Elemental Profile in Chicken Egg Components and Associated Human Health Risk Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Egg Collection and Sample Preparation
2.2. Health Risk Assessment
2.3. Statistical Analysis
3. Results and Discussion
3.1. Elemental Determinations
3.2. Health Risk Assessment
3.3. Chemometric Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Voica, C.; Rosu, C.; Iordache, A.M.; Pistea, I.C.; Miricioiu, M.G. The Romanian Consumer Among Education, Information, Health Risk, Food Quality and Ethics. Rev. Chim. 2020, 71, 436–442. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, H. Health risk from veterinary antimicrobial use in China’s food animal production and its reduction. Environ. Pollut. 2016, 219, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cheng, H.; Tao, S. Environmental and human health challenges of industrial livestock and poultry farming in China and their mitigation. Environ. Int. 2017, 107, 111–130. [Google Scholar] [CrossRef]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef]
- Dunn, I.C. Breeding strategies to improve the egg’s natural defense. Worlds Poult. Sci. J. 2004, 60, 458–468. [Google Scholar] [CrossRef]
- Dunn, I.C. Poultry Breeding for Egg Quality. In Improving the Safety and Quality of Eggs and Products; Nys, Y., Bain, M., Van Immerseel, F., Eds.; Woodhead Publ. Ltd.: Cambridge, UK, 2011; Volume 1, pp. 245–260. [Google Scholar]
- Jagadeesh Babu, A.; Swetha, C.S.; Supriya, R.A.; Suganya, G.; Sasikala, K.; Surendra, R.; Yeshwanth Srinivas, K.A. Study on the Levels of Heavy Metals in Poultry Eggs in Chittoor District of Andhra Pradesh, India. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1113–1121. [Google Scholar] [CrossRef]
- Nisianakis, P.; Giannenas, I.; Gavriil, A.; Kontopidis, G.; Kyriazakis, I. Variation in trace element contents among chicken, turkey, duck, goose, and pigeon eggs analyzed by inductively coupled plasma mass spectrometry (ICP-MS). Biol. Trace Elem. Res. 2009, 128, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.Z.A.; Siddique, A.A.; Hossain, S.M.A.; Kazi, A.I.; Ahsan, A.A.; Ahmed, S.; Zaman, M.M. Determination of essential and toxic metals in meats, meat products and eggs by spectrophotometric method. J. Bangladesh Chem. Soc. 2011, 24, 165–172. [Google Scholar] [CrossRef]
- Abdulkhaliq, A.; Swaileh, K.M.; Hussein, R.M.; Matani, M. Levels of metals (Cd, Pb, Cu and Fe) in cow’s milk, dairy products and hen’s eggs from the West Bank, Palestine. Int. Food Res. J. 2012, 19, 1089–1094. [Google Scholar]
- AL-Ashmawy, M.A.M. Trace elements residues in the table eggs rolling in the Mansoura City markets Egypt. Int. Food Res. J. 2013, 20, 1783–1787. [Google Scholar]
- Hu, Y.; Zhang, W.; Chen, G.; Cheng, H.; Tao, S. Public health risk of trace metals in fresh chicken meat products on the food markets of a major production region in southern China. Environ. Pollut. 2018, 234, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Sobhanardakani, S. Assessment of levels and health risk of heavy metals (Pb, Cd, Cr, and Cu) in commercial hen’s eggs from the city of Hamedan. Pollution 2017, 3, 669–677. [Google Scholar]
- U.S. Department of Agriculture USDA National Nutrient Database. Available online: https://data.nal.usda.gov/search/type/dataset (accessed on 14 June 2022).
- Food and Agriculture Organization. Report of a Sub-Committee of the 2011 FAO Consultation on “Protein Quality Evaluation in Human Nutrition”: The Assessment of Amino Acid Digestibility in Foods for Humans and including a Collation of Published Ileal Amino Acid Digestibility Data for Human Foods; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Food and Agriculture Organization of the United Nations. United Nations University Protein and Amino Acid Requirements in Human Nutrition; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Sathiya, R.; Banumathy, V.; Pazhanisamy, C. Comparative analysis of quail and chicken meat and egg. J. Emerg. Technol. Innov. Res. 2019, 6, 462–470. [Google Scholar]
- Kovas-Nolan, J.; Phillips, M.; Mine, Y. Advances in the value of eggs and egg components for human health. J. Agric. Food Chem. 2005, 53, 8421–8431. [Google Scholar] [CrossRef]
- Fernandez, M.L. Effects of eggs on plasma lipoproteins in healthy populations. Food Funct. 2010, 1, 156–160. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Dietary Protein Quality Evaluation in Human Nutrition; Food and Nutrition Paper. 92; FAO: Rome, Italy, 2013. [Google Scholar]
- Puglisi, M.J.; Fernandez, M.L. The Health Benefits of Egg Protein. Nutrients 2022, 14, 2904. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 Establishing a Common Organisation of the Markets in Agricultural Products and Repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32013R1308 (accessed on 2 October 2023).
- Available online: https://agriculture.ec.europa.eu/farming/animal-products/eggs_en (accessed on 2 October 2023).
- Giucă, A.D.; Necula, D.M. The Evolution of Egg Production and Consumption at the Level of Romania in the Period 2016–2020. In Agrarian Economy and Rural Development—Realities and Perspectives for Romania, 12th ed.; International Symposium; The Research Institute for Agricultural Economy and Rural Development (ICEADR): Bucharest, Romania, 2021; pp. 62–67. [Google Scholar]
- Available online: https://www.businessagricol.ro/studiu-privind-piata-oualor/ (accessed on 1 October 2023).
- Grace, E.J.; MacFarlane, G.R. Assessment of the bioaccumulation of metals to chicken eggs from residential backyards. Sci. Total Environ. 2016, 563–564, 256–260. [Google Scholar] [CrossRef]
- Cross, S.J.; Taylor, E.R. Human Exposure to Soil Contaminants through the Consumption of Home-Grown Produce; Contaminated Sites Monograph Series, No. 6; South Australian Health Commission: Adelaide, Australia, 1996. [Google Scholar]
- Van Overmeire, I.; Pussemier, L.; Hanot, V.; De Temmerman, L.; Hoenig, M.; Goeyens, L. Chemical contamination of free-range eggs from Belgium. Food Addit. Contam. 2006, 23, 1109–1122. [Google Scholar] [CrossRef]
- Waegeneers, N.; De Steur, H.; De Temmerman, L.; Van Steenwinkel, S.; Gellynck, X.; Viaene, J. Transfer of soil contaminants to home-produced eggs and preventive measures to reduce contamination. Sci. Total Environ. 2009, 407, 4438–4446. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.L.; Jin, G.F.; Gouda, M.; Jin, Y.G.; Ma, M.H. Characterization and classification of volatiles from different breeds of eggs by SPME-GC-MS and chemometrics. Food Res. Int. 2019, 116, 767–777. [Google Scholar] [CrossRef]
- York, J.L.; Magnuson, R.H.; Schug, K.A. On-line sample preparation for multiclass vitamin, hormone, and mycotoxin determination in chicken egg yolk using LC-MS/MS. Food Chem. 2020, 326, 126939. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.D.; Wang, Q.H.; Ma, M.H.; Ma, Y.X.; Vong, C.N. Prediction and visualization of S-ovalbumin content in egg whites using hyperspectral images. Int. J. Food Prop. 2019, 22, 1077–1086. [Google Scholar] [CrossRef]
- Mi, S.; Shang, K.; Zhang, C.-H.; Fan, Y.-Q. Characterization and discrimination of selected chicken eggs in China’s retail market based on multi-element and lipidomics analysis. Food Res. Int. 2019, 126, 108668. [Google Scholar] [CrossRef] [PubMed]
- Aboonajmi, M.; Saberi, A.; Abbasian Najafabadi, T.; Kondo, N.S. Quality assessment of poultry egg based on visible-near infrared spectroscopy and radial basis function networks. Int. J. Food Prop. 2016, 19, 1163–1172. [Google Scholar] [CrossRef]
- Rock, L. The use of stable isotope techniques in egg authentication schemes: A review. Trends Food Sci. Technol. 2012, 28, 62–68. [Google Scholar] [CrossRef]
- Bay, L.J.; Harn Chan, J.S.; Walczyk, T. Optimization of analytical strategies by Monte Carlo simulation: A case study in eggs for tracing their geographical origin using stable isotope signatures. Forensic Chem. 2018, 11, 32–37. [Google Scholar] [CrossRef]
- Rogers, K.M.; Van Ruth, S.; Alewijn, M.; Philips, A.; Rogers, P. Verification of egg farming systems from The Netherlands and New Zealand using stable isotopes. J. Agric. Food Chem. 2015, 63, 8372–8380. [Google Scholar] [CrossRef]
- Perez-Alvarez, E.P.; Garcia, R.; Barrulas, P.; Dias, C.; Cabrita, M.J.; Garde-Cerdan, T. Classification of wines according to several factors by ICP-MS multi-elemental analysis. Food Chem. 2019, 270, 273–280. [Google Scholar] [CrossRef]
- Griboff, J.; Baroni, M.V.; Horacek, M.; Wunderlin, D.A.; Monferran, M.V. Multilemental+isotopic fingerprint enables linking soil, water, forage and milk composition, assessing the geographical origin of Argentinian milk. Food Chem. 2019, 283, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Voica, C.; Nechita, C.; Iordache, A.M.; Roba, C.; Zgavarogea, R.; Ionete, R. ICP–MS assessment of essential and toxic trace elements in foodstuffs with different geographic origins available in Romanian supermarkets. Molecules 2021, 26, 7081. [Google Scholar] [CrossRef]
- Cristea, G.; Voica, C.; Feher, I.; Puscas, R.; Magdas, D.A. Isotopic and elemental characterization of Romanian pork meat in corroboration with advanced chemometric methods: A first exploratory study. Meat Sci. 2022, 189, 108825. [Google Scholar] [CrossRef]
- Hoseini, H.; Abedi, A.-S.; Mohammadi-Nasrabadi, F.; Salmani, Y.; Esfarjan, F. Risk assessment of lead and cadmium concentrations in hen’s eggs using Monte Carlo simulations. Food Sci. Nutr. 2023, 1, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.; Roy, D.; Hasan, M.M.; Ahmed, K.S.; Sarker, S.; Hossain, M.M.; Shajahan, M. Intake of toxic metals through dietary eggs consumption and its potential health risk assessment on the peoples of the capital city Dhaka Bangladesh. Arab. J. Chem. 2023, 16, 105104. [Google Scholar] [CrossRef]
- Hashemi, M.; Sadeghi, A.; Saghi, M.; Aminzare, M.; Raeisi, M.; Rezayi, M.; Sany, S.B.T. Health Risk Assessment for Human Exposure to Trace Metals and Arsenic via Consumption of Hen Egg Collected from Largest Poultry Industry in Iran. Biol. Trace Elem. Res. 2019, 188, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.; Kumar Singh, A.K. Heavy metals in eggs and chicken and the associated human health risk assessment in the mining areas of Singhbhum copper belt, India. Arch. Environ. Occup. Health 2019, 74, 161–170. [Google Scholar] [CrossRef]
- Fakayode, S.O.; Olu-OWOLABI, I.B. Trace Metal Content and Estimated Daily Human Intake from Chicken Eggs in Ibadan, Nigeria. Arch. Environ. Health Int. J. 2003, 58, 245–251. [Google Scholar] [CrossRef]
- Korish, M.A.; Attia, Y.A. Evaluation of Heavy Metal Content in Feed, Litter, Meat, Meat Products, Liver, and Table Eggs of Chickens. Animals 2020, 10, 727. [Google Scholar] [CrossRef]
- Shaheen, N.; Ahmed, M.K.; Islam, M.S.; Habibullah-Al-Mamun, M.; Tukun, A.B.; Islam, S.; MARahim, A.T. Health risk assessment of trace elements via dietary intake of ‘non-piscine protein source’ foodstuffs (meat, milk and egg) in Bangladesh. Environ. Sci. Pollut. Res. 2016, 23, 7794–7806. [Google Scholar] [CrossRef]
- Burger, J. Heavy metals in avian eggshells: Another excretion method. J. Toxicol. Environ. Health. 1994, 41, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, G.L. Methods of Analysis by the US Geological Survey National Water Quality Laboratory; Preparation Procedure for Aquatic Biological Material Determined for Trace Metals (No. 96-362); US Geological Survey: Branch of Information Services: Reston, VA, USA, 1996; pp. 1–42. [Google Scholar]
- US-EPA. Risk Assessment Guidance for Superfund. Human Health Evaluation Manual (Vol. I. Part A). EPA/540/1-89/1989, 291p. Available online: https://www.epa.gov/sites/production/files/2015-09/documents/rags_a.pdf (accessed on 30 September 2023).
- National Institute of Statistics (NIS). Social Trends, 2020, 252p. Available online: https://insse.ro/cms/sites/default/files/field/publicatii/social_trends_in_2020_0.pdf (accessed on 30 September 2023).
- FAO/WHO. Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods. 12th Session Utrecht, The Netherlands, 12–16 March 2018. Working Document for Information and Use in Discussions Related to Contaminants and Toxins in the GSCTFF (Prepared by Japan and the Netherlands). CF12/INF01, 169p. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-735-12%252FWD%252Fcf12_INF01x.pdf (accessed on 7 July 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 3 March 2023).
- Rohart, F.; Gautier, B.; Singh, A.; Lê Cao, K.A. mixOmics: An R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 2017, 13, e1005752. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Martín-Gómez, A.; Rodríguez-Hernández, P.; Cardador, M.J.; Vega-Márquez, B.; Rodríguez-Estévez, V.; Arce, L. Guidelines to build PLS-DA chemometric classification models using a GC-IMS method: Dry-cured ham as a case of study. Talanta 2023, 7, 100175. [Google Scholar] [CrossRef]
- Borràs, E.; Ferré, J.; Boqué, R.; Mestres, M.; Aceña, L.; Calvo, A.; Busto, O. Olive oil sensory defects classification with data fusion of instrumental techniques and multivariate analysis (PLS-DA). Food Chem. 2016, 203, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Manzanares, N.; Martín-Gómez, A.; Jurado-Campos, N.; Garrido-Delgado, R.; Arce, C.; Arce, L. Target vs. spectral fingerprint data analysis of Iberian ham samples for avoiding labelling fraud using headspace—Gas chromatography–ion mobility spectrometry. Food Chem. 2018, 246, 65–73. [Google Scholar] [CrossRef]
- Xie, S.; Hai, C.; He, S.; Lu, H.; Xu, L.; Fu, H. Discrimination of Free-Range and Caged Eggs by Chemometrics Analysis of the Elemental Profiles of Eggshell. J. Anal. Meth. Chem. 2023, 1271409. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Herron, S.; Daniel, T. Overcoming the challenges of phosphorus-based management challenges in poultry farming. J. Soil Water Conserv. 2007, 62, 375–389. [Google Scholar]
- National Research Council (US) Committee on Diet and Health. Diet and Health: Implications for Reducing Chronic Disease Risk; National Academies Press (US): Washington, DC, USA, 1989. [Google Scholar]
- Trziszka, T.; Dobrzański, Z.; Chojnacka, K.; Bubel, A.; Beń, H.; Korczyński, M.; Konkol, D.; Tronina, W. Assessment of Macro-, Micro-, Trace, and Ultratrace Element Concentration in Green-Legged Partridge Hens’ Eggs from a Free-Range System. Agriculture 2021, 11, 473. [Google Scholar] [CrossRef]
- Bashir, L.; Ossai, P.C.; Shittu, O.K.; Abubakar, A.N.; Caleb, T. Comparison of the Nutritional Value of Egg Yolk and Egg Albumin from Domestic Chicken, Guinea Fowl and Hybrid Chicken. Am. J. Exp. Agric. 2015, 6, 310–316. [Google Scholar] [CrossRef]
- Suttle, N.F. Mineral Nutrition of Livestock, 4th ed.; CABI: Wallingford, UK, 2010; pp. 1–547. [Google Scholar]
- Abbasi, F.; Fakhur-un-Nisa, T.; Liu, J.; Luo, X.; Abbasi, I.M.R. Low digestibility of phytate phosphorus, their impacts on the environment, and phytase opportunity in the poultry industry. Environ. Sci. Pollut. Res. 2019, 26, 9469–9479. [Google Scholar] [CrossRef]
- Abbasi, F.; Jingbo, L.; Hongfu, Z.; Xiaoyun, S.; Xuegang, L. Effects of dietary total phosphorus concentration and casein supplementation on the determination of true phosphorus digestibility for broiler chickens. Ital. J. Anim. Sci. 2018, 17, 135–144. [Google Scholar] [CrossRef]
- Camden, B.J.; Morel, P.C.H.; Thomas, D.V.; Ravindran, V.; Bedford, M.R. Effectiveness of exogenous microbial phytase in improving the bio availabilities of phosphorus and other nutrients in maize-soya-bean meal diets for broilers. J. Anim. Sci. 2001, 73, 289–297. [Google Scholar] [CrossRef]
- Bouvarel, I.; Nys, Y.; Lescoat, P. Hen Nutrition for Sustained Egg Quality. In Improving the Safety and Quality of Eggs and Egg Products; Nys, Y., Bain, M., Van Immerseel, F., Eds.; Egg Chemistry, Production and Consumption; Woodhead Publ Ltd.: Cambridge, UK, 2011; Volume 1, pp. 261–299. [Google Scholar]
- Nys, Y.; Le Roy, N. Calcium Homeostasis and Eggshell Biomineralization in Female Chicken. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: London, UK, 2018; Volume 1, pp. 361–382. [Google Scholar]
- Gautron, G.; Guyot, N.; Brionne, A.; Réhault-Godbert, S. Bioactive Minor Egg Components. In Eggs as Functional Foods and Nutraceuticals for Human Health; Wu, J., Ed.; Royal Society of Chemistry: Alberta, QC, Canada, 2019; p. 406. [Google Scholar]
- FAO. Gateway to Poultry Production and Products. Food and Agriculture Organization of the United Nations. 2020. Available online: https://www.fao.org/poultry-production-products/en/ (accessed on 29 September 2023).
- Ajala, E.; Eletta, O.; Ajala, M.; Oyeniyi, S. Characterization and evaluation of chicken eggshell for use as a bioresource. Arid Zone J. Eng. Technol. Environ. 2018, 14, 26–40. [Google Scholar]
- Waheed, M.; Yousaf, M.; Shehzad, A.; Inam-Ur-Raheem, M.; Khan, M.K.I.; Khan, M.R.; Ahmed, N.; Aadil, R.M. Channelling eggshell waste to valuable and utilizable products: A comprehensive review. Trends Food Sci. Technol. 2020, 106, 78–90. [Google Scholar] [CrossRef]
- Arif, S.S.; Pasha, I.; Iftikhar, H.; Mehak, F.; Sultana, R. Effects of eggshell powder supplementation on nutritional and sensory attributes of biscuits. Czech J. Food Sci. 2022, 40, 26–32. [Google Scholar] [CrossRef]
- Al-Salimi, M.S.S.; Kassem, A.S.; Saeed, A.A.M. Mineral and nutritional contents of hen’s eggs from different sources in Aden Governorate-Yemen. Arid Int. J. Sci. Technol. 2022, 5, 66–90. [Google Scholar] [CrossRef]
- Kiliç, A.S.; Paksoy, N.; Dinç, H.; Durmaz, H. Determination of 17 Elements in Free Range Hen Eggs with ICP-MS. Appl. Ecol. Environ. Res. 2019, 17, 8369–8380. [Google Scholar] [CrossRef]
- Attia, Y.A.; Al-Harthi, M.A.; Shiboob, M.M. Evaluation of Quality and Nutrient Contents of Table Eggs from Different Sources in the Retail Market. Ital. J. Anim. Sci. 2014, 13, 3294. [Google Scholar] [CrossRef]
- Gall, E.J.; Boyd, R.S.; Rajakaruna, N. Transfer of heavy through terrestrial food webs; a review. Environ. Monit. Assess. 2015, 187, 201. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Gochfeld, M. Cadmium and Lead in common Terns (Aves: Sterna hirundo): Relationship between levels in parents and eggs. Environ. Assess. 1991, 16, 253–258. [Google Scholar] [CrossRef]
- Demirulus, H. The Heavy Metal Content in Chicken Eggs Consumed in Van Lake Territory. Ekoloji. 2013, 22, 19–25. [Google Scholar] [CrossRef]
- Abduljaleel, S.A.; Shuhaimi-Othman, M. Metals Concentrations in Eggs of Domestic Avian and Estimation of Health Risk from Eggs Consumption. J. Biol. Sci. 2011, 11, 448–453. [Google Scholar] [CrossRef]
- Saeed, A.A.M.; Alkhader, S.K.; Al-Salim, M.S. ICP-OES Analysis of Some Nonessential Trace Elements in Hen’s Eggs. J. Pure Appl. Sci. 2022, 21, 28–34. [Google Scholar] [CrossRef]
- Dobrzański, Z.; Chojnacka, K.; Trziszka, T.; Opaliński, S.; Bobak, L.; Konkol, D.; Korczyński, M. The Effect of Dietary Humic Preparations on the Content of Essential and Non-Essential Chemical Elements in Hen Eggs. Animals 2020, 10, 1252. [Google Scholar] [CrossRef]
- Alasfar, R.H.; Isaifan, R.J. Aluminum Environmental Pollution: The Silent Killer. Environ. Sci. Pollut. Res. 2021, 28, 44587–44597. [Google Scholar] [CrossRef]
- Kaya-Umucalilar, H.D.; Halidoglu, S.; Ipek, H. Effect of Dietary Vitamin A and Zinc on Egg Yield and Some Blood Parameters of Laying Hens. Turkish J. Vet. Anim. Sci. 2001, 25, 763–769. [Google Scholar]
- Berman, E. Toxic Metals and Their Analysis; Heyden and San Ltd.: London, UK, 1980. [Google Scholar]
- Takahashi, A. Role of Zinc and Copper in Erythropoiesis in Patients on Hemodialysis. J. Ren. Nutr. 2022, 32, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.H.; Alibhai, Y. Teratogenicity of metals to chick embryos. J. Toxicol. Environ. Health 1990, 30, 23–31. [Google Scholar] [CrossRef]
- Nevin, V. Toxicoloy; No.73; Ankara University Faculty of Farmacy Press: Ankara, Turkey, 1996. [Google Scholar]
- Yazdanparast, T.; Strezov, V.; Wieland, P.; Lai, Y.-J.; Jacob, D.E.; Taylor, M.P. Lead poisoning of backyard chickens: Implications for urban gardening and food production. Environ. Pollut. 2022, 310, 119798. [Google Scholar] [CrossRef] [PubMed]
- Zheng, N.; Wang, Q.; Zhang, X.; Zheng, D.; Zhang, Z.; Zhang, S. Population health risk due to dietary intake of heavy metals in the industrial area of Huludao city, China. Sci. Total Environ. 2007, 387, 96–104. [Google Scholar] [CrossRef]
- Noël, L.; Chekri, R.; Millour, S.; Vastel, C.; Kadar, A.; Sirot, V.; Leblanc, J.-C.; Guérin, T. Li, Cr, Mn, Co, Ni, Cu, Zn, Se and Mo levels in foodstuffs from the Second French TDS. Food Chem. 2012, 132, 1502–1513. [Google Scholar] [CrossRef]
- Aliu, H.; Dizman, S.; Sinani, A.; Hodoll, G. Comparative Study of Heavy Metal Concentration in Eggs Originating from Industrial Poultry Farms and Free-Range Hens in Kosovo. J. Food Qual. 2021, 2021, 6615289. [Google Scholar] [CrossRef]
- Langard, S.; Vigander, T. Occurrence of lung cancer in workers producing chromium pigments. Br. J. Ind. Med. 1983, 40, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.R.; Powell, S.; Johnston, S.; Shelton, J.L.; Bidner, T.D.; Valdez, F.R.; Southern, L.L. The effect of chromium propionate on growth performance and carcass traits in broilers. J. Appl. Poult. Res. 2008, 17, 476–481. [Google Scholar] [CrossRef]
- Aendo, P.; Netvichian, R.; Tippayalak, S.; Sanguankiat, A.; Khuntamoon, T.; Songserm, T.; Tulayakul, P. Health risk contamination of heavy metals in yolk and albumen of duck eggs collected in central and western Thailand. Biol. Trace Elem. Res. 2018, 184, 501–507. [Google Scholar] [CrossRef]
- Marín, S.; Pardo, O.; Sánchez, A.; Sanchis, Y.; Vélez, D.; Devesa, V.; Font, G.; Yusà, V. Assessment of metal levels in foodstuffs from the Region of Valencia (Spain). Toxicol. Rep. 2018, 5, 654–670. [Google Scholar] [CrossRef]
- Siddiqui, I.; Nazami, S.S.; Ahmed Khan, F.; Bhutto, S.; Tahir, M.; Munshi, A.B.; Syed, N. Determination of some heavy metals in hen eggs using ICP-AES technique. Pak. J. Biochem. Mol. Biol. 2011, 44, 133–136. [Google Scholar]
- Zakir Hossain, S.M.; Brennan, J.D. Β-galactosidase-based colorimertric paper sensor for determination of heavy metals. Anal. Chem. 2011, 83, 8772–8778. [Google Scholar] [CrossRef]
- IARC. Cadmium, Nickel, Some Epoxides, Miscellaneous Industrial Chemicals and General Considerations on Volatile Anaesthetices (International Agency for Research on Cancer). IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Man 11. International Agency for Research on Cancer, Lyon, 1976, pp. 39–74. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Cadmium-Nickel-Some-Epoxides-Miscellaneous-Industrial-Chemicals-And-General-Considerations-On-Volatile-Anaesthetics-1976 (accessed on 23 August 2023).
- Iwegbue, C.M.A.; Nwozo, S.O.; Overah, C.L.; Ossai, E.K.; Mkpado, C.I.; Osazuwa, O.; Nwajei, G.E. Concentrations of selected metals in chicken eggs from commercial farms in Southern Nigeria. Toxicol. Environ. Chem. 2012, 94, 1152–1163. [Google Scholar] [CrossRef]
- Ysart, G.; Miller, P.; Croasdale, M.; Crews, H.; Robb, P.; Baxter, M.; De L’Argy, C.; Harrison, N. 1997 UK total diet study-dietary exposures to aluminum, arsenic, cadmium, chromium, copper, lead, mercury, nickel, selenium, tin and zinc. Food Addit. Contam. 2000, 17, 775–786. [Google Scholar] [CrossRef]
- Uluozlu, O.D.; Tuzen, M.; Mendil, D.; Soylak, M. Assessment of trace element contents of chicken products from Turkey. J. Hazard. Mater. 2009, 163, 982–987. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Khafaga, A.F.; Noreldin, A.E.; Arif, M.; Chaudhry, M.T.; Abdel-Daim, M.M. Impacts of rare earth elements on animal health and production: Highlights of cerium and lanthanum. Sci. Total Environ. 2019, 672, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Shi, L.; Hou, G.; Zhou, H.; Yue, W.; Zhang, C.; Ren, Y. Effect of rare earth elements on feed digestibility, rumen fermentation and purine derivates in sheep. Ital. J. Anim. Sci. 2014, 13, 357–362. [Google Scholar] [CrossRef][Green Version]
- Aendo, P.; Thongyuan, S.; Songserm, T.; Tulayakul, P. Carcinogenic and non-carcinogenic risk assessment of heavy metals contamination in duck eggs and meat as a warning scenario in Thailand. Sci. Total Environ. 2019, 689, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.M.; Wen, K.; Xue, Y.; Liu, L.; Geng, T.Y.; Gong, D.Q.; Yu, L. Probing the effects of dietary selenised glucose on the selenium concentration, quality, and antioxidant activity of eggs and production performances of laying hens. Animal 2021, 15, 100374. [Google Scholar] [CrossRef]

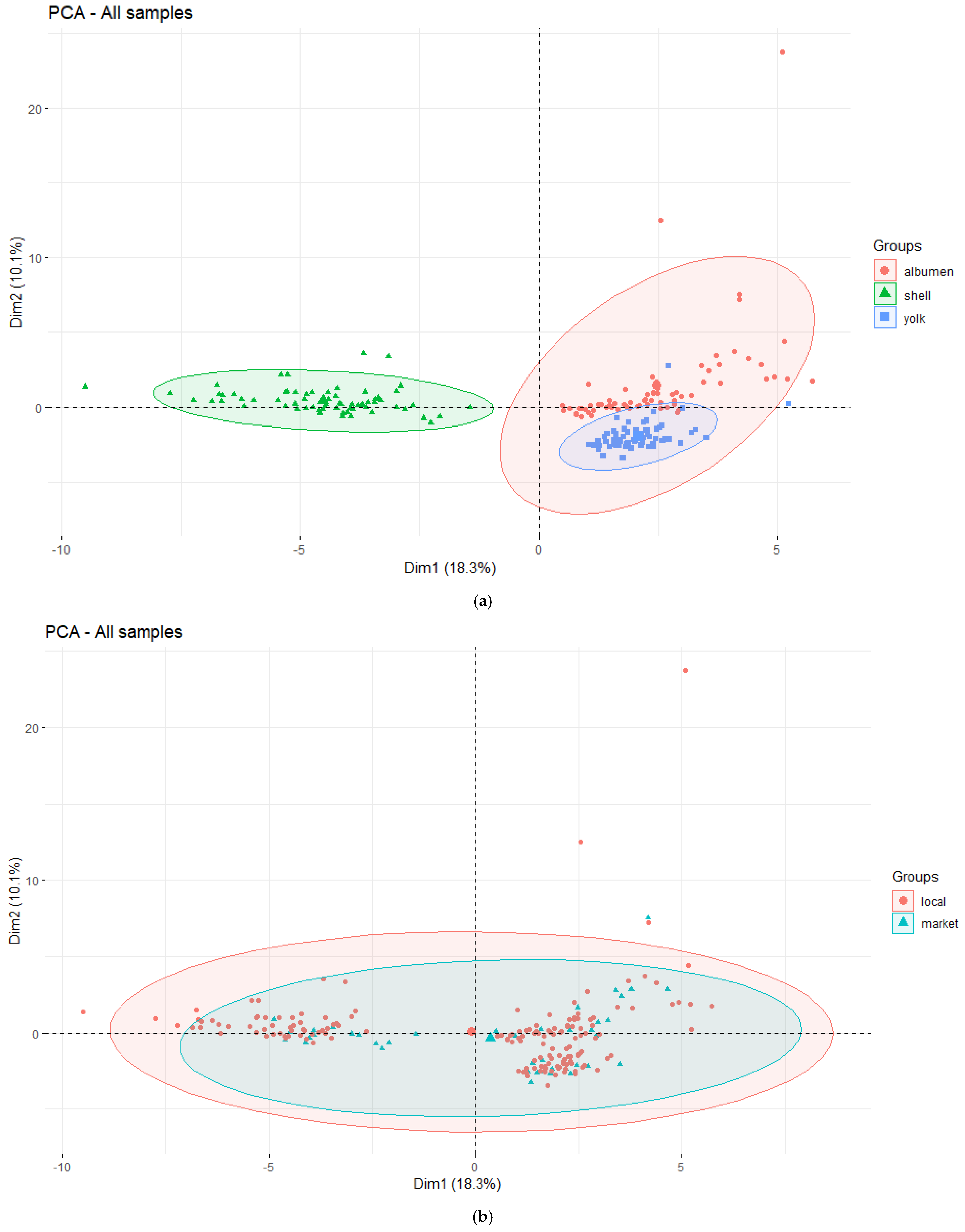
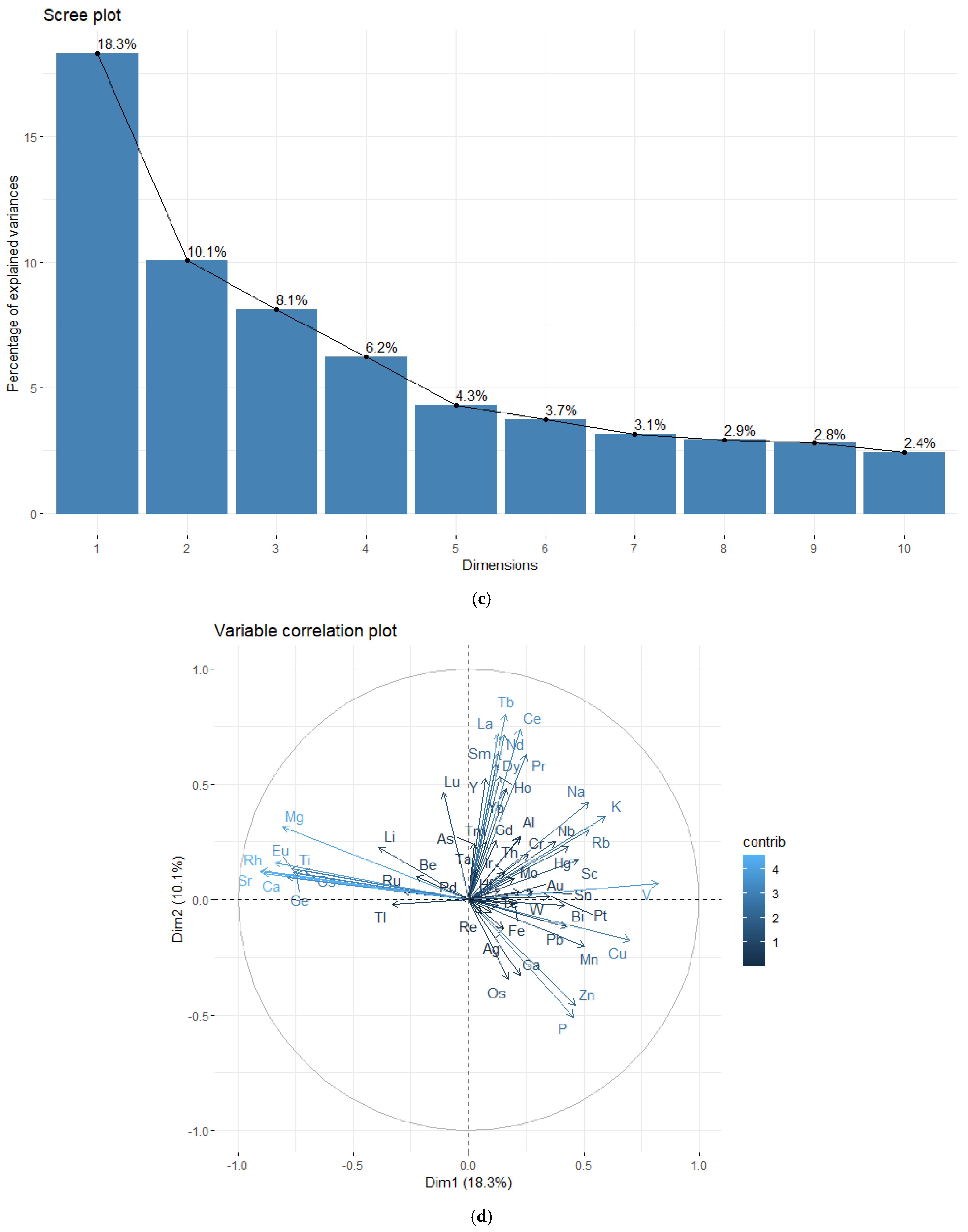

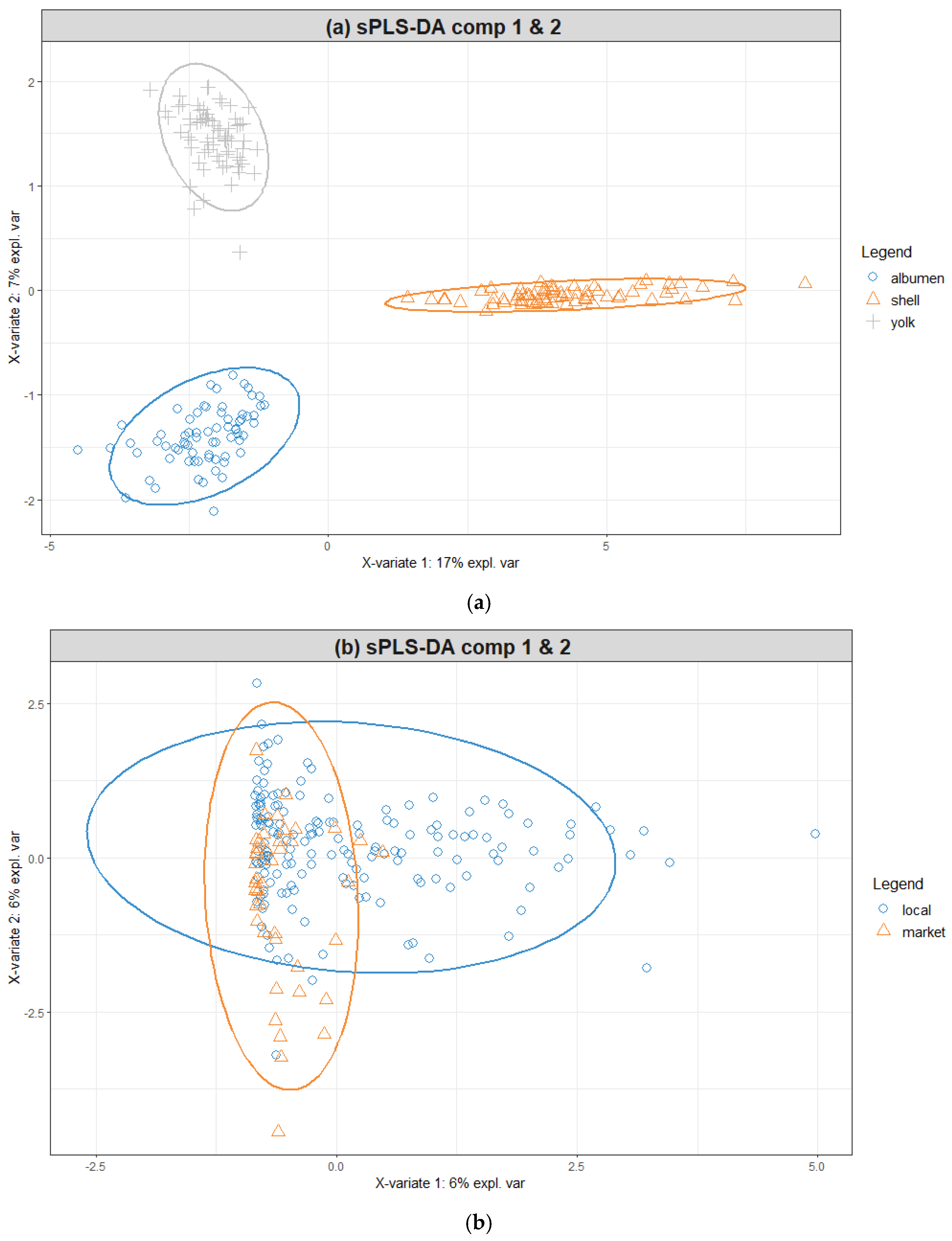

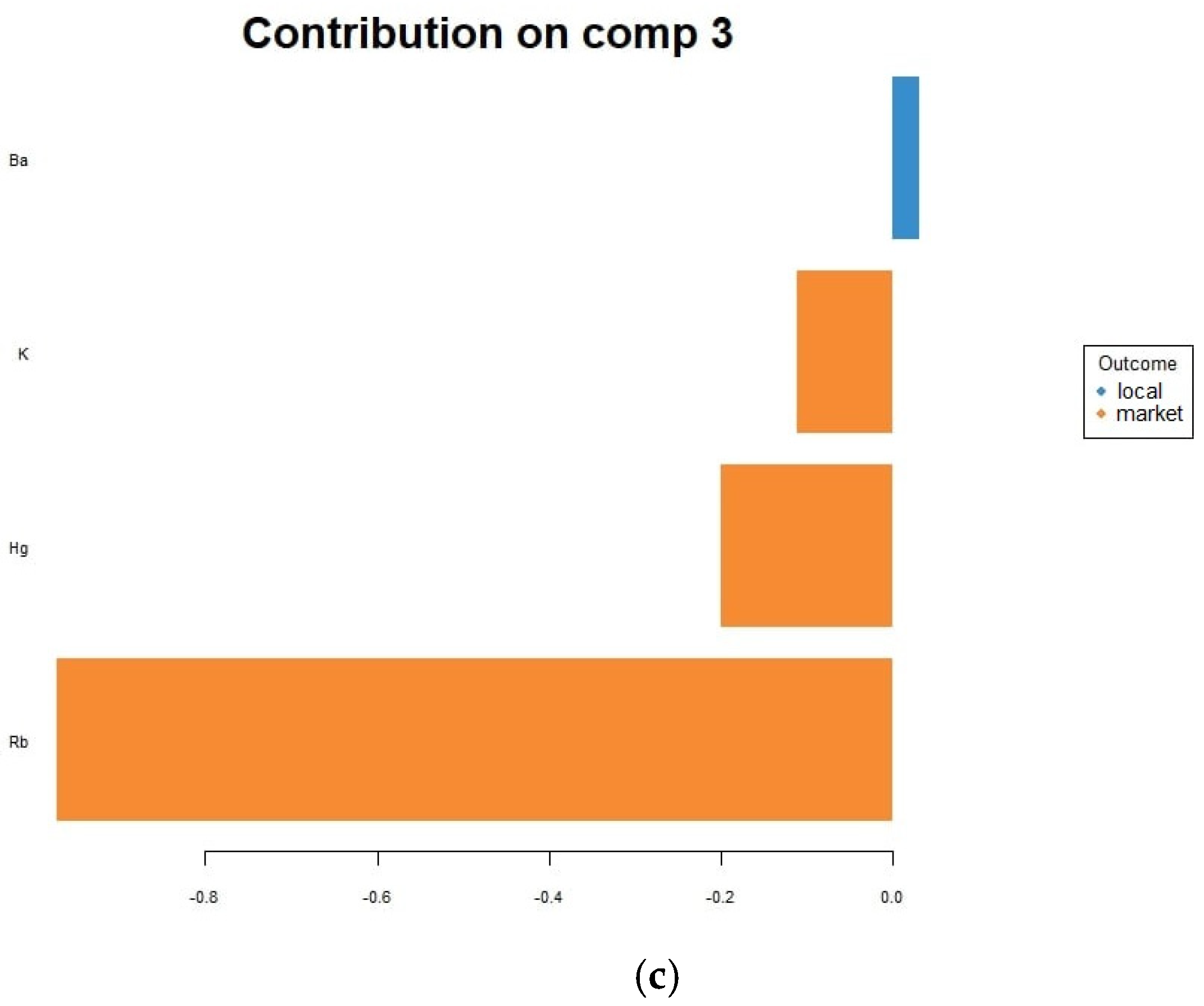
| Elements | Element Concentration (mg/kg) | |||
|---|---|---|---|---|
| Egg | Minimum | Maximum | Mean | |
| Na | egg white | 5900.00 | 15,373.56 | 10,266.37 |
| yolk | 156.75 | 1200.11 | 629.06 | |
| eggshell | 300.22 | 1183.84 | 653.64 | |
| Mg | egg white | 290.55 | 965.85 | 639.20 |
| yolk | 53.10 | 230.45 | 176.03 | |
| eggshell | 738.57 | 3000.70 | 1488.01 | |
| P | egg white | 220.38 | 1400.02 | 665.93 |
| yolk | 1331.11 | 5000.44 | 3856.23 | |
| eggshell | 230.29 | 873.81 | 521.04 | |
| K | egg white | 526.95 | 6977.12 | 4151.84 |
| yolk | 280.72 | 921.95 | 593.43 | |
| eggshell | 85.56 | 346.10 | 156.72 | |
| Ca | egg white | 130.11 | 2928.81 | 453.49 |
| yolk | 438.15 | 1843.34 | 1225.18 | |
| eggshell | 40,813.66 | 139,519.80 | 74,643.56 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voica, C.; Cristea, G.; Iordache, A.M.; Roba, C.; Curean, V. Elemental Profile in Chicken Egg Components and Associated Human Health Risk Assessment. Toxics 2023, 11, 900. https://doi.org/10.3390/toxics11110900
Voica C, Cristea G, Iordache AM, Roba C, Curean V. Elemental Profile in Chicken Egg Components and Associated Human Health Risk Assessment. Toxics. 2023; 11(11):900. https://doi.org/10.3390/toxics11110900
Chicago/Turabian StyleVoica, Cezara, Gabriela Cristea, Andreea Maria Iordache, Carmen Roba, and Victor Curean. 2023. "Elemental Profile in Chicken Egg Components and Associated Human Health Risk Assessment" Toxics 11, no. 11: 900. https://doi.org/10.3390/toxics11110900
APA StyleVoica, C., Cristea, G., Iordache, A. M., Roba, C., & Curean, V. (2023). Elemental Profile in Chicken Egg Components and Associated Human Health Risk Assessment. Toxics, 11(11), 900. https://doi.org/10.3390/toxics11110900









