Neurotoxicity of Benzotriazole Ultraviolet Stabilizers in Teleost Fishes: A Review
Abstract
1. Introduction: Ultraviolet Stabilizers, Non-Negligible Additives in Plastics
2. Objectives of the Review
3. Occurrence of Ultraviolet Stabilizers in Aquatic Environments
4. Neurotoxicity of Ultraviolet Stabilizers in Fish
4.1. Molecular and Biochemical Indicators of Neurotoxicity
4.2. Behavioral Indicators of Neurotoxicity
5. Biomarkers of Toxicity: Neurotoxic Indicators?
6. Conclusions
- (1)
- In-depth mechanistic studies on the central nervous system of zebrafish are needed to address neurotoxicity. Validation of specific neurotoxicity pathways relevant for BUVS exposure is needed.
- (2)
- Broader scope of behavioral assays related to the dopaminergic systems, such as anxiety-related and fear-related behaviors, given that the exploration of novel tank environments by fish is altered with exposures.
- (3)
- Histopathology of the central nervous system is needed following exposure to these chemicals, given evidence for neuronal damage, apoptosis, and neurodegeneration.
- (4)
- Ecologically important species would broaden the scope and environmental relevance of laboratory-based studies, as most studies are conducted using zebrafish. Nevertheless, the zebrafish model has proven useful for developmental toxicity studies for plasticizers and has improved our understanding of toxicity mechanisms in fish.
- (5)
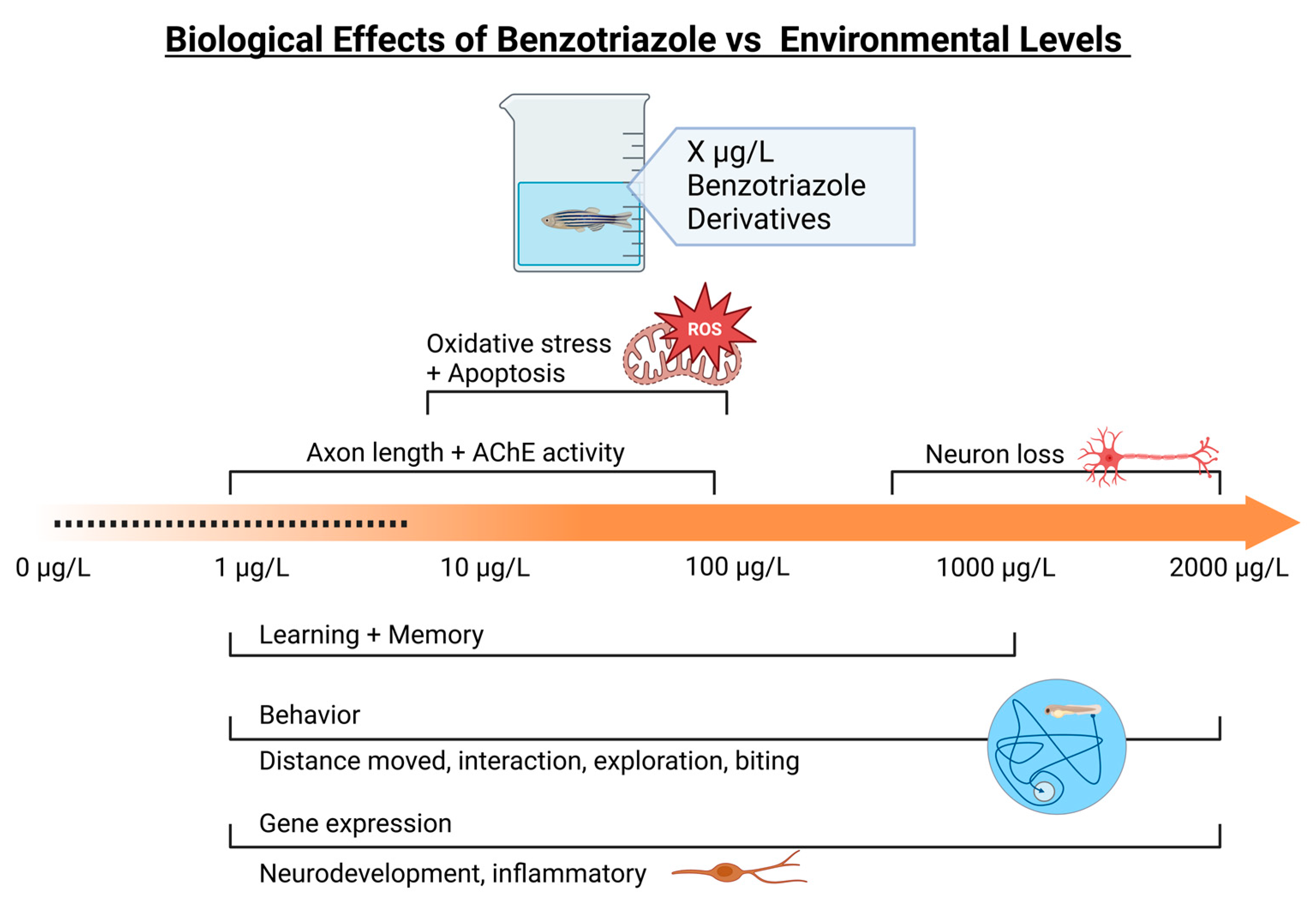
- Based on our review, several studies report neurological responses above environmental levels (Figure 5), although there are experimental data that correspond to environmental levels.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Do, A.T.N.; Ha, Y.; Kwon, J.H. Leaching of microplastic-associated additives in aquatic environments: A critical review. Environ. Pollut. 2022, 305, 119258. [Google Scholar] [CrossRef]
- Rahman, M.; Brazel, C.S. The plasticizer market: An assessment of traditional plasticizers and research trends to meet new challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Bernard, L.; Bourdeaux, D.; Pereira, B.; Azaroual, N.; Barthelemy, C.; Breysse, C.; Chennell, P.; Cueff, R.; Dine, T.; Eljezi, T.; et al. Analysis of plasticizers in PVC medical devices: Performance comparison of eight analytical methods. Talanta 2017, 162, 604–611. [Google Scholar] [CrossRef]
- Billings, A.; Jones, K.C.; Pereira, M.G.; Spurgeon, D.J. Plasticisers in the terrestrial environment: Sources, occurrence and fate. Environ. Chem. 2021, 18, 111–130. [Google Scholar] [CrossRef]
- Jia, P.Y.; Xia, H.Y.; Tang, K.H.; Zhou, Y.H. Plasticizers Derived from Biomass Resources: A Short Review. Polymers 2018, 10, 1303. [Google Scholar] [CrossRef]
- Wormuth, M.; Scheringer, M.; Vollenweider, M.; Hungerbuhler, K. What are the sources of exposure to eight frequently used phthalic acid esters in Europeans? Risk Anal. 2006, 26, 803–824. [Google Scholar] [CrossRef] [PubMed]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Shim, W.J.; Han, G.M.; Jang, M.; Song, Y.K.; Hong, S.H. Benzotriazole-type ultraviolet stabilizers and antioxidants in plastic marine debris and their new products. Sci. Total Environ. 2017, 579, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-W.; Isobe, T.; Ramaswamy, B.R.; Chang, K.-H.; Amano, A.; Miller, T.M.; Siringan, F.P.; Tanabe, S. Contamination and bioaccumulation of benzotriazole ultraviolet stabilizers in fish from Manila Bay, the Philippines using an ultra-fast liquid chromatography-tandem mass spectrometry. Chemosphere 2011, 85, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Montesdeoca-Esponda, S.; Vega-Morales, T.; Sosa-Ferrera, Z.; Santana-Rodriguez, J.J. Extraction and determination methodologies for benzotriazole UV stabilizers in personal-care products in environmental and biological samples. Trac.-Trends Anal. Chem. 2013, 51, 23–32. [Google Scholar] [CrossRef]
- Chisvert, A.; Leon-Gonzalez, Z.; Tarazona, I.; Salvador, A.; Giokas, D. An overview of the analytical methods for the determination of organic ultraviolet filters in biological fluids and tissues. Anal. Chim. Acta 2012, 752, 11–29. [Google Scholar] [CrossRef]
- Sanchez-Quiles, D.; Tovar-Sanchez, A. Are sunscreens a new environmental risk associated with coastal tourism? Environ. Int. 2015, 83, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Sakuragi, Y.; Takada, H.; Sato, H.; Kubota, A.; Terasaki, M.; Takeuchi, S.; Ikeda-Araki, A.; Watanabe, Y.; Kitamura, S.; Kojima, H. An analytical survey of benzotriazole UV stabilizers in plastic products and their endocrine-disrupting potential via human estrogen and androgen receptors. Sci. Total Environ. 2021, 800, 149374. [Google Scholar] [CrossRef] [PubMed]
- Khare, A.; Jadhao, P.; Kawre, S.; Kanade, G.; Patil, M.; Vaidya, A.N.; Kumar, A.R. Occurrence, spatio-temporal variation and ecological risk assessment of benzotriazole ultraviolet stabilizers (BUVs) in water and sediment of rivers in central India. Sci. Total Environ. 2023, 882, 163381. [Google Scholar] [CrossRef] [PubMed]
- OECD. HPV Database. Available online: https://hpvchemicals.oecd.org/UI/Search.aspx (accessed on 31 October 2023).
- Fujita, K.K.; Doering, J.A.; Stock, E.; Lu, Z.; Montina, T.; Wiseman, S. Effects of dietary 2-(2H-benzotriazol-2-yl)-4-methylphenol (UV-P) exposure on Japanese medaka (Oryzias latipes) in a short-term reproduction assay. Aquat. Toxicol. 2022, 248, 106206. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liang, X.; Liu, W.; Zhao, Y.; Yang, H.; Li, W.; Adamovsky, O.; Martyniuk, C.J. Elucidating mechanisms of immunotoxicity by benzotriazole ultraviolet stabilizers in zebrafish (Danio rerio): Implication of the AHR-IL17/IL22 immune pathway. Environ. Pollut. 2020, 262, 114291. [Google Scholar] [CrossRef]
- Kim, H.; Kim, B.; Shin, Y.-J.; Kim, J.; Kim, H.-j.; Kim, K.; Kim, P.; Park, K. Effect of benzotriazole on oxidative stress response and transcriptional gene expression in Oryzias latipes and Danio rerio embryo. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 252, 109222. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Otero, X.L.; Fernández, E.V.; Vieira, L.R.; Fernandes, J.O.; Cunha, S.C.; Guilhermino, L. Are microplastics contributing to pollution-induced neurotoxicity? A pilot study with wild fish in a real scenario. Heliyon 2023, 9, e13070. [Google Scholar] [CrossRef]
- Lu, Z.; Smyth, S.A.; Peart, T.E.; De Silva, A.O. Occurrence and fate of substituted diphenylamine antioxidants and benzotriazole UV stabilizers in various Canadian wastewater treatment processes. Water Res. 2017, 124, 158–166. [Google Scholar] [CrossRef]
- Awonaike, B.; Lei, Y.D.; Parajulee, A.; Wania, F. Phase partitioning, transport and sources of Benzotriazole Ultraviolet Stabilizers during a runoff event. Water Res. X 2021, 13, 100115. [Google Scholar] [CrossRef]
- Langford, K.H.; Reid, M.J.; Fjeld, E.; Øxnevad, S.; Thomas, K.V. Environmental occurrence and risk of organic UV filters and stabilizers in multiple matrices in Norway. Environ. Int. 2015, 80, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, T.; Sakane, F.; Kinoshita, C.; Sato, K.; Mizukawa, K.; Takada, H. COVID-19-derived plastic debris contaminating marine ecosystem: Alert from a sea turtle. Mar. Pollut. Bull. 2022, 175, 113389. [Google Scholar] [CrossRef]
- Ngoc Do, A.T.; Ha, Y.; Kang, H.J.; Kim, J.M.; Kwon, J.H. Equilibrium leaching of selected ultraviolet stabilizers from plastic products. J. Hazard. Mater. 2022, 427, 128144. [Google Scholar] [CrossRef] [PubMed]
- Montesdeoca-Esponda, S.; del Toro-Moreno, A.; Sosa-Ferrera, Z.; Juan Santana-Rodriguez, J. Development of a sensitive determination method for benzotriazole UV stabilizers in enviromental water samples with stir bar sorption extraction and liquid desorption prior to ultra-high performance liquid chromatography with tandem mass spectrometry. J. Sep. Sci. 2013, 36, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Guerra, R.B.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, A.; Furton, K.G.; Juan Santana-Rodriguez, J. Rapid monitoring of residual UV-stabilizers in seawater samples from beaches using fabric phase sorptive extraction and UHPLC-MS/MS. Chemosphere 2016, 164, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Murata, S.; Filatreau, J. Occurrence and concentrations of benzotriazole UV stabilizers in marine organisms and sediments from the Ariake Sea, Japan. Environ. Sci. Technol. 2009, 43, 6920–6926. [Google Scholar] [CrossRef] [PubMed]
- Ruan, T.; Liu, R.; Fu, Q.; Thanh, W.; Wang, Y.; Song, S.; Wang, P.; Teng, M.; Jiang, G. Concentrations and Composition Profiles of Benzotriazole UV Stabilizers in Municipal Sewage Sludge in China. Environ. Sci. Technol. 2012, 46, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Chang, K.H.; Prudente, M.; Viet, P.H.; Takahashi, S.; Tanabe, S.; Kunisue, T.; Isobe, T. Occurrence of benzotriazole ultraviolet stabilizers (BUVSs) in human breast milk from three Asian countries. Sci. Total Environ. 2019, 655, 1081–1088. [Google Scholar] [CrossRef]
- Denghel, H.; Goen, T. Determination of the UV absorber 2-(2H-benzotriazol-2-yl)-4,6-di-tert-pentylphenol (UV 328) and its oxidative metabolites in human urine by dispersive liquid-liquid microextraction and GC-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1144, 122071. [Google Scholar] [CrossRef]
- Kim, J.W.; Isobe, T.; Malarvannan, G.; Sudaryanto, A.; Chang, K.H.; Prudente, M.; Tanabe, S. Contamination of benzotriazole ultraviolet stabilizers in house dust from the Philippines: Implications on human exposure. Sci. Total Environ. 2012, 424, 174–181. [Google Scholar] [CrossRef]
- Lu, Z.; De Silva, A.O.; Zhou, W.; Tetreault, G.R.; de Solla, S.R.; Fair, P.A.; Houde, M.; Bossart, G.; Muir, D.C.G. Substituted diphenylamine antioxidants and benzotriazole UV stabilizers in blood plasma of fish, turtles, birds and dolphins from North America. Sci. Total Environ. 2019, 647, 182–190. [Google Scholar] [CrossRef]
- Montesdeoca-Esponda, S.; Esther Torres-Padron, M.; Novak, M.; Krchova, L.; Sosa-Ferrera, Z.; Juan Santana-Rodriguez, J. Occurrence of benzotriazole UV stabilizers in coastal fishes. J. Environ. Manag. 2020, 269, 110805. [Google Scholar] [CrossRef]
- Nakata, H.; Shinohara, R.-I.; Nakazawa, Y.; Isobe, T.; Sudaryanto, A.; Subramanian, A.; Tanabe, S.; Zakaria, M.P.; Zheng, G.J.; Lam, P.K.S.; et al. Asia-Pacific mussel watch for emerging pollutants: Distribution of synthetic musks and benzotriazole UV stabilizers in Asian and US coastal waters. Mar. Pollut. Bull. 2012, 64, 2211–2218. [Google Scholar] [CrossRef]
- Wick, A.; Jacobs, B.; Kunkel, U.; Heininger, P.; Ternes, T.A. Benzotriazole UV stabilizers in sediments, suspended particulate matter and fish of German rivers: New insights into occurrence, time trends and persistency. Environ. Pollut. 2016, 212, 401–412. [Google Scholar] [CrossRef]
- Asimakopoulos; Alexandros, G.; Ajibola, A.; Kannan, K.; Thomaidis, N.S. Occurrence and removal efficiencies of benzotriazoles and benzothiazoles in a wastewater treatment plant in Greece. Sci. Total Environ. 2013, 452, 163–171. [Google Scholar]
- Carpinteiro, I.; Ramil, M.; Rodriguez, I.; Nogueira, J.M.F. Combining stir-bar sorptive extraction and large volume injection-gas chromatography-mass spectrometry for the determination of benzotriazole UV stabilizers in wastewater matrices. J. Sep. Sci. 2012, 35, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Castilloux, A.D.; Houde, M.; Gendron, A.; De Silva, A.; Soubaneh, Y.D.; Lu, Z. Distribution and Fate of Ultraviolet Absorbents and IndustrialAntioxidants in the St. Lawrence River, Quebec, Canada. Environ. Sci. Technol. 2022, 56, 5009–5019. [Google Scholar] [CrossRef] [PubMed]
- Fenni, F.; Sunyer-Caldu, A.; Mansour, H.B.; Diaz-Cruz, M.S. Contaminants of emerging concern in marine areas: First evidence of UV filters and paraben preservatives in seawater and sediment on the eastern coast of Tunisia. Environ. Pollut. 2022, 309, 119749. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.X.; Cheng, Y.X.; Wu, D.; Fan, L.; Zhao, J.H.; Xiong, Q.; Chen, Q.L.; Liu, Y.S.; Ying, G.G. Continuous input of organic ultraviolet filters and benzothiazoles threatens the surface water and sediment of two major rivers in the Pearl River Basin. Sci. Total Environ. 2021, 798, 149299. [Google Scholar] [CrossRef] [PubMed]
- Kahle, M.; Buerge, I.J.; Muller, M.D.; Poiger, T. Hydrophilic anthropogenic markers for quantification of wastewater contamination in ground- and surface waters. Environ. Toxicol. Chem. 2009, 28, 2528–2536. [Google Scholar] [CrossRef] [PubMed]
- Karthikraj, R.; Kannan, K. Mass loading and removal of benzotriazoles, benzothiazoles, benzophenones, and bisphenols in Indian sewage treatment plants. Chemosphere 2017, 181, 216–223. [Google Scholar] [CrossRef]
- Liu, Y.-S.; Ying, G.-G.; Shareef, A.; Kookana, R.S. Simultaneous determination of benzotriazoles and ultraviolet filters in ground water, effluent and biosolid samples using gas chromatography-tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 5328–5335. [Google Scholar] [CrossRef]
- Lu, J.; Li, H.; Luo, Z.; Lin, H.; Yang, Z. Occurrence, distribution, and environmental risk of four categories of personal care products in the Xiangjiang River, China. Environ. Sci. Pollut. Res. 2018, 25, 27524–27534. [Google Scholar] [CrossRef]
- Lu, Z.; De Silva, A.O.; Peart, T.E.; Cook, C.J.; Tetreault, G.R. Tissue Distribution of Substituted Diphenylamine Antioxidants and Benzotriazole Ultraviolet Stabilizers in White Sucker (Catostomus commersonii) from an Urban Creek in Canada. Environ. Sci. Technol. Lett. 2017, 4, 433–438. [Google Scholar] [CrossRef]
- Lu, Z.; De Silva, A.O.; Peart, T.E.; Cook, C.J.; Tetreault, G.R.; Servos, M.R.; Muir, D.C.G. Distribution, Partitioning and Bioaccumulation of Substituted Diphenylamine Antioxidants and Benzotriazole UV Stabilizers in an Urban Creek in Canada. Environ. Sci. Technol. 2016, 50, 9089–9097. [Google Scholar] [CrossRef]
- Montesdeoca-Esponda, S.; Álvarez-Raya, C.; Torres-Padrón, M.E.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Monitoring and environmental risk assessment of benzotriazole UV stabilizers in the sewage and coastal environment of Gran Canaria (Canary Islands, Spain). J. Environ. Manag. 2019, 233, 567–575. [Google Scholar] [CrossRef]
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Santana-Rodriguez, J.J. On-line solid-phase extraction coupled to ultra-performance liquid chromatography with tandem mass spectrometry detection for the determination of benzotriazole UV stabilizers in coastal marine and wastewater samples. Anal. Bioanal. Chem. 2012, 403, 867–876. [Google Scholar] [CrossRef]
- Pei, J.; Hu, J.; Zhang, R.; Liu, N.; Yu, W.; Yan, A.; Han, M.; Liu, H.; Huang, X.; Yu, K. Occurrence, bioaccumulation and ecological risk of organic ultraviolet absorbers in multiple coastal and offshore coral communities of the South China Sea. Sci. Total Environ. 2023, 868, 161611. [Google Scholar] [CrossRef]
- Serra-Roig, M.P.; Jurado, A.; Díaz-Cruz, M.S.; Vázquez-Suñé, E.; Pujades, E.; Barceló, D. Occurrence, fate and risk assessment of personal care products in river–groundwater interface. Sci. Total Environ. 2016, 568, 829–837. [Google Scholar] [CrossRef]
- Song, S.; Ruan, T.; Wang, T.; Liu, R.; Jiang, G. Occurrence and removal of benzotriazole ultraviolet stabilizers in a wastewater treatment plant in China. Environ. Sci.-Process. Impacts 2014, 16, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Han, X.; Li, G.; Tian, S.; Yang, Y.; Zhong, F.; Han, Y.; Yang, J. Occurrence, distribution and ecological risk of ultraviolet absorbents in water and sediment from Lake Chaohu and its inflowing rivers, China. Ecotoxicol. Environ. Saf. 2018, 164, 540–547. [Google Scholar] [CrossRef]
- Vimalkumar, K.; Arun, E.; Krishna-Kumar, S.; Poopal, R.K.; Nikhil, N.P.; Subramanian, A.; Babu-Rajendran, R. Occurrence of triclocarban and benzotriazole ultraviolet stabilizers in water, sediment, fish from Indian rivers. Sci. Total Environ. 2018, 625, 1351–1360. [Google Scholar] [CrossRef]
- Vimalkumar, K.; Mayilsamy, M.; Arun, E.; Gobinath, B.; Prasanth, S.; Nikhil, P.N.; Krishna-Kumar, S.; Srimurali, S.; Mkandawire, M.; Babu-Rajendran, R. Screening of antimicrobials, fragrances, UV stabilizers, plasticizers and preservatives in sewage treatment plants (STPs) and their risk assessment in India. Chemosphere 2022, 308, 136452. [Google Scholar] [CrossRef]
- Wang, W.; Lee, I.-S.; Oh, J.-E. Specific-accumulation and trophic transfer of UV filters and stabilizers in marine food web. Sci. Total Environ. 2022, 825, 154079. [Google Scholar] [CrossRef]
- Zhang, Z.-Y.; Xu, Y.-H.; Chen, K.-Y.; Zhang, M.-H.; Meng, C.-Y.; Wang, X.-S.; Wang, M.-M. Flower-like molybdenum disulfide/cobalt ferrite composite for the extraction of benzotriazole UV stabilizers in environmental samples. Microchim. Acta 2023, 190, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-L.; Chen, Y.; Yang, G.-P.; Chen, R. Simultaneous determination of benzothiazoles, benzotriazoles, and benzotriazole UV absorbers by solid-phase extraction-gas chromatography-mass spectrometry. Environ. Sci. Pollut. Res. 2023, 30, 45315–45330. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, Z.-F.; Xu, L.; Liu, L.-Y.; Song, W.-W.; Zhu, F.-J.; Li, Y.-F.; Ma, W.-L. Occurrence and fate of benzotriazoles UV filters in a typical residential wastewater treatment plant in Harbin, China. Environ. Pollut. 2017, 227, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, Y.; Pei, Y.; Wang, Y.; Li, M.; Chen, H.; Liang, X.; Martyniuk, C.J. Biotransformation, metabolic response, and toxicity of UV-234 and UV-326 in larval zebrafish (Danio rerio). Environ. Int. 2023, 174, 107896. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Adamovsky, O.; Souders, C.L., 2nd; Martyniuk, C.J. Biological effects of the benzotriazole ultraviolet stabilizers UV-234 and UV-320 in early-staged zebrafish (Danio rerio). Environ. Pollut. 2019, 245, 272–281. [Google Scholar] [CrossRef]
- Tao, J.; Bai, C.; Chen, Y.; Zhou, H.; Liu, Y.; Shi, Q.; Pan, W.; Dong, H.; Li, L.; Xu, H. Environmental relevant concentrations of benzophenone-3 induced developmental neurotoxicity in zebrafish. Sci. Total Environ. 2020, 721, 137686. [Google Scholar] [CrossRef]
- Bai, C.; Dong, H.; Tao, J.; Chen, Y.; Xu, H.; Lin, J.; Huang, C.; Dong, Q. Lifetime exposure to benzophenone-3 at an environmentally relevant concentration leads to female–biased social behavior and cognition deficits in zebrafish. Sci. Total Environ. 2023, 857, 159733. [Google Scholar] [CrossRef]
- Hemalatha, D.; Rangasamy, B.; Nataraj, B.; Maharajan, K.; Narayanasamy, A.; Ramesh, M. Transcriptional, biochemical, and histological alterations in adult zebrafish (Danio rerio) exposed to benzotriazole ultraviolet stabilizer-328. Sci. Total Environ. 2020, 739, 139851. [Google Scholar] [CrossRef]
- Sandoval-Gío, J.J.; Noreña-Barroso, E.; Escalante-Herrera, K.; Rodríguez-Fuentes, G. Effect of benzophenone-3 to acetylcholinesterase and antioxidant system in zebrafish (Danio rerio) embryos. Bull. Environ. Contam. Toxicol. 2021, 107, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.L.P.; Luchiari, A.C. Effects of oxybenzone on zebrafish behavior and cognition. Sci. Total Environ. 2022, 808, 152101. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Q.; Jing, M.; Jia, X.; Tian, L.; Tao, J. Environmentally relevant concentrations of organic (benzophenone-3) and inorganic (titanium dioxide nanoparticles) UV filters co-exposure induced neurodevelopmental toxicity in zebrafish. Ecotoxicol. Environ. Saf. 2023, 249, 114343. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, S.; Jiang, X.; Ren, Q.; Deng, H.; Paudel, Y.N.; Wang, B.; Liu, K.; Jin, M. Benzoresorcinol induces developmental neurotoxicity and injures exploratory, learning and memorizing abilities in zebrafish. Sci. Total Environ. 2022, 834, 155268. [Google Scholar] [CrossRef]
- Huang, Y.; Law, J.C.-F.; Lam, T.-K.; Leung, K.S.-Y. Risks of organic UV filters: A review of environmental and human health concern studies. Sci. Total Environ. 2021, 755, 142486. [Google Scholar] [CrossRef]
- Liang, X.; Martyniuk, C.J.; Zha, J.; Wang, Z. Brain quantitative proteomic responses reveal new insight of benzotriazole neurotoxicity in female Chinese rare minnow (Gobiocypris rarus). Aquat. Toxicol. 2016, 181, 67–75. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Tong, T.; Liu, R.; Yan, S.; Liang, X.; Martyniuk, C.J.; Zha, J. Comparative toxicogenomics of benzotriazole ultraviolet stabilizers at environmental concentrations in Asian clam (Corbicula fluminea): Insight into molecular networks and behavior. Josuurnal Hazard. Mater. 2023, 447, 130811. [Google Scholar] [CrossRef]
- Dos Santos, B.; Ivantsova, E.; Guzman, A.P.; Martyniuk, C.J. Critical review of the toxicity mechanisms of bisphenol F in zebrafish (Danio rerio): Knowledge gaps and future directions. Chemosphere 2022, 297, 134132. [Google Scholar] [CrossRef] [PubMed]
- Ivantsova, E.; Martyniuk, C.J. A synthesis on the sub-lethal toxicity of atenolol, a beta-blocker, in teleost fish. Environ. Toxicol. Pharmacol. 2023, 102, 104236. [Google Scholar] [CrossRef]
- Davis, A.P.; Wiegers, T.C.; Johnson, R.J.; Sciaky, D.; Wiegers, J.; Mattingly, C.J. Comparative Toxicogenomics Database (CTD): Update 2023. Nucleic Acids Res. 2023, 51, D1257–D1262. [Google Scholar] [CrossRef]
- Zuin, M.; Rosta, V.; Trentini, A.; Bosi, C.; Zuliani, G.; Cervellati, C. Paraoxonase 1 activity in patients with Alzheimer disease: Systematic review and meta-analysis. Chem. -Biol. Interact. 2023, 382, 110601. [Google Scholar] [CrossRef]
- Meyer, W.K.; Jamison, J.; Richter, R.; Woods, S.E.; Partha, R.; Kowalczyk, A.; Kronk, C.; Chikina, M.; Bonde, R.K.; Crocker, D.E. Ancient convergent losses of Paraoxonase 1 yield potential risks for modern marine mammals. Science 2018, 361, 591–594. [Google Scholar] [CrossRef]
- Xu, L.; Shao, A.; Zhao, Y.; Wang, Z.; Zhang, C.; Sun, Y.; Deng, J.; Chou, L.L. Neurotoxicity of silver nanoparticles in rat brain after intragastric exposure. J. Nanosci. Nanotechnol. 2015, 15, 4215–4223. [Google Scholar] [CrossRef]
- Jablonska, B.; Aguirre, A.; Vandenbosch, R.; Belachew, S.; Berthet, C.; Kaldis, P.; Gallo, V. Cdk2 is critical for proliferation and self-renewal of neural progenitor cells in the adult subventricular zone. J. Cell Biol. 2007, 179, 1231–1245. [Google Scholar] [CrossRef]
- Rothhammer, V.; Borucki, D.M.; Tjon, E.C.; Takenaka, M.C.; Chao, C.-C.; Ardura-Fabregat, A.; De Lima, K.A.; Gutiérrez-Vázquez, C.; Hewson, P.; Staszewski, O. Microglial control of astrocytes in response to microbial metabolites. Nature 2018, 557, 724–728. [Google Scholar] [CrossRef]
- He, T.T.; Liang, B.; Liu, W.H.; Shin, P.K.S.; Wu, R.S.S. Estrogenic potential of benzotriazole on marine medaka (Oryzias melastigma). Ecotoxicol. Environ. Saf. 2012, 80, 327–332. [Google Scholar]
- Fent, K.; Chew, G.; Li, J.; Gomez, E. Benzotriazole UV-stabilizers and benzotriazole: Antiandrogenic activity in vitro and activation of aryl hydrocarbon receptor pathway in zebrafish eleuthero-embryos. Sci. Total Environ. 2014, 482–483, 125–136. [Google Scholar] [CrossRef]
- Shore, E.A.; Huber, K.E.; Garrett, A.D.; Pespeni, M.H. Four plastic additives reduce larval growth and survival in the sea urchin Strongylocentrotus purpuratus. Mar. Pollut. Bull. 2022, 175, 113385. [Google Scholar] [CrossRef]
- He, T.; Tsui, M.M.; Mayfield, A.B.; Liu, P.J.; Chen, T.H.; Wang, L.H.; Fan, T.Y.; Lam, P.K.; Murphy, M.B. Organic ultraviolet filter mixture promotes bleaching of reef corals upon the threat of elevated seawater temperature. Sci. Total Environ. 2023, 876, 162744. [Google Scholar] [CrossRef]
- Apel, C.; Joerss, H.; Ebinghaus, R. Environmental occurrence and hazard of organic UV stabilizers and UV filters in the sediment of European North and Baltic Seas. Chemosphere 2018, 212, 254–261. [Google Scholar] [CrossRef]
- Apel, C.; Tang, J.; Ebinghaus, R. Environmental occurrence and distribution of organic UV stabilizers and UV filters in the sediment of Chinese Bohai and Yellow Seas. Environ. Pollut. 2018, 235, 85–94. [Google Scholar] [CrossRef]
- Avagyan, R.; Luongo, G.; Thorsen, G.; Ostman, C. Benzothiazole, benzotriazole, and their derivates in clothing textiles-a potential source of environmental pollutants and human exposure. Environ. Sci. Pollut. Res. 2015, 22, 5842–5849. [Google Scholar] [CrossRef]
- Carpinteiro, I.; Abuin, B.; Rodriguez, I.; Cela, R.; Ramil, M. Headspace solid-phase microextraction followed by gas chromatography tandem mass spectrometry for the sensitive determination of benzotriazole UV stabilizers in water samples. Anal. Bioanal. Chem. 2010, 397, 829–839. [Google Scholar] [CrossRef]
- Kim, J.-W.; Ramaswamy, B.R.; Chang, K.-H.; Isobe, T.; Tanabe, S. Multiresidue analytical method for the determination of antimicrobials, preservatives, benzotriazole UV stabilizers, flame retardants and plasticizers in fish using ultra high performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 3511–3520. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Su, W.; Liang, W.; Zhu, B.; Li, T.; Ruan, T.; Jiang, G. Occurrence and Temporal Trends of Benzotriazole UV Stabilizers in Mollusks (2010–2018) from the Chinese Bohai Sea Revealed by Target, Suspect, and Nontarget Screening Analysis. Environ. Sci. Technol. 2022, 56, 16759–16767. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xing, X.; An, D.; Sun, J.; Tang, Z. Occurrence and distribution of organic ultraviolet absorbents in sediments from small urban rivers, Tianjin, China: Implications for risk management. Ecotoxicol. Environ. Saf. 2022, 230, 113120. [Google Scholar] [CrossRef]
- Liu, R.; Ruan, T.; Wang, T.; Song, S.; Guo, F.; Jiang, G. Determination of nine benzotriazole UV stabilizers in environmental water samples by automated on-line solid phase extraction coupled with high-performance liquid chromatography–tandem mass spectrometry. Talanta 2014, 120, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Mizukawa, A.; Molins-Delgado, D.; de Azevedo, J.C.R.; Fernandes, C.V.S.; Diaz-Cruz, S.; Barcelo, D. Sediments as a sink for UV filters and benzotriazoles: The case study of Upper Iguacu watershed, Curitiba (Brazil). Environ. Sci. Pollut. Res. 2017, 24, 18284–18294. [Google Scholar] [CrossRef]
- Molins-Delgado, D.; Díaz-Cruz, M.S.; Barceló, D. Removal of polar UV stabilizers in biological wastewater treatments and ecotoxicological implications. Chemosphere 2015, 119, S51–S57. [Google Scholar] [CrossRef] [PubMed]
- Molins-Delgado, D.; Távora, J.; Díaz-Cruz, M.S.; Barceló, D. UV filters and benzotriazoles in urban aquatic ecosystems: The footprint of daily use products. Sci. Total Environ. 2017, 601–602, 975–986. [Google Scholar] [CrossRef]
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, A.; Furton, K.G.; Santana-Rodriguez, J.J. Fabric phase sorptive extraction followed by UHPLC-MS/MS for the analysis of benzotriazole UV stabilizers in sewage samples. Anal. Bioanal. Chem. 2015, 407, 8137–8150. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Shinohara, R.-I.; Murata, S.; Watanabe, M. Detection of benzotriazole UV stabilizers in the blubber of marine mammals by gas chromatography-high resolution mass spectrometry (GC-HRMS). J. Environ. Monit. 2010, 12, 2088–2092. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Zhong, F.; Cheng, J.; Nie, Z.; Han, X.; Han, Y.; Yang, Y. Concentrations and tissue-specific distributions of organic ultraviolet absorbents in wild fish from a large subtropical lake in China. Sci. Total Environ. 2019, 647, 1305–1313. [Google Scholar] [CrossRef]
- Yao, L.; Zhao, J.-L.; Liu, Y.-S.; Yang, Y.-Y.; Liu, W.-R.; Ying, G.-G. Simultaneous determination of 24 personal care products in fish muscle and liver tissues using QuEChERS extraction coupled with ultra pressure liquid chromatography-tandem mass spectrometry and gas chromatography-mass spectrometer analyses. Anal. Bioanal. Chem. 2016, 408, 8177–8193. [Google Scholar] [CrossRef]
- Campos, D.; Gravato, C.; Quintaneiro, C.; Golovko, O.; Žlábek, V.; Soares, A.M.V.M.; Pestana, J.L.T. Toxicity of organic UV-filters to the aquatic midge Chironomus riparius. Ecotoxicol. Environ. Saf. 2017, 143, 210–216. [Google Scholar] [CrossRef]
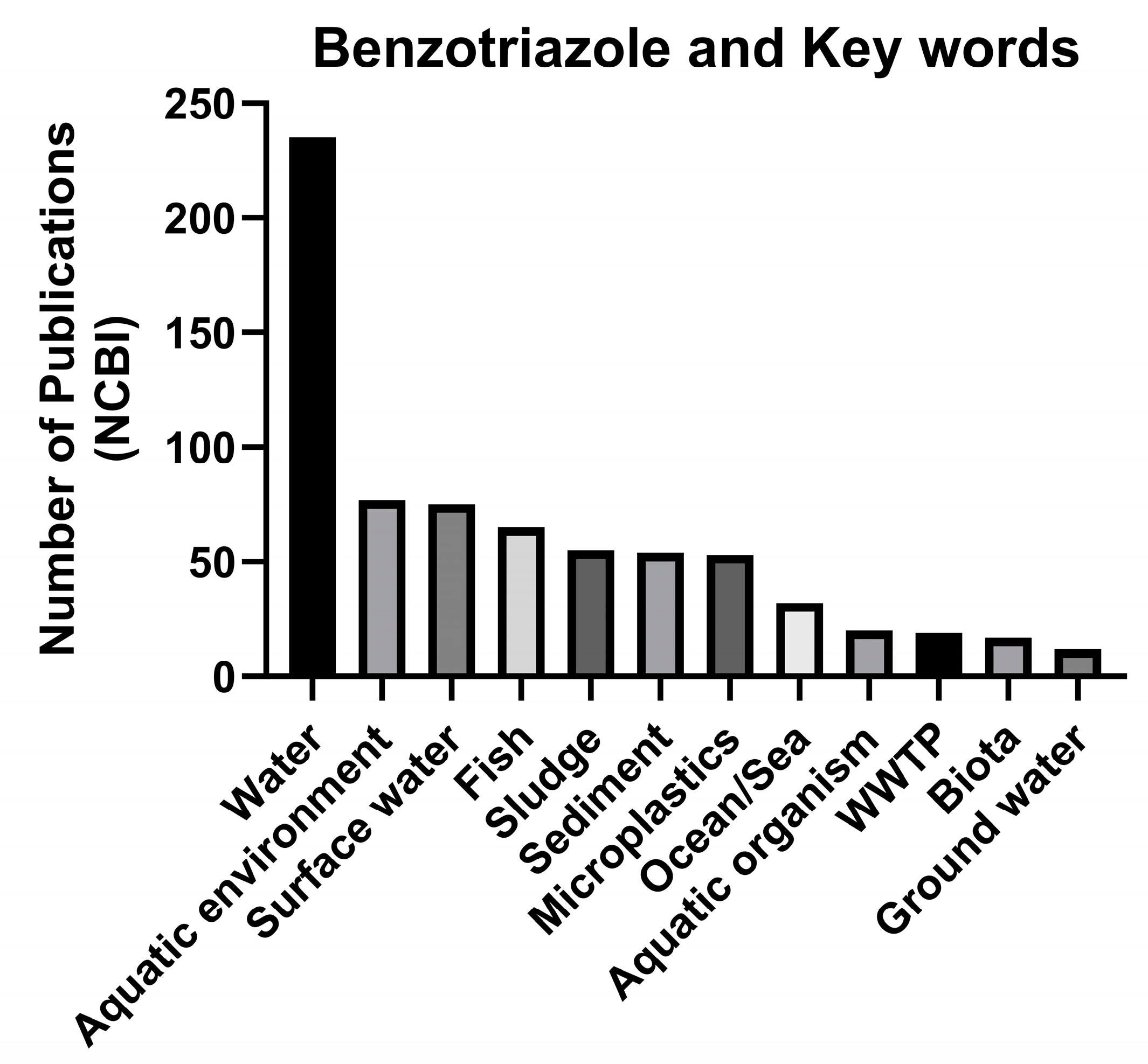
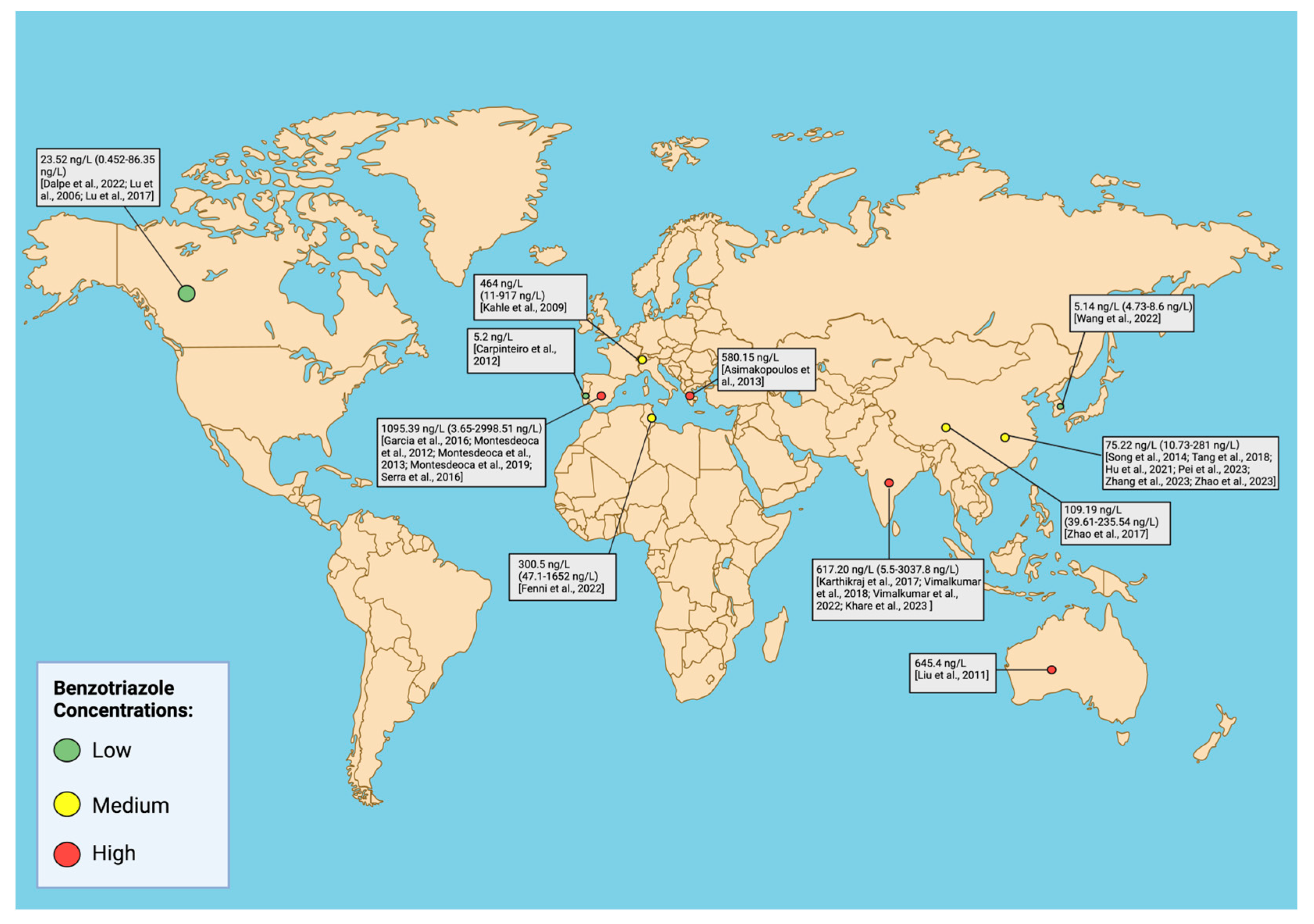
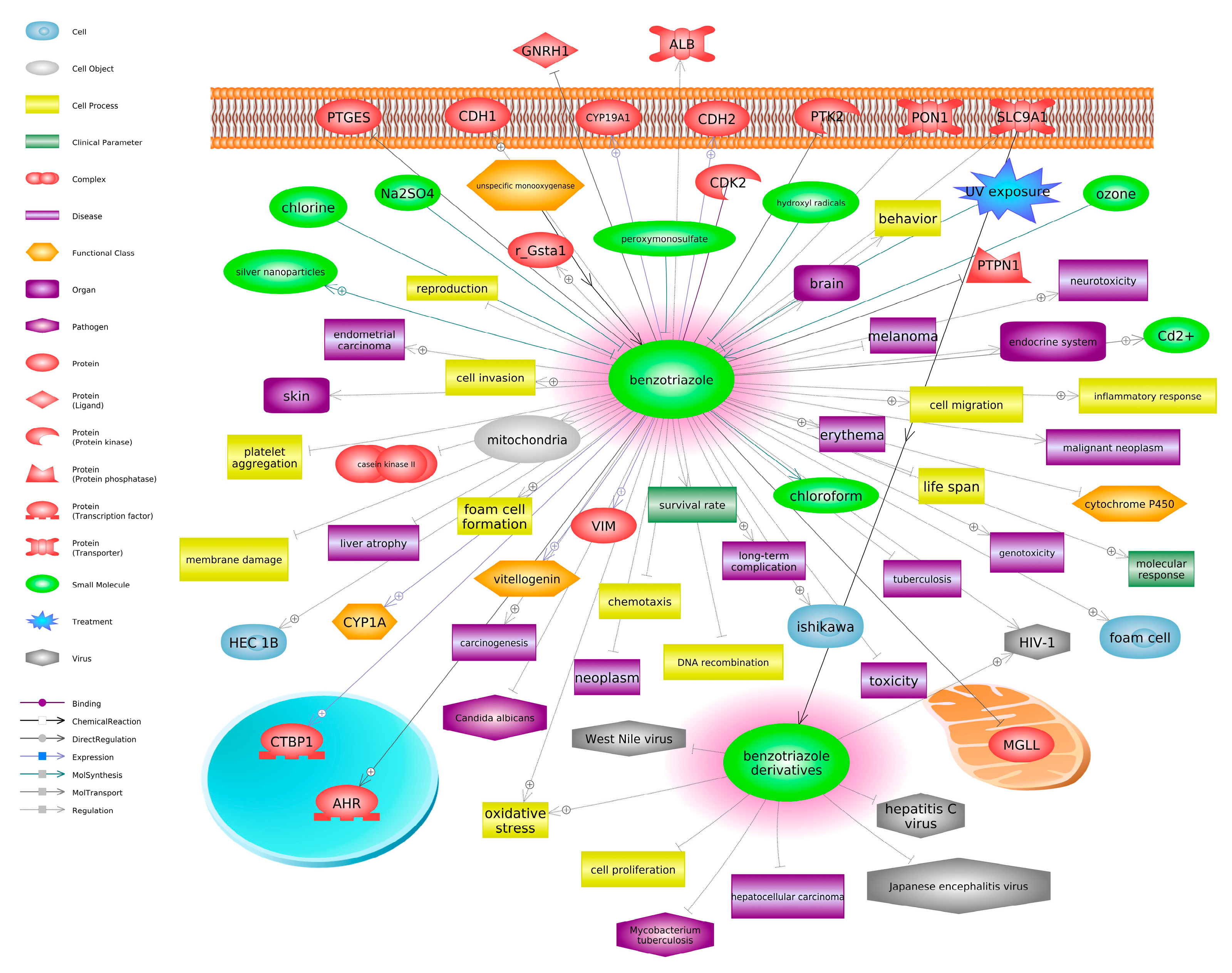
| Chemicals | Dose | Life Stage | Exposure Period | Endpoint | Results | Reference |
|---|---|---|---|---|---|---|
| UV-234,UV-326 | 1, 10, 100µg/L | Embryos | 7d | AchE activity | Upregulated at 10 and 100 μg/L | Zhang et al., 2023 [57] |
| Locomotor response | Both compounds induced hyperactivity in the dark cycle via swimming distance, acceleration, and mobile activity. | |||||
| Neurotrophic factors | igf1 and sdf1a were inhibited 1.65- to 2.26-fold and 2.15- to 2.19-fold, respectively, with UV-234; mmp9, fgf2, and sdf1a increased with 1 and 100μg/L UV-326; igf1 decreased with 10 μg/L exposure UV-326 | |||||
| Spontaneous tail coiling (STC) | Inhibited 2.08–6.25-fold | |||||
| Pro-inflammatory gene expression | tnfα decreased in all treatment. il1β decreased with 100 μg/L UV-234 and increased with 100 μg/ L UV-326; il6 increased with 100 μg/ L UV-326 | |||||
| UV-234, UV-320 | 0.01, 0.1, 1 µM | Embryos | 6d | Locomotor response | UV-234 altered activity in both light/dark periods; Hyperactivity was induced in fish pre-adapted to darkness with 1 µM UV-320; 1 µM UV-320 increased distance moved in the dark phase; 0.1 µM UV-320 increased distance moved in the light phase | Liang et al., 2019 [58] |
| BP3 | 1, 10, 100 µg/L | Embryos and larvae | 4d | Axonal Growth | Decreased relative axon length in 27 hpf larvae. | Tao et al., 2020 [59] |
| Touch response | Decreased in 27 hpf larvae with 10 μg/L | |||||
| Locomotor response | Increased swimming distance and average swimming speed in the dark period with 10 µg/L | |||||
| Spontaneous movement | Increased frequency of bending at 21 hpf (10 and 100 μg/L) and 24 hpf (10 μg/L) | |||||
| Social behaviors | Nearest neighbor distance and the inter-individual distance increased; Mean attacks and time spent in the mirror area decreased | |||||
| BP3 | 10 µg/L | Adults | 150d | Social preference | Reduced prosocial behaviors | Bai et al., 2023 [60] |
| Mirror biting test | Reduction of biting behavior in females | |||||
| T–maze test | Impaired learning and memory regardless of sex | |||||
| Body length, weight, brain weight, brain dopamine and acetylcholine | Reduced female brain weight and dopamine level | |||||
| Cell proliferation in the telencephalon | Neurogenesis inhibited in the telencephalon | |||||
| Cell apoptosis in the telencephalon | Apoptotic cells increased in the female telencephalon | |||||
| BP3 | 2 mg/L | Larvae | 5d | Enteric neuron number and related gene expression | BP-3 could impede ENS zebrafish development via the MAPK/ERK signaling pathway | Hemalatha et al., 2020 [61] |
| BP3 | 1, 10 µg/L | Embryos | 3d | AChE | Inhibited by both concentrations | Sandoval-Gío et al., 2021 [62] |
| BP3 | 10, 100, 1000 µg/L | Adults | 15d | Novel tank test | Reduced locomotion and decreased anxiety-like behavior | Moreira et al., 2022 [63] |
| Shoal preference | Reduced interaction and time near the shoal | |||||
| Mirror test | Reduced interactions with the mirror image; thus, impairing proper aggressive response | |||||
| T-maze | Reduced exploration of the novel arm; thus, jeopardizing the ability to retain information | |||||
| BP3, nano-Tio2 | 10 µg/L BP3; 100 µg/L nano-Tio2 (separately and combined) | Embryos | 1d | Spontaneous movement | Increased in single and coexposure groups at 24 hpf | Sun et al., 2023 [64] |
| Touch response | Decreased in co-exposure at 30 hpf | |||||
| Axonal growth | Single and coexposure inhibited axonal growth, and induced apoptosis and ROS generation | |||||
| BP1 | 0.8, 1, 1.2, 1.6, 2.4 µg/mL | Larvae | 4d | CNS | Abnormal brain structure and neuron loss | Song et al., 2022 [65] |
| DA neurons | Decreased the number in the midbrain | |||||
| 6d | Locomotor capacity | Suppressed velocity and movement distance; altered expression of neurodevelopment related genes | ||||
| BP1 | 1, 10, 100, 1000 µg/L | Adults | 14d | T-maze tests | Inhibited spatial working memory | |
| Tank diving tests | Increase in proportion of bottom swimming duration/distance to total duration/distance, indicating a decrease of exploratory behavior |
| Name | Expanded # of Entities | Overlap | Percent Overlap | Hit Type |
|---|---|---|---|---|
| Complement Activation in Alzheimer’s Disease | 36 | 10 | 27 | Disease |
| MPB-Related Complement Cascade Activation | 39 | 10 | 25 | Disease |
| Positive Acute Phase Proteins Synthesis | 609 | 20 | 3 | Biological Process |
| Trophoblast Damage in Infertility (Hypothesis) | 61 | 10 | 16 | Disease |
| CD46/CD55/CD59 Inhibit Complement Mediated Lysis of Cancer Cells | 50 | 10 | 20 | Pathological Process |
| Complement Activation in Glomerulonephritis | 63 | 10 | 15 | Disease |
| Complement System Defects in Systemic Lupus Erythematosis | 73 | 10 | 13 | Disease |
| Complement Activation by Lectin | 68 | 10 | 14 | Biological Process |
| Extraocular Muscles Weakness in Myasthenia Gravis | 84 | 10 | 11 | Disease |
| Complement Classical Pathway | 71 | 10 | 14 | Biological Process |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Ivantsova, E.; Liang, X.; Martyniuk, C.J. Neurotoxicity of Benzotriazole Ultraviolet Stabilizers in Teleost Fishes: A Review. Toxics 2024, 12, 125. https://doi.org/10.3390/toxics12020125
Li M, Ivantsova E, Liang X, Martyniuk CJ. Neurotoxicity of Benzotriazole Ultraviolet Stabilizers in Teleost Fishes: A Review. Toxics. 2024; 12(2):125. https://doi.org/10.3390/toxics12020125
Chicago/Turabian StyleLi, Mengli, Emma Ivantsova, Xuefang Liang, and Christopher J. Martyniuk. 2024. "Neurotoxicity of Benzotriazole Ultraviolet Stabilizers in Teleost Fishes: A Review" Toxics 12, no. 2: 125. https://doi.org/10.3390/toxics12020125
APA StyleLi, M., Ivantsova, E., Liang, X., & Martyniuk, C. J. (2024). Neurotoxicity of Benzotriazole Ultraviolet Stabilizers in Teleost Fishes: A Review. Toxics, 12(2), 125. https://doi.org/10.3390/toxics12020125








