 ± 1.40 j | 159.57 ± 1.26 c |
| Peak 5 | 326.79 ± 4.88 d | 344.19 ± 0.67 e | 346.04 ± 0.78 e | 352.59 ± 2.05 f | 240.44 ± 0.56 a | 312.34 ± 1.46 c | 383.56 ± 1.59 h | 377.75 ± 0.93 g | 379.11 ± 1.05 g | 298.32 ± 0.70 b | 391.66 ± 0.52 i |
| Peak 6 | 16.79 ± 0.71 b | 17.95 ± 0.45 b | 16.78 ± 0.35 b | 16.67 ± 0.41 b | 16.49 ± 0.12 b | 11.76 ± 0.31 a | 16.67 ± 0.62 b | 17.65 ± 0.87 b | 16.98 ± 0.56 b | 21.49 ± 2.05 c | 20.79 ± 1.39 c |
| Peak 7 | 62.06 ± 0.87 e | 24.98 ± 0.64 a | 57.52 ± 4.00 d | 24.97 ± 0.11 a | 25.55 ± 0.54 a | 31.60 ± 1.08 c | 24.33 ± 0.58 a | 25.00 ± 0.52 a | 23.82 ± 0.72 a | 28.54 ± 0.83 b | 25.22 ± 0.17 a |
| Peak 8 | 332.11 ± 1.40 b | 331.53 ± 0.28 b | 328.87 ± 0.87 b | 368.61 ± 1.31 d | 368.50 ± 0.93 d | 369.62 ± 1.09 d | 317.94 ± 1.40 a | 357.46 ± 1.14 c | 386.72 ± 6.74 e | 385.49 ± 2.10 e | 444.96 ± 1.61 f |
| Code | P1 | P2 | P3 | P4 | P5 | P6 | P7 | P8 | P9 | P10 | P11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TP | PSC | 164.08 ± 9.58 f | 144.92 ± 9.66 cde | 147.85 ± 3.72 def | 133.35 ± 3.16 bcd | 140.63 ± 8.54 bcde | 143.32 ± 5.19 bcde | 137.07 ± 6.21 bcde | 126.23 ± 7.71 ab | 153.33 ± 23.43 ef | 114.57 ± 1.11 a | 129.01 ± 6.78 abc |
| PSK | 3.10 ± 0.28 bc | 2.68 ± 0.10 ab | 2.91 ± 0.27 bc | 3.14 ± 0.24 bc | 2.35 ± 0.27 a | 3.70 ± 0.61 d | 3.02 ± 0.12 bc | 3.26 ± 0.17 cd | 3.04 ± 0.28 bc | 3.35 ± 0.11 cd | 2.93 ± 0.22 bc | |
| DPPH assay | PSC | 32.38 ± 2.51 bc | 29.25 ± 0.78 ab | 31.59 ± 0.66 abc | 29.12 ± 1.76 ab | 31.91 ± 2.94 bc | 30.95 ± 1.76 abc | 29.92 ± 1.10 ab | 30.69 ± 1.61 abc | 34.57 ± 3.63 c | 30.07 ± 1.58 ab | 27.82 ± 2.15 a |
| PSK | 0.40 ± 0.03 b | 0.36 ± 0.01 a | 0.46 ± 0.01 cd | 0.43 ± 0.05 bc | 0.40 ± 0.02 b | 0.40 ± 0.01 b | 0.41 ± 0.01 b | 0.48 ± 0.02 d | 0.46 ± 0.02 cd | 0.54 ± 0.01 e | 0.45 ± 0.01 cd | |
| FRAP assay | PSC | 8.38 ± 0.78 bd | 7.57 ± 0.50 abc | 8.95 ± 0.18 d | 7.21 ± 0.46 a | 7.69 ± 0.55 abc | 7.59 ± 0.11 abc | 7.67 ± 0.18 abc | 7.44 ± 0.16 ab | 8.18 ± 0.84 bcd | 7.45 ± 0.35 ab | 7.27 ± 0.37 ab |
| PSK | 0.32 ± 0.01 a | 0.35 ± 0.02 ab | 0.38 ± 0.02 bcde | 0.42 ± 0.02 def | 0.34 ± 0.02 ab | 0.36 ± 0.00 abc | 0.37 ± 0.01 abcd | 0.44 ± 0.05 f | 0.43 ± 0.05 ef | 0.53 ± 0.01 g | 0.40 ± 0.02 cdef | |
| ABTS assay | PSC | 32.82 ± 2.13 cd | 28.98 ± 0.97 bc | 33.00 ± 2.97 d | 26.74 ± 2.38 ab | 27.58 ± 2.16 ab | 29.00 ± 1.46 bc | 27.89 ± 0.55 ab | 25.95 ± 1.27 ab | 32.11 ± 3.54 cd | 24.18 ± 2.13 a | 25.56 ± 1.67 ab |
| PSK | 1.06 ± 0.09 de | 0.88 ± 0.04 ab | 1.07 ± 0.03 de | 1.08 ± 0.09 de | 0.84 ± 0.05 a | 1.05 ± 0.04 cde | 1.04 ± 0.02 cde | 1.06 ± 0.07 de | 1.02 ± 0.05 cd | 1.13 ± 0.00 e | 0.95 ± 0.03 bc |
| Code | (β- + γ-) Tocopherols (μg g−1) | δ-Tocopherol (μg g−1) | Total Tocopherols (μg g−1) |
|---|---|---|---|
| P1 | 268.16 ± 2.33 h | 8.54 ± 0.22 f | 276.70 ± 2.56 g |
| P2 | 255.95 ± 1.95 g | 6.09 ± 0.09 cd | 262.04 ± 2.02 f |
| P3 | 248.38 ± 7.94 g | 4.83 ± 0.22 a | 253.21 ± 8.17 f |
| P4 | 230.21 ± 4.21 f | 5.65 ± 0.14 abc | 235.86 ± 4.31 e |
| P5 | 245.40 ± 5.09 g | 5.02 ± 0.55 ab | 250.42 ± 5.17 f |
| P6 | 160.88 ± 5.77 a | 6.95 ± 0.05 de | 167.83 ± 5.83 a |
| P7 | 173.43 ± 7.83 c | 5.63 ± 0.12 abc | 179.06 ± 7.95 a |
| P8 | 164.57 ± 14.25 ab | 6.02 ± 0.49 bcd | 170.59 ± 14.74 a |
| P9 | 196.42 ± 2.97 d | 7.56 ± 1.62 e | 203.98 ± 4.22 b |
| P10 | 221.43 ± 6.05 ef | 5.06 ± 0.16 abc | 226.49 ± 6.21 de |
| P11 | 215.23 ± 1.23 e | 5.19 ± 0.07 abc | 220.42 ± 1.30 d |
| Range | 160.88–268.16 | 4.83–8.54 | 167.83–276.70 |
| Average | 216.37 ± 37.05 | 6.05 ± 1.23 | 222.42 ± 37.08 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, L.-P.; Men, S.-Q.; Liu, Z.-A.; Tong, N.-N.; Imran, M.; Shu, Q.-Y. Fatty Acid Composition, Phytochemistry, Antioxidant Activity on Seed Coat and Kernel of Paeonia ostii from Main Geographic Production Areas. Foods 2020, 9, 30. https://doi.org/10.3390/foods9010030
Peng L-P, Men S-Q, Liu Z-A, Tong N-N, Imran M, Shu Q-Y. Fatty Acid Composition, Phytochemistry, Antioxidant Activity on Seed Coat and Kernel of Paeonia ostii from Main Geographic Production Areas. Foods. 2020; 9(1):30. https://doi.org/10.3390/foods9010030
Chicago/Turabian StylePeng, Li-Ping, Si-Qi Men, Zheng-An Liu, Ning-Ning Tong, Muhammad Imran, and Qing-Yan Shu. 2020. "Fatty Acid Composition, Phytochemistry, Antioxidant Activity on Seed Coat and Kernel of Paeonia ostii from Main Geographic Production Areas" Foods 9, no. 1: 30. https://doi.org/10.3390/foods9010030
APA StylePeng, L.-P., Men, S.-Q., Liu, Z.-A., Tong, N.-N., Imran, M., & Shu, Q.-Y. (2020). Fatty Acid Composition, Phytochemistry, Antioxidant Activity on Seed Coat and Kernel of Paeonia ostii from Main Geographic Production Areas. Foods, 9(1), 30. https://doi.org/10.3390/foods9010030





