Simulation and Characterization of Nanoplastic Dissolution under Different Food Consumption Scenarios
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Scenario Setup
2.3. Preparation of Colloidal Silver
2.4. Characterization of NPs
2.5. Data Analysis
3. Results and Discussion
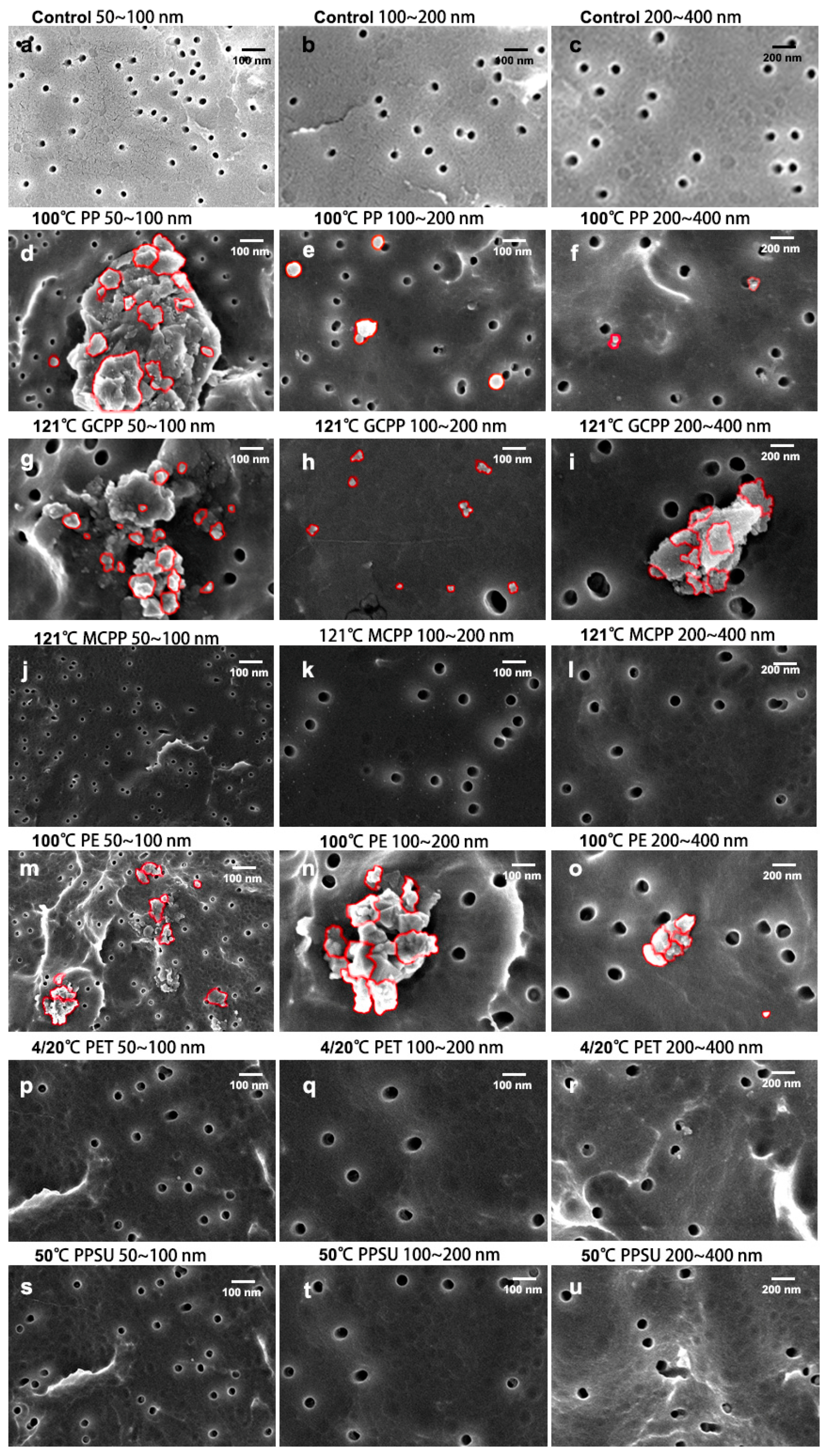
3.1. Morphology Analysis of NPs
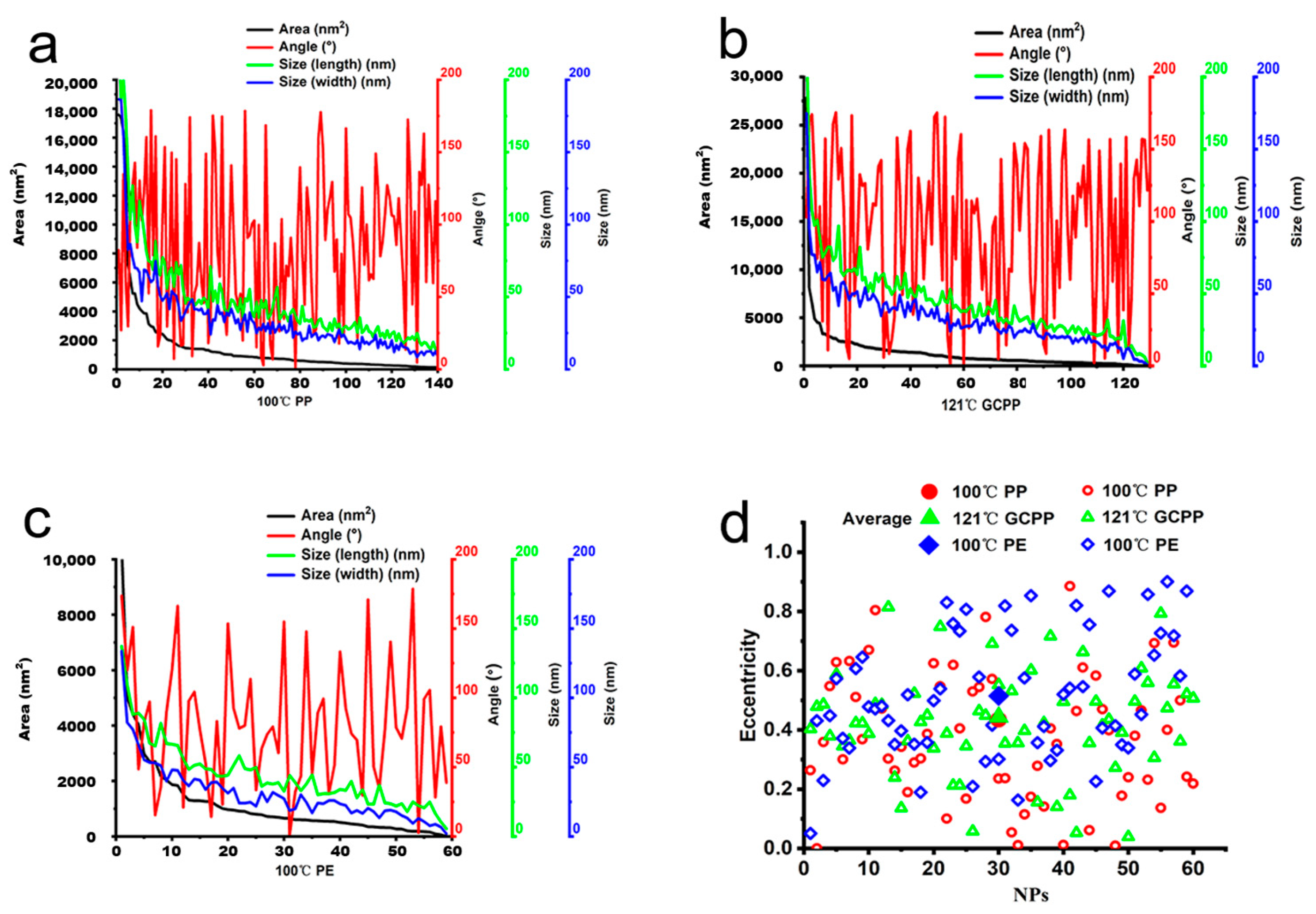
3.2. Measurement Parameters of NP Particles
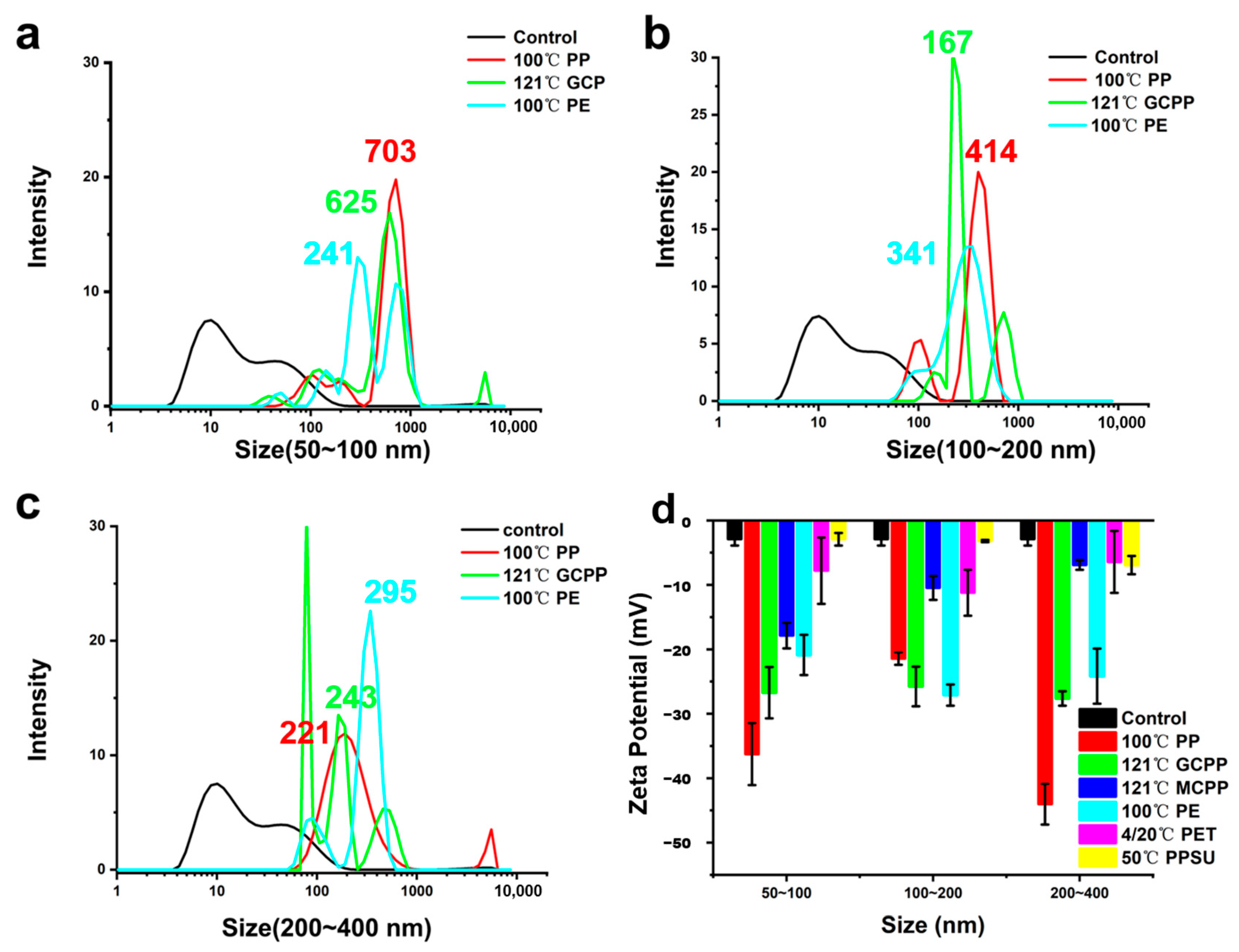
3.3. Particle Size Distribution and Zeta Potential of the NPs
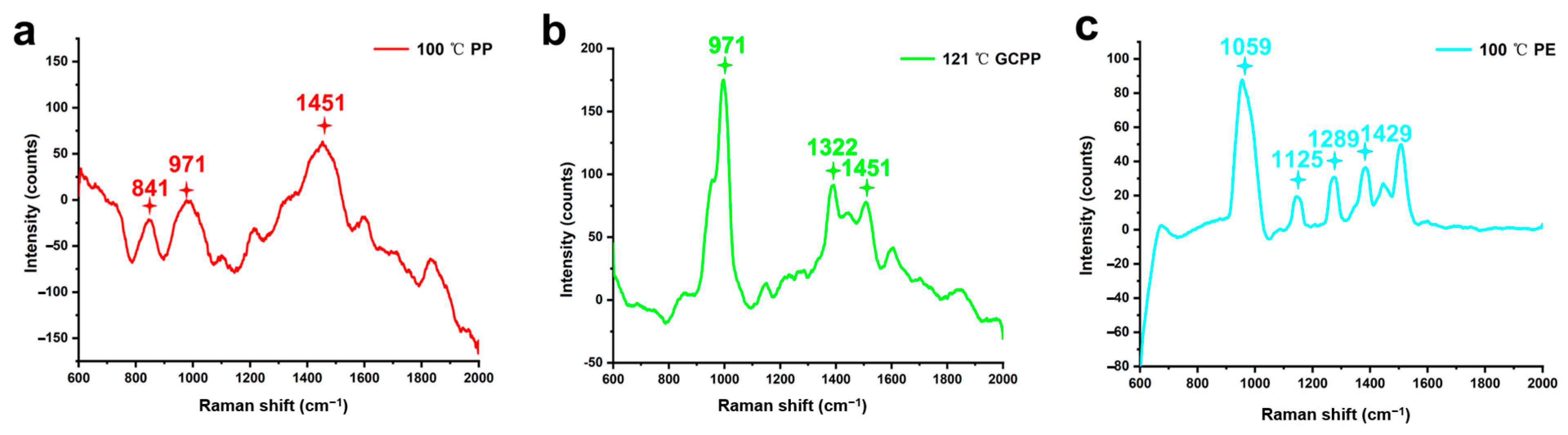
3.4. SERS Spectroscopy Analysis of the NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yates, J.; Deeney, M.; Rolker, H.B.; White, H.; Kalamatianou, S.; Kadiyala, S. A systematic scoping review of environmental, food security and health impacts of food system plastics. Nat. Food 2021, 2, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Enfrin, M.; Dumee, L.F.; Lee, J. Nano/microplastics in water and wastewater treatment processes—Origin, impact and potential solutions. Water Res. 2019, 161, 621–638. [Google Scholar] [CrossRef] [PubMed]
- Julienne, F.; Delorme, N.; Lagarde, F. From macroplastics to microplastics: Role of water in the fragmentation of polyethylene. Chemosphere 2019, 236, 124409. [Google Scholar] [CrossRef]
- Pramanik, B.K.; Pramanik, S.K.; Monira, S. Understanding the fragmentation of microplastics into nano-plastics and removal of nano/microplastics from wastewater using membrane, air flotation and nano-ferrofluid processes. Chemosphere 2021, 282, 131053. [Google Scholar] [CrossRef] [PubMed]
- Cerasa, M.; Teodori, S.; Pietrelli, L. Searching Nanoplastics: From Sampling to Sample Processing. Polymers 2021, 13, 3658. [Google Scholar] [CrossRef]
- Cai, H.W.; Xu, E.G.; Du, F.N.; Li, R.L.; Liu, J.F.; Shi, H.H. Analysis of environmental nanoplastics: Progress and challenges. Chem. Eng. J. 2021, 410, 128208. [Google Scholar] [CrossRef]
- Huang, D.L.; Tao, J.X.; Cheng, M.; Deng, R.; Chen, S.; Yin, L.S.; Li, R.J. Microplastics and nanoplastics in the environment: Macroscopic transport and effects on creatures. J. Hazard. Mater. 2021, 407, 124399. [Google Scholar] [CrossRef]
- Bradney, L.; Wijesekara, H.; Palansooriya, K.N.; Obadamudalige, N.; Bolan, N.S.; Ok, Y.S.; Rinklebe, J.; Kim, K.H.; Kirkham, M.B. Particulate plastics as a vector for toxic trace-element uptake by aquatic and terrestrial organisms and human health risk. Environ. Int. 2019, 131, 104937. [Google Scholar] [CrossRef]
- Hernandez, L.M.; Xu, E.G.; Larsson, H.C.E.; Tahara, R.; Maisuria, V.B.; Tufenkji, N. Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea. Environ. Sci. Technol. 2019, 53, 12300–12310. [Google Scholar] [CrossRef]
- Liebezeit, G.; Liebezeit, E. Origin of Synthetic Particles in Honeys. Pol. J. Food Nutr. Sci. 2015, 65, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; Van den Eede, G. Review of micro- and nanoplastic contamination in the food chain. Food Addit. Contam. Part A 2019, 36, 639–673. [Google Scholar] [CrossRef]
- Winkler, A.; Santo, N.; Ortenzi, M.A.; Bolzoni, E.; Bacchetta, R.; Tremolada, P. Does mechanical stress cause microplastic release from plastic water bottles? Water Res. 2019, 166, 115082. [Google Scholar] [CrossRef] [PubMed]
- Fadare, O.O.; Wan, B.; Guo, L.H.; Zhao, L.X. Microplastics from consumer plastic food containers: Are we consuming it? Chemosphere 2020, 253, 126787. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Z.; Shi, Y.H.; Yang, L.M.; Xiao, L.W.; Kehoe, D.K.; Gun’ko, Y.K.; Boland, J.J.; Wang, J.J. Microplastic release from the degradation of polypropylene feeding bottles during infant formula preparation. Nat. Food 2020, 1, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, V.P.; Joseph, A.; Goel, S. Microplastics and other harmful substances released from disposable paper cups into hot water. J. Hazard. Mater. 2021, 404, 124118. [Google Scholar] [CrossRef]
- Guo, X.; Lin, H.L.; Xu, S.P.; He, L.L. Recent Advances in Spectroscopic Techniques for the Analysis of Microplastics in Food. J. Agric. Food Chem. 2022, 70, 1410–1422. [Google Scholar] [CrossRef]
- Schmid, P.; Welle, F. Chemical Migration from Beverage Packaging Materials—A Review. Beverages 2020, 6, 37. [Google Scholar] [CrossRef]
- Eckardt, M.; Greb, A.; Simat, T.J. Polyphenylsulfone (PPSU) for baby bottles: A comprehensive assessment on polymer-related non-intentionally added substances (NIAS). Food Addit. Contam. A 2018, 35, 1421–1437. [Google Scholar] [CrossRef]
- von der Esch, E.; Lanzinger, M.; Kohles, A.J.; Schwaferts, C.; Weisser, J.; Hofmann, T.; Glas, K.; Elsner, M.; Ivleva, N.P. Simple Generation of Suspensible Secondary Microplastic Reference Particles via Ultrasound Treatment. Front. Chem. 2020, 8, 169. [Google Scholar] [CrossRef] [Green Version]
- Anger, P.M.; von der Esch, E.; Baumann, T.; Elsner, M.; Niessner, R.; Ivleva, N.P. Raman microspectroscopy as a tool for microplastic particle analysis. TrAC Trends Anal. Chem. 2018, 109, 214–226. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Formation of microscopic particles during the degradation of different polymers. Chemosphere 2016, 161, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Leopold, N.; Lendl, B. A new method for fast preparation of highly surface-enhanced Raman scattering (SERS) active silver colloids at room temperature by reduction of silver nitrate with hydroxylamine hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Chen, H.E.; Xu, L.H.; Yu, K.; Wei, F.; Zhang, M. Release of microplastics from disposable cups in daily use. Sci. Total Environ. 2023, 854, 158606. [Google Scholar] [CrossRef]
- Aliakbarzadeh, F.; Rafiee, M.; Khodagholi, F.; Khorramizadeh, M.R.; Manouchehri, H.; Eslami, A.; Sayehmiri, F.; Mohseni-Bandpei, A. Adverse effects of polystyrene nanoplastic and its binary mixtures with nonylphenol on zebrafish nervous system: From oxidative stress to impaired neurotransmitter system. Environ. Pollut. 2023, 317, 120587. [Google Scholar] [CrossRef]
- Liu, G.Q.; Wang, J.; Wang, M.J.; Ying, R.R.; Li, X.W.; Hu, Z.W.; Zhang, Y. Disposable plastic materials release microplastics and harmful substances in hot water. Sci. Total Environ. 2022, 818, 151685. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Magni, S.; Tretola, M.; Luciano, A.; Ferrari, L.; Bernardi, C.E.M.; Lin, P.; Ottoboni, M.; Binelli, A.; Pinotti, L. Packaging contaminants in former food products: Using Fourier Transform Infrared Spectroscopy to identify the remnants and the associated risks. J. Hazard. Mater. 2023, 448, 130888. [Google Scholar] [CrossRef]
- Zhou, G.Y.; Wu, Q.D.; Tang, P.; Chen, C.; Cheng, X.; Wei, X.F.; Ma, J.; Liu, B.C. How many microplastics do we ingest when using disposable drink cups? J. Hazard. Mater. 2023, 441, 129982. [Google Scholar] [CrossRef]
- Goral, K.D.; Guler, H.G.; Larsen, B.E.; Carstensen, S.; Christensen, E.D.; Kerpen, N.B.; Schlurmann, T.; Fuhrman, D.R. Settling velocity of microplastic particles having regular and irregular shapes. Environ. Res. 2023, 228, 115783. [Google Scholar] [CrossRef]
- Wang, Z.; Dou, M.; Ren, P.J.; Sun, B.; Jia, R.P.; Zhou, Y.Z. Settling velocity of irregularly shaped microplastics under steady and dynamic flow conditions. Environ. Sci. Pollut. Res. 2021, 28, 62116–62132. [Google Scholar] [CrossRef]
- Wang, X.J.; Bolan, N.; Tsang, D.C.W.; Sarkar, B.; Bradney, L.; Li, Y. A review of microplastics aggregation in aquatic environment: Influence factors, analytical methods, and environmental implications. J. Hazard. Mater. 2021, 402, 123496. [Google Scholar] [CrossRef]
- Liu, L.; Song, J.; Zhang, M.; Jiang, W. Aggregation and Deposition Kinetics of Polystyrene Microplastics and Nanoplastics in Aquatic Environment. Bull. Environ. Contam. Toxicol. 2021, 107, 741–747. [Google Scholar] [CrossRef]
- Xiang, Q.Q.; Zhou, Y.; Tan, C.X. Toxicity Effects of Polystyrene Nanoplastics with Different Sizes on Freshwater Microalgae Chlorella vulgaris. Molecules 2023, 28, 3958. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.Y.; Wu, I.H.; Tsai, C.Y.; Kirankumar, R.; Hsieh, S.C. Detecting the release of plastic particles in packaged drinking water under simulated light irradiation using surface-enhanced Raman spectroscopy. Anal. Chim. Acta 2022, 1198, 339516. [Google Scholar] [CrossRef] [PubMed]
- Dzhagan, V.; Mazur, N.; Smirnov, O.; Yeshchenko, O.; Isaieva, O.; Kovalenko, M.; Vuichyk, M.; Skoryk, M.; Pirko, Y.; Yemets, A.; et al. SERS application of Ag nanoparticles synthesized with aqueous fungi extract. J. Nanopart. Res. 2023, 25, 37. [Google Scholar] [CrossRef]
- Zhou, X.J.; Wang, J.; Ren, J.F. Analysis of Microplastics in Takeaway Food Containers in China Using FPA-FTIR Whole Filter Analysis. Molecules 2022, 27, 2646. [Google Scholar] [CrossRef] [PubMed]
- Zangmeister, C.D.; Radney, J.G.; Benkstein, K.D.; Kalanyan, B. Common Single-Use Consumer Plastic Products Release Trillions ofSub-100 nm Nanoparticles per Liter into Water during Normal Use. Environ. Sci. Technol. 2022, 56, 5448–5455. [Google Scholar] [CrossRef]
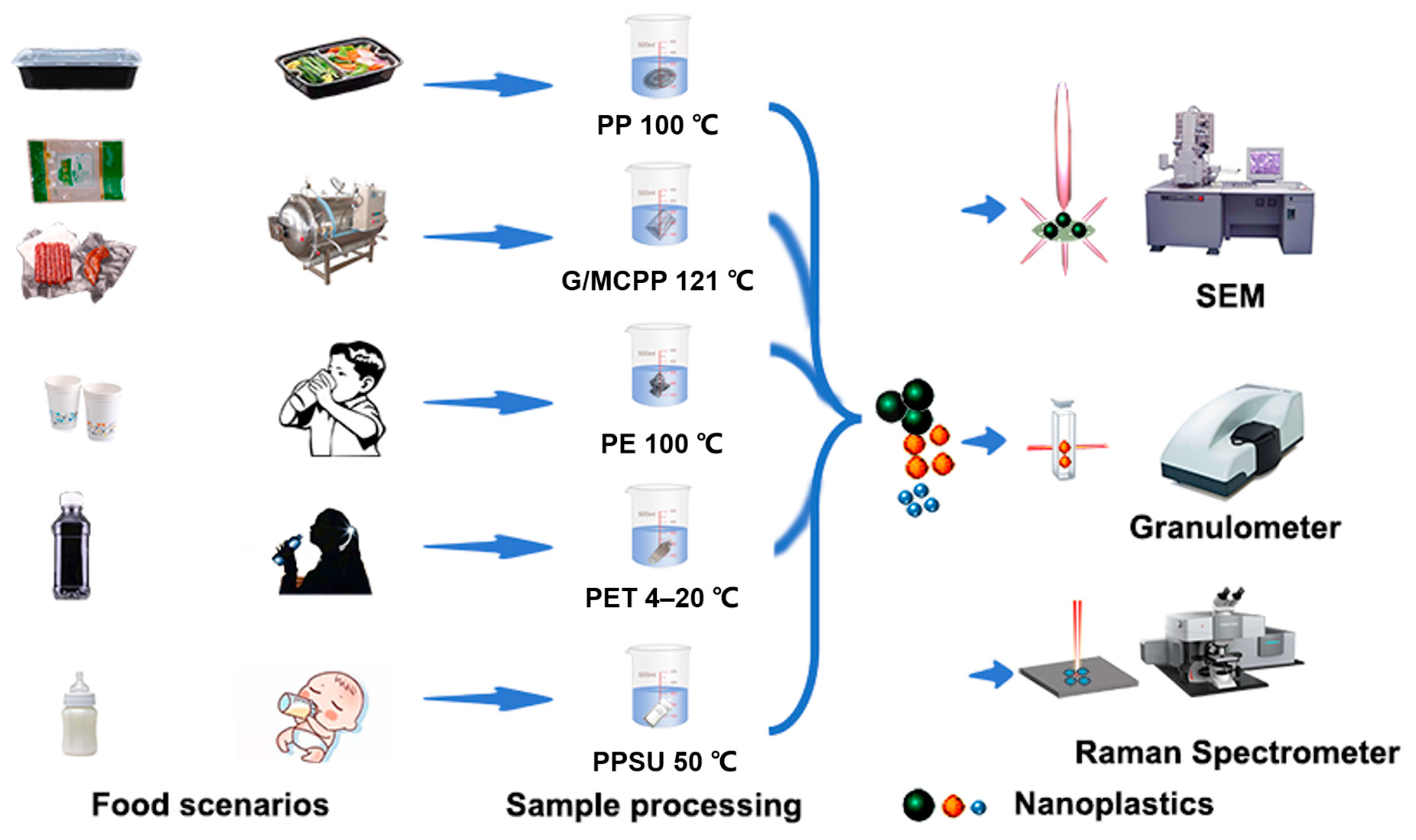

| Materials | Simulation | Reasons for Selection | Reachable Temperature | Experiment Condition | |
|---|---|---|---|---|---|
| Temperature | Time | ||||
| PP | Common packaging for hot food with plastic boxes | These six plastic materials are common in food packaging and food consumption scenarios, including industrial food production (GCPP/MCPP), daily foods, beverages (PP/PE/PET), and infant foods (PPSU) | 60–100 °C | 100 °C | 20 min |
| GCPP | Industrial production prepackaging foods, 121 °C high-temperature sterilization process | 100–121 °C | 121 °C | 20 min | |
| MCPP | 100–121 °C | 121 °C | 20 min | ||
| PE | Drinking hot water with embedded PE disposable paper cup, packaging hot food with PE plastic bags | 50–100 °C | 100 °C | Natural cooling | |
| PET | Common water and beverage plastic bottles | 4–20 °C | 4/20 °C | 20 min | |
| PPSU | Used for dissolving milk powder for infants | 50 °C | 50 °C | 20 min | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, Z.; Lu, X.; Zhang, H.; Jia, Z. Simulation and Characterization of Nanoplastic Dissolution under Different Food Consumption Scenarios. Toxics 2023, 11, 550. https://doi.org/10.3390/toxics11070550
Wang Y, Wang Z, Lu X, Zhang H, Jia Z. Simulation and Characterization of Nanoplastic Dissolution under Different Food Consumption Scenarios. Toxics. 2023; 11(7):550. https://doi.org/10.3390/toxics11070550
Chicago/Turabian StyleWang, Ying, Zhongtang Wang, Xin Lu, Hongyan Zhang, and Zhenzhen Jia. 2023. "Simulation and Characterization of Nanoplastic Dissolution under Different Food Consumption Scenarios" Toxics 11, no. 7: 550. https://doi.org/10.3390/toxics11070550








