Chemical Compositional Changes in Over-Oxidized Fish Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Oils
2.2. Oxidation Conditions and Incubations
2.3. Primary Indicators of Oxidative Quality
2.4. Moisture
2.5. Induction Time
2.6. EPA and DHA
2.7. Fatty Acid Classes
2.8. Tocopherols
2.9. Volatiles
2.10. Isoprostanoids
2.11. Oxysterols
2.12. Data Analysis
3. Results
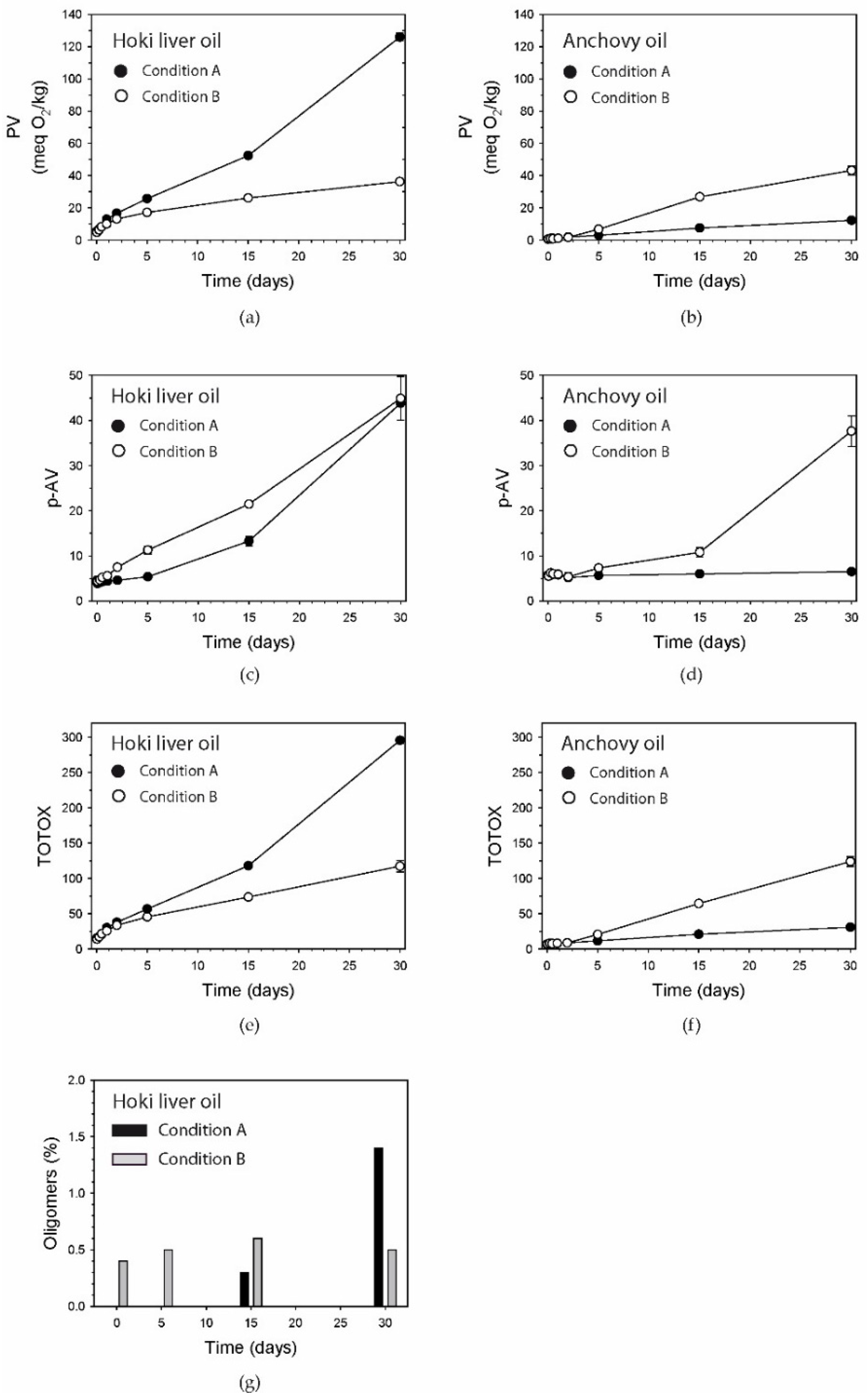
3.1. Peroxide Value
3.2. p-Anisidine Value
3.3. TOTOX Number
3.4. Oligomers
3.5. Acid Value
3.6. Moisture Content
3.7. Induction Time
3.8. Tocopherols
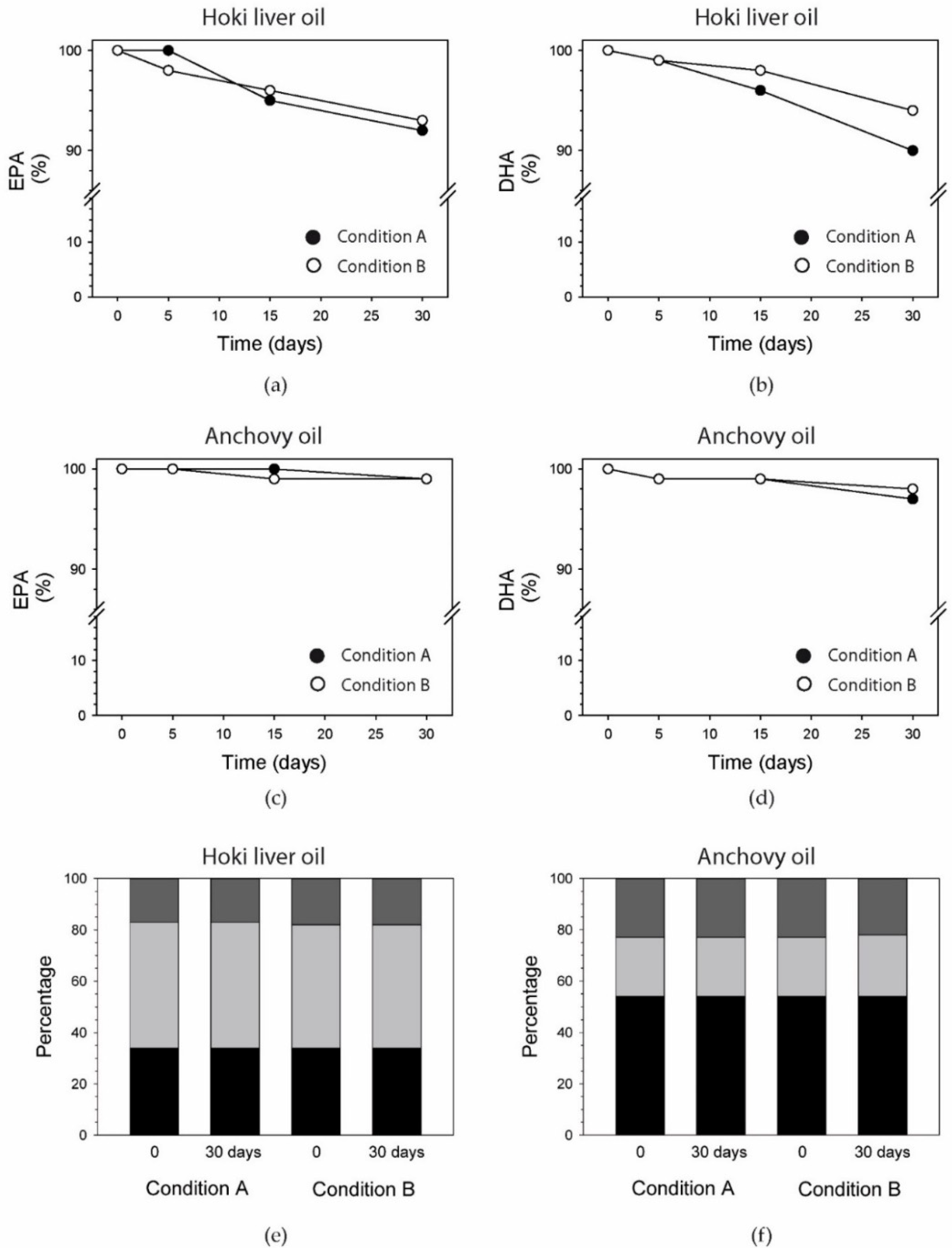
3.9. EPA and DHA
3.10. Fatty Acids
3.11. Volatiles
3.12. Isoprostanoids and Isofuranoids
3.13. Oxysterols
3.14. Correlations
4. Discussion
4.1. Hoki Liver Oil Over-Oxidation
4.2. Contrasting Distinct Oxidation Conditions
4.3. Comparison of Oxidation in Different Fish Oils
4.4. Specific Groups of Secondary Oxidation Products and Biological Activity
4.4.1. Volatiles
4.4.2. Isoprostanoids
4.4.3. Oxysterols
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- AbuMweis, S.; Jew, S.; Tayyem, R.; Agraib, L. Eicosapentaenoic acid and docosahexaenoic acid containing supplements modulate risk factors for cardiovascular disease: A meta-analysis of randomised placebo-control human clinical trials. J. Hum. Nutr. Diet. 2018, 31, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.E.; Van Elswyk, M.; Alexander, D.D. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: A meta-analysis of randomized controlled trials. Am. J. Hypertens. 2014, 27, 885–896. [Google Scholar] [CrossRef]
- Ellulu, M.S.; Khaza’ai, H.; Abed, Y.; Rahmat, A.; Ismail, P.; Ranneh, Y. Role of fish oil in human health and possible mechanism to reduce the inflammation. Inflammopharmacol. 2015, 23, 79–89. [Google Scholar] [CrossRef]
- Harris, W.S. Fish oil supplementation: Evidence for health benefits. Cleve. Clin. J. Med. 2004, 71, 208–221. [Google Scholar] [CrossRef]
- Oomen, C.M.; Feskens, E.J.M.; Räsänen, L.; Fidanza, F.; Nissinen, A.M.; Menotti, A.; Kok, F.J.; Kromhout, D. Fish consumption and coronary heart disease mortality in Finland, Italy, and The Netherlands. Am. J. Epidemiol. 2000, 151, 999–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruxton, C.H.S.; Reed, S.C.; Simpson, M.J.A.; Millington, K.J. The health benefits of omega-3 polyunsaturated fatty acids: A review of the evidence. J. Hum. Nutr. Diet. 2004, 17, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Marine omega-3 (n-3) fatty acids for cardiovascular health: An update for 2020. Int. J. Mol. Sci. 2020, 21, 1–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmonds, L.A.; Sullivan, T.R.; Skubisz, M.; Middleton, P.F.; Best, K.P.; Yelland, L.N.; Quinlivan, J.; Zhou, S.J.; Liu, G.; McPhee, A.J.; et al. Omega-3 fatty acid supplementation in pregnancy-baseline omega-3 status and early preterm birth: Exploratory analysis of a randomised controlled trial. BJOG 2020, 127, 975–981. [Google Scholar] [CrossRef]
- Hashim, A.F.; Hamed, S.F.; Abdel Hamid, H.A.; Abd-Elsalam, K.A.; Golonka, I.; Musial, W.; El-Sherbiny, I.M. Antioxidant and antibacterial activities of omega-3 rich oils/curcumin nanoemulsions loaded in chitosan and alginate-based microbeads. Int. J. Biol. Macromol. 2019, 140, 682–696. [Google Scholar] [CrossRef]
- Aguilera, C.M.; Mesa, M.D.; Ramirez-Tortosa, M.C.; Quiles, J.L.; Gil, A. Virgin olive and fish oils enhance the hepatic antioxidant defence system in atherosclerotic rabbits. Clin. Nutr. 2003, 22, 379–384. [Google Scholar] [CrossRef]
- Song, J.; Inoue, Y.; Miyazawa, T. Oxidative stability of docosahexaenoic acid-containing oils in the form of phospholipids, triacylglycerols, and ethyl esters. Biosci. Biotechnol. Biochem. 1997, 61, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K. Prevention of fish oil oxidation. J. Oleo Sci. 2019, 68, 1–11. [Google Scholar]
- Schaich, K.M. Lipid oxidation: Theoretical aspects. In Bailey’s Industrial Fat and Oil Products, 6th ed.; John Wiley: New York, NY, USA, 2005; Volume 1, pp. 269–355. [Google Scholar]
- Aidos, I.; Jacobsen, C.; Jensen, B.; Luten, J.B.; Padt, A.; van der Boom, R.M. Volatile oxidation products formed in crude herring oil under accelerated oxidative conditions. Eur. J. Lipid Sci. Technol. 2002, 104, 808–818. [Google Scholar] [CrossRef]
- Aidos, I.; Lourenço, S.; Van der Padt, A.; Luten, J.B.; Boom, R.M. Stability of crude herring oil produced from fresh byproducts: Influence of temperature during storage. J. Food Sci. 2002, 67, 3314–3320. [Google Scholar] [CrossRef]
- Sang, W.; Jin, Z.T. Lipid oxidation of fish liver oil as affected by light, antioxidants and temperature. J. Food Process. Preserv. 2003, 28, 1–10. [Google Scholar] [CrossRef]
- Drusch, S.; Groß, N.; Schwarz, K. Efficient stabilization of bulk fish oil rich in long-chain polyunsaturated fatty acids. Eur. J. Lipid Sci. Technol. 2008, 110, 351–359. [Google Scholar] [CrossRef]
- GOED. Goed Voluntary Monograph v7.1. Available online: https://goedomega3.com/goed-monograph (accessed on 6 August 2019).
- Bannenberg, G.; Mallon, C.; Edwards, H.; Yeadon, D.; Yan, K.; Johnson, H.; Ismail, A. Omega-3 long-chain polyunsaturated fatty acid content and oxidation state of fish oil supplements in New Zealand. Sci. Rep. 2017, 7, 1488. [Google Scholar] [CrossRef] [Green Version]
- De Boer, A.A.; Ismail, A.; Marshall, K.; Bannenberg, G.; Yan, K.L.; Rowe, W.J. Examination of marine and vegetable oil oxidation data from a multi-year, third-party database. Food Chem. 2018, 254, 249–255. [Google Scholar] [CrossRef]
- Bannenberg, G.; Rice, H.B.; Bernasconi, A.; Ferrari, A.; Mallon, C.; Navarrete, L.; Hughes, R.; Igarashi, J.; Persons, K.; Latynski, L.; et al. Ingredient label claim compliance and oxidative quality of EPA/DHA omega-3 retail products in the U.S. J. Food Comp. Anal. 2020, 88, 103435. [Google Scholar] [CrossRef]
- Heller, M.; Gemming, L.; Tung, C.; Grant, R. Oxidation of fish oil supplements in Australia. Int. J. Food Sci. Nutr. 2019, 70, 540–550. [Google Scholar] [CrossRef]
- Sprague, M.; Cooper, S.; Tocher, D.R.; Betancor, M.B. Encapsulated fish oil products available in the UK meet regulatory guidelines with respect to EPA + DHA contents and oxidative status. Eur. J. Lipid Sci. Technol. 2018, 120, 1800105. [Google Scholar] [CrossRef] [Green Version]
- Killeen, D.P.; Card, A.; Gordon, K.C.; Perry, N.B. First use of handheld raman spectroscopy to analyze omega-3 fatty acids in intact fish oil capsules. Appl. Spectrosc. 2020, 74, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Damerau, A.; Ahonen, E.; Kortesniemi, M.; Puganen, A.; Tarvainen, M.; Linderborg, K.M. Evaluation of the composition and oxidative status of omega-3 fatty acid supplements on the finnish market using NMR and SPME-GC-MS in comparison with conventional methods. Food Chem. 2020, 330, 127194. [Google Scholar] [CrossRef] [PubMed]
- Ottestad, I.; Retterstol, K.; Myhrstad, M.C.; Andersen, L.F.; Vogt, G.; Nilsson, A.; Borge, G.I.; Nordvi, B.; Bronner, K.W.; Ulven, S.M.; et al. Intake of oxidised fish oil does not affect circulating levels of oxidised LDL or inflammatory markers in healthy subjects. Nutr. Metab. Cardiovasc. Dis. 2013, 23, e3–e4. [Google Scholar] [CrossRef]
- Ottestad, I.; Vogt, G.; Retterstol, K.; Myhrstad, M.C.; Haugen, J.E.; Nilsson, A.; Ravn-Haren, G.; Nordvi, B.; Bronner, K.W.; Andersen, L.F.; et al. Oxidised fish oil does not influence established markers of oxidative stress in healthy human subjects: A randomised controlled trial. Br. J. Nutr. 2012, 108, 315–326. [Google Scholar] [CrossRef] [Green Version]
- Albert, B.B.; Vickers, M.H.; Gray, C.; Reynolds, C.M.; Segovia, S.A.; Derraik, J.G.B.; Lewandowski, P.A.; Garg, M.L.; Cameron-Smith, D.; Hofman, P.L.; et al. Oxidized fish oil in rat pregnancy causes high newborn mortality and increases maternal insulin resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, 497–504. [Google Scholar] [CrossRef] [Green Version]
- Mason, R.P.; Sherratt, S.C.R. Omega-3 fatty acid fish oil dietary supplements contain saturated fats and oxidized lipids that may interfere with their intended biological benefits. Biochem. Biophys. Res. Commun. 2017, 483, 425–429. [Google Scholar] [CrossRef]
- Morton, J. Study Reveals Effects of “Off” Fish Oil. New Zealand Herald. 22 July 2016. Available online: https://www.nzherald.co.nz/nz/study-reveals-effects-of-off-fish-oil/KVCV45M5YGLD5R7WJHIKPBSUGU/ (accessed on 19 October 2020).
- GOED. Annual Report 2019. Available online: https://goedomega3.com/storage/app/media/annual%20reports/GOED%20Annual%20Report%20-%202019.pdf (accessed on 27 August 2020).
- Kato, S.; Shimizu, N.; Hanzawa, Y.; Otoki, Y.; Ito, J.; Kimura, F.; Takekoshi, S.; Sakaino, M.; Sano, T.; Eitsuka, T.; et al. Determination of triacylglycerol oxidation mechanisms in canola oil using liquid chromatography-tandem mass spectrometry. NPJ Sci. Food 2018, 2, 1. [Google Scholar] [CrossRef]
- Shin, E.-C.; Jang, H.-J.; Lee, H.-I.; An, H.-J.; Lee, Y.-B. Optimization of headspace analysis of volatile compounds from oxidized fish oil. Prev. Nutr. Food Sci. 2003, 8, 315–320. [Google Scholar] [CrossRef]
- Lee, H.; Kizito, S.A.; Weese, S.J.; Craig-Schmidt, M.C.; Lee, Y.; Wei, C.-I.; An, H. Analysis of headspace volatile and oxidized volatile compounds in DHA-enriched fish oil on accelerated oxidative storage. J. Food Sci. 2003, 68, 2169–2177. [Google Scholar] [CrossRef]
- Comporti, M. Lipid peroxidation and biogenic aldehydes: From the identification of 4-hydroxynonenal to further achievements in biopathology. Free Radic. Res. 1998, 28, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Jamil, J.; Bankhele, P.; Salvi, A.; Mannix, J.E.; Oger, C.; Guy, A.; Galano, J.M.; Durand, T.; Njie-Mbye, Y.F.; Ohia, S.E.; et al. Role of the non-enzymatic metabolite of eicosapentaenoic acid, 5-epi-5-F3t-isoprostane in the regulation of [(3)H]D-aspartate release in isolated bovine retina. Neurochem. Res. 2014, 39, 2360–2369. [Google Scholar] [CrossRef] [PubMed]
- Joumard-Cubizolles, L.; Lee, J.C.-Y.; Vigor, C.; Leung, H.H.; Bertrand-Michel, J.; Galano, J.-M.; Mazur, A.; Durand, T.; Gladine, C. Insight into the contribution of isoprostanoids to the health effects of omega-3 PUFAs. Prostagladins Other Lipid Mediat. 2017, 133, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Alkazemi, D.; Jackson, R.L., 2nd; Chan, H.M.; Kubow, S. Increased F3-isoprostanes in the Canadian Inuit population could be cardioprotective by limiting F2-isoprostane production. J. Clin. Endocrinol. Metab. 2016, 101, 3264–3271. [Google Scholar] [CrossRef] [Green Version]
- Miller, E.; Morel, A.; Saso, L.; Saluk, J. Isoprostanes and neuroprostanes as biomarkers of oxidative stress in neurodegenerative diseases. Oxid. Med. Cell Longev. 2014, 2014, 572491. [Google Scholar] [CrossRef]
- Fessel, J.P.; Porter, N.A.; Moore, K.P.; Sheller, J.R.; Roberts, L.J. Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. Proc. Natl. Acad. Sci. USA 2002, 99, 16713–16718. [Google Scholar] [CrossRef] [Green Version]
- Cuyamendous, C.; Leung, K.S.; Durand, T.; Lee, J.C.; Oger, C.; Galano, J.M. Synthesis and discovery of phytofurans: Metabolites of alpha-linolenic acid peroxidation. Chem. Commun. 2015, 51, 15696–15699. [Google Scholar] [CrossRef]
- Song, W.L.; Lawson, J.A.; Reilly, D.; Rokach, J.; Chang, C.T.; Giasson, B.; FitzGerald, G.A. Neurofurans, novel indices of oxidant stress derived from docosahexaenoic acid. J. Biol. Chem. 2008, 283, 6–16. [Google Scholar] [CrossRef] [Green Version]
- Brzeska, M.; Szymczyk, K.; Szterk, A. Current knowledge about oxysterols: A review. J. Food Sci. 2016, 81, R2299–R2308. [Google Scholar] [CrossRef]
- Otaegui-Arrazola, A.; Menendez-Carreno, M.; Ansorena, D.; Astiasaran, I. Oxysterols: A world to explore. Food Chem. Toxicol. 2010, 48, 3289–3303. [Google Scholar] [CrossRef]
- Sottero, B.; Gamba, P.; Gargiulo, S.; Leonarduzzi, G.; Poli, G. Cholesterol oxidation products and disease: An emerging topic of interest in medicinal chemistry. Curr. Med. Chem. 2009, 16, 685–705. [Google Scholar] [CrossRef]
- Griffiths, W.J.; Wang, Y. Oxysterols as lipid mediators: Their biosynthetic genes, enzymes and metabolites. Prostaglandins Other Lipid Mediat. 2020, 147, 106381. [Google Scholar] [CrossRef] [PubMed]
- Muccioli, G.G.; Lizard, G.; Iuliano, L. Current trends in oxysterols & related sterols. Biochimie 2018, 153, 1–2. [Google Scholar] [PubMed]
- AOCS. Official Methods and Recommended Practices of the American Oil Chemists’ Society; American Oil Chemists’ Society: Champaign, IL, USA, 1998. [Google Scholar]
- McCoy, M.; McGinley, M.; Klein, M.; Rivera, B. Oligomer Composition of Over-the-Counter Omega-3 Fish Oil Capsules by Gel Permeation Chromatography (Ph. Eur. Monograph 1912). Available online: https://www.nzherald.co.nz/nz/study-reveals-effects-of-off-fish-oil/KVCV45M5YGLD5R7WJHIKPBSUGU/ (accessed on 19 October 2020).
- Council of Europe. European Pharmacopoeia, 9th ed.; Omega-3 Acid Triglycerides. Monograph 07/2012:1352; Council of Europe: Strasbourg, France, 2016; pp. 3209–3211. [Google Scholar]
- Metrohm. Oxidation Stability of Oils and Fats—Rancimat Method. Available online: https://www.metrohm.com/en/applications/AB-204 (accessed on 26 August 2020).
- USP. Fats and Fixed Oils <401>; United States Pharmacopeial Convention: Rockville, MD, USA, 2017; Volume USP 40. [Google Scholar]
- Gimeno, E.; Castellote, A.I.; Lamuela-Raventós, R.M.; de laTorre, M.C.; López-Sabater, M.C. Rapid determination of vitamin E in vegetable oils by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2000, 881, 251–254. [Google Scholar] [CrossRef]
- Shen, Z.; Augustin, M.A.; Sanguansri, L.; Cheng, L.J. Oxidative stability of microencapsulated fish oil powders stabilized by blends of chitosan, modified starch, and glucose. J. Agric. Food Chem. 2010, 58, 4487–4493. [Google Scholar] [CrossRef] [PubMed]
- Polari, J.J.; Garci-Aguirre, D.; Olmo-Garcia, L.; Carrasco-Pancorbo, A.; Wang, S.C. Impact of industrial hammer mill rotor speed on extraction efficiency and quality of extra virgin olive oil. Food Chem. 2018, 242, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Huang, S.H.; Jenner, A.M.; Halliwell, B. Measurement of F2-isoprostanes, hydroxyeicosatetraenoic products, and oxysterols from a single plasma sample. Free Radic. Biol. Med. 2008, 44, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Yonny, M.E.; Torresi, A.R.; Cuyamendous, C.; Réversat, G.; Oger, C.; Galano, J.-M.; Durand, T.; Vigor, C.; Nazareno, M.A. Thermal stress in melon plants: Phytoprostanes and phytofurans as oxidative stress biomarkers and the effect of antioxidant supplementation. J. Agric. Food Chem. 2016, 64, 8296–8304. [Google Scholar] [CrossRef]
- Mutemberezi, V.; Masquelier, J.; Guillemot-Legris, O.; Muccioli, G.G. Development and validation of an HPLC-MS method for the simultaneous quantification of key oxysterols, endocannabinoids, and ceramides: Variations in metabolic syndrome. Anal. Bioanal. Chem. 2016, 408, 733–745. [Google Scholar] [CrossRef]
- Tengku-Rozaina, T.M.; Birch, E.J. Enrichment of omega-3 fatty acids of refined hoki oil. J. Am. Oil Chem. Soc. 2013, 90, 1111–1119. [Google Scholar] [CrossRef]
- Kaya, Y.; Turan, H. Fatty acids composition of anchovy (Engraulis enrasicolus l. 1758) oil produced in Sinop-Turkey. Fish. Sci. 2008, 2, 693–697. [Google Scholar]
- Schaich, K.M. Challenges in elucidating lipid oxidation mechanisms: When, where, and how do products arise? In Lipid Oxidation: Challenges in Food Systems; Nienaber, U., Logan, A., Pan, X., Eds.; American Oil Chemists Society: Champaign, IL, USA, 2013; pp. 1–52. [Google Scholar]
- Andersson, K.; Lingnert, H. Kinetic studies of oxygen dependence during initial lipid oxidation in rapeseed oil. J. Food Sci. 1999, 64, 262–266. [Google Scholar] [CrossRef]
- Kolev, N.I. Solubilty of O2, N2, H2 and CO2 in water. In Multiphase Flow Dynamics; Springer: Berlin/Heidelberg, Germany, 2012; Volume 4, pp. 209–239. [Google Scholar]
- Ke, P.J.; Ackman, R.G. Bunsen coefficient for oxygen in marine oils at various temperatures determined by an exponential dilution method with a polarographic oxygen electrode. J. Am. Oil Chem. Soc. 1973, 50, 429–435. [Google Scholar] [CrossRef]
- European Pharmacopoeia Organization. Monograph—Fish Oil, Rich in Omega-3 Acids; Council of Europe: Strasbourg, France, 2012. [Google Scholar]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Food Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Miyashita, K.; Takagi, T. Study on the oxidative rate and prooxidant activity of free fatty acids. J. Am. Oil Chem. Soc. 1986, 63, 1380–1384. [Google Scholar] [CrossRef]
- SeaDragon (Nelson, New Zealand). Personal Communication, 2020.
- Frankel, E.N. Antioxidants in lipid foods and their impact on food quality. Food Chem. 1996, 57, 51–55. [Google Scholar] [CrossRef]
- Kulås, E.; Ackman, R.G. Different tocopherols and the relationship between two methods for determination of primary oxidation products in fish oil. J. Agric. Food Chem. 2001, 49, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, E.; Brandon, E.F.; Blokland, M.H.; Guillen, M.D. Fate in digestion in vitro of several food components, including some toxic compounds coming from omega-3 and omega-6 lipids. Food Chem. Toxicol. 2011, 49, 115–124. [Google Scholar] [CrossRef]
- Eckl, P.M.; Bresgen, N. Genotoxicity of lipid oxidation compounds. Free Radic. Biol. Med. 2017, 111, 244–252. [Google Scholar] [CrossRef]
- Grootveld, M.; Atherton, M.D.; Sheerin, A.N.; Hawkes, J.; Blake, D.R.; Richens, T.E.; Silwood, C.J.; Lynch, E.; Claxson, A.W. In vivo absorption, metabolism, and urinary excretion of alpha,beta-unsaturated aldehydes in experimental animals. Relevance to the development of cardiovascular diseases by the dietary ingestion of thermally stressed polyunsaturate-rich culinary oils. J. Clin. Investig. 1998, 101, 1210–1218. [Google Scholar] [CrossRef] [Green Version]
- Tew, K.D.; Townsend, D.M. Glutathione-S-transferases as determinants of cell survival and death. Antioxid. Redox Signal. 2012, 17, 1728–1737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, T.; Bryant, B.P. Multiple cation channels mediate increases in intracellular calcium induced by the volatile irritant, trans-2-pentenal in rat trigeminal neurons. Cell. Mol. Neurobiol. 2010, 30, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, B.M.; Agerbo, P.; Jensen, B.; Børresen, T.; Hølmer, G. Inhibition of microsomal glucose 6-phosphatase by unsaturated aliphatic aldehydes and ketones. Chem. Biol. Interact. 1992, 81, 209–218. [Google Scholar] [CrossRef]
- Shibata, A.; Uemura, M.; Hosokawa, M.; Miyashita, K. Acrolein as a major volatile in the early stages of fish oil tag oxidation. J. Oleo Sci. 2018, 67, 515–524. [Google Scholar] [CrossRef] [Green Version]
- Nieva-Echevarría, B.; Goicoechea, E.; Guillén, M.D. Polyunsaturated lipids and vitamin A oxidation during cod liver oil in vitro gastrointestinal digestion. Antioxidant effect of added BHT. Food Chem. 2017, 232, 733–743. [Google Scholar] [CrossRef]
- Morrow, J.D.; Hill, K.E.; Burk, R.F.; Nammour, T.M.; Badr, K.F.; Roberts, L.J. A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc. Natl. Acad. Sci. USA 1990, 87, 9383–9387. [Google Scholar] [CrossRef] [Green Version]
- Nourooz-Zadeh, J.; Halliwell, B.; Anggård, E.E. Evidence for the formation of F3-isoprostanes during peroxidation of eicosapentaenoic acid. Biochem. Biophys. Res. Commun. 1997, 236, 467–472. [Google Scholar] [CrossRef]
- Jahn, U.; Galano, J.M.; Durand, T. Beyond prostaglandins–chemistry and biology of cyclic oxygenated metabolites formed by free-radical pathways from polyunsaturated fatty acids. Angew. Chem. Int. Ed. Engl. 2008, 47, 5894–5955. [Google Scholar] [CrossRef]
- Pavlickova, T.; Bultel-Ponce, V.; Guy, A.; Rocher, A.; Reversat, G.; Vigor, C.; Durand, T.; Galano, J.M.; Jahn, U.; Oger, C. First total syntheses of novel non-enzymatic polyunsaturated fatty acid metabolites and their identification in edible oils. Chem. A Eur. J. 2020, 26, 10090–10098. [Google Scholar] [CrossRef]
- Galano, J.-M.; Lee, Y.Y.; Oger, C.; Vigor, C.; Vercauteren, J.; Durand, T.; Giera, M.; Lee, J.C. Isoprostanes, neuroprostanes, and phytoprostanes: An overview of 25 years of research in chemistry and biology. Prog. Lipid Res. 2017, 68, 83–108. [Google Scholar] [CrossRef] [PubMed]
- Rogers, M.S.; Wang, C.C.; Lau, T.K.; Xiao, X.; Zhou, X.G.; Fok, T.F.; Chu, K.O.; Pang, C.P. Relationship between isoprostane concentrations, metabolic acidosis, and morbid neonatal outcome. Clin. Chem. 2005, 51, 1271–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chafer-Pericas, C.; Cernada, M.; Rahkonen, L.; Stefanovic, V.; Andersson, S.; Vento, M. Preliminary case control study to establish the correlation between novel peroxidation biomarkers in cord serum and the severity of hypoxic ischemic encephalopathy. Free Radic. Biol. Med. 2016, 97, 244–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.X.; O’Mara, P.W.; Poole, S.D.; Brown, N.; Ehinger, N.J.; Slaughter, J.C.; Paria, B.C.; Aschner, J.L.; Reese, J. Isoprostanes as physiological mediators of transition to newborn life: Novel mechanisms regulating patency of the term and preterm ductus arteriosus. Pediatr. Res. 2012, 72, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosviel, R.; Joumard-Cubizolles, L.; Chinetti-Gbaguidi, G.; Bayle, D.; Copin, C.; Hennuyer, N.; Duplan, I.; Staels, B.; Zanoni, G.; Porta, A.; et al. DHA-derived oxylipins, neuroprostanes and protectins, differentially and dose-dependently modulate the inflammatory response in human macrophages: Putative mechanisms through PPAR activation. Free Radic. Biol. Med. 2016, 103, 146–154. [Google Scholar] [CrossRef]
- Roy, J.; Oger, C.; Thireau, J.; Roussel, J.; Mercier-Touzet, O.; Faure, D.; Pinot, E.; Farah, C.; Taber, D.F.; Cristol, J.P.; et al. Nonenzymatic lipid mediators, neuroprostanes, exert the antiarrhythmic properties of docosahexaenoic acid. Free Radic. Biol. Med. 2015, 86, 269–278. [Google Scholar] [CrossRef] [Green Version]
- Lacampagne, A.; Jean-Yves, L.G.; Bultel-Ponce, V.; Galano, J.-M.; Guy, A.; Durand, T.; Oger, C.; Matecki, S.; Dridi, H.; Thireau, J.; et al. Methods and pharmaceutical compositions for the treatment of disorders or diseases associated with ryanodine receptor dysfunction. Patent WO2015197562A1, 30 December 2015. [Google Scholar]
- Lee, Y.Y.; Galano, J.M.; Leung, H.H.; Balas, L.; Oger, C.; Durand, T.; Lee, J.C. Nonenzymatic oxygenated metabolite of docosahexaenoic acid, 4(RS)-4-F4t-neuroprostane, acts as a bioactive lipid molecule in neuronal cells. FEBS Lett. 2020, 594, 1797–1808. [Google Scholar] [CrossRef]
- Tengku-Rozaina, T.M.; John Birch, E.J. Physicochemical characterisation and oxidative stability of refined hoki oil, unrefined hoki oil and unrefined tuna oil. Int. J. Food Sci. Technol. 2013, 48, 2331–2339. [Google Scholar] [CrossRef]
- Ismail, A.; (KD Pharma, Bexbach, Germany). Personal Communication, 2020.
- Valenzuela, A.; Sanhueza, J.; Nieto, S. Cholesterol oxidation: Health hazard and the role of antioxidants in prevention. Biol. Res. 2003, 36, 291–302. [Google Scholar] [CrossRef] [Green Version]
- Javitt, N.B. Oxysterols: Novel biologic roles for the 21st century. Steroids 2008, 73, 149–157. [Google Scholar] [CrossRef]
- Mutemberezi, V.; Guillemot-Legris, O.; Muccioli, G.G. Oxysterols: From cholesterol metabolites to key mediators. Prog. Lipid Res. 2016, 64, 152–169. [Google Scholar] [CrossRef]
- Carvalho, J.F.; Silva, M.M.; Moreira, J.N.; Simões, S.; E Melo, M.L.S. Selective cytotoxicity of oxysterols through structural modulation on rings A and B. Synthesis, in vitro evaluation, and sar. J. Med. Chem. 2011, 54, 6375–6393. [Google Scholar] [CrossRef] [PubMed]
- Steffen, Y.; Wiswedel, I.; Peter, D.; Schewe, T.; Sies, H. Cytotoxicity of myeloperoxidase/nitrite-oxidized low-density lipoprotein toward endothelial cells is due to a high 7beta-hydroxycholesterol to 7-ketocholesterol ratio. Free Radic. Biol. Med. 2006, 41, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wasowicz, E.; Kummerow, F.A. Failure of vitamin E to protect cultured human arterial smooth muscle cells against oxysterol-induced cytotoxicity. J. Am. Coll. Nutr. 1995, 14, 169–175. [Google Scholar] [CrossRef]
- Ringseis, R.; Eder, K. Dietary oxidized cholesterol increases expression and activity of antioxidative enzymes and reduces the concentration of glutathione in the liver of rats. Int. J. Vitam. Nutr. Res. 2004, 74, 86–92. [Google Scholar] [CrossRef]
- Basciano, H.; Miller, A.E.; Naples, M.; Baker, C.; Kohen, R.; Xu, E.; Su, Q.; Allister, E.M.; Wheeler, M.B.; Adeli, K. Metabolic effects of dietary cholesterol in an animal model of insulin resistance and hepatic steatosis. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E462–E473. [Google Scholar] [CrossRef] [PubMed]
- Khorrami, A.; Ghanbarzadeh, S.; Mahmoudi, J.; Nayebi, A.M.; Maleki-Dizaji, N.; Garjani, A. Investigation of the memory impairment in rats fed with oxidized-cholesterol-rich diet employing passive avoidance test. Drug Res. 2015, 65, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.C.; Sears, S.B.; Sadovsky, Y. The influence of ligand-activated LXR on primary human trophoblasts. Placenta 2014, 35, 919–924. [Google Scholar] [CrossRef] [Green Version]
- Aye, I.L.; Waddell, B.J.; Mark, P.J.; Keelan, J.A. Oxysterols inhibit differentiation and fusion of term primary trophoblasts by activating liver X receptors. Placenta 2011, 32, 183–191. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Rice, H.B.; Bannenberg, G.; Harwood, M.; Ismail, A. Determining the potential effects of oxidized fish oils in pregnant women requires a more systematic approach. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R263. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phung, A.S.; Bannenberg, G.; Vigor, C.; Reversat, G.; Oger, C.; Roumain, M.; Galano, J.-M.; Durand, T.; Muccioli, G.G.; Ismail, A.; et al. Chemical Compositional Changes in Over-Oxidized Fish Oils. Foods 2020, 9, 1501. https://doi.org/10.3390/foods9101501
Phung AS, Bannenberg G, Vigor C, Reversat G, Oger C, Roumain M, Galano J-M, Durand T, Muccioli GG, Ismail A, et al. Chemical Compositional Changes in Over-Oxidized Fish Oils. Foods. 2020; 9(10):1501. https://doi.org/10.3390/foods9101501
Chicago/Turabian StylePhung, Austin S., Gerard Bannenberg, Claire Vigor, Guillaume Reversat, Camille Oger, Martin Roumain, Jean-Marie Galano, Thierry Durand, Giulio G. Muccioli, Adam Ismail, and et al. 2020. "Chemical Compositional Changes in Over-Oxidized Fish Oils" Foods 9, no. 10: 1501. https://doi.org/10.3390/foods9101501
APA StylePhung, A. S., Bannenberg, G., Vigor, C., Reversat, G., Oger, C., Roumain, M., Galano, J.-M., Durand, T., Muccioli, G. G., Ismail, A., & Wang, S. C. (2020). Chemical Compositional Changes in Over-Oxidized Fish Oils. Foods, 9(10), 1501. https://doi.org/10.3390/foods9101501




