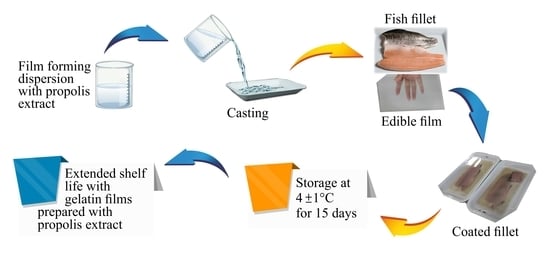
Potential of Propolis Extract as a Natural Antioxidant and Antimicrobial in Gelatin Films Applied to Rainbow Trout (Oncorhynchus mykiss) Fillets
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Propolis Extraction and Preparation of Gelatin Films
2.3. Total Phenolic Content and Antioxidant Activity
2.4. Physicochemical Analysis
2.5. Microbiological Analysis
2.6. Sensory Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic Content and Antioxidant Capacity
3.2. Physicochemical Properties
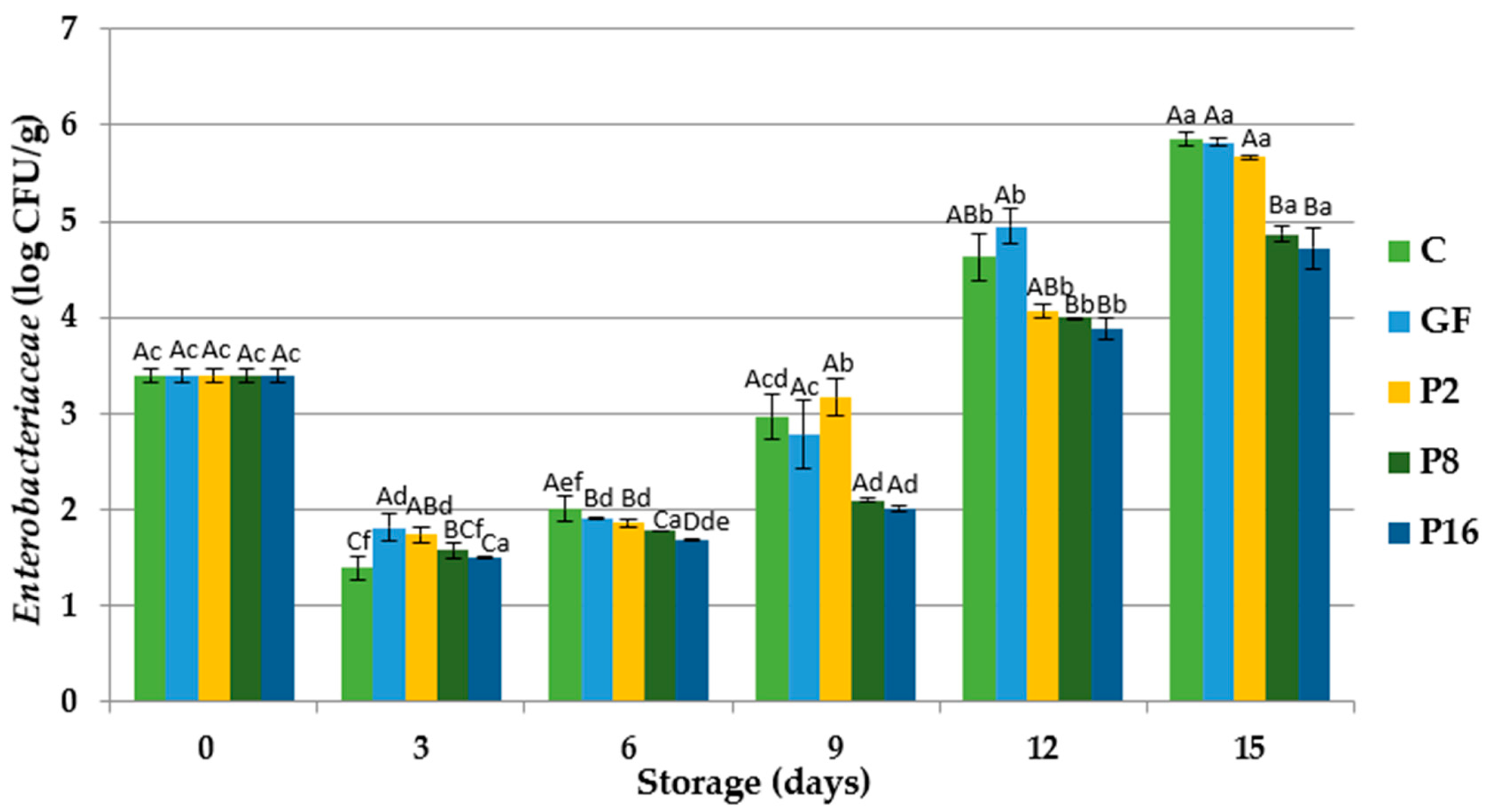
3.3. Changes in Microbiological Quality
3.4. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sampels, S. The effects of processing technologies and preparation on the final quality of fish products. Trends Food Sci. Technol. 2011, 44, 131–146. [Google Scholar] [CrossRef]
- Nisar, T.; Yang, X.; Alim, A.; Iqbal, M.; Wanga, Z.C.; Guo, Y. Physicochemical responses and microbiological changes of bream (Megalobrama ambycephala) to pectin based coatings enriched with clove essential oil during refrigeration. Int. J. Biol. Macromol. 2019, 124, 156–1166. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Ebadi, Z.; Khodanazary, A.; Hosseini, S.M.; Zanguee, N. The shelf life extension of refrigerated Nemipterus japonicus fillets by chitosan coating incorporated with propolis extract. Int. J. Biol. Macromol. 2019, 139, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Jin, T.Z.; Yadav, M.P.; Yang, R. Antimicrobial property and microstructure of micro-emulsion edible composite films against Listeria. Int. J. Food Microbiol. 2015, 208, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
- Yazgan, H.; Burgut, A.; Durmus, M.; Kosker, A.R. The impacts of water and ethanolic extracts of propolis on vacuum packaged sardine fillets inoculated with Morganella psychrotolerans during chilly storage. J. Food Saf. 2020, 40, e12767. [Google Scholar] [CrossRef]
- Duman, M.; Ozpolat, E. Effects of water extract of propolis on fresh shibuta (Barbus grypus) fillets during chilled storage. Food Chem. 2015, 189, 80–85. [Google Scholar] [CrossRef]
- Piedrahíta Márquez, D.G.; Fuenmayor, C.A.; Suarez Mahecha, H. Effect of chitosan-propolis edible coatings on stability of refrigerated cachama (Piaractus brachypomus) vacuum-packed fish fillets. Packag. Technol. Sci. 2018, 32, 143–153. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Sumpavapol, P.; Nirmal, N.P. Quality changes of sea bass slices wrapped with gelatin film incorporated with lemongrass essential oil. Int. J. Food Microbiol. 2012, 155, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Estaca, J.; Montero, P.; Fernandez-Martin, F.; Aleman, A.; Gomez-Guillen, M.C. Physical and chemical properties of tuna-skin and bovine-hide gelatin films with added aqueous oregano and rosemary extracts. Food Hydrocoll. 2009, 23, 1334–1341. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolourization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Schormuller, J. Handbuch der Lebensmittelchemie; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1968; Volume III/2. [Google Scholar]
- AOAC (Association of Official Analytical Chemists). Official Methods of Analyses of Association of Analytical Chemist, 15th ed.; AOAC: Washington, DC, USA, 1990. [Google Scholar]
- AOCS (American Oil Chemists’ Society). AOCS Official Method Cd 19-90. 2-Thiobarbituric acid value. Direct Method. In Official Methods and Recommended Practices of the American Oil Chemists’ Society, 5th ed.; Firestone, D., Ed.; AOCS: Champaign, IL, USA, 1998. [Google Scholar]
- Amerina, M.A.; Pangborn, R.V.; Roessler, E.B. Principles of Sensory Evaluation of Food; Academic Press: New York, NY, USA, 1965; p. 602. [Google Scholar]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic composition and antioxidant activity of propolis from various regions of Poland. Nat. Prod. Res. 2015, 29, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Aliyazıcıoglu, R.; Sahin, H.; Erturk, O.; Ulusoy, E.; Kolayli, S. Properties of phenolic composition and biological activity of propolis from Turkey. Int. J. Food Prop. 2013, 16, 277–287. [Google Scholar] [CrossRef]
- Ozdal, T.; Ceylan, F.D.; Eroglu, N.; Kaplan, M.; Olgun, E.O.; Capanoglu, E. Investigation of antioxidant capacity, bioaccessibility and LC-MS/MS phenolic profile of Turkish propolis. Food Res. Int. 2019, 122, 528–536. [Google Scholar] [CrossRef]
- Chaillou, L.L.; Nazareno, M.A. Bioactivity of propolis from Santiago del Estero, Argentina, related to their chemical composition. LWT-Food Sci. Technol. 2009, 42, 1322–1427. [Google Scholar] [CrossRef]
- Ciftci-Yilmaz, S.; Azman, Z.N.; Gunduz, E.; Grenman, R. Evaluating antioxidant capacity of different propolis samples from konya, turkey and their inhibitory effect on head and neck cancer cells. bioRxiv 2017. preprint. [Google Scholar]
- Moreira, L.; Dias, L.G.; Pereira, J.A.; Estevinho, L. Antioxidant properties, total phenols and pollen analysis of propolis samples from Portugal. Food Chem. Toxicol. 2008, 46, 3482–3485. [Google Scholar] [CrossRef]
- Mello, B.C.B.S.; Hubinger, M.D. Antioxidant activity and polyphenol contents in Brazilian green propolis extracts prepared with the use of ethanol and water as solvent in different pH values. Int. J. Food Sci. Technol. 2012, 47, 2510–2518. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Biesaga, M. Analysis of phenolic acids and flavonoids in honey. Trends Anal. Chem. 2009, 28, 893–902. [Google Scholar] [CrossRef]
- Küçük, M.; Kolayli, S.; Karaoglu, Ş.; Ulusoy, E.; Baltacı, C.; Candan, F. Biological activities and chemical composition of three honeys of different types from Anatolia. Food Chem. 2007, 100, 526–534. [Google Scholar] [CrossRef]
- Ahn, M.-R.; Kumazawa, S.; Usui, Y.; Nakamura, J.; Matsuka, M.; Zhu, F.; Nakayama, T. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 2007, 101, 1383–1392. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Konteles, S.J.; Troullidou, E.; Mourtzinos, I.; Karathanos, V. Chemical composition, antioxidant activity and antimicrobial properties of propolis extract from Greece and Cyprus. Food Chem. 2009, 116, 452–461. [Google Scholar] [CrossRef]
- Osés, S.M.; Marcos, P.; Azofra, P.; de Pablo, A.; Fernández-Muíño, M.A.; Sancho, M.T. Phenolic profile, antioxidant capacities and enzymatic inhibitory activities of propolis from different geographical areas: Needs for analytical harmonization. Antioxidants 2020, 9, 75. [Google Scholar] [CrossRef]
- Serra-Bonvehí, J.; Lacalle-Gutiérrez, A. Antioxidant activity and total phenolics of propolis from the Basque country (Northeastern Spain). J. Am. Oil Chem. Soc. 2011, 88, 1387–1395. [Google Scholar] [CrossRef]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of bioactive compounds potential and antioxidant activity of brown, green and red propolis from Brazilian northeast region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographical origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Ozogul, Y.; Yuvka, Y.; Ucar, Y.; Durmus, M.; Kosker, A.R.; Oz, M.; Ozogul, F. Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme and sage) on sensory, chemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. LWT-Food Sci. Technol. 2017, 75, 677–684. [Google Scholar] [CrossRef]
- Oz, M.; Dikel, S.; Durmus, M.; Ozogul, Y. Effects of black cumin oil (Nigella sativa) on sensory, chemical and microbiological properties of rainbow trout during 23 days of storage at 2 ± 1 °C. J. Aquat. Food Prod. Technol. 2017, 26, 665–674. [Google Scholar] [CrossRef]
- Volpe, M.G.; Sianoa, F.; Paolucci, M.; Sacco, A.; Sorrentino, A.; Malinconico, M.; Varricchio, E. Active edible coating effectiveness in shelf-life enhancement of trout (Oncorhynchus mykiss) fillets. LWT-Food Sci. Technol. 2015, 60, 615–622. [Google Scholar] [CrossRef]
- Ludorf, W.; Meyer, V. Fish and Fish Products; Verlag Paul Parey: Berlin, Germany, 1973; p. 308. [Google Scholar]
- Alparslan, Y.; Baygar, T.; Baygar, T.; Hasanhocaoglu, H.; Metin, C. Effects of gelatin-based edible films enriched with laurel essential oil on the quality of rainbow trout (Oncorhynchus mykiss) fillets during refrigerated storage. Food Technol. Biotechnol. 2014, 52, 325–333. [Google Scholar]
- Lopez-Caballero, M.E.; Martinez-Alvarez, O.; Gomez-Guillen, M.C.; Montero, P. Quality of thawed deepwater pink shrimp (Parapenaeus longirostris) treated with melanosis- inhibiting formulations during chilled storage. Int. J. Food Sci. Technol. 2007, 42, 1029–1038. [Google Scholar] [CrossRef]
- Pastoriza, L.; Sampedro, G.; Herrera, J.J.; Cabo, M.L. Effect of modified atmosphere packaging on shelf life of iced fresh hake slices. J. Sci. Food Agric. 1996, 71, 541–547. [Google Scholar] [CrossRef]
- EEC. Total volatile basic nitrogen (TVB-N) limits values for certain categories of fishery products and specifying the analysis methods to be used. Commission Decision 95/149/EEC of 8 March 1995. Off. J. Eur. Commun. L 1995, 97, 84–87. [Google Scholar]
- Goulas, A.E.; Kontominas, M.G. Effect of salting and smoking-method on the keeping quality of chub mackerel (Scomber japonicus): Biochemical and sensory attributes. Food Chem. 2005, 93, 511–520. [Google Scholar] [CrossRef]
- Nowzari, F.; Shabanpour, B.; Ojagh, S.M. Comparison of chitosan–gelatin composite and bilayer coating and film effect on the quality of refrigerated rainbow trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef]
- Ojagh, S.M.; Rezaei, M.; Razavi, S.H.; Hosseini, S.M.H. Effect of chitosan coatings enriched with cinnamon oil on the quality of refrigerated rainbow trout. Food Chem. 2010, 120, 193–198. [Google Scholar] [CrossRef]
- Sun, X.; Guo, X.; Ji, M.; Wu, J.; Zhu, W.; Wang, J.; Cheng, C.; Chen, L.; Zhang, Q. Preservative effects of fish gelatin coating enriched with CUR/βCD emulsion on grass carp (Ctenopharyngodon idellus) fillets during storage at 4 °C. Food Chem. 2019, 272, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Ucak, I. Physicochemical and antimicrobial effects of gelatin-based edible films incorporated with garlic peel extract on the rainbow trout fillets. Prog. Nutr. 2019, 21, 232–240. [Google Scholar]
- Alsaggaf, M.S.; Moussa, S.H.; Tayel, A.A. Application of fungal chitosan incorporated with pomegranate peel extract as edible coating for microbiological, chemical and sensorial quality enhancement of Nile tilapia fillets. Int. J. Biol. Macromol. 2017, 99, 499–505. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, X.; Li, D. Chitosan films and coatings containing essential oils: The antioxidant and antimicrobial activity, and application in food systems. Food Res. Int. 2016, 89, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Shahbazi, Y. Shelf-life extension and quality attributes of sauced silver carp fillet: A comparison among direct addition, edible coating and biodegradable film. LWT-Food Sci. Technol. 2018, 87, 122–133. [Google Scholar] [CrossRef]
- Jeon, Y.; Kamil, J.Y.V.A.; Shahidi, F. Chitosan as an edible film for quality preservation of herring and Atlantic cod. J. Agric. Food Chem. 2002, 50, 5167–5178. [Google Scholar] [CrossRef]
- Ibrahim Sallam, K. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control 2007, 18, 566–575. [Google Scholar] [CrossRef]
- Reis, A.S.; Diedrich, C.; Moura, C.; Pereira, D.; de Florio Almeida, C.; Silva, L.D. Physico-chemical characteristics of microencapsulated propolis co-product extract and its effect on storage stability of burger meat during storage at −15 °C. LWT-Food Sci. Technol. 2017, 76, 306–313. [Google Scholar] [CrossRef]
- Spinelli, S.; Conte, A.; Lecce, L.; Incoronato, A.L.; del Nobile, M.A. Microencapsulated propolis to enhance the antioxidant properties of fresh fish burgers. J. Food Process. Eng. 2015, 38, 527–535. [Google Scholar] [CrossRef]
- Pobiega, K.; Kraśniewska, K.; Gniewosz, M. Application of propolis in antimicrobial and antioxidative protection of food quality- a review. Trends Food Sci. Technol. 2019, 83, 53–62. [Google Scholar] [CrossRef]
- Kurek-Gorecka, A.; Rzepecka-Stojko, A.; Gorecki, M.; Stojko, J.; Sosada, M.; Swierczek-Zieba, G. Structure and antioxidant activity of polyphenols derived from propolis. Molecules 2013, 19, 78–101. [Google Scholar] [CrossRef] [PubMed]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Gorecka, A.; Gorecki, M.; Kabala-Dzik, A.; Kubina, R.; Mozdzierz, A.; Buszman, E. Polyphenols from bee pollen: Structure, absorption, metabolism and biological activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef] [PubMed]
- ICMSF. Microorganisms in Foods; The International Commission on Microbiological Specifications for Foods of the International Union of Biological Societies; Blackwell: Oxford, UK, 1986; pp. 181–196. [Google Scholar]
- Jouki, M.; Yazdi, F.T.; Mortazavi, S.A.; Koocheki, A.; Khazaei, N. Effect of quince seed mucilage edible films incorporated with oregano or thyme essential oil on shelf life extension of refrigerated rainbow trout fillets. Int. J. Food Microbiol. 2014, 174, 88–97. [Google Scholar] [CrossRef]
- Petruzzi, L.; Rosaria Corbo, M.; Campaniello, D.; Speranza, B.; Sinigaglia, M.; Bevilacqua, A. Antifungal and Antibacterial Effect of Propolis: A Comparative Hit for Food-Borne Pseudomonas, Enterobacteriaceae and Fungi. Foods 2020, 9, 559. [Google Scholar] [CrossRef]
- Kahramanoglu, I.; Okatan, V.; Wan, C. Biochemical Composition of Propolis and Its Efficacy in Maintaining Postharvest Storability of Fresh Fruits and Vegetables. J. Food Qual. 2020, 2020, 8869624. [Google Scholar] [CrossRef]
- Jasour, M.S.; Rahimabadi, E.Z.; Ehsani, A.; Rahnama, M.; Arshadi, A. Effects of refrigerated storage on fillet lipid quality of rainbow trout (Oncorhynchus mykiss) supplemented by a-tocopheryl acetate through diet and direct addition after slaughtering. Int. J. Food Process. Technol. 2011, 2, 2–5. [Google Scholar] [CrossRef]
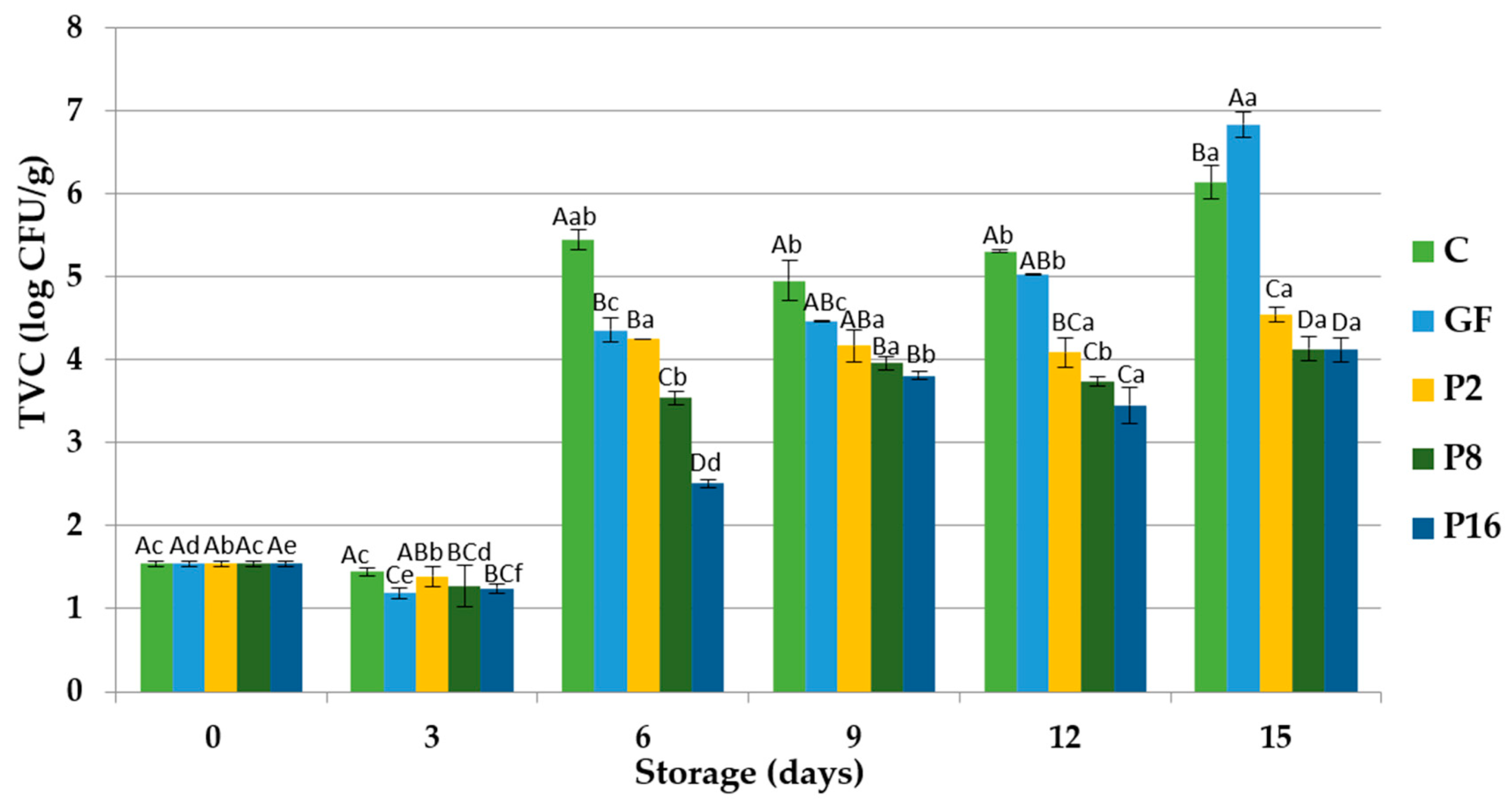
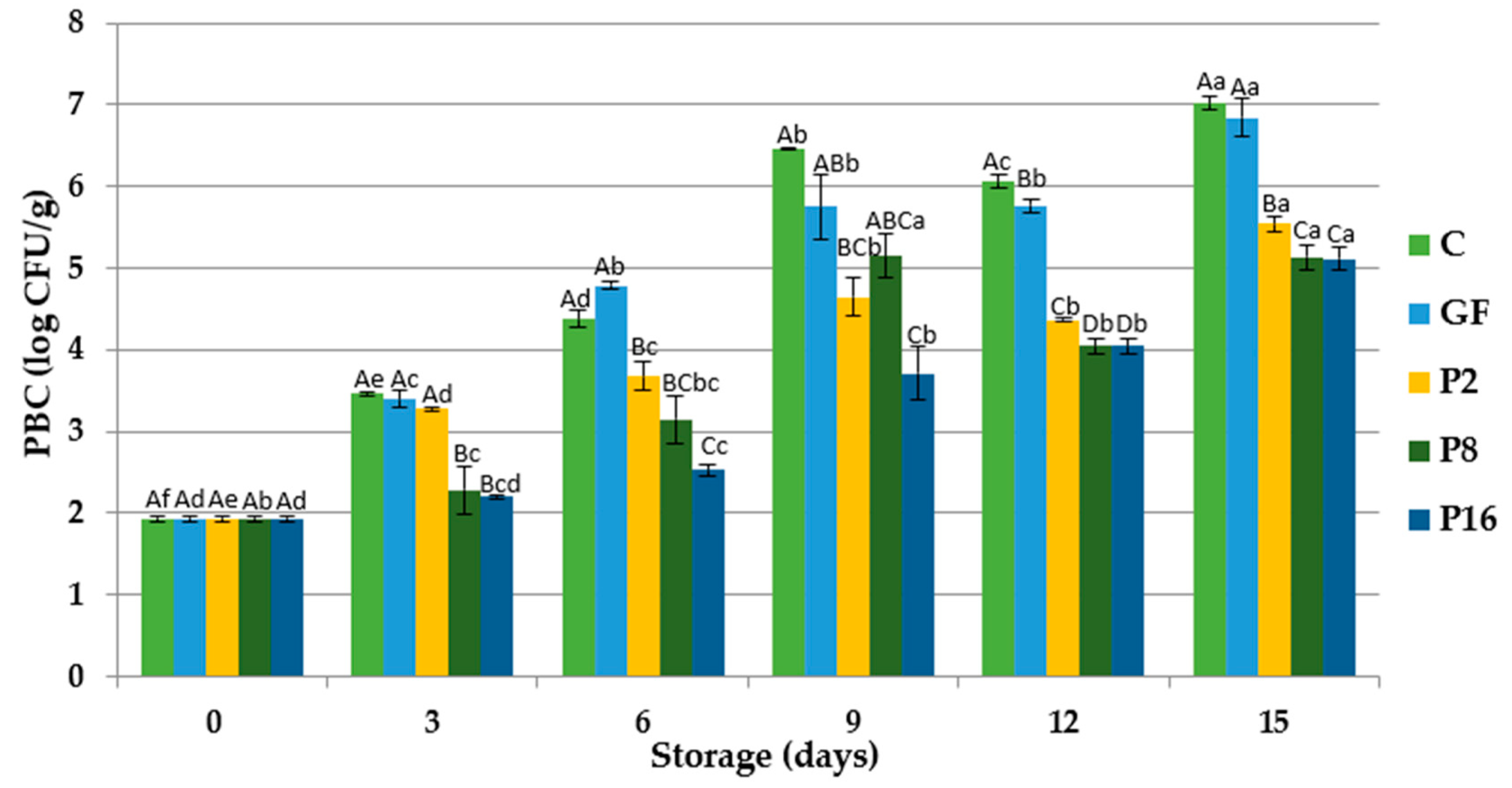
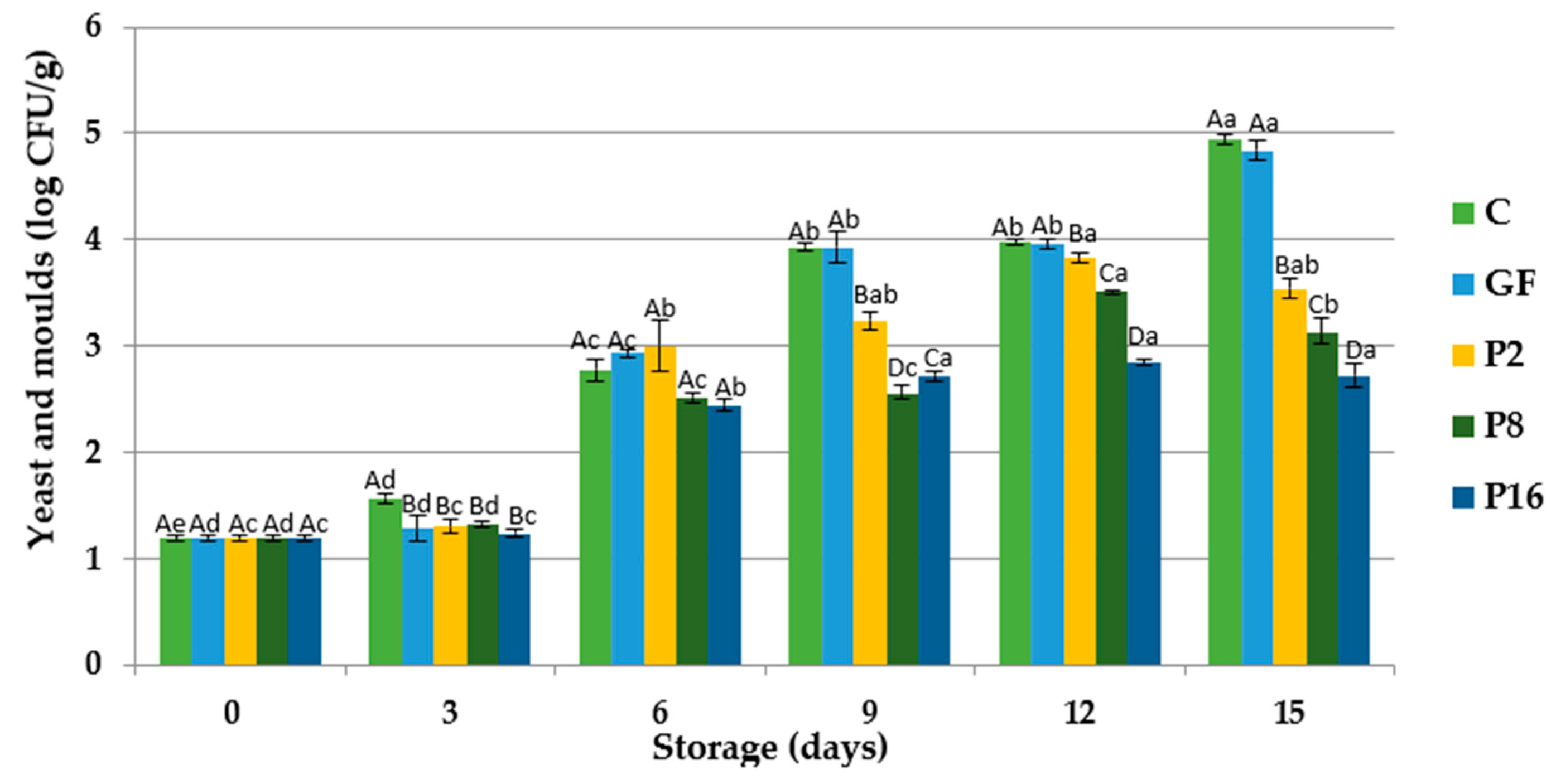
| Storage Period (Days) | C | GF | P2 | P8 | P16 | |
|---|---|---|---|---|---|---|
| pH | 0 | 6.482 ± 0.054 Ac | 6.482 ± 0.054 Ac | 6.482 ± 0.054 Ae | 6.482 ± 0.054 Abc | 6.482 ± 0.054 Ac |
| 3 | 6.713 ± 0.041 Ab | 6.679 ± 0.052 Ab | 6.644 ± 0.022 ABc | 6.548 ± 0.047 BCc | 6.458 ± 0.079 Cbc | |
| 6 | 6.733 ± 0.040 Ab | 6.703 ± 0.000 Ab | 6.680 ± 0.027 Ac | 6.577 ± 0.078 Bbc | 6.554 ± 0.011 Bb | |
| 9 | 6.730 ± 0.102 Ab | 6.615 ± 0.068 Ab | 6.571 ± 0.024 Bd | 6.582 ± 0.013 Bb | 6.579 ± 0.014 Bb | |
| 12 | 7.504 ± 0.014 Aa | 7.493 ± 0.092 Aa | 7.309 ± 0.021 Bb | 6.836 ± 0.067 Ca | 6.713 ± 0.000 Da | |
| 15 | 7.548 ± 0.019 Aa | 7.524 ± 0.036 Aa | 7.527 ± 0.017 Aa | 6.880 ± 0.000 Ba | 6.769 ± 0.147 Ca | |
| TVB-N (mg N/100 g) | 0 | 16.804 ± 0.000 Ae | 16.804 ± 0.000 Ae | 16.804 ± 0.000 Ad | 16.804 ± 0.000 Ae | 16.804 ± 0.000 Ae |
| 3 | 28.003 ± 0.000 Ad | 21.700 ± 0.991 Ad | 20.303 ± 8.911 Ac | 18.904 ± 0.992 Ad | 19.647 ± 0.944 Ad | |
| 6 | 35.702 ± 0.967 Ac | 32.203 ± 3.955 ABc | 28.001 ± 0.004 Bb | 27.302 ± 2.974 Bc | 25.204 ± 1.982 Bcd | |
| 9 | 40.601 ± 0.000 Ab | 39.200 ± 1.983 Ab | 34.302 ± 0.989 Bcb | 32.904 ± 0.985 Bb | 30.802 ± 1.977 Bcd | |
| 12 | 43.400 ± 1.978 Ab | 44.801 ± 0.000 Ab | 35.704 ± 0.985 Bb | 34.300 ± 0.987 BCb | 32.203 ± 0.000 Cb | |
| 15 | 53.903 ± 2.966 Aa | 52.504 ± 4.954 Aa | 49.700 ± 2.967 ABa | 42.001 ± 1.983 BCa | 44.100 ± 0.988 Ca | |
| PV (meq O2/kg) | 0 | 2.004 ± 0.002 Ad | 2.004 ± 0.002 Ac | 2.004 ± 0.002 Ad | 2.004 ± 0.002 Ac | 2.004 ± 0.002 Ac |
| 3 | 5.002 ± 0.000 Ac | 5.504 ± 0.707 Ab | 2.004 ± 0.001 Bd | 1.994 ± 0.001 Bc | 1.974 ± 0.065 Bc | |
| 6 | 6.504 ± 0.714 Ab | 5.002 ± 0.002 Bb | 3.502 ± 0.714 Bc | 2.503 ± 0.714 Cc | 1.943 ± 0.074 Cc | |
| 9 | 5.002 ± 0.002 Bc | 6.502 ± 0.710 Aa | 5.466 ± 0.704 Ab | 3.978 ± 0.001 Cb | 3.967 ± 0.000 Cb | |
| 12 | 7.001 ± 0.000 Ab | 6.978 ± 0.033 Aa | 6.003 ± 0.002 Aab | 5.477 ± 0.720 BCa | 4.502 ± 0.712 Cab | |
| 15 | 8.503 ± 0.713 Aa | 7.002 ± 0.001 Ba | 6.956 ± 0.007 Bab | 5.505 ± 0.708 Ca | 5.001 ± 0.000 Ca | |
| TBARS (mg MDA/kg) | 0 | 0.882 ± 0.0438 Ad | 0.882 ± 0.038 Ad | 0.882 ± 0.038 Ac | 0.882 ± 0.038 Ad | 0.882 ± 0.038 Ad |
| 3 | 1.248 ± 0.010 Ac | 0.862 ± 0.112 Bd | 0.824 ± 0.022 Bc | 0.790 ± 0.040 Be | 0.777 ± 0.023 Be | |
| 6 | 1.217 ± 0.092 Ac | 1.114 ± 0.030 Ac | 0.912 ± 0.082 Bc | 1.277 ± 0.000 Cf | 0.704 ± 0.052 Cf | |
| 9 | 1.474 ± 0.024 Ab | 1.730 ± 0.000 Aa | 1.023 ± 0.024 Bb | 0.975 ± 0.000 Cc | 0.912 ± 0.064 Cc | |
| 12 | 1.523 ± 0.017 Aab | 1.493 ± 0.001 Bb | 1.460 ± 0.014 Ba | 1.252 ± 0.001 Cb | 1.088 ± 0.012 Db | |
| 15 | 1.588 ± 0.016 Aa | 1.558 ± 0.033 Ab | 1.488 ± 0.000 Ba | 1.417 ± 0.024 Ca | 1.284 ± 0.015 Da |
| Storage Period (Days) | C | GF | P2 | P8 | P16 | |
|---|---|---|---|---|---|---|
| Odor | 0 | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa |
| 3 | 8.002 ± 0.530 Bb | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 8.902 ± 0.354 Aa | 9.000 ± 0.000 Aa | |
| 6 | 8.124 ± 0.831 Bb | 8.750 ± 0.461 Aa | 9.000 ± 0.000 Aa | 8.752 ± 0.458 Aa | 9.000 ± 0.000 Aa | |
| 9 | 3.865 ± 0.832 Cc | 3.372 ± 0.923 Cb | 5.622 ± 1.064 Bb | 7.869 ± 0.643 Ab | 7.623 ± 0.521 Ab | |
| 12 | 1.124 ± 0.347 Cd | 1.366 ± 0.522 Cc | 3.002 ± 0.533 Bc | 5.251 ± 1.580 Ac | 5.119 ± 0.825 Ac | |
| 15 | 1.002 ± 0.000 Bd | 1.001 ± 0.000 Bc | 1.248 ± 0.458 Bd | 2.123 ± 0.636 Ad | 2.247 ± 0.458 Ad | |
| Texture | 0 | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa |
| 3 | 8.253 ± 0.461 Bb | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 8.872 ± 0.354 Aa | 9.000 ± 0.000 Aa | |
| 6 | 6.747 ± 0.892 Bc | 7.754 ± 0.462 Ab | 9.000 ± 0.000 Aa | 8.620 ± 0.522 Aa | 9.000 ± 0.000 Aa | |
| 9 | 3.124 ± 0.988 Dd | 2.251 ± 0.891 Ec | 5.124 ± 0.350 Cb | 8.502 ± 0.531 Aa | 7.374 ± 0.520 Bb | |
| 12 | 1.369 ± 0.743 BCe | 1.254 ± 0.457 Cd | 2.367 ± 0.521 Bc | 5.371 ± 1.766 Ab | 5.117 ± 0.991 Ac | |
| 15 | 1.002 ± 0.000 Be | 1.003 ± 0.001 Bd | 1.115 ± 0.347 Bd | 2.502 ± 0.763 Ac | 2.503 ± 0.527 Ad | |
| Color | 0 | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa |
| 3 | 7.872 ± 0.636 Bb | 8.874 ± 0.458 Aab | 8.871 ± 0.348 Aa | 8.869 ± 0.354 Aa | 9.000 ± 0.000 Aa | |
| 6 | 7.001 ± 0.758 Bc | 8.253 ± 0.888 Ab | 8.623 ± 0.464 Aa | 8.754 ± 0.521 Aa | 8.752 ± 0.457 Aa | |
| 9 | 2.001 ± 0.933 Dd | 3.372 ± 0.921 Cc | 5.368 ± 0.922 Bb | 8.002 ± 0.758 Aa | 7.621 ± 0.523 Aa | |
| 12 | 1.004 ± 0.002 Ce | 1.367 ± 0.524 Cd | 2.503 ± 2.002 Bc | 4.617 ± 2.000 Ab | 4.118 ± 0.612 Ab | |
| 15 | 1.002 ± 0.000 Be | 1.002 ± 0.000 Bd | 1.117 ± 0.351 Bd | 2.503 ± 0.531 Ac | 2.254 ± 0.463 Ac | |
| Appearance | 0 | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Ad |
| 3 | 8.000 ± 0.762 Bb | 8.754 ± 0.461 Aab | 8.753 ± 0.462 Aa | 8.868 ± 0.345 Aa | 9.000 ± 0.000 Aa | |
| 6 | 7.124 ± 0.643 Bc | 8.252 ± 0.887 Ab | 8.754 ± 0.461 Aa | 8.621 ± 0.743 Aab | 8.747 ± 0.464 Aa | |
| 9 | 2.868 ± 0.638 Cd | 3.121 ± 0.644 Cc | 5.747 ± 0.710 Ba | 7.872 ± 0.827 Ad | 7.503 ± 0.527 Aa | |
| 12 | 1.124 ± 0.352 Ce | 1.124 ± 0.353 Ce | 3.365 ± 0.922 Bc | 5.370 ± 1.602 Ac | 4.619 ± 1.192 Ac | |
| 15 | 1.000 ± 0.000 Be | 1.000 ± 0.000 Be | 1.254 ± 0.463 Bd | 2.366 ± 0.521 Ad | 2.254 ± 0.463 Ad | |
| Overall acceptance | 0 | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa |
| 3 | 8.000 ± 0.532 Bb | 9.000 ± 0.000 Aa | 9.000 ± 0.000 Aa | 8.874 ± 0.351 Aa | 9.000 ± 0.000 Aa | |
| 6 | 7.000 ± 0.760 Bc | 8.754 ± 0.457 Aa | 9.000 ± 0.000 Aa | 8.867 ± 0.354 Aa | 9.000 ± 0.000 Aa | |
| 9 | 3.000 ± 0.930 Dd | 3.365 ± 0.743 Db | 6.000 ± 0.761 Cb | 8.370 ± 0.744 Aa | 7.500 ± 0.530 Bb | |
| 12 | 1.124 ± 0.351 Ce | 1.369 ± 0.524 Cc | 3.000 ± 9.533 Bc | 4.866 ± 1.887 Ab | 4.754 ± 0.461 Ac | |
| 15 | 1.000 ± 0.000 Be | 1.000 ± 0.000 Bc | 1.252 ± 0.464 Bd | 2.374 ± 0.521 Ac | 2.503 ± 0.532 Ad |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ucak, I.; Khalily, R.; Carrillo, C.; Tomasevic, I.; Barba, F.J. Potential of Propolis Extract as a Natural Antioxidant and Antimicrobial in Gelatin Films Applied to Rainbow Trout (Oncorhynchus mykiss) Fillets. Foods 2020, 9, 1584. https://doi.org/10.3390/foods9111584
Ucak I, Khalily R, Carrillo C, Tomasevic I, Barba FJ. Potential of Propolis Extract as a Natural Antioxidant and Antimicrobial in Gelatin Films Applied to Rainbow Trout (Oncorhynchus mykiss) Fillets. Foods. 2020; 9(11):1584. https://doi.org/10.3390/foods9111584
Chicago/Turabian StyleUcak, Ilknur, Rowida Khalily, Celia Carrillo, Igor Tomasevic, and Francisco J. Barba. 2020. "Potential of Propolis Extract as a Natural Antioxidant and Antimicrobial in Gelatin Films Applied to Rainbow Trout (Oncorhynchus mykiss) Fillets" Foods 9, no. 11: 1584. https://doi.org/10.3390/foods9111584
APA StyleUcak, I., Khalily, R., Carrillo, C., Tomasevic, I., & Barba, F. J. (2020). Potential of Propolis Extract as a Natural Antioxidant and Antimicrobial in Gelatin Films Applied to Rainbow Trout (Oncorhynchus mykiss) Fillets. Foods, 9(11), 1584. https://doi.org/10.3390/foods9111584