Characteristics of DOM and Their Relationships with Potentially Toxic Elements in the Inner Mongolia Section of the Yellow River, China
Abstract
1. Introduction
2. Materials and Methods
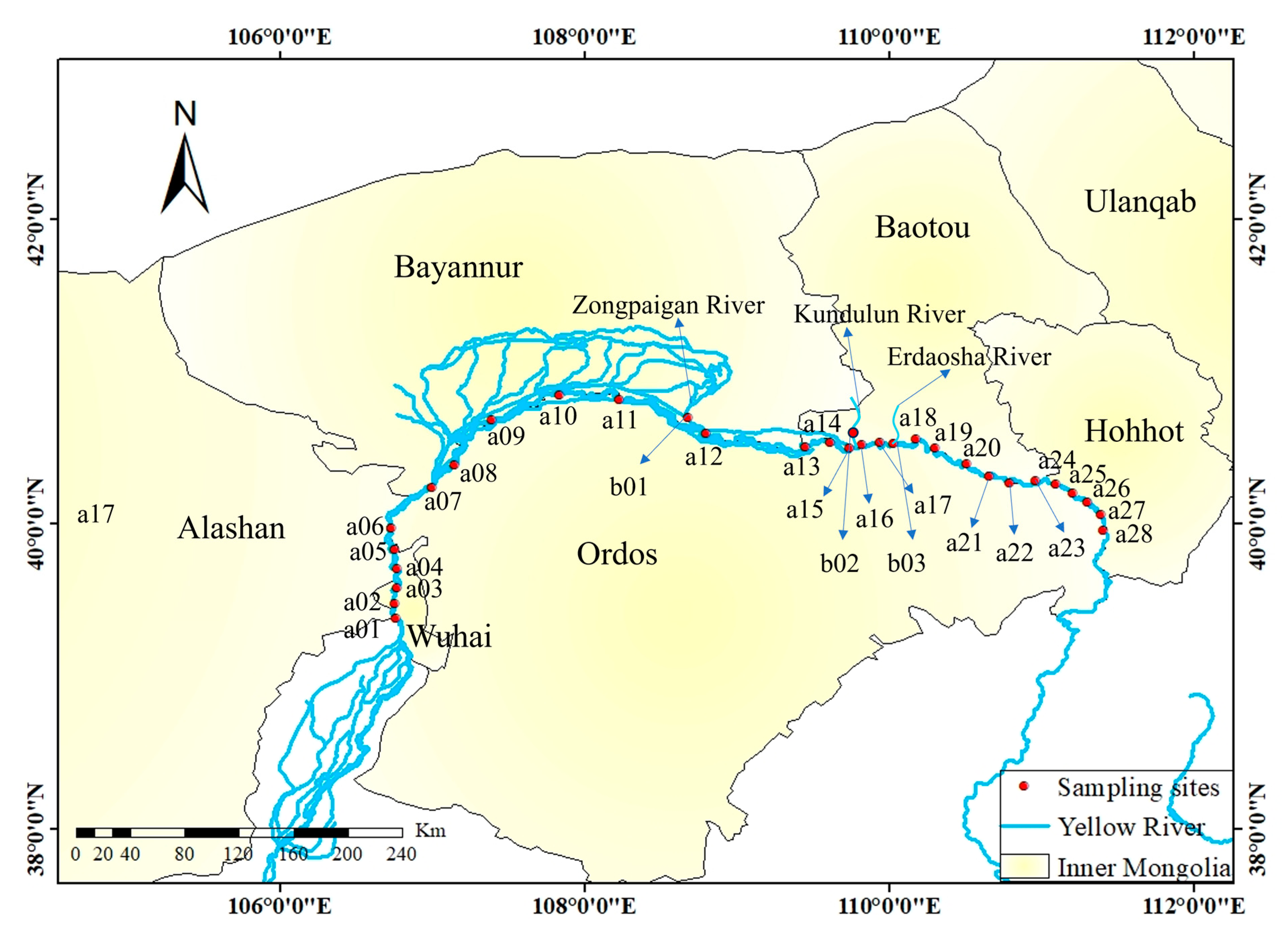
2.1. Study Area and Sampling Sites
2.2. DOM Fluorescence
2.3. FT-ICR MS
2.4. Potentially Toxic Elements
2.5. Statistical Analyses
3. Results and Discussion
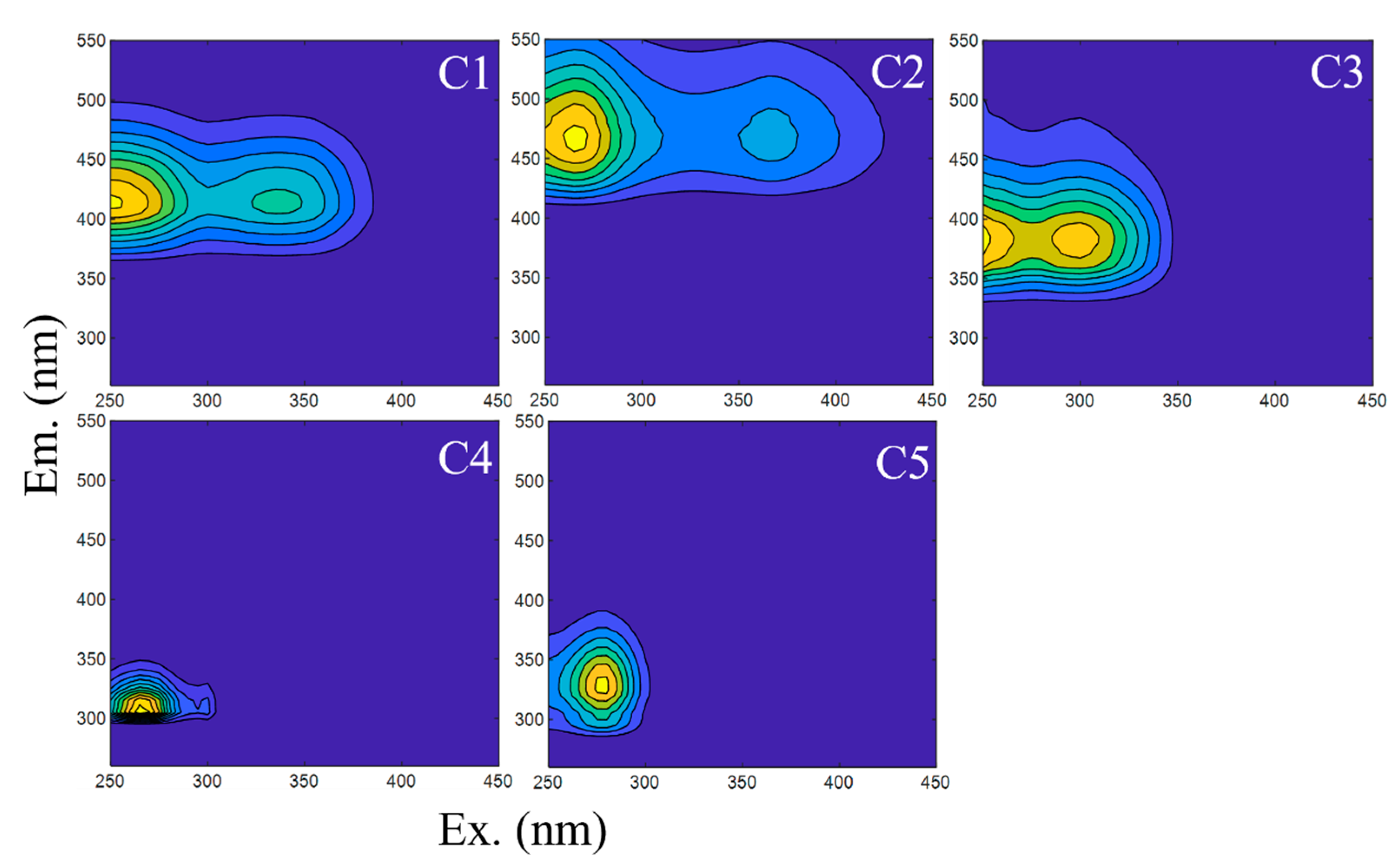
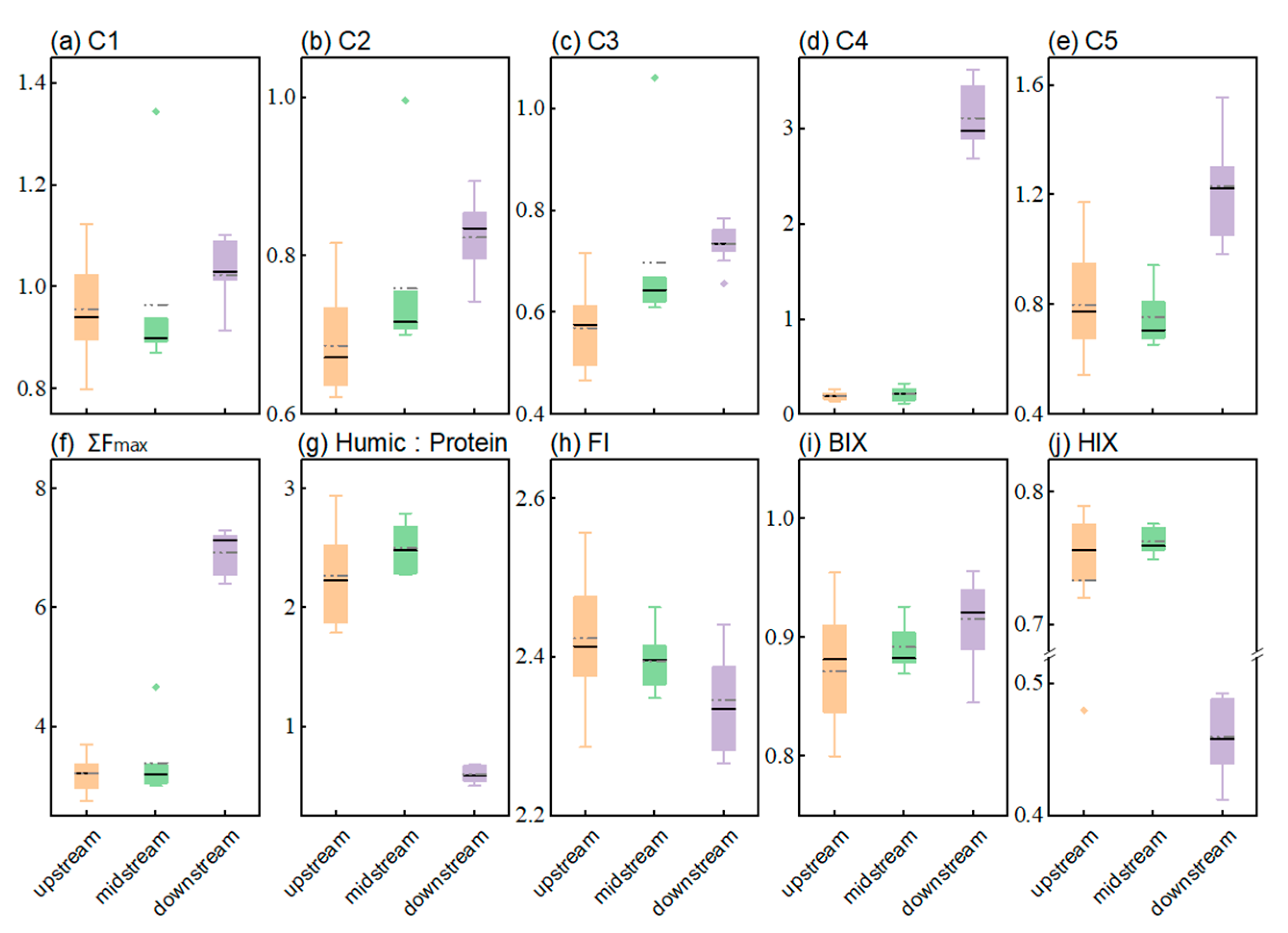
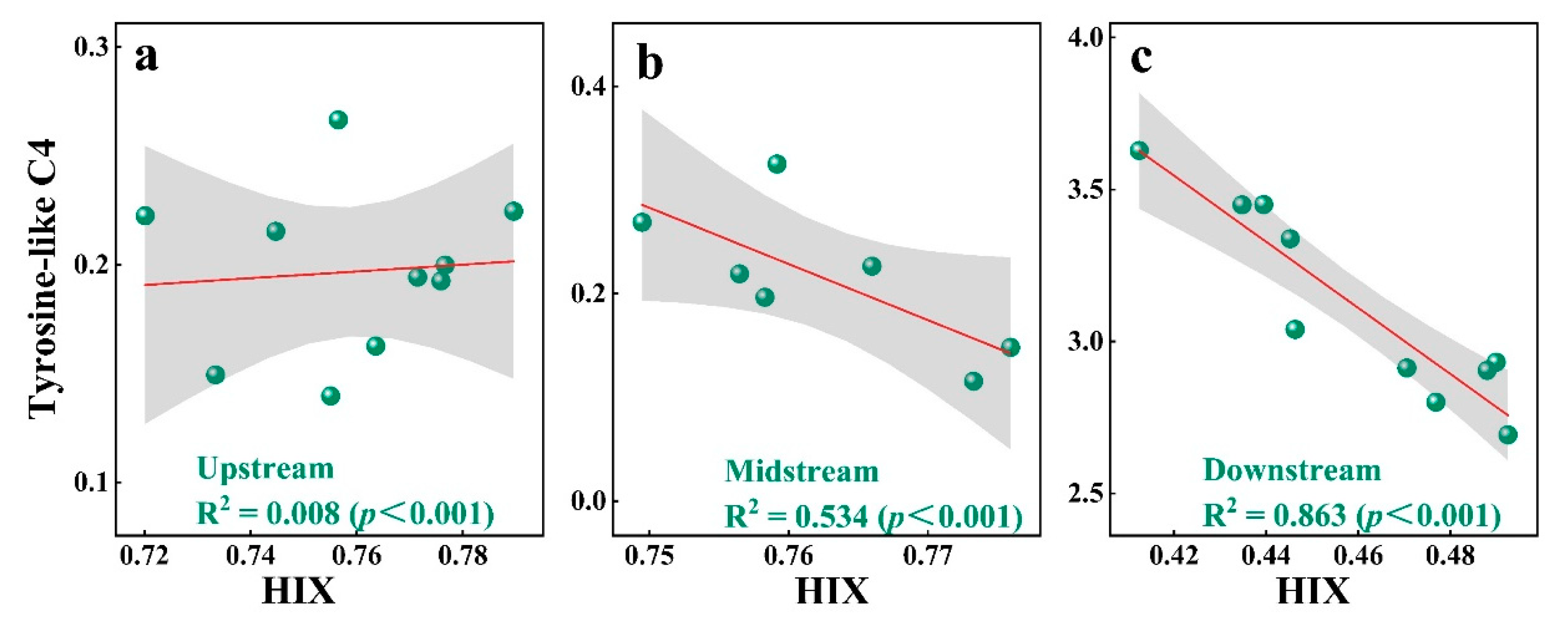
3.1. Fluorescence Characteristics of DOM
3.2. Molecular Characteristics of DOM
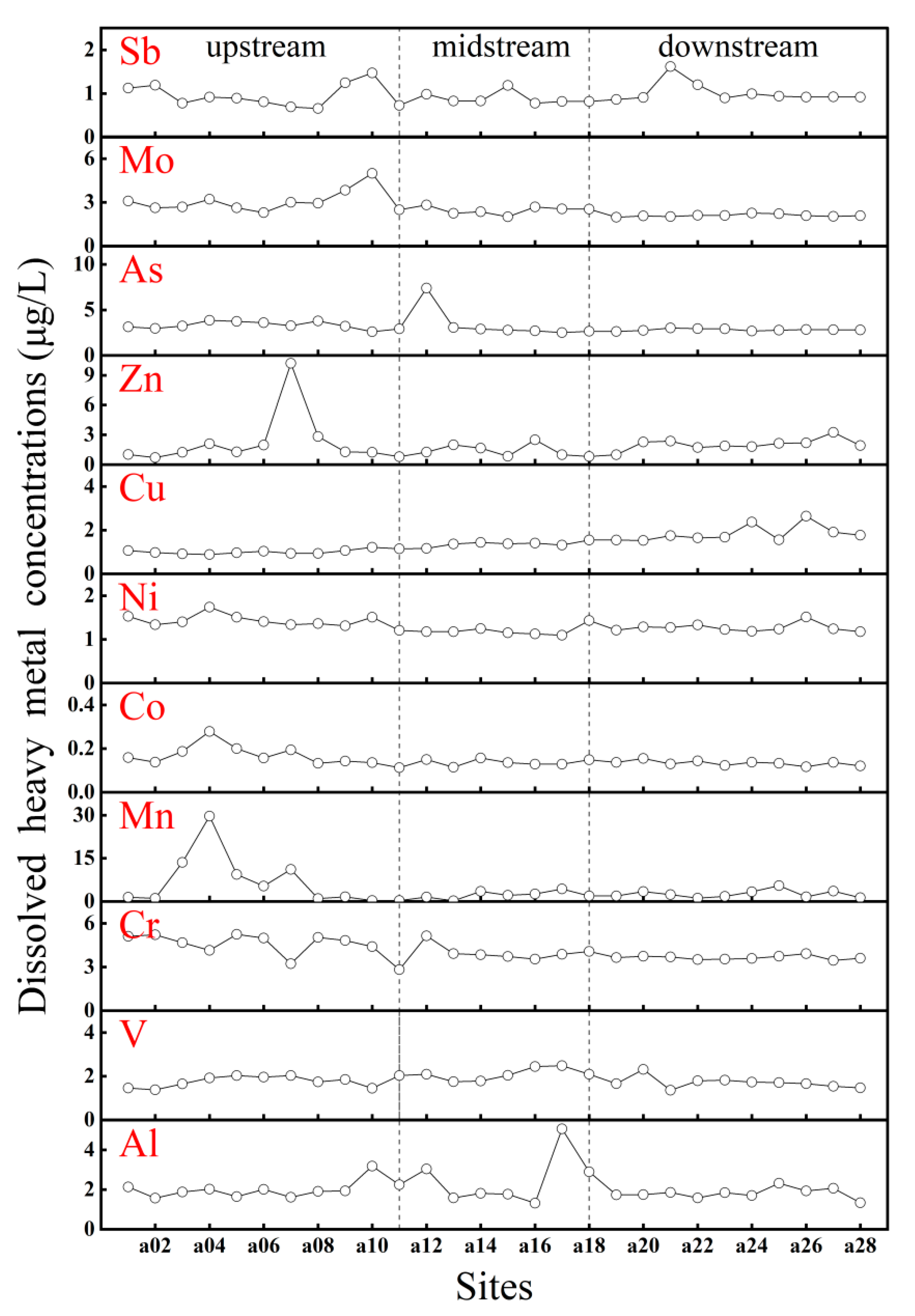
3.3. Concentrations and Distribution Characteristics of PTEs in Water
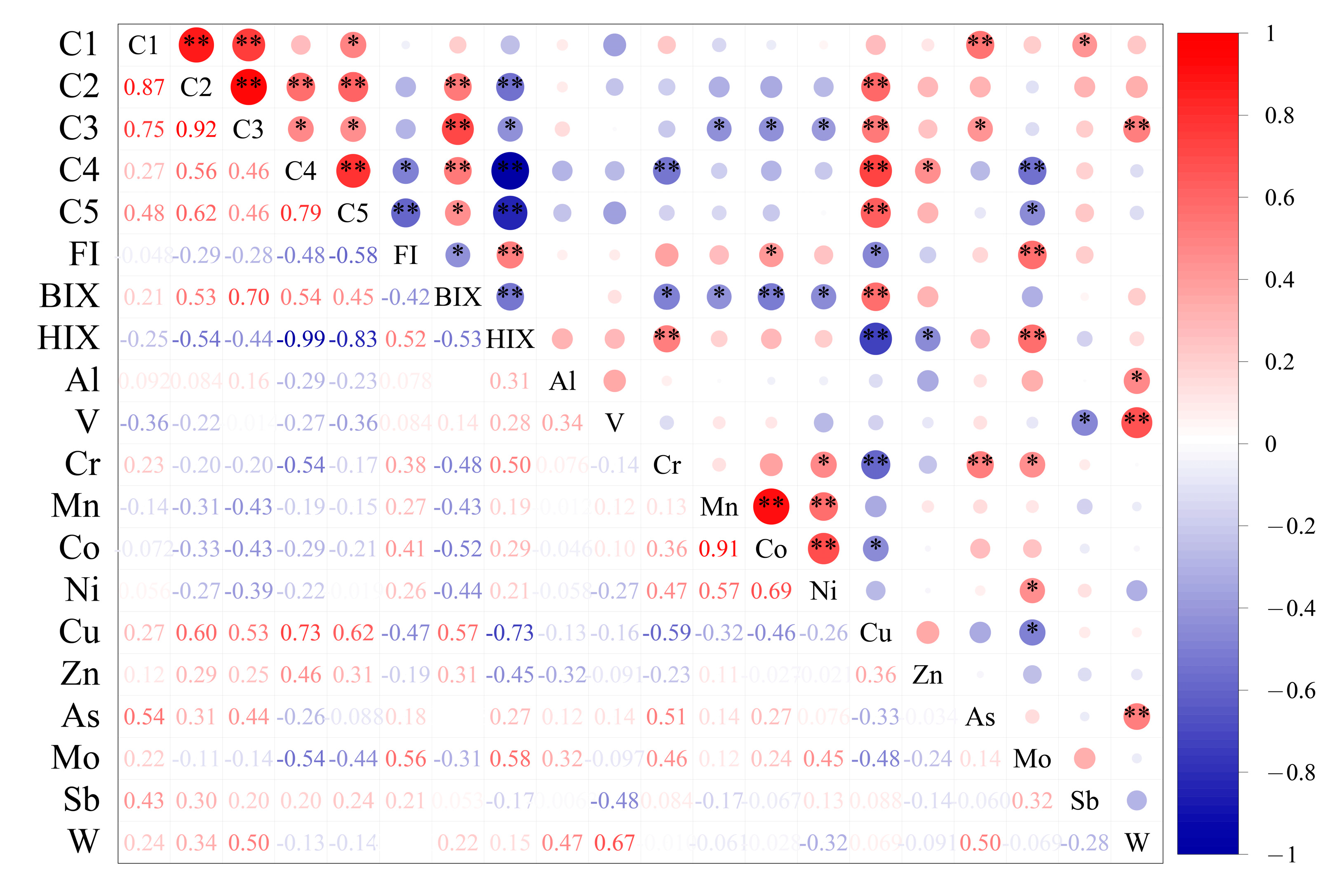
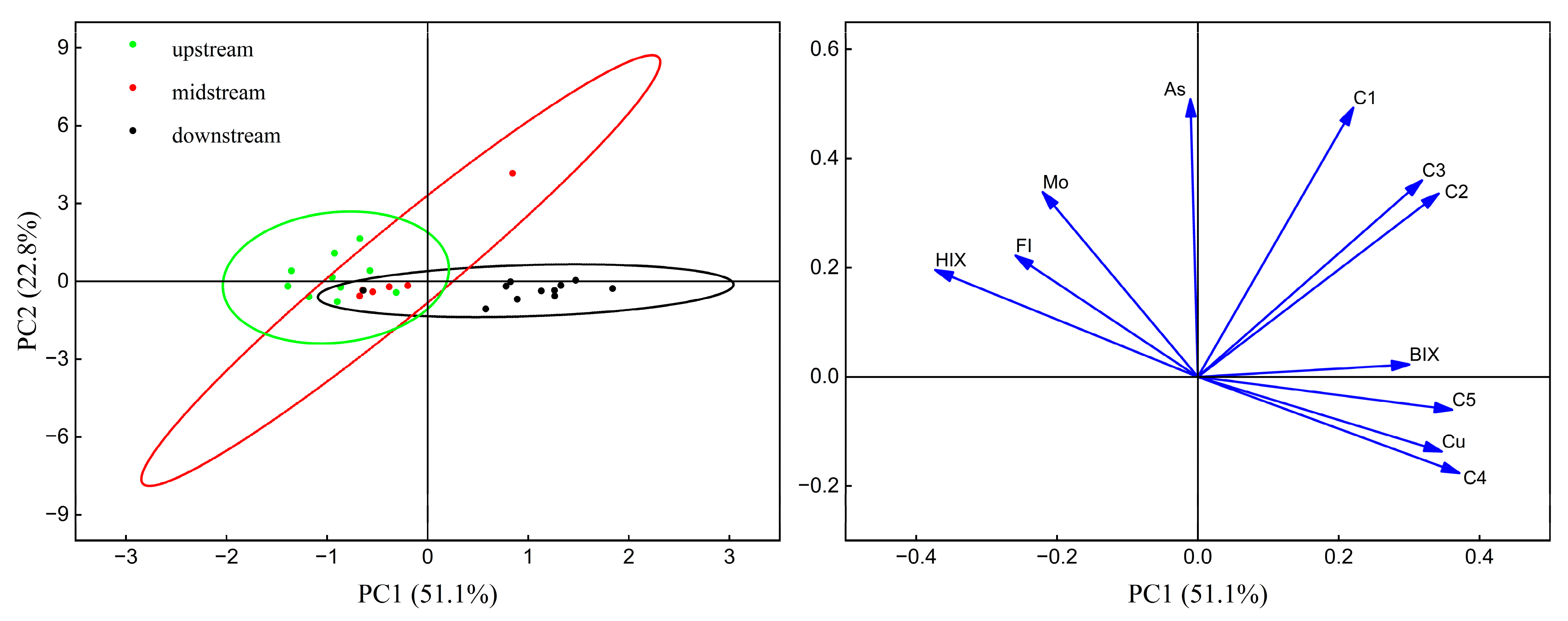
3.4. Implication for Interaction and Source of DOM and PTEs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOM | dissolved organic matter |
| IMYR | Inner Mongolia section of the Yellow River |
| PTEs | potentially toxic elements |
| PARAFAC | parallel factor analysis |
| FI | fluorescence index |
| BIX | biological index |
| HIX | humification index |
| AImod | modified aromaticity index |
| DBE | double bond equivalents |
| IOS | island of stability |
| MLBL | molecular lability boundary for more labile contributions |
| FT-ICR MS | Fourier transform ion cyclotron resonance mass spectrometry |
References
- Qian, C.; Chen, W.; Gong, B.; Wang, L.-F.; Yu, H.-Q. Diagnosis of the unexpected fluorescent contaminants in quantifying dissolved organic matter using excitation-emission matrix fluorescence spectroscopy. Water Res. 2019, 163, 114873. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Shang, Y.; Wen, Z.; Jacinthe, P.-A.; Liu, G.; Lyu, L.; Fang, C. Characterization of CDOM in saline and freshwater lakes across China using spectroscopic analysis. Water Res. 2019, 150, 403–417. [Google Scholar] [CrossRef]
- Thurman, E.M. Organic Geochemistry of Natural Waters; Springer Science & Business Media: Berlin, Germany, 2012; Volume 2. [Google Scholar]
- Wen, Z.; Shang, Y.; Song, K.; Liu, G.; Hou, J.; Lyu, L.; Tao, H.; Li, S.; He, C.; Shi, Q.; et al. Composition of dissolved organic matter (DOM) in lakes responds to the trophic state and phytoplankton community succession. Water Res. 2022, 224, 119073. [Google Scholar] [CrossRef] [PubMed]
- Al-Reasi, H.A.; Wood, C.M.; Smith, D.S. Characterization of freshwater natural dissolved organic matter (DOM): Mechanistic explanations for protective effects against metal toxicity and direct effects on organisms. Environ. Int. 2013, 59, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Jiang, Y.; Tian, Y.; Hou, Z.; He, K.; Fu, L.; Xu, H. Impact of land use on the DOM composition in different seasons in a subtropical river flowing through a region undergoing rapid urbanization. J. Clean. Prod. 2019, 212, 1224–1231. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, E.; Sun, C.; Li, M.; Gao, L.; Fan, L. Using fluorescence index (FI) of dissolved organic matter (DOM) to identify non-point source pollution: The difference in FI between soil extracts and wastewater reveals the principle. Sci. Total. Environ. 2023, 862, 160848. [Google Scholar] [CrossRef] [PubMed]
- He, X.-S.; Zhang, Y.-L.; Liu, Z.-H.; Wei, D.; Liang, G.; Liu, H.-T.; Xi, B.-D.; Huang, Z.-B.; Ma, Y.; Xing, B.-S. Interaction and coexistence characteristics of dissolved organic matter with toxic metals and pesticides in shallow groundwater. Environ. Pollut. 2020, 258, 113736. [Google Scholar] [CrossRef] [PubMed]
- Dvorski, S.E.-M.; Gonsior, M.; Hertkorn, N.; Uhl, J.; Müller, H.; Griebler, C.; Schmitt-Kopplin, P. Geochemistry of Dissolved Organic Matter in a Spatially Highly Resolved Groundwater Petroleum Hydrocarbon Plume Cross-Section. Environ. Sci. Technol. 2016, 50, 5536–5546. [Google Scholar] [CrossRef] [PubMed]
- Kozyatnyk, I.; Bouchet, S.; Björn, E.; Haglund, P. Fractionation and size-distribution of metal and metalloid contaminants in a polluted groundwater rich in dissolved organic matter. J. Hazard. Mater. 2016, 318, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Jaffé, R. Characterizing the Interactions between Trace Metals and Dissolved Organic Matter Using Excitation−Emission Matrix and Parallel Factor Analysis. Environ. Sci. Technol. 2008, 42, 7374–7379. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Yao, X.; Ren, H.; Liu, L.; Deng, H.; Shao, K. Regional-scale investigation of the molecular weight distribution and metal-binding behavior of dissolved organic matter from a shallow macrophytic lake using multispectral techniques. J. Hazard. Mater. 2022, 439, 129532. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Han, X.; Liu, C.-Q.; Guo, L.; Ding, H.; Lang, Y. Differences in the spectroscopic characteristics of wetland dissolved organic matter binding with Fe3+, Cu2+, Cd2+, Cr3+ and Zn2+. Sci. Total. Environ. 2021, 800, 149476. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.; Yang, Z.; Biao, P.; Jing, W.; Liu, D. Spatial–temporal distribution characteristics and traceability analysis of organic matter in Shahe Reservoir (Beijing, China). Sci. Rep. 2023, 13, 21524. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Liu, J.; Zhang, X.; Ng, W.J.; Liu, Y. Generation of dissolved organic matter and byproducts from activated sludge during contact with sodium hypochlorite and its implications to on-line chemical cleaning in MBR. Water Res. 2016, 104, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mei, X.; Ma, J.; Grasmick, A.; Wu, Z. Potential Foulants and Fouling Indicators in MBRs: A Critical Review. Sep. Sci. Technol. 2013, 48, 22–50. [Google Scholar] [CrossRef]
- Zhang, W.-H.; Zhao, D.-L.; Wang, X.-Y.; Hao, Q.-Z.; Li, X.-M.; Gao, P.-C. Absorption and Three Dimensional Fluorescence Spectra of CDOM in the Water of the Taibaishan Nature Reserve. Environ. Sci. 2020, 41, 4958–4969, (In Chinese with English abstract). [Google Scholar]
- Kikuchi, T.; Fujii, M.; Terao, K.; Jiwei, R.; Lee, Y.P.; Yoshimura, C. Correlations between aromaticity of dissolved organic matter and trace metal concentrations in natural and effluent waters: A case study in the Sagami River Basin, Japan. Sci. Total. Environ. 2017, 576, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, D.H.; Lee, B.-M.; Hur, J. Characterizing treated wastewaters of different industries using clustered fluorescence EEM–PARAFAC and FT-IR spectroscopy: Implications for downstream impact and source identification. Chemosphere 2015, 127, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Matsui, K.; Newton, R.J.; Guo, L. Disproportionate Changes in Composition and Molecular Size Spectra of Dissolved Organic Matter between Influent and Effluent from a Major Metropolitan Wastewater Treatment Plant. ACS EST Water 2022, 2, 216–225. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Bro, R. Characterizing dissolved organic matter fluorescence with parallel factor analysis: A tutorial. Limnol. Oceanogr. Methods 2008, 6, 572–579. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Lechtenfeld, O.J.; Kattner, G.; Flerus, R.; McCallister, S.L.; Schmitt-Kopplin, P.; Koch, B.P. Molecular transformation and degradation of refractory dissolved organic matter in the Atlantic and Southern Ocean. Geochim. Cosmochim. Acta 2014, 126, 321–337. [Google Scholar] [CrossRef]
- D’Andrilli, J.; Cooper, W.T.; Foreman, C.M.; Marshall, A.G. An ultrahigh-resolution mass spectrometry index to estimate natural organic matter lability. Rapid Commun. Mass Spectrom. 2015, 29, 2385–2401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, L.; Zhou, L.; Zhang, Y.; Peng, K.; Gong, Z.; Jang, K.-S.; Spencer, R.G.; Jeppesen, E.; Brookes, J.D.; et al. Key factors driving dissolved organic matter composition and bioavailability in lakes situated along the Eastern Route of the South-to-North Water Diversion Project, China. Water Res. 2023, 233, 119782. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Chu, J.; Luo, C.; Chen, H. Revealing the characteristics of dissolved organic matter in urban runoff at three typical regions via optical indices and molecular composition. J. Environ. Sci. 2021, 108, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Mostovaya, A.; Hawkes, J.A.; Dittmar, T.; Tranvik, L.J. Molecular Determinants of Dissolved Organic Matter Reactivity in Lake Water. Front. Earth Sci. 2017, 5, 106. [Google Scholar] [CrossRef]
- Koch, B.P.; Dittmar, T. From mass to structure: An aromaticity index for high-resolution mass data of natural organic matter. Rapid Commun. Mass Spectrom. 2006, 20, 926–932. [Google Scholar] [CrossRef]
- Chen, J.; Liu, M.; Wang, F.; Ding, Y.; Fan, D.; Wang, H. Accumulation and migration of particulate trace metals by artificial flood event of the Yellow River: From Xiaolangdi reservoir to estuary. Sci. Total. Environ. 2024, 912, 168614. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, C.; Jia, B.; Fu, X. Spatial variation of event-based suspended sediment dynamics in the middle Yellow River basin, China. Geomorphology 2022, 401, 108115. [Google Scholar] [CrossRef]
- Liu, S.; Yu, F.; Lang, T.; Ji, Y.; Fu, Y.; Zhang, J.; Ge, C. Spatial distribution of heavy metal contaminants: The effects of water-sediment regulation in the Henan section of the Yellow River. Sci. Total. Environ. 2023, 892, 164568. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Bai, J.; Gao, Y.; Zhang, G.; Lu, Q.; Jia, J. Heavy metal contamination in soils from freshwater wetlands to salt marshes in the Yellow River Estuary, China. Sci. Total. Environ. 2021, 774, 145072. [Google Scholar] [CrossRef]
- Su, Z.; Wang, K.; Yang, F.; Zhuang, T. Antibiotic pollution of the Yellow River in China and its relationship with dissolved organic matter: Distribution and Source identification. Water Res. 2023, 235, 119867. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-P.; Zhao, Y.; Zhao, M.M.; Wu, J.-H.; Wang, K.-B. Potential health risk assessment of HFRs, PCBs, and OCPs in the Yellow River basin. Environ. Pollut. 2021, 275, 116648. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Lei, Y.; Liu, L.; Yao, H. Water scarcity risk through trade of the Yellow River Basin in China. Ecol. Indic. 2023, 154, 110893. [Google Scholar] [CrossRef]
- Zhao, J.; Kou, L.; Wang, H.; He, X.; Xiong, Z.; Liu, C.; Cui, H. Carbon Emission Prediction Model and Analysis in the Yellow River Basin Based on a Machine Learning Method. Sustainability 2022, 14, 6153. [Google Scholar] [CrossRef]
- Li, C.; Xiong, Y.; Cui, Z.; Huang, Q.; Xu, X.; Han, W.; Huang, G. Effect of irrigation and fertilization regimes on grain yield, water and nitrogen productivity of mulching cultivated maize (Zea mays L.) in the Hetao Irrigation District of China. Agric. Water Manag. 2020, 232, 106065. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, L.; Yang, L.; Zhang, F.; Norse, D.; Zhu, Z. Agricultural Non-Point Source Pollution in China: Causes and Mitigation Measures. AMBIO 2012, 41, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.; Wang, L.; Pan, B.; Guan, W.; Sun, X.; Cai, A. Distribution features and controls of heavy metals in surface sediments from the riverbed of the Ningxia-Inner Mongolian reaches, Yellow River, China. Chemosphere 2016, 144, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Kothawala, D.N.; Murphy, K.R.; Stedmon, C.A.; Weyhenmeyer, G.A.; Tranvik, L.J. Inner filter correction of dissolved organic matter fluorescence. Limnol. Oceanogr. Methods 2013, 11, 616–630. [Google Scholar] [CrossRef]
- Lawaetz, A.J.; Stedmon, C.A. Fluorescence Intensity Calibration Using the Raman Scatter Peak of Water. Appl. Spectrosc. 2009, 63, 936–940. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.R.; Stedmon, C.A.; Wenig, P.; Bro, R. OpenFluor—An online spectral library of auto-fluorescence by organic compounds in the environment. Anal. Methods 2014, 6, 658–661. [Google Scholar] [CrossRef]
- Cory, R.M.; McKnight, D.M. Fluorescence Spectroscopy Reveals Ubiquitous Presence of Oxidized and Reduced Quinones in Dissolved Organic Matter. Environ. Sci. Technol. 2005, 39, 8142–8149. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, Q.; Huang, W.-L.; Feng, L.; Wang, Y.-H.; Xie, Z.; Yong, S.-S.; Zhang, S.; Jiang, B.; Zheng, Y.; et al. Spectroscopic and molecular-level characteristics of dissolved organic matter in the Pearl River Estuary, South China. Sci. Total. Environ. 2020, 710, 136307. [Google Scholar] [CrossRef] [PubMed]
- McKnight, D.M.; Boyer, E.W.; Westerhoff, P.K.; Doran, P.T.; Kulbe, T.; Andersen, D.T. Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 2001, 46, 38–48. [Google Scholar] [CrossRef]
- Ohno, T. Fluorescence Inner-Filtering Correction for Determining the Humification Index of Dissolved Organic Matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef] [PubMed]
- Parlanti, E.; Wörz, K.; Geoffroy, L.; Lamotte, M. Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org. Geochem. 2000, 31, 1765–1781. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, Z.-T.; Liu, Y.-C.; Wang, Y.-H.; Zhang, S.; He, C.; Shi, Q.; Zeng, H.-X.; Wang, J.-J. Spectroscopic and Molecular-Level Characteristics of Dissolved Organic Matter in a Highly Polluted Urban River in South China. ACS Earth Space Chem. 2019, 3, 2033–2044. [Google Scholar] [CrossRef]
- Dittmar, T.; Koch, B.; Hertkorn, N.; Kattner, G. A simple and efficient method for the solid-phase extraction of dissolved organic matter (SPE-DOM) from seawater. Limnol. Oceanogr. Methods 2008, 6, 230–235. [Google Scholar] [CrossRef]
- Coward, E.K.; Ohno, T.; Sparks, D.L. Direct Evidence for Temporal Molecular Fractionation of Dissolved Organic Matter at the Iron Oxyhydroxide Interface. Environ. Sci. Technol. 2018, 53, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Kellerman, A.M.; Arellano, A.; Podgorski, D.C.; Martin, E.E.; Martin, J.B.; Deuerling, K.M.; Bianchi, T.S.; Spencer, R.G.M. Fundamental drivers of dissolved organic matter composition across an Arctic effective precipitation gradient. Limnol. Oceanogr. 2020, 65, 1217–1234. [Google Scholar] [CrossRef]
- Spencer, R.G.; Guo, W.; Raymond, P.A.; Dittmar, T.; Hood, E.; Fellman, J.; Stubbins, A. Source and biolability of ancient dissolved organic matter in glacier and lake ecosystems on the Tibetan Plateau. Geochim. Cosmochim. Acta 2014, 142, 64–74. [Google Scholar] [CrossRef]
- Hertkorn, N.; Benner, R.; Frommberger, M.; Schmitt-Kopplin, P.; Witt, M.; Kaiser, K.; Kettrup, A.; Hedges, J.I. Characterization of a major refractory component of marine dissolved organic matter. Geochim. Cosmochim. Acta 2006, 70, 2990–3010. [Google Scholar] [CrossRef]
- Coble, P.G. Marine Optical Biogeochemistry: The Chemistry of Ocean Color. Chem. Rev. 2007, 107, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Coble, P.G. Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 1996, 51, 325–346. [Google Scholar] [CrossRef]
- Yamashita, Y.; Scinto, L.J.; Maie, N.; Jaffé, R. Dissolved Organic Matter Characteristics Across a Subtropical Wetland’s Landscape: Application of Optical Properties in the Assessment of Environmental Dynamics. Ecosystems 2010, 13, 1006–1019. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S. Resolving the variability in dissolved organic matter fluorescence in a temperate estuary and its catchment using PARAFAC analysis. Limnol. Oceanogr. 2005, 50, 686–697. [Google Scholar] [CrossRef]
- Murphy, K.R.; Hambly, A.; Singh, S.; Henderson, R.K.; Baker, A.; Stuetz, R.; Khan, S.J. Organic Matter Fluorescence in Municipal Water Recycling Schemes: Toward a Unified PARAFAC Model. Environ. Sci. Technol. 2011, 45, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.; Lead, J.; Baker, A. Characterisation of the fluorescence from freshwater, planktonic bacteria. Water Res. 2006, 40, 2075–2083. [Google Scholar] [CrossRef]
- Baker, A. Fluorescence Excitation−Emission Matrix Characterization of Some Sewage-Impacted Rivers. Environ. Sci. Technol. 2001, 35, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Carstea, E.M.; Bridgeman, J.; Baker, A.; Reynolds, D.M. Fluorescence spectroscopy for wastewater monitoring: A review. Water Res. 2016, 95, 205–219. [Google Scholar] [CrossRef]
- Baker, A.; Inverarity, R. Protein-like fluorescence intensity as a possible tool for determining river water quality. Hydrol. Process. 2004, 18, 2927–2945. [Google Scholar] [CrossRef]
- Gu, N.; Song, Q.; Yang, X.; Yu, X.; Li, X.; Li, G. Fluorescence characteristics and biodegradability of dissolved organic matter (DOM) leached from non-point sources in southeastern China. Environ. Pollut. 2020, 258, 113807. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zhou, D.; Fan, W.; Huo, M.; Crittenden, J.C.; Yu, Z.; Ju, P.; Wang, Y. The effectiveness of coagulation for water reclamation from a wastewater treatment plant that has a long hydraulic and sludge retention times: A case study. Chemosphere 2016, 157, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, G.; Wang, H. Current state of sludge production, management, treatment and disposal in China. Water Res. 2015, 78, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Ni, B.-J.; Zeng, R.J.; Fang, F.; Xie, W.-M.; Sheng, G.-P.; Yu, H.-Q. Fractionating soluble microbial products in the activated sludge process. Water Res. 2010, 44, 2292–2302. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.-B.; Peng, D.-C.; Hou, Y.-P.; Li, H.-J.; Pei, L.-Y.; Yu, L.-F. The important implications of particulate substrate in determining the physicochemical characteristics of extracellular polymeric substances (EPS) in activated sludge. Water Res. 2014, 58, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Qu, F.; Sun, L.; Liang, H.; Han, Z.; Chang, H.; Shao, S.; Li, G. Relationship between soluble microbial products (SMP) and effluent organic matter (EfOM): Characterized by fluorescence excitation emission matrix coupled with parallel factor analysis. Chemosphere 2015, 121, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Huang, G.; Yang, X.; Li, Z.; Li, J.; Cao, J.; Wang, Z.; Sun, L. Identifying the sources and fate of anthropogenically impacted dissolved organic matter (DOM) in urbanized rivers. Water Res. 2013, 47, 5027–5039. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Wei, M.; Yao, L.; Li, M. Relationship between non-point source pollution and fluorescence fingerprint of riverine dissolved organic matter is season dependent. Sci. Total. Environ. 2022, 823, 153617. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-P.; Huang, W.-L.; Yang, M.-T.; Liu, Y.; Bowden, R.D.; Simpson, M.J.; Lajtha, K.; Tian, L.-Q.; Wang, J.-J. Chlorination of soil-derived dissolved organic matter: Long term nitrogen deposition does not increase terrestrial precursors of toxic disinfection byproducts. Water Res. 2020, 185, 116271. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Harir, M.; Gonsior, M.; Enrich-Prast, A.; Schmitt-Kopplin, P.; Bastviken, D.; Hertkorn, N. Molecular differences between water column and sediment pore water SPE-DOM in ten Swedish boreal lakes. Water Res. 2020, 170, 115320. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Riedel, T.; Niggemann, J.; Vähätalo, A.V.; Dittmar, T.; Jaffé, R. Linking the Molecular Signature of Heteroatomic Dissolved Organic Matter to Watershed Characteristics in World Rivers. Environ. Sci. Technol. 2015, 49, 13798–13806. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Li, P.; He, C.; Wang, Y.; Shi, Q. Eutrophication and watershed characteristics shape changes in dissolved organic matter chemistry along two river-estuarine transects. Water Res. 2022, 214, 118196. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Pang, Y.; He, C.; Li, P.; Xiao, S.; Sun, Y.; Pan, Q.; Zhang, Y.; Shi, Q.; He, D. Optical and molecular signatures of dissolved organic matter in Xiangxi Bay and mainstream of Three Gorges Reservoir, China: Spatial variations and environmental implications. Sci. Total. Environ. 2019, 657, 1274–1284. [Google Scholar] [CrossRef] [PubMed]
- He, D.; He, C.; Li, P.; Zhang, X.; Shi, Q.; Sun, Y. Optical and Molecular Signatures of Dissolved Organic Matter Reflect Anthropogenic Influence in a Coastal River, Northeast China. J. Environ. Qual. 2019, 48, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Gonsior, M.; Zwartjes, M.; Cooper, W.J.; Song, W.; Ishida, K.P.; Tseng, L.Y.; Jeung, M.K.; Rosso, D.; Hertkorn, N.; Schmitt-Kopplin, P. Molecular characterization of effluent organic matter identified by ultrahigh resolution mass spectrometry. Water Res. 2011, 45, 2943–2953. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wang, K.; Sun, Y.; Zhou, Y.; Yang, S.; Li, Y.; He, C.; Shi, Q.; He, D. Linking the unique molecular complexity of dissolved organic matter to flood period in the Yangtze River mainstream. Sci. Total. Environ. 2021, 764, 142803. [Google Scholar] [CrossRef] [PubMed]
- Kellerman, A.M.; Guillemette, F.; Podgorski, D.C.; Aiken, G.R.; Butler, K.D.; Spencer, R.G.M. Unifying Concepts Linking Dissolved Organic Matter Composition to Persistence in Aquatic Ecosystems. Environ. Sci. Technol. 2018, 52, 2538–2548. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, Y.; Zhang, Y.; Wu, Y.; Jang, K.-S.; Spencer, R.G.M.; Brookes, J.D.; Jeppesen, E. Hydrological Controls on Dissolved Organic Matter Composition throughout the Aquatic Continuum of the Watershed of Selin Co, the Largest Lake on the Tibetan Plateau. Environ. Sci. Technol. 2023, 57, 4668–4678. [Google Scholar] [CrossRef]
- Zaharescu, D.G.; Hooda, P.S.; Soler, A.P.; Fernandez, J.; Burghelea, C.I. Trace metals and their source in the catchment of the high altitude Lake Respomuso, Central Pyrenees. Sci. Total. Environ. 2009, 407, 3546–3553. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Yu, M.; Yuan, Q.; Meng, Y.; Qie, Y.; Shang, Z.; Luan, F.; Zhang, D. Spatial distribution, pollution assessment, and source identification of heavy metals in the Yellow River. J. Hazard. Mater. 2022, 436, 129309. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Liu, Y. Study on heavy metals and ecological risk assessment from Gansu, Ningxia and Inner Mongolia sections of the Yellow River, China. Spectrosc. Spect. Anal. 2013, 33, 3249–3254. [Google Scholar]
- Guo, X.; Xie, X.; Liu, Y.; Wang, C.; Yang, M.; Huang, Y. Effects of digestate DOM on chemical behavior of soil heavy metals in an abandoned copper mining areas. J. Hazard. Mater. 2020, 393, 122436. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, C.-C.; Li, J.-Q.; Wang, P.; Ou, J.-Q.; Cui, J.-R. Interactions between copper(II) and DOM in the urban stormwater runoff: Modeling and characterizations. Environ. Technol. 2018, 39, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ma, X.; Yang, X.; Xu, H. Influence of heavy metal ions on the spectra and charge characteristics of DOM of municipal sewage secondary effluent. Water Sci. Technol. 2018, 77, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
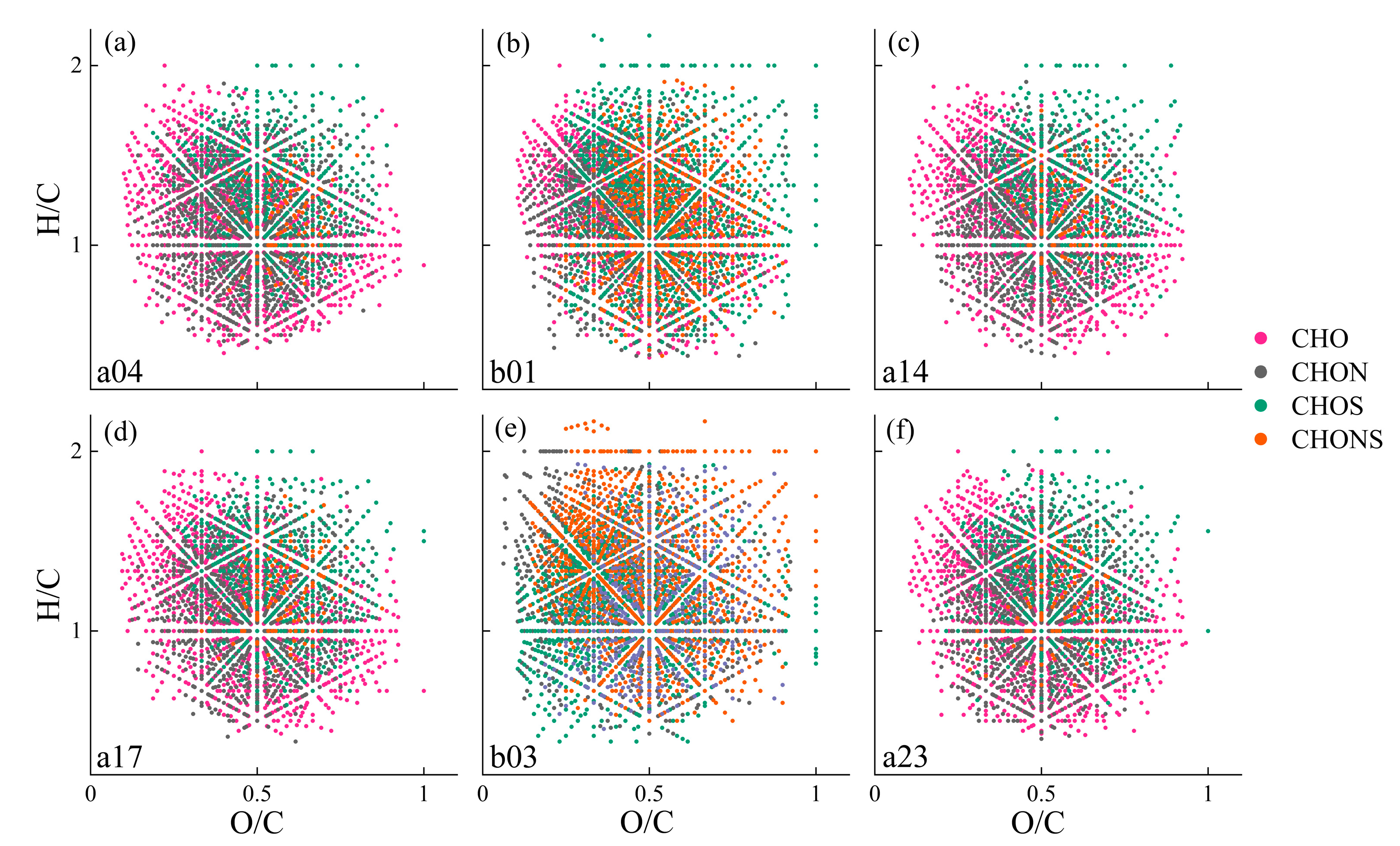
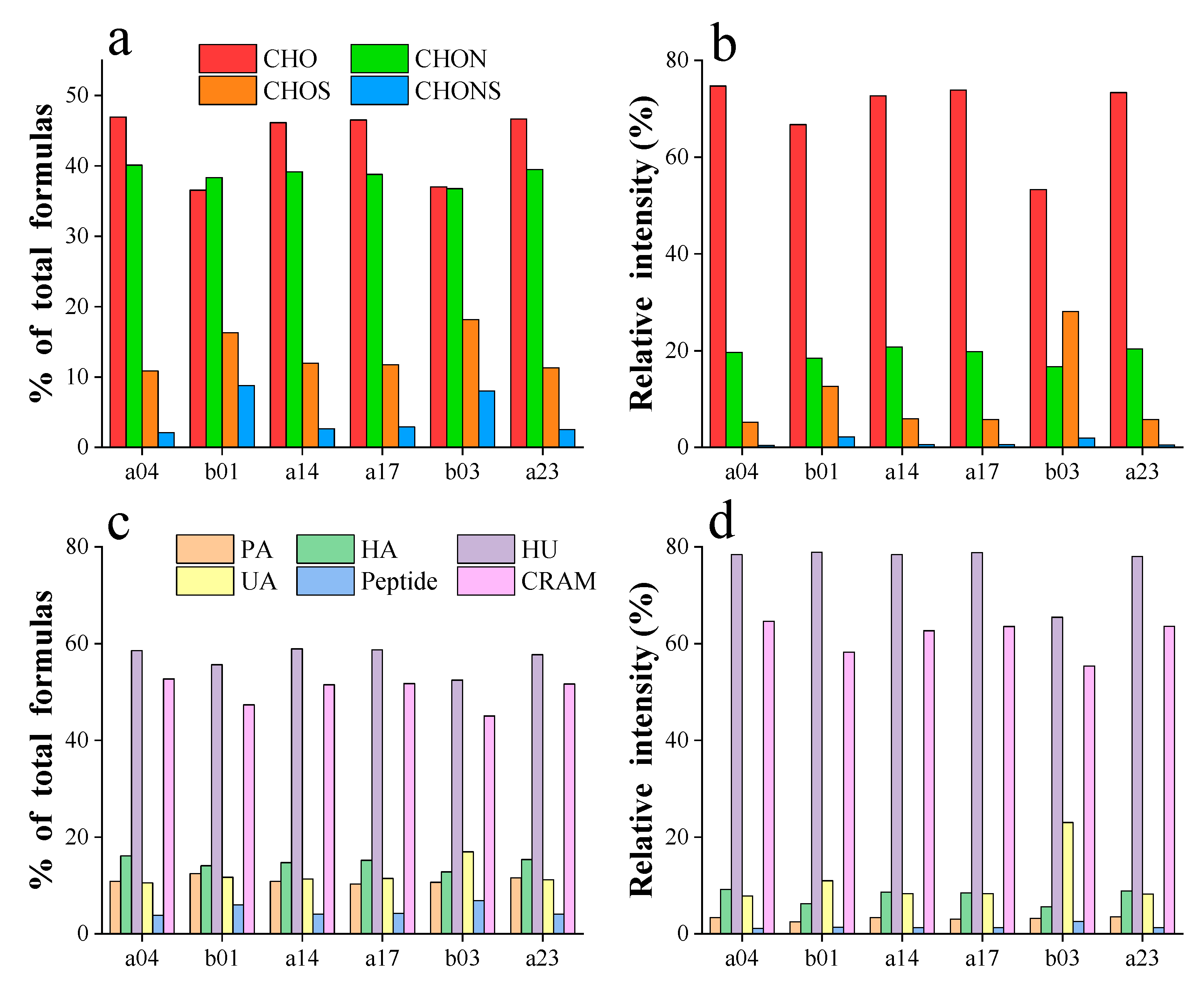
| a04 | b01 | a14 | a17 | b03 | a23 | |
|---|---|---|---|---|---|---|
| Number of formulas | 4402 | 6719 | 4026 | 4194 | 6620 | 4166 |
| Average MW (Da) | 365.39 | 366.17 | 359.04 | 359.65 | 346.92 | 359.79 |
| Average H/C | 1.20 | 1.25 | 1.21 | 1.21 | 1.33 | 1.21 |
| Average O/C | 0.50 | 0.52 | 0.51 | 0.51 | 0.45 | 0.51 |
| Average DBE | 7.97 | 7.47 | 7.73 | 7.72 | 6.61 | 7.78 |
| Average AImod | 0.33 | 0.28 | 0.32 | 0.32 | 0.26 | 0.32 |
| MLBL (%) | 14.5 | 17.8 | 15.5 | 15.7 | 24.1 | 15.3 |
| IOS (%) | 7.3 | 6.8 | 7.5 | 7.4 | 6.1 | 7.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Jiang, J.; Zhu, Y.; Zhou, Q.; Bing, X.; Tan, Y.; Wang, Y.; Zhang, R. Characteristics of DOM and Their Relationships with Potentially Toxic Elements in the Inner Mongolia Section of the Yellow River, China. Toxics 2024, 12, 250. https://doi.org/10.3390/toxics12040250
Wang K, Jiang J, Zhu Y, Zhou Q, Bing X, Tan Y, Wang Y, Zhang R. Characteristics of DOM and Their Relationships with Potentially Toxic Elements in the Inner Mongolia Section of the Yellow River, China. Toxics. 2024; 12(4):250. https://doi.org/10.3390/toxics12040250
Chicago/Turabian StyleWang, Kuo, Juan Jiang, Yuanrong Zhu, Qihao Zhou, Xiaojie Bing, Yidan Tan, Yuyao Wang, and Ruiqing Zhang. 2024. "Characteristics of DOM and Their Relationships with Potentially Toxic Elements in the Inner Mongolia Section of the Yellow River, China" Toxics 12, no. 4: 250. https://doi.org/10.3390/toxics12040250
APA StyleWang, K., Jiang, J., Zhu, Y., Zhou, Q., Bing, X., Tan, Y., Wang, Y., & Zhang, R. (2024). Characteristics of DOM and Their Relationships with Potentially Toxic Elements in the Inner Mongolia Section of the Yellow River, China. Toxics, 12(4), 250. https://doi.org/10.3390/toxics12040250







