Emerging Contaminants in the Effluent of Wastewater Should Be Regulated: Which and to What Extent?
Abstract
:1. Introduction
2. Methodology
2.1. Methods of Data Collection
2.2. Risk Assessment Methods
2.3. Water Quality Criteria Derivation Method
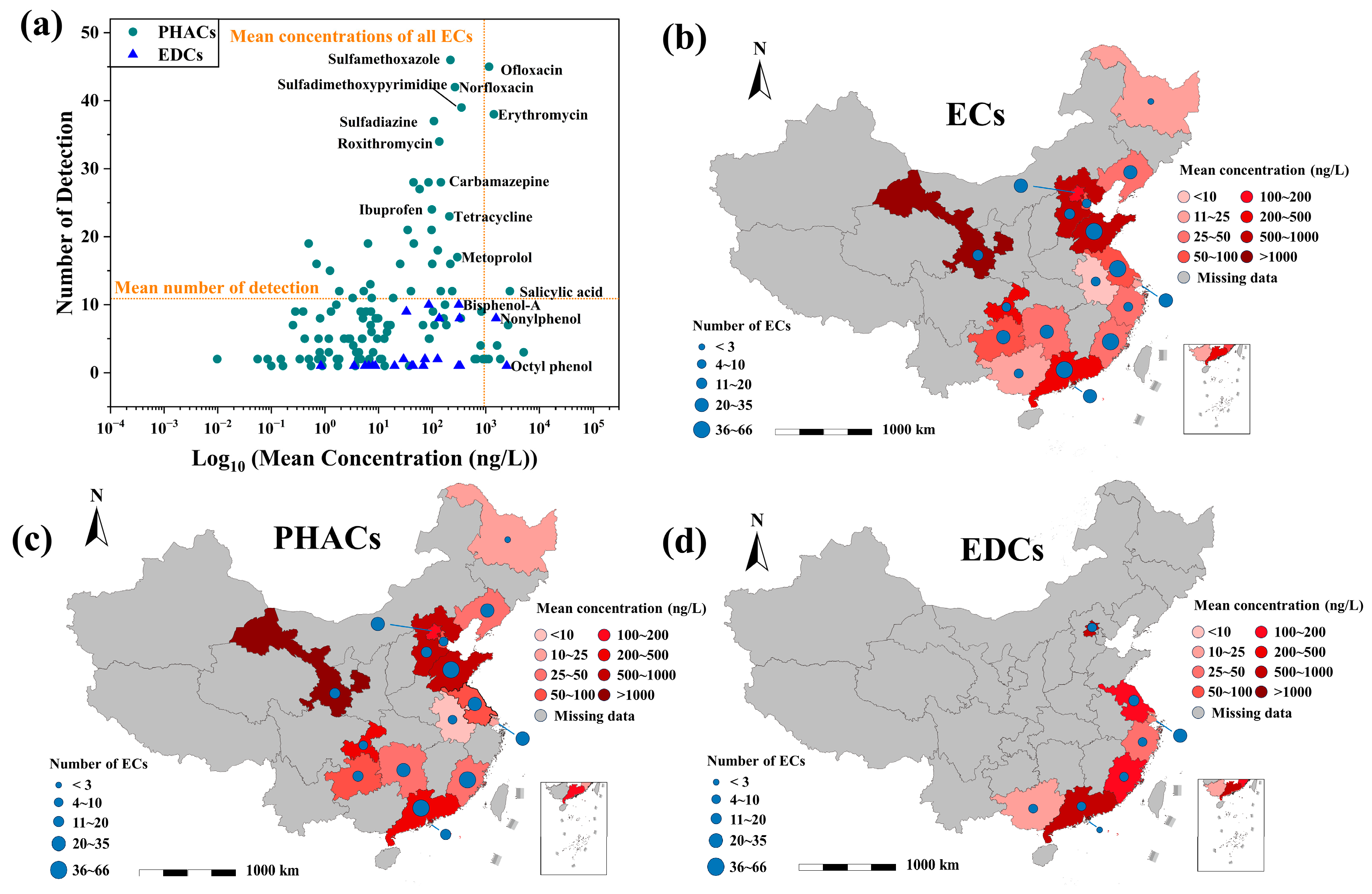
3. Exposure and Risk of ECs
3.1. Exposure
3.2. Risk
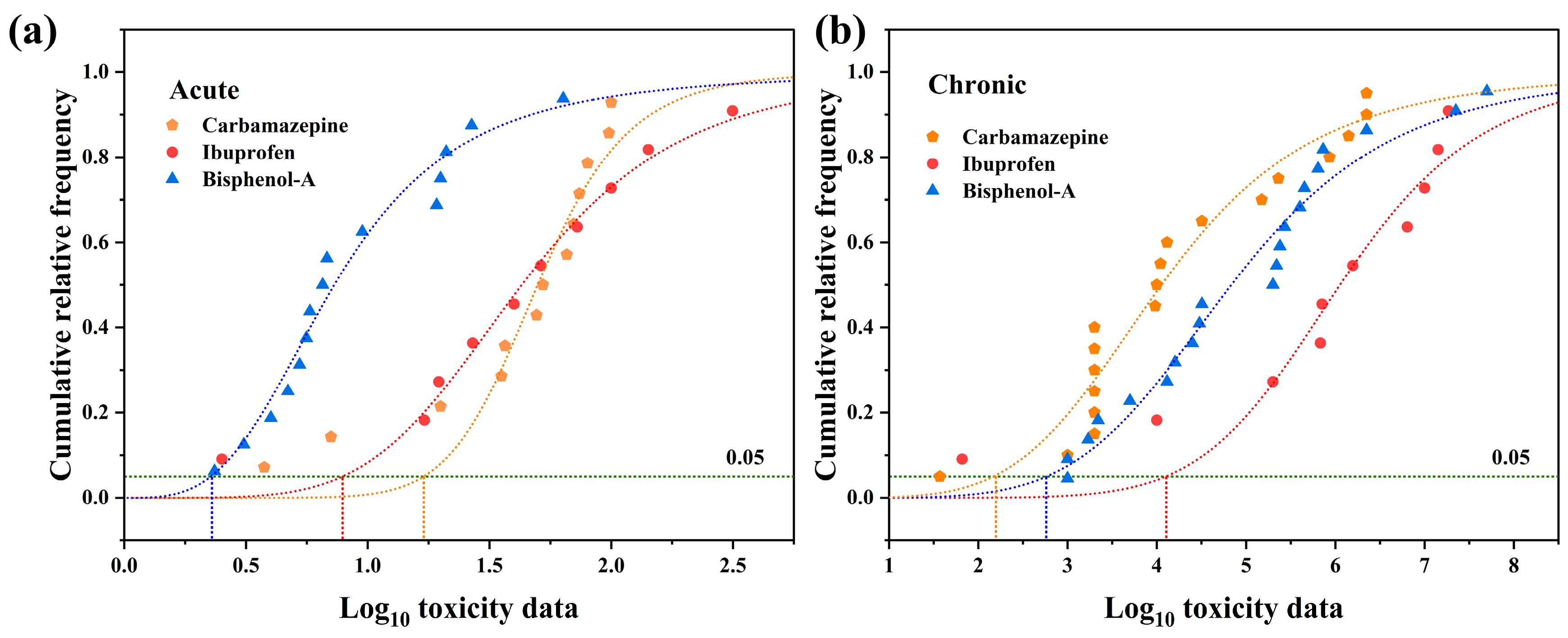
4. The Derivation Water Quality Criteria of ECs
5. Future Prospects
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ramírez-Malule, H.; Quiñones-Murillo, D.H.; Manotas-Duque, D. Emerging Contaminants as Global Environmental Hazards. A Bibliometr. Analysis. Emerg. Contam. 2020, 6, 179–193. [Google Scholar] [CrossRef]
- Puri, M.; Gandhi, K.; Kumar, M.S. Emerging Environmental Contaminants: A Global Perspective on Policies and Regulations. J. Environ. Manag. 2023, 332, 117344. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging Contaminants of High Concern for the Environment: Current Trends and Future Research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef]
- Guo, J.; Tu, K.; Chou, L.; Zhang, Y.; Wei, S.; Zhang, X.; Yu, H.; Shi, W. Deep Mining of Reported Emerging Contaminants in China’s Surface Water in the Past Decade: Exposure, Ecological Effects and Risk Assessment. Water Res. 2023, 243, 120318. [Google Scholar] [CrossRef] [PubMed]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The Removal of Pharmaceuticals, Personal Care Products, Endocrine Disruptors and Illicit Drugs during Wastewater Treatment and Its Impact on the Quality of Receiving Waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Moldovan, Z.; Schmutzer, G.; Tusa, F.; Calin, R.; Alder, A.C. An Overview of Pharmaceuticals and Personal Care Products Contamination along the River Somes Watershed, Romania. J. Environ. Monit. 2007, 9, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Wang, B.; Fu, C.; Dong, R.; Huang, J.; Deng, S.; Wang, Y.; Yu, G. Pharmaceuticals and Personal Care Products (PPCPs) in Urban and Suburban Rivers of Beijing, China: Occurrence, Source Apportionment and Potential Ecological Risk. Environ. Sci.: Process. Impacts 2016, 18, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Xu, J.; Keller, A.A.; He, L.; Gu, Y.; Zheng, W.; Sun, D.; Lu, Z.; Huang, J.; Huang, X.; et al. Occurrence and Risk Assessment of Emerging Contaminants in a Water Reclamation and Ecological Reuse Project. Sci. Total Environ. 2020, 744, 140977. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, S.; Vasiloglou, V.; Kungolos, A. A Multicriteria Analysis Application for Evaluating the Possibility of Reusing Wastewater for Irrigation Purposes in a Greek Region. Desalination Water Treat. 2012, 39, 262–270. [Google Scholar] [CrossRef]
- Lin, S.H.; Cheng, K.W. A New Sequencing Batch Reactor for Treatment of Municipal Sewage Wastewater for Agricultural Reuse. Desalination 2001, 133, 41–51. [Google Scholar] [CrossRef]
- Bauer, S.; Linke, H.J.; Wagner, M. Optimizing Water-Reuse and Increasing Water-Saving Potentials by Linking Treated Industrial and Municipal Wastewater for a Sustainable Urban Development. Water Sci. Technol. 2020, 81, 1927–1940. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Hu, Y.; Zhu, Q.; Gao, J.; Liao, C.; Jiang, G. Co-Exposure and Health Risks of Several Typical Endocrine Disrupting Chemicals in General Population in Eastern China. Environ. Res. 2022, 204, 112366. [Google Scholar] [CrossRef] [PubMed]
- Futran Fuhrman, V.; Tal, A.; Arnon, S. Why Endocrine Disrupting Chemicals (EDCs) Challenge Traditional Risk Assessment and How to Respond. J. Hazard. Mater. 2015, 286, 589–611. [Google Scholar] [CrossRef] [PubMed]
- Posthuma, L.; Suter, G.W.; Traas, T.P. Species Sensitivity Distributions in Ecotoxicology; Taylor & Francis Ltd.: London, UK, 2001. [Google Scholar] [CrossRef]
- Canadian Council of Minister of the Environment (CCME). Canadian Environmental Quality Guidelines (CEQGs) Provide Science-Based Goals for the Quality of Aquatic and Terrestrial Ecosystems; Canadian Council of Minister of the Environment: Manitoba, ON, Canada, 2018. [Google Scholar]
- Niyogi, S.; Wood, C.M. Biotic Ligand Model, a Flexible Tool for Developing Site-Specific Water Quality Guidelines for Metals. Environ. Sci. Technol. 2004, 38, 6177–6192. [Google Scholar] [CrossRef] [PubMed]
- USEPA. Guidelines for Deriving Numerical National Water Quality Criteria for the Protection of Aquatic Organisms and Their Uses. Available online: https://www.epa.gov/wqc/guidelines-deriving-numerical-national-water-quality-criteria-protection-aquatic-organisms-and (accessed on 22 March 2024).
- Bu, Q.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Pharmaceuticals and Personal Care Products in the Aquatic Environment in China: A Review. J. Hazard. Mater. 2013, 262, 189–211. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Ecology and Environment of People’s Republic of China. Technical Guideline for Deriving Water Quality Criteria for Freshwater Organisms; Ministry of Ecology and Environment of People’s Republic of China: Beijing, China, 2022.
- National Ecological Environment Benchmark Expert Committee. National Ecological Environment Benchmark Calculation Software Species Sensitivity Distribution Method; Ministry of Ecology and Environment, People’s Republic of China: Beijing, China, 2021.
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Varela Della Giustina, S.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic Residues in Final Effluents of European Wastewater Treatment Plants and Their Impact on the Aquatic Environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-W.; Rodriguez, J.M. Occurrence and Removal of Selected Pharmaceuticals and Personal Care Products in Three Wastewater-Treatment Plants. Arch. Environ. Contam. Toxicol. 2014, 66, 538–548. [Google Scholar] [CrossRef]
- Balakrishna, K.; Rath, A.; Praveenkumarreddy, Y.; Guruge, K.S.; Subedi, B. A Review of the Occurrence of Pharmaceuticals and Personal Care Products in Indian Water Bodies. Ecotoxicol. Environ. Saf. 2017, 137, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, K.G.; Meyer, M.T. Occurrence of Antibiotics in Wastewater Treatment Facilities in Wisconsin, USA. Sci. Total Environ. 2006, 361, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Crouse, B.A.; Ghoshdastidar, A.J.; Tong, A.Z. The Presence of Acidic and Neutral Drugs in Treated Sewage Effluents and Receiving Waters in the Cornwallis and Annapolis River Watersheds and the Mill CoveSewage Treatment Plant in Nova Scotia, Canada. Environ. Res. 2012, 112, 92–99. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, Y.; Lu, S.; Yan, P.; Sui, Q. Recent Advances in Pharmaceuticals and Personal Care Products in the Surface Water and Sediments in China. Front. Environ. Sci. Eng. 2016, 10, 2. [Google Scholar] [CrossRef]
- Tran, N.H.; Hoang, L.; Nghiem, L.D.; Nguyen, N.M.H.; Ngo, H.H.; Guo, W.; Trinh, Q.T.; Mai, N.H.; Chen, H.; Nguyen, D.D.; et al. Occurrence and Risk Assessment of Multiple Classes of Antibiotics in Urban Canals and Lakes in Hanoi, Vietnam. Sci. Total Environ. 2019, 692, 157–174. [Google Scholar] [CrossRef]
- Meng, F.; Sun, S.; Geng, J.; Ma, L.; Jiang, J.; Li, B.; Yabo, S.D.; Lu, L.; Fu, D.; Shen, J.; et al. Occurrence, Distribution, and Risk Assessment of Quinolone Antibiotics in Municipal Sewage Sludges throughout China. J. Hazard. Mater. 2023, 453, 131322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, Z.; Zhao, M.; He, J.; Zhang, X.; Hao, F.; Du, P. Occurrence, Removal, Emission and Environment Risk of 32 Antibiotics and Metabolites in Wastewater Treatment Plants in Wuhu, China. Sci. Total Environ. 2023, 899, 165681. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Lin, C.; Lei, K.; Xin, M.; Gu, X.; Lian, M.; Wang, B.; Liu, X.; Ouyang, W.; He, M. Profiling of the Spatiotemporal Distribution, Risks, and Prioritization of Antibiotics in the Waters of Laizhou Bay, Northern China. J. Hazard. Mater. 2022, 424, 127487. [Google Scholar] [CrossRef]
- Sun, C.; Hu, E.; Liu, S.; Wen, L.; Yang, F.; Li, M. Spatial Distribution and Risk Assessment of Certain Antibiotics in 51 Urban Wastewater Treatment Plants in the Transition Zone between North and South China. J. Hazard. Mater. 2022, 437, 129307. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.d.J.S.; Kulzer, J.; Lima, P. da R.P. de; Barbosa, S.C.; Primel, E.G. Updated Knowledge, Partitioning and Ecological Risk of Pharmaceuticals and Personal Care Products in Global Aquatic Environments. Environ. Sci. Process. Impacts 2022, 24, 1982–2008. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, R.; Wang, Y.; Pan, X.; Tang, J.; Zhang, G. Occurrence and Distribution of Antibiotics in the Beibu Gulf, China: Impacts of River Discharge and Aquaculture Activities. Mar. Environ. Res. 2012, 78, 26–33. [Google Scholar] [CrossRef]
- Lin, T.; Yu, S.; Chen, W. Occurrence, Removal and Risk Assessment of Pharmaceutical and Personal Care Products (PPCPs) in an Advanced Drinking Water Treatment Plant (ADWTP) around Taihu Lake in China. Chemosphere 2016, 152, 1–9. [Google Scholar] [CrossRef]
- Wu, C.; Huang, X.; Witter, J.D.; Spongberg, A.L.; Wang, K.; Wang, D.; Liu, J. Occurrence of Pharmaceuticals and Personal Care Products and Associated Environmental Risks in the Central and Lower Yangtze River, China. Ecotoxicol. Environ. Saf. 2014, 106, 19–26. [Google Scholar] [CrossRef]
- Liu, J.; Lu, G.; Xie, Z.; Zhang, Z.; Li, S.; Yan, Z. Occurrence, Bioaccumulation and Risk Assessment of Lipophilic Pharmaceutically Active Compounds in the Downstream Rivers of Sewage Treatment Plants. Sci. Total Environ. 2015, 511, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, S.; Xu, X.-R.; Zhou, G.-J.; Liu, S.-S.; Yue, W.-Z.; Sun, K.-F.; Ying, G.-G. Antibiotics in the Coastal Environment of the Hailing Bay Region, South China Sea: Spatial Distribution, Source Analysis and Ecological Risks. Mar. Pollut. Bull. 2015, 95, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence and Risk Assessment of Pharmaceutically Active Compounds in Wastewater Treatment Plants. A Case Study: Seville City (Spain). Environ. Int. 2007, 33, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Kondor, A.C.; Molnár, É.; Vancsik, A.; Filep, T.; Szeberényi, J.; Szabó, L.; Maász, G.; Pirger, Z.; Weiperth, A.; Ferincz, Á.; et al. Occurrence and Health Risk Assessment of Pharmaceutically Active Compounds in Riverbank Filtrated Drinking Water. J. Water Process Eng. 2021, 41, 102039. [Google Scholar] [CrossRef]
- Wang, C.; Lu, Y.; Wang, C.; Xiu, C.; Cao, X.; Zhang, M.; Song, S. Distribution and Ecological Risks of Pharmaceuticals and Personal Care Products with Different Anthropogenic Stresses in a Coastal Watershed of China. Chemosphere 2022, 303, 135176. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, B.; Paxéus, N.; Giudice, R.L.; Pollio, A.; Garric, J. Ecotoxicological Impact of Pharmaceuticals Found in Treated Wastewaters: Study of Carbamazepine, Clofibric Acid, and Diclofenac. Ecotoxicol. Environ. Saf. 2003, 55, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Shen, Y.; Jiang, L.; Jiang, B.; Li, Y.; Yuan, Q.; Zhang, Y. Occurrence and Ecological-Risk Levels of Antibiotic Pollution in the Coastal Waters of Eastern China. Environ. Sci. Pollut. Res. 2023, 30, 71371–71381. [Google Scholar] [CrossRef]
- Choi, K.; Kim, Y.; Jung, J.; Kim, M.-H.; Kim, C.-S.; Kim, N.-H.; Park, J. Occurrences and Ecological Risks of Roxithromycin, Trimethoprim, and Chloramphenicol in the Han River, Korea. Environ. Toxicol. Chem. 2008, 27, 711–719. [Google Scholar] [CrossRef]
- Yu, X.; Yu, F.; Li, Z.; Zhan, J. Occurrence, Distribution, and Ecological Risk Assessment of Pharmaceuticals and Personal Care Products in the Surface Water of the Middle and Lower Reaches of the Yellow River (Henan Section). J. Hazard. Mater. 2023, 443, 130369. [Google Scholar] [CrossRef]
- Sun, S.; Chen, Y.; Lin, Y.; An, D. Occurrence, Spatial Distribution, and Seasonal Variation of Emerging Trace Organic Pollutants in Source Water for Shanghai, China. Sci. Total Environ. 2018, 639, 1–7. [Google Scholar] [CrossRef]
- Acuña, V.; Ginebreda, A.; Mor, J.R.; Petrovic, M.; Sabater, S.; Sumpter, J.; Barceló, D. Balancing the Health Benefits and Environmental Risks of Pharmaceuticals: Diclofenac as an Example. Environ. Int. 2015, 85, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Gopal, C.M.; Bhat, K.; Ramaswamy, B.R.; Kumar, V.; Singhal, R.K.; Basu, H.; Udayashankar, H.N.; Vasantharaju, S.G.; Praveenkumarreddy, Y.; Shailesh; et al. Seasonal Occurrence and Risk Assessment of Pharmaceutical and Personal Care Products in Bengaluru Rivers and Lakes, India. J. Environ. Chem. Eng. 2021, 9, 105610. [Google Scholar] [CrossRef]
- Tauxe-Wuersch, A.; De Alencastro, L.F.; Grandjean, D.; Tarradellas, J. Occurrence of Several Acidic Drugs in Sewage Treatment Plants in Switzerland and Risk Assessment. Water Res. 2005, 39, 1761–1772. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.; Khan, K.N.; Rasool, S.; Mustafa, G.; Saif-Ur-Rehman, M.; Nazar, M.F.; Sun, Q.; Yu, C.-P. Occurrence and Ecological Risk Assessment of Fluoroquinolone Antibiotics in Hospital Waste of Lahore, Pakistan. Environ. Toxicol. Pharmacol. 2016, 42, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nasir, F.A.; Praveena, S.M.; Aris, A.Z. Public Awareness Level and Occurrence of Pharmaceutical Residues in Drinking Water with Potential Health Risk: A Study from Kajang (Malaysia). Ecotoxicol. Environ. Saf. 2019, 185, 109681. [Google Scholar] [CrossRef]
- de Souza, R.C.; Godoy, A.A.; Kummrow, F.; dos Santos, T.L.; Brandão, C.J.; Pinto, E. Occurrence of Caffeine, Fluoxetine, Bezafibrate and Levothyroxine in Surface Freshwater of São Paulo State (Brazil) and Risk Assessment for Aquatic Life Protection. Environ. Sci. Pollut. Res. 2021, 28, 20751–20761. [Google Scholar] [CrossRef] [PubMed]
- Komori, K.; Suzuki, Y.; Minamiyama, M.; Harada, A. Occurrence of Selected Pharmaceuticals in River Water in Japan and Assessment of Their Environmental Risk. Environ. Monit. Assess. 2013, 185, 4529–4536. [Google Scholar] [CrossRef]
- Singh, V.; Suthar, S. Occurrence, Seasonal Variations, and Ecological Risk of Pharmaceuticals and Personal Care Products in River Ganges at Two Holy Cities of India. Chemosphere 2021, 268, 129331. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wen, J.; He, B.; Wang, J.; Hu, X.; Liu, J. Occurrence of Caffeine in the Freshwater Environment: Implications for Ecopharmacovigilance. Environ. Pollut. 2020, 263, 114371. [Google Scholar] [CrossRef]
- Ilechukwu, I.; Okonkwo, C.J.; Olusina, T.A.; Mpock, J.A.; Ilechukwu, C. Occurrence and Risk Assessment of Selected Pharmaceuticals in Water and Sediments of Usuma Dam, Abuja, Nigeria. Int. J. Environ. Anal. Chem. 2023, 103, 4398–4410. [Google Scholar] [CrossRef]
- Qu, H.; Barrett, H.; Wang, B.; Han, J.; Wang, F.; Gong, W.; Wu, J.; Wang, W.; Yu, G. Co-Occurrence of Antiseptic Triclocarban and Chiral Anti-Inflammatory Ibuprofen in Environment: Association between Biological Effect in Sediment and Risk to Human Health. J. Hazard. Mater. 2021, 407, 124871. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, N.; Tuhkanen, T.; Kronberg, L. Occurrence of Acidic Pharmaceuticals in Raw and Treated Sewages and in Receiving Waters. Water Res. 2005, 39, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Sörengård, M.; Campos-Pereira, H.; Ullberg, M.; Lai, F.Y.; Golovko, O.; Ahrens, L. Mass Loads, Source Apportionment, and Risk Estimation of Organic Micropollutants from Hospital and Municipal Wastewater in Recipient Catchments. Chemosphere 2019, 234, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Godoy, A.A.; Kummrow, F.; Pamplin, P.A.Z. Occurrence, Ecotoxicological Effects and Risk Assessment of Antihypertensive Pharmaceutical Residues in the Aquatic Environment—A Review. Chemosphere 2015, 138, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhao, Y.; Fent, K. Cardiovascular Drugs and Lipid Regulating Agents in Surface Waters at Global Scale: Occurrence, Ecotoxicity and Risk Assessment. Sci. Total Environ. 2020, 729, 138770. [Google Scholar] [CrossRef]
- Mijangos, L.; Ziarrusta, H.; Ros, O.; Kortazar, L.; Fernández, L.A.; Olivares, M.; Zuloaga, O.; Prieto, A.; Etxebarria, N. Occurrence of Emerging Pollutants in Estuaries of the Basque Country: Analysis of Sources and Distribution, and Assessment of the Environmental Risk. Water Res. 2018, 147, 152–163. [Google Scholar] [CrossRef]
- Peng, X.; Ou, W.; Wang, C.; Wang, Z.; Huang, Q.; Jin, J.; Tan, J. Occurrence and Ecological Potential of Pharmaceuticals and Personal Care Products in Groundwater and Reservoirs in the Vicinity of Municipal Landfills in China. Sci. Total Environ. 2014, 490, 889–898. [Google Scholar] [CrossRef]
- Ashfaq, M.; Nawaz Khan, K.; Saif Ur Rehman, M.; Mustafa, G.; Faizan Nazar, M.; Sun, Q.; Iqbal, J.; Mulla, S.I.; Yu, C.-P. Ecological Risk Assessment of Pharmaceuticals in the Receiving Environment of Pharmaceutical Wastewater in Pakistan. Ecotoxicol. Environ. Saf. 2017, 136, 31–39. [Google Scholar] [CrossRef]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Parrella, A.; Previtera, L.; Rubino, M. Ecotoxicity of Naproxen and Its Phototransformation Products. Sci. Total Environ. 2005, 348, 93–101. [Google Scholar] [CrossRef]
- Korkmaz, N.E.; Savun-Hekimoğlu, B.; Aksu, A.; Burak, S.; Caglar, N.B. Occurrence, Sources and Environmental Risk Assessment of Pharmaceuticals in the Sea of Marmara, Turkey. Sci. Total Environ. 2022, 819, 152996. [Google Scholar] [CrossRef]
- Cao, J.; Shi, J.; Han, R.; Li, Y.; Yang, Z. Seasonal Variations in the Occurrence and Distribution of Estrogens and Pharmaceuticals in the Zhangweinanyun River System. Chin. Sci. Bull. 2010, 55, 3138–3144. [Google Scholar] [CrossRef]
- Wang, D.; Luo, Z.; Zhang, X.; Lin, L.; Du, M.; Du Laing, G.; Yan, C. Occurrence, Distribution and Risk Assessment of Estrogenic Compounds for Three Source Water Types in Ningbo City, China. Environ. Earth Sci. 2015, 74, 5961–5969. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, L.; Chang, A.C. Seasonal Variation of Endocrine Disrupting Compounds, Pharmaceuticals and Personal Care Products in Wastewater Treatment Plants. Sci. Total Environ. 2013, 442, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Fan, G.; Yu, W.; Yang, S.; Zhou, J.; Luo, J. Occurrence and Risk Assessment of Steroid Estrogens in Environmental Water Samples: A Five-Year Worldwide Perspective. Environ. Pollut. 2020, 267, 115405. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Lu, G.; Jiang, R.; Yan, Z.; Li, Y. Occurrence, Toxicity and Ecological Risk of Bisphenol A Analogues in Aquatic Environment—A Review. Ecotoxicol. Environ. Saf. 2021, 208, 111481. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Q.; Hu, L.; Lu, G.; Li, Y. Occurrence of Estrogens in Water, Sediment and Biota and Their Ecological Risk in Northern Taihu Lake in China. Environ. Geochem. Health 2015, 37, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhu, Q.; Yan, X.; Liao, C.; Jiang, G. Occurrence, Fate and Risk Assessment of BPA and Its Substituents in Wastewater Treatment Plant: A Review. Environ. Res. 2019, 178, 108732. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Luo, X.; Shu, S.; Ding, J.; Zhang, G.; Wang, Z.; Zou, H.; Zhang, Y. Impact of Rainfall on the Occurrence, Spatiotemporal Distribution, and Partition Trend of Micropollutants in Taihu Lake, China: Bisphenol A and 4-Nonylphenol as Examples. Ecotoxicol. Environ. Saf. 2020, 204, 111064. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Sui, Y.; Feng, J.; Wang, X.; Li, X.; Jiang, S.; Zhang, Z.; Zi, J.; Sun, T.; Gao, Y.; et al. Environmental Occurrence and Ecological Risk of Bisphenol A in Erhai Lake Basin Away From Industrial Regions in China. Pol. J. Environ. Stud. 2020, 30, 841–850. [Google Scholar] [CrossRef]
- Catenza, C.J.; Farooq, A.; Shubear, N.S.; Donkor, K.K. A Targeted Review on Fate, Occurrence, Risk and Health Implications of Bisphenol Analogues. Chemosphere 2021, 268, 129273. [Google Scholar] [CrossRef]
- Zhong, M.; Wang, T.; Zhao, W.; Huang, J.; Wang, B.; Blaney, L.; Bu, Q.; Yu, G. Emerging Organic Contaminants in Chinese Surface Water: Identification of Priority Pollutants. Engineering 2022, 11, 111–125. [Google Scholar] [CrossRef]
- Wu, J.; Shi, D.; Wang, S.; Yang, X.; Zhang, H.; Zhang, T.; Zheng, L.; Zhang, Y. Derivation of Water Quality Criteria for Carbamazepine and Ecological Risk Assessment in the Nansi Lake Basin. Int. J. Environ. Res. Public Health 2022, 19, 10875. [Google Scholar] [CrossRef]
- Huang, Q.; Bu, Q.; Zhong, W.; Shi, K.; Cao, Z.; Yu, G. Derivation of Aquatic Predicted No-Effect Concentration (PNEC) for Ibuprofen and Sulfamethoxazole Based on Various Toxicity Endpoints and the Associated Risks. Chemosphere 2018, 193, 223–229. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Liu, J.; Ji, G.; Shi, L.; Xu, J.; Yang, J. Deriving the Freshwater Quality Criteria of BPA, BPF and BPAF for Protecting Aquatic Life. Ecotoxicol. Environ. Saf. 2018, 164, 713–721. [Google Scholar] [CrossRef]
- Wang, Y.; Na, G.; Zong, H.; Ma, X.; Yang, X.; Mu, J.; Wang, L.; Lin, Z.; Zhang, Z.; Wang, J.; et al. Applying Adverse Outcome Pathways and Species Sensitivity–Weighted Distribution to Predicted-No-Effect Concentration Derivation and Quantitative Ecological Risk Assessment for Bisphenol A and 4-Nonylphenol in Aquatic Environments: A Case Study on Tianjin City, China. Environ. Toxicol. Chem. 2018, 37, 551–562. [Google Scholar] [CrossRef]
- Zhou, Y.; Meng, J.; Zhang, M.; Chen, S.; He, B.; Zhao, H.; Li, Q.; Zhang, S.; Wang, T. Which Type Of pollutants Need to be Controlled with Priority in Wastewater Treatment Plants: Traditional or Emerging Pollutants? Environ. Int. 2019, 131, 104982. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Liu, W.-R.; Liu, Y.-S.; Zhao, J.-L.; Zhang, Q.-Q.; Zhang, M.; Zhang, J.-N.; Jiang, Y.-X.; Zhang, L.-J.; Ying, G.-G. Suitability of Pharmaceuticals and Personal Care Products (PPCPs) and Artificial Sweeteners (ASs) as Wastewater Indicators in the Pearl River Delta, South China. Sci. Total Environ. 2017, 590–591, 611–619. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, L.-L.; Hong, C.-Y.; Bing, Y.-X.; Xu, Z.-C. Seasonal Occurrence, Removal and Risk Assessment of 10 Pharmaceuticals in 2 Sewage Treatment Plants of Guangdong, China. Environ. Technol. 2019, 40, 458–469. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, D.; Cao, Y.; Yu, X.; Hui, Y.; Li, W.; Wang, D. Seasonal changes and ecological risk assessment of pharmaceutical and personal care products in the effluents of wastewater treatment plants in Beijin. Acta Sci. Circumstantiae 2021, 41, 2922–2932. [Google Scholar] [CrossRef]
- Shao, T.; Ben, W.; Su, D.; Zhang, H.; Hou, P.; Qiang, Z.; Zhang, Y. Quantitative determination of typical pharmaceuticals and their metabolites in municipal wastewater treatment plants. Acta Sci. Circumstantiae 2020, 40, 2136–2141. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Zhang, S.; Xiang, F.; Tang, T.; Yang, Y. Pollution Level and Ecological Risk of Typical Antibiotics in Guiyang Wastewater Treatment Plants. Environ. Sci. 2019, 40, 3249–3256. [Google Scholar] [CrossRef]
- Ye, P.; You, W.; Yang, B.; Chen, Y.; Wang, L.; Zhao, J.; Ying, G. Pollution Characteristics and Removal of Typical Pharmaceuticals in Hospital Wastewater and Municipal Wastewater Treatment Plants. Environ. Sci. 2021, 42, 2928–2936. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, H.; Du, J.; Qu, Y.; Shen, C.; Tan, F.; Chen, J.; Quan, X. Occurrence, Removal, and Risk Assessment of Antibiotics in 12 Wastewater Treatment Plants from Dalian, China. Environ. Sci. Pollut. Res. 2017, 24, 16478–16487. [Google Scholar] [CrossRef]
- Yao, L.; Chen, Z.-Y.; Dou, W.-Y.; Yao, Z.-K.; Duan, X.-C.; Chen, Z.-F.; Zhang, L.-J.; Nong, Y.-J.; Zhao, J.-L.; Ying, G.-G. Occurrence, Removal and Mass Loads of Antiviral Drugs in Seven Wastewater Treatment Plants with Various Treatment Processes. Water Res. 2021, 207, 117803. [Google Scholar] [CrossRef]
- Ashfaq, M.; Li, Y.; Wang, Y.; Chen, W.; Wang, H.; Chen, X.; Wu, W.; Huang, Z.; Yu, C.-P.; Sun, Q. Occurrence, Fate, and Mass Balance of Different Classes of Pharmaceuticals and Personal Care Products in an Anaerobic-Anoxic-Oxic Wastewater Treatment Plant in Xiamen, China. Water Res. 2017, 123, 655–667. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, Y.; Wang, B.; Deng, S.; Huang, J.; Wang, Y.; Yu, G. Occurrence, Elimination, Enantiomeric Distribution and Intra-Day Variations of Chiral Pharmaceuticals in Major Wastewater Treatment Plants in Beijing, China. Environ. Pollut. 2018, 239, 473–482. [Google Scholar] [CrossRef]
- Gao, L.; Shi, Y.; Li, W.; Niu, H.; Liu, J.; Cai, Y. Occurrence of Antibiotics in Eight Sewage Treatment Plants in Beijing, China. Chemosphere 2012, 86, 665–671. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Liang, H.; Gao, D. Occurrence and Fate of Typical Antibiotics in Wastewater Treatment Plants in Harbin, North-East China. Front. Environ. Sci. Eng. 2019, 13, 34. [Google Scholar] [CrossRef]
- Gan, X.; Yan, Q.; Gao, X.; Zhang, Y.; Zi, C.; Peng, X.; Guo, J. Occurrence and Fate of Typical Antibiotics in a Wastewater Treatment Plant in Southwest China. Environ. Sci. 2014, 35, 1817–1823. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Y.; Dong, W.; Li, M.; Lu, S.; Wang, W.; Fan, Y. Occurrence and Fate of Ten Sulfonamide Antibiotics in Typical Wastewater Treatment Plants in the City of Jinan of Northeastern China. Desalination Water Treat. 2020, 206, 340–348. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; Liu, J.; Jia, L.; Wang, Q. Occurrence and fate of sulfonamide and β-lactam antibiotics in wastewater treatment plants in Handan. Environ. Chem. 2018, 37, 1738–1745. [Google Scholar]
- Wu, M.; Xiang, J.; Que, C.; Chen, F.; Xu, G. Occurrence and Fate of Psychiatric Pharmaceuticals in the Urban Water System of Shanghai, China. Chemosphere 2015, 138, 486–493. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, J.; Zhou, S.; Wu, P.; Tsang, Y.F. Occurrence and Fate of Antibiotics in a Wastewater Treatment Plant and Their Biological Effects on Receiving Waters in Guizhou. Process Saf. Environ. Prot. 2018, 113, 483–490. [Google Scholar] [CrossRef]
- Du, J.; Fan, Y.; Qian, X. Occurrence and Behavior of Pharmaceuticals in Sewage Treatment Plants in Eastern China. Front. Environ. Sci. Eng. 2015, 9, 725–730. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Hu, A.; Rashid, A.; Ashfaq, M.; Wang, Y.; Wang, H.; Luo, H.; Yu, C.-P.; Sun, Q. Monitoring, Mass Balance and Fate of Pharmaceuticals and Personal Care Products in Seven Wastewater Treatment Plants in Xiamen City, China. J. Hazard. Mater. 2018, 354, 81–90. [Google Scholar] [CrossRef]
- Lin, H.; Li, H.; Chen, L.; Li, L.; Yin, L.; Lee, H.; Yang, Z. Mass Loading and Emission of Thirty-Seven Pharmaceuticals in a Typical Municipal Wastewater Treatment Plant in Hunan Province, Southern China. Ecotoxicol. Environ. Saf. 2018, 147, 530–536. [Google Scholar] [CrossRef]
- Ke, R.; Jiang, Y.; Huang, Q.; Chen, L. Investigative Screening of Pharmaceuticals in a Municipal Wastewater Treatment Plant in Shanghai. Asian J. Ecotoxicol. 2014, 9, 1146–1155. [Google Scholar]
- Yin, Z.Y.; Min, L.; Jin, L.; Wang, F.; Sun, H. HPLC-MS/MS determination of eight PPCPs in sewage and sludge from three types of sewage treatment plants. Environ. Chem. 2018, 37, 1720–1727. [Google Scholar] [CrossRef]
- Chen, L.; Fu, W.; Tan, Y.; Zhang, X. Emerging Organic Contaminants and Odorous Compounds in Secondary Effluent Wastewater: Identification and Advanced Treatment. J. Hazard. Mater. 2021, 408, 124817. [Google Scholar] [CrossRef]
- Li, Y.; Niu, X.; Yao, C.; Yang, W.; Lu, G. Distribution, Removal, and Risk Assessment of Pharmaceuticals and Their Metabolites in Five Sewage Plants. Int. J. Environ. Res. Public Health 2019, 16, 4729. [Google Scholar] [CrossRef]
- Leung, H.W.; Minh, T.B.; Murphy, M.B.; Lam, J.C.W.; So, M.K.; Martin, M.; Lam, P.K.S.; Richardson, B.J. Distribution, Fate and Risk Assessment of Antibiotics in Sewage Treatment Plants in Hong Kong, South China. Environ. Int. 2012, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chunhui, Z.; Liangliang, W.; Xiangyu, G.; Xudan, H. Antibiotics in WWTP Discharge into the Chaobai River, Beijing. Arch. Environ. Prot. 2016, 42, 48–57. [Google Scholar] [CrossRef]
- Wang, K.; Zhuang, T.; Su, Z.; Chi, M.; Wang, H. Antibiotic Residues in Wastewaters from Sewage Treatment Plants and Pharmaceutical Industries: Occurrence, Removal and Environmental Impacts. Sci. Total Environ. 2021, 788, 147811. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-L.; Zhang, Z.-F.; Ma, W.-L.; Liu, L.-Y.; Song, W.-W.; Li, Y.-F. An Evaluation on the Intra-Day Dynamics, Seasonal Variations and Removal of Selected Pharmaceuticals and Personal Care Products from Urban Wastewater Treatment Plants. Sci. Total Environ. 2018, 640–641, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.G.B.; Liu, S.; Ying, G.-G.; Zheng, G.J.S.; Lee, J.H.W.; Leung, K.M.Y. The Occurrence and Ecological Risks of Endocrine Disrupting Chemicals in Sewage Effluents from Three Different Sewage Treatment Plants, and in Natural Seawater from a Marine Reserve of Hong Kong. Mar. Pollut. Bull. 2014, 85, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Gong, J.; Zhou, K.; Deng, L.; Chen, J.; Guo, L.; Jiang, M.; Lin, J.; Tang, H.; Liu, X. Removal Efficiencies and Risk Assessment of Endocrine-Disrupting Chemicals at Two Wastewater Treatment Plants in South China. Ecotoxicol. Environ. Saf. 2021, 225, 112758. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Huang, Z.; Wang, Y.; Li, Z.; Ye, X.; Yang, S.; Huang, Z. Pollution characteristics and ecological risk assessment of typical EDCs and PPCPs in a municipal sewage treatment plant in Huizhou. Water Wastewater 2017, 53, 152–154. [Google Scholar] [CrossRef]
- Zhou, Y.; Zha, J.; Xu, Y.; Lei, B.; Wang, Z. Occurrences of Six Steroid Estrogens from Different Effluents in Beijing, China. Environ. Monit. Assess. 2012, 184, 1719–1729. [Google Scholar] [CrossRef]
- Xu, G.; Ma, S.; Tang, L.; Sun, R.; Xiang, J.; Xu, B.; Bao, Y.; Wu, M. Occurrence, Fate, and Risk Assessment of Selected Endocrine Disrupting Chemicals in Wastewater Treatment Plants and Receiving River of Shanghai, China. Environ. Sci. Pollut. Res. 2016, 23, 25442–25450. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, N.; Llewellyn, N.R.; Tao, H. Occurrence and Removal of Free and Conjugated Estrogens in Wastewater and Sludge in Five Sewage Treatment Plants. Environ. Sci. Process. Impacts 2014, 16, 262–270. [Google Scholar] [CrossRef]
- Ashfaq, M.; Li, Y.; Wang, Y.; Qin, D.; Rehman, M.S.U.; Rashid, A.; Yu, C.-P.; Sun, Q. Monitoring and Mass Balance Analysis of Endocrine Disrupting Compounds and Their Transformation Products in an Anaerobic-Anoxic-Oxic Wastewater Treatment System in Xiamen, China. Chemosphere 2018, 204, 170–177. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, W.; Zheng, X.; Wang, X.; Huang, X. Fate and Removal of Typical Pharmaceuticals and Personal Care Products by Three Different Treatment Processes. Sci. Total Environ. 2013, 447, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Wang, Y.; Li, Y.; Ashfaq, M.; Dai, L.; Xie, X.; Yu, C.-P. Fate and Mass Balance of Bisphenol Analogues in Wastewater Treatment Plants in Xiamen City, China. Environ. Pollut. 2017, 225, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Lu, C.; Huang, N.; Zhong, M.; Teng, Y.; Tian, Y.; Ye, K.; Liang, L.; Hu, Z. Exploration of the Effect of Simultaneous Removal of EDCs in the Treatment Process of Different Types of Wastewater. Water Sci. Technol. 2022, 87, 436–453. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, K.; Shen, Y.; Wu, M. Evaluation of Estrogenic Activity in Surface Water and Municipal Wastewater in Shanghai, China. Bull. Environ. Contam. Toxicol. 2011, 87, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yan, W.; Huang, W.; Miao, L.; Zhong, L. Endocrine-Disrupting Chemicals in the Pearl River Delta and Coastal Environment: Sources, Transfer, and Implications. Environ. Geochem. Health 2014, 36, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Liu, J.; Huang, B.; Wang, X.; Luan, T.; Yuan, K. Assessment of the Potential Ecological Risk of Residual Endocrine-Disrupting Chemicals from Wastewater Treatment Plants. Sci. Total Environ. 2020, 714, 136689. [Google Scholar] [CrossRef]
- Qiang, Z.; Dong, H.; Zhu, B.; Qu, J.; Nie, Y. A Comparison of Various Rural Wastewater Treatment Processes for the Removal of Endocrine-Disrupting Chemicals (EDCs). Chemosphere 2013, 92, 986–992. [Google Scholar] [CrossRef]
- Triebskorn, R.; Casper, H.; Scheil, V.; Schwaiger, J. Ultrastructural Effects of Pharmaceuticals (Carbamazepine, Clofibric Acid, Metoprolol, Diclofenac) in Rainbow Trout (Oncorhynchus mykiss) and Common Carp (Cyprinus carpio). Anal. Bioanal. Chem. 2007, 387, 1405–1416. [Google Scholar] [CrossRef]
- Zhu, B.; Liu, L.; Gong, Y.-X.; Ling, F.; Wang, G.-X. Triazole-Induced Toxicity in Developing Rare Minnow (Gobiocypris rarus) Embryos. Environ. Sci. Pollut. Res. Int. 2014, 21, 13625–13635. [Google Scholar] [CrossRef]
- Guo, J.; Bai, Y.; Chen, Z.; Mo, J.; Li, Q.; Sun, H.; Zhang, Q. Transcriptomic Analysis Suggests the Inhibition of DNA Damage Repair in Green Alga Raphidocelis Subcapitata Exposed to Roxithromycin. Ecotoxicol. Environ. Saf. 2020, 201, 110737. [Google Scholar] [CrossRef]
- Huggett, D.B.; Brooks, B.W.; Peterson, B.; Foran, C.M.; Schlenk, D. Toxicity of Select Beta Adrenergic Receptor-Blocking Pharmaceuticals (B-Blockers) on Aquatic Organisms. Arch. Environ. Contam. Toxicol. 2002, 43, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Baran, W.; Sochacka, J.; Wardas, W. Toxicity and Biodegradability of Sulfonamides and Products of Their Photocatalytic Degradation in Aqueous Solutions. Chemosphere 2006, 65, 1295–1299. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.; Kho, Y.; Kim, P.-G.; Ji, K. Thyroid Endocrine Disruption in Male Zebrafish Following Exposure to Binary Mixture of Bisphenol AF and Sulfamethoxazole. Environ. Toxicol. Pharmacol. 2016, 48, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.F.; Huggett, D.B.; Hutchinson, T.H.; Hetheridge, M.J.; McCormack, P.; Kinter, L.B.; Ericson, J.F.; Constantine, L.A.; Sumpter, J.P. The Value of Repeating Studies and Multiple Controls: Replicated 28-day Growth Studies of Rainbow Trout Exposed to Clofibric Acid. Enviro Toxic. Chem. 2010, 29, 2831–2839. [Google Scholar] [CrossRef] [PubMed]
- Lower, N. The Effects of Contaminants on Various Life-Cycle Stages of Atlantic Salmon (Salmo salar L.). Ph.D. Thesis, University of Portsmouth, Portsmouth, UK, 2008. [Google Scholar]
- Ebringer, L.; Krajcovic, J.; Polónyi, J.; Lahitová, N.; Doupovcová, M.; Dobias, J. Tetracycline Reduces Fluoroquinolones-Induced Bleaching of Euglena Gracilis. Mutat. Res. 1996, 340, 141–149. [Google Scholar] [CrossRef]
- Giusti, A.; Ducrot, V.; Joaquim-Justo, C.; Lagadic, L. Testosterone Levels and Fecundity in the Hermaphroditic Aquatic Snail Lymnaea Stagnalis Exposed to Testosterone and Endocrine Disruptors. Environ. Toxicol. Chem. 2013, 32, 1740–1745. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, R.E.; Duncan, R.R. Seashore Paspalum (Paspalum Vaginatuh Swartz) Seminal Root Response to Calcium (45 ca2+) Absorption Modifiers. J. Plant Nutr. 1994, 17, 1385–1392. [Google Scholar] [CrossRef]
- Zhang, X.; Hecker, M.; Tompsett, A.R.; Park, J.-W.; Jones, P.D.; Newsted, J.; Au, D.; Kong, R.; Wu, R.S.S.; Giesy, J.P. Responses of the Medaka HPG Axis PCR Array and Reproduction to Prochloraz and Ketoconazole. Environ. Sci. Technol. 2008, 42, 6762–6769. [Google Scholar] [CrossRef]
- Ayanda, O.; Oniye, S.; Auta, J.; Ajibola, V.; Bello, O. Responses of the African Catfish Clarias Gariepinus to Long-Term Exposure to Glyphosate- and Paraquat-Based Herbicides. Afr. J. Aquat. Sci. 2015, 40, 261–267. [Google Scholar] [CrossRef]
- Crago, J.; Klaper, R. Place-Based Screening of Mixtures of Dominant Emerging Contaminants Measured in Lake Michigan Using Zebrafish Embryo Gene Expression Assay. Chemosphere 2018, 193, 1226–1234. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L. Pharmaceuticals in the Environment: Sources, Fate, Effects and Risks. Waste Manag. 2003, 23, 193. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, D.; Duan, X.; Zhang, Y.; Chen, D.; Gong, Z.; Liu, C. Perfluorooctane Sulfonate Promotes Doxycycline-Induced Liver Tumor Progression in Male KrasV12 Transgenic Zebrafish. Environ. Res. 2021, 196, 110962. [Google Scholar] [CrossRef] [PubMed]
- Cunha, E.; Machado, J. Parturition in Anodonta Cygnea Induced by Selective Serotonin Reuptake Inhibitors (SSRIs). Can. J. Zool. 2011, 79, 95–100. [Google Scholar] [CrossRef]
- Divo, A.A.; Geary, T.G.; Jensen, J.B. Oxygen- and Time-Dependent Effects of Antibiotics and Selected Mitochondrial Inhibitors on Plasmodium Falciparum in Culture. Antimicrob. Agents Chemother. 1985, 27, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, F.; Merante, A.; Pontieri, F.E.; Margotta, V.; Venturini, G.; Palladini, G. Opioid-Dopamine Interaction in Planaria: A Behavioral Study. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1999, 124, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Mennillo, E.; Pretti, C.; Cappelli, F.; Luci, G.; Intorre, L.; Meucci, V.; Arukwe, A. Novel Organ-Specific Effects of Ketoprofen and Its Enantiomer, Dexketoprofen on Toxicological Response Transcripts and Their Functional Products in Salmon. Aquat. Toxicol. 2020, 229, 105677. [Google Scholar] [CrossRef] [PubMed]
- Bereketoglu, C.; Pradhan, A.; Olsson, P.-E. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) Cause Male-Biased Sex Differentiation in Zebrafish. Aquat. Toxicol. 2020, 223, 105476. [Google Scholar] [CrossRef] [PubMed]
- Lebreton, M.; Sire, S.; Carayon, J.-L.; Malgouyres, J.-M.; Vignet, C.; Géret, F.; Bonnafé, E. Low Concentrations of Oxazepam Induce Feeding and Molecular Changes in Radix Balthica Juveniles. Aquat. Toxicol. 2021, 230, 105694. [Google Scholar] [CrossRef]
- Macphee, C.; Ruelle, R. Lethal Effects of 1888 Chemicals upon 4 Species of Fish from Western North America. Research technical Compeltion Report, Project A-013-IDA. Idaho Waters Digital Library, University of Idaho Library Digital Collections, 1969. Available online: https://www.lib.uidaho.edu/digital/iwdl/items/iwdl-196913.html (accessed on 15 June 2023).
- Baldisserotto, B.; Brinn, R.P.; Brandão, F.R.; Gomes, L.C.; Abreu, J.S.; McComb, D.M.; Marcon, J.L. Ion Flux and Cortisol Responses of Cardinal Tetra, Paracheirodon axelrodi (Schultz, 1956), to Additives (Tetracycline, Tetracycline + Salt or Amquel®) Used during Transportation: Contributions to Amazonian Ornamental Fish Trade. J. Appl. Ichthyol. 2014, 30, 86–92. [Google Scholar] [CrossRef]
- Gustafson, A.-L.; Stedman, D.B.; Ball, J.; Hillegass, J.M.; Flood, A.; Zhang, C.X.; Panzica-Kelly, J.; Cao, J.; Coburn, A.; Enright, B.P.; et al. Inter-Laboratory Assessment of a Harmonized Zebrafish Developmental Toxicology Assay—Progress Report on Phase I. Reprod. Toxicol. 2012, 33, 155–164. [Google Scholar] [CrossRef]
- Yang, L.-H.; Ying, G.-G.; Su, H.-C.; Stauber, J.L.; Adams, M.S.; Binet, M.T. Growth-Inhibiting Effects of 12 Antibacterial Agents and Their Mixtures on the Freshwater Microalga Pseudokirchneriella Subcapitata. Environ. Toxicol. Chem. 2008, 27, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Bulut, C.; Kubilay, A.; Hanol Bektaş, Z.; Birden, B. Histopathological Effects of Formaldehyde (CH2O) on Rainbow Trout (Oncorhynchus mykiss Walbaum, 1792). J. Limnol. Freshw. Fish. Res. 2015, 1, 43. [Google Scholar]
- Park, K.; Kwak, I.-S. Gene Expression of Ribosomal Protein mRNA in Chironomus riparius: Effects of Endocrine Disruptor Chemicals and Antibiotics. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 156, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gong, Z. Fluorescent Transgenic Zebrafish Tg(Nkx2.2a:mEGFP) Provides a Highly Sensitive Monitoring Tool for Neurotoxins. PLoS ONE 2013, 8, e55474. [Google Scholar] [CrossRef] [PubMed]
- Rocco, L.; Frenzilli, G.; Fusco, D.; Peluso, C.; Stingo, V. Evaluation of Zebrafish DNA Integrity after Exposure to Pharmacological Agents Present in Aquatic Environments. Ecotoxicol. Environ. Saf. 2010, 73, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, K.; Nagase, H.; Ozawa, M.; Endoh, Y.S.; Goto, K.; Hirata, K.; Miyamoto, K.; Yoshimura, H. Evaluation of Antimicrobial Agents for Veterinary Use in the Ecotoxicity Test Using Microalgae. Chemosphere 2004, 57, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Choi, K.; Kim, J.; Ji, K.; Kim, S.; Ahn, B.; Yun, J.; Choi, K.; Khim, J.S.; Zhang, X.; et al. Endocrine Disruption and Consequences of Chronic Exposure to Ibuprofen in Japanese Medaka (Oryzias latipes) and Freshwater Cladocerans Daphnia magna and Moina Macrocopa. Aquat. Toxicol. 2010, 98, 256–264. [Google Scholar] [CrossRef]
- Tojo, J.; Santamariña, M.; Ubeira, F.; Leiro, J. Efficacy of Antiprotozoal Drugs against Gyrodactylosis in Rainbow Trout (Oncorhynchus mykiss). Bull. Eur. Assoc. Fish Pathol. 1993, 13, 79–82. [Google Scholar]
- Steinkey, D.; Lari, E.; Woodman, S.G.; Luong, K.H.; Wong, C.S.; Pyle, G.G. Effects of Gemfibrozil on the Growth, Reproduction, and Energy Stores of Daphnia magna in the Presence of Varying Food Concentrations. Chemosphere 2018, 192, 75–80. [Google Scholar] [CrossRef]
- Oggier, D.M.; Weisbrod, C.J.; Stoller, A.M.; Zenker, A.K.; Fent, K. Effects of Diazepam on Gene Expression and Link to Physiological Effects in Different Life Stages in Zebrafish Danio rerio. Environ. Sci. Technol. 2010, 44, 7685–7691. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Sun, Z.; Liu, Z.; Xue, Y. Effects of Clotrimazole and Amiodarone on Early Development of Amphibian (Xenopus tropicalis). Toxicol. Environ. Chem. 2012, 94, 128–135. [Google Scholar] [CrossRef]
- Nesbitt, R. Effects of Chronic Exposure to Ibuprofen and Naproxen on Florida Flagfish (Jordanella floridae) over One Complete Life-Cycle. Ph.D. Thesis, Ontario Tech University, Oshawa, ON, Canada, 2011. [Google Scholar]
- Li, Y.; Ma, Y.; Yang, L.; Duan, S.; Zhou, F.; Chen, J.; Liu, Y.; Zhang, B. Effects of Azithromycin on Feeding Behavior and Nutrition Accumulation of Daphnia magna under the Different Exposure Pathways. Ecotoxicol. Environ. Saf. 2020, 197, 110573. [Google Scholar] [CrossRef]
- Brain, R.A.; Johnson, D.J.; Richards, S.M.; Sanderson, H.; Sibley, P.K.; Solomon, K.R. Effects of 25 Pharmaceutical Compounds to Lemna Gibba Using a Seven-Day Static-Renewal Test. Environ. Toxicol. Chem. 2004, 23, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Nandurkar, H.P.; Zambare, S.P. Effect of Tetracycline and Chloramphenicol on Protein Contents in Different Tissues of Freshwater Bivalve, Parreysia cylindrica (Annandale & Prashad). J. Am. Chem. Soc. 2012, 18, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Reda, R.M.; Ibrahim, R.E.; Ahmed, E.-N.G.; El-Bouhy, Z.M. Effect of Oxytetracycline and Florfenicol as Growth Promoters on the Health Status of Cultured Oreochromis niloticus. Egypt. J. Aquat. Res. 2013, 39, 241–248. [Google Scholar] [CrossRef]
- Han, G.H.; Hur, H.G.; Kim, S.D. Ecotoxicological Risk of Pharmaceuticals from Wastewater Treatment Plants in Korea: Occurrence and Toxicity to Daphnia magna. Environ. Toxicol. Chem. 2006, 25, 265–271. [Google Scholar] [CrossRef]
- Białk-Bielińska, A.; Stolte, S.; Arning, J.; Uebers, U.; Böschen, A.; Stepnowski, P.; Matzke, M. Ecotoxicity Evaluation of Selected Sulfonamides. Chemosphere 2011, 85, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Overturf, M.D.; Overturf, C.L.; Baxter, D.; Hala, D.N.; Constantine, L.; Venables, B.; Huggett, D.B. Early Life-Stage Toxicity of Eight Pharmaceuticals to the Fathead Minnow, Pimephales promelas. Arch. Environ. Contam. Toxicol. 2012, 62, 455–464. [Google Scholar] [CrossRef]
- Pascoe, D.; Karntanut, W.; Müller, C.T. Do Pharmaceuticals Affect Freshwater Invertebrates? A Study with the Cnidarian Hydra vulgaris. Chemosphere 2003, 51, 521–528. [Google Scholar] [CrossRef]
- Parolini, M.; Quinn, B.; Binelli, A.; Provini, A. Cytotoxicity Assessment of Four Pharmaceutical Compounds on the Zebra Mussel (Dreissena polymorpha) Haemocytes, Gill and Digestive Gland Primary Cell Cultures. Chemosphere 2011, 84, 91–100. [Google Scholar] [CrossRef]
- Schoenfuss, H.L.; Furlong, E.T.; Phillips, P.J.; Scott, T.-M.; Kolpin, D.W.; Cetkovic-Cvrlje, M.; Lesteberg, K.E.; Rearick, D.C. Complex Mixtures, Complex Responses: Assessing Pharmaceutical Mixtures Using Field and Laboratory Approaches. Environ. Toxicol. Chem. 2016, 35, 953–965. [Google Scholar] [CrossRef]
- Zounková, R.; Klimešová, Z.; Nepejchalová, L.; Hilscherová, K.; Bláha, L. Complex Evaluation of Ecotoxicity and Genotoxicity of Antimicrobials Oxytetracycline and Flumequine Used in Aquaculture. Environ. Toxicol. Chem. 2011, 30, 1184–1189. [Google Scholar] [CrossRef]
- Calleja, M.C.; Persoone, G.; Geladi, P. Comparative Acute Toxicity of the First 50 Multicentre Evaluation of In Vitro Cytotoxicity Chemicals to Aquatic Non-Vertebrates. Arch. Environ. Contam. Toxicol. 1994, 26, 69–78. [Google Scholar] [CrossRef]
- Galus, M.; Kirischian, N.; Higgins, S.; Purdy, J.; Chow, J.; Rangaranjan, S.; Li, H.; Metcalfe, C.; Wilson, J.Y. Chronic, Low Concentration Exposure to Pharmaceuticals Impacts Multiple Organ Systems in Zebrafish. Aquat. Toxicol. 2013, 132–133, 200–211. [Google Scholar] [CrossRef]
- Parolini, M.; Pedriali, A.; Binelli, A. Application of a Biomarker Response Index for Ranking the Toxicity of Five Pharmaceutical and Personal Care Products (PPCPs) to the Bivalve Dreissena polymorpha. Arch. Environ. Contam. Toxicol. 2013, 64, 439–447. [Google Scholar] [CrossRef]
- Lister, A.L.; Van Der Kraak, G. An Investigation into the Role of Prostaglandins in Zebrafish Oocyte Maturation and Ovulation. Gen. Comp. Endocrinol. 2008, 159, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.; Gagné, F.; Blaise, C. An Investigation into the Acute and Chronic Toxicity of Eleven Pharmaceuticals (and Their Solvents) Found in Wastewater Effluent on the Cnidarian, Hydra Attenuata. Sci. Total Environ. 2008, 389, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Lützhøft, H.H.; Halling-Sørensen, B.; Jørgensen, S.E. Algal Toxicity of Antibacterial Agents Applied in Danish Fish Farming. Arch. Environ. Contam. Toxicol. 1999, 36, 1–6. [Google Scholar] [CrossRef]
- Nunes, B.; Antunes, S.C.; Gomes, R.; Campos, J.C.; Braga, M.R.; Ramos, A.S.; Correia, A.T. Acute Effects of Tetracycline Exposure in the Freshwater Fish Gambusia Holbrooki: Antioxidant Effects, Neurotoxicity and Histological Alterations. Arch. Environ. Contam. Toxicol. 2015, 68, 371–381. [Google Scholar] [CrossRef]
- Wollenberger, L.; Halling-Sørensen, B.; Kusk, K.O. Acute and Chronic Toxicity of Veterinary Antibiotics to Daphnia magna. Chemosphere 2000, 40, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Pruvot, B.; Quiroz, Y.; Voncken, A.; Jeanray, N.; Piot, A.; Martial, J.A.; Muller, M. A Panel of Biological Tests Reveals Developmental Effects of Pharmaceutical Pollutants on Late Stage Zebrafish Embryos. Reprod. Toxicol. 2012, 34, 568–583. [Google Scholar] [CrossRef] [PubMed]
- Ando, T.; Nagase, H.; Eguchi, K.; Hirooka, T.; Nakamura, T.; Miyamoto, K.; Hirata, K. A Novel Method Using Cyanobacteria for Ecotoxicity Test of Veterinary Antimicrobial Agents. Environ. Toxicol. Chem. 2007, 26, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Selcer, K.W.; Verbanic, J.D. Vitellogenin of the Northern Leopard Frog (Rana pipiens): Development of an ELISA Assay and Evaluation of Induction after Immersion in Xenobiotic Estrogens. Chemosphere 2014, 112, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.-Q.; Xu, W.; Liang, X.; Jing, Z.; Pan, C.-G.; Tian, F. The Synthetic Progestin Norethindrone Causes Thyroid Endocrine Disruption in Adult Zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 236, 108819. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kang, C.-W.; Su, C.-K.; Okubo, K.; Nagahama, Y. Screening Estrogenic Activity of Environmental Contaminants and Water Samples Using a Transgenic Medaka Embryo Bioassay. Chemosphere 2012, 88, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, K.L.; Cummings, R.I.; Hutchinson, T.H.; Scholze, M.; Brighty, G.; Sumpter, J.P.; Tyler, C.R. Relative Potencies and Combination Effects of Steroidal Estrogens in Fish. Environ. Sci. Technol. 2003, 37, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Kang, J.; Yu, Y.; Zha, J.; Li, W.; Wang, Z.; Wang, Y.; Wen, Y. Long-Term Exposure Investigating the Estrogenic Potency of Estriol in Japanese Medaka (Oryzias latipes). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2014, 160, 86–92. [Google Scholar] [CrossRef] [PubMed]
- EG and G BIONOMICS. Initial Submission: The Chronic Toxicity of s-900d to the Water Flea (Daphnia Magna) with Cover Letter Dated 081492, USEPA: Health and Environmental Research on Line. 1992. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/1325554 (accessed on 15 June 2023).
- Zhang, X.; Xiong, L.; Liu, Y.; Deng, C.; Mao, S. Histopathological and Estrogen Effect of Pentachlorophenol on the Rare Minnow (Gobiocypris rarus). Fish. Physiol. Biochem. 2014, 40, 805–816. [Google Scholar] [CrossRef]
- Kazeto, Y.; Place, A.R.; Trant, J.M. Effects of Endocrine Disrupting Chemicals on the Expression of CYP19 Genes in Zebrafish (Danio rerio) Juveniles. Aquat. Toxicol. 2004, 69, 25–34. [Google Scholar] [CrossRef]
- Villeneuve, D.L.; Garcia-Reyero, N.; Escalon, B.L.; Jensen, K.M.; Cavallin, J.E.; Makynen, E.A.; Durhan, E.J.; Kahl, M.D.; Thomas, L.M.; Perkins, E.J.; et al. Ecotoxicogenomics to Support Ecological Risk Assessment: A Case Study with Bisphenol A in Fish. Environ. Sci. Technol. 2012, 46, 51–59. [Google Scholar] [CrossRef]
- Gagnaire, B.; Gagné, F.; André, C.; Blaise, C.; Abbaci, K.; Budzinski, H.; Dévier, M.-H.; Garric, J. Development of Biomarkers of Stress Related to Endocrine Disruption in Gastropods: Alkali-Labile Phosphates, Protein-Bound Lipids and Vitellogenin-like Proteins. Aquat. Toxicol. 2009, 92, 155–167. [Google Scholar] [CrossRef]
- Bean, R.M.; Gibson, C.I.; Anderson, D.R. Biocide By-Products in Aquatic Environments; Pacific Northwest National Lab.: Richland, WA, USA, 1981. [Google Scholar] [CrossRef]
- Croteau, M.C.; Davidson, M.; Duarte-Guterman, P.; Wade, M.; Popesku, J.T.; Wiens, S.; Lean, D.R.S.; Trudeau, V.L. Assessment of Thyroid System Disruption in Rana pipiens Tadpoles Chronically Exposed to UVB Radiation and 4-Tert-Octylphenol. Aquat. Toxicol. 2009, 95, 81–92. [Google Scholar] [CrossRef]
- Jarvis, A.L.; Bernot, M.J.; Bernot, R.J. The Effects of the Psychiatric Drug Carbamazepine on Freshwater Invertebrate Communities and Ecosystem Dynamics. Sci. Total Environ. 2014, 496, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Melvin, S.D.; Cameron, M.C.; Lanctôt, C.M. Individual and Mixture Toxicity of Pharmaceuticals Naproxen, Carbamazepine, and Sulfamethoxazole to Australian Striped Marsh Frog Tadpoles (Limnodynastes peronii). J. Toxicol. Environ. Health A 2014, 77, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Velisek, J.; Zlabek, V.; Grabic, R.; Machova, J.; Kolarova, J.; Randak, T. Hepatic Antioxidant Status and Hematological Parameters in Rainbow Trout, Oncorhynchus mykiss, after Chronic Exposure to Carbamazepine. Chem. Biol. Interact. 2010, 183, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Fraz, S.; Lee, A.H.; Wilson, J.Y. Gemfibrozil and Carbamazepine Decrease Steroid Production in Zebrafish Testes (Danio rerio). Aquat. Toxicol. 2018, 198, 1–9. [Google Scholar] [CrossRef]
- Dordio, A.V.; Belo, M.; Martins Teixeira, D.; Palace Carvalho, A.J.; Dias, C.M.B.; Picó, Y.; Pinto, A.P. Evaluation of Carbamazepine Uptake and Metabolization by Typha Spp., a Plant with Potential Use in Phytotreatment. Bioresour. Technol. 2011, 102, 7827–7834. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zha, J.; Liang, X.; Li, J.; Wang, Z. Effects of the Human Antiepileptic Drug Carbamazepine on the Behavior, Biomarkers, and Heat Shock Proteins in the Asian Clam Corbicula fluminea. Aquat. Toxicol. 2014, 155, 1–8. [Google Scholar] [CrossRef]
- Oetken, M.; Nentwig, G.; Löffler, D.; Ternes, T.; Oehlmann, J. Effects of Pharmaceuticals on Aquatic Invertebrates. Part I. The Antiepileptic Drug Carbamazepine. Arch. Environ. Contam. Toxicol. 2005, 49, 353–361. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, M.; Lin, K.; Sun, W.; Xiong, B.; Guo, M.; Cui, X.; Fu, R. Eco-Toxicological Effect of Carbamazepine on Scenedesmus obliquus and Chlorella pyrenoidosa. Environ. Toxicol. Pharmacol. 2012, 33, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, K.; Garcia, S.N.; Huggett, D.B.; DeAngelis, D.L.; La Point, T.W. Chronic Effects of Carbamazepine on Life-History Strategies of Ceriodaphnia dubia in Three Successive Generations. Arch. Environ. Contam. Toxicol. 2013, 64, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, R.; Wang, M.; Zha, J. Carbamazepine at Environmentally Relevant Concentrations Caused DNA Damage and Apoptosis in the Liver of Chinese Rare Minnows (Gobiocypris rarus) by the Ras/Raf/ERK/P53 Signaling Pathway. Environ. Pollut. 2021, 270, 116245. [Google Scholar] [CrossRef]
- Gust, M.; Gagné, F.; Berlioz-Barbier, A.; Besse, J.P.; Buronfosse, T.; Tournier, M.; Tutundjian, R.; Garric, J.; Cren-Olivé, C. Caged Mudsnail Potamopyrgus Antipodarum (Gray) as an Integrated Field Biomonitoring Tool: Exposure Assessment and Reprotoxic Effects of Water Column Contamination. Water Res. 2014, 54, 222–236. [Google Scholar] [CrossRef]
- Weigt, S.; Huebler, N.; Strecker, R.; Braunbeck, T.; Broschard, T.H. Zebrafish (Danio rerio) Embryos as a Model for Testing Proteratogens. Toxicology 2011, 281, 25–36. [Google Scholar] [CrossRef]
- DeLorenzo, M.E.; Fleming, J. Individual and Mixture Effects of Selected Pharmaceuticals and Personal Care Products on the Marine Phytoplankton Species Dunaliella tertiolecta. Arch. Environ. Contam. Toxicol. 2008, 54, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Jos, A.; Repetto, G.; Rios, J.C.; Hazen, M.J.; Molero, M.L.; del Peso, A.; Salguero, M.; Fernández-Freire, P.; Pérez-Martín, J.M.; Cameán, A. Ecotoxicological Evaluation of Carbamazepine Using Six Different Model Systems with Eighteen Endpoints. Toxicol. Vitr. 2003, 17, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, K.; Jung, J.; Park, S.; Kim, P.-G.; Park, J. Aquatic Toxicity of Acetaminophen, Carbamazepine, Cimetidine, Diltiazem and Six Major Sulfonamides, and Their Potential Ecological Risks in Korea. Environ. Int. 2007, 33, 370–375. [Google Scholar] [CrossRef]
- Cleuvers, M. Aquatic Ecotoxicity of Pharmaceuticals Including the Assessment of Combination Effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef]
- Li, Z.-H.; Zlabek, V.; Velisek, J.; Grabic, R.; Machova, J.; Kolarova, J.; Li, P.; Randak, T. Acute Toxicity of Carbamazepine to Juvenile Rainbow Trout (Oncorhynchus mykiss): Effects on Antioxidant Responses, Hematological Parameters and Hepatic EROD. Ecotoxicol. Environ. Saf. 2011, 74, 319–327. [Google Scholar] [CrossRef]
- Richards, S.M.; Cole, S.E. A Toxicity and Hazard Assessment of Fourteen Pharmaceuticals to Xenopus laevis Larvae. Ecotoxicology 2006, 15, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Kaza, M.; Nałącz-Jawecki, G.; Sawicki, J. The Toxicity of Selected Pharmaceuticals to the Aquatic Plant Lemna Minor. Fresenius Environ. Bull. 2007, 16, 524–531. [Google Scholar]
- Dordio, A.; Ferro, R.; Teixeira, D.; Palace, A.J.; Pinto, A.P.; Dias, C.M.B. Study on the Use of Typha Spp. for the Phytotreatment of Water Contaminated with Ibuprofen. Int. J. Environ. Anal. Chem. 2011, 91, 654–667. [Google Scholar] [CrossRef]
- Saravanan, M.; Devi, K.U.; Malarvizhi, A.; Ramesh, M. Effects of Ibuprofen on Hematological, Biochemical and Enzymological Parameters of Blood in an Indian Major Carp, Cirrhinus mrigala. Environ. Toxicol. Pharmacol. 2012, 34, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Laita, L.; Calvo, L.; Bes, M.; Fillat, M.; Peleato, M. Effects of Benzene and Several Pharmaceuticals on the Growth and Microcystin Production in Microcystis Aeruginosa PCC 7806. Limnetica 2015, 34, 237–246. [Google Scholar] [CrossRef]
- Flippin, J.L.; Huggett, D.; Foran, C.M. Changes in the Timing of Reproduction Following Chronic Exposure to Ibuprofen in Japanese Medaka, Oryzias latipes. Aquat. Toxicol. 2007, 81, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Pounds, N.; Maclean, S.; Webley, M.; Pascoe, D.; Hutchinson, T. Acute and Chronic Effects of Ibuprofen in the Mollusc Planorbis carinatus (Gastropoda: Planorbidae). Ecotoxicol. Environ. Saf. 2008, 70, 47–52. [Google Scholar] [CrossRef]
- Kim, J.-W.; Ishibashi, H.; Yamauchi, R.; Ichikawa, N.; Takao, Y.; Hirano, M.; Koga, M.; Arizono, K. Acute Toxicity of Pharmaceutical and Personal Care Products on Freshwater Crustacean (Thamnocephalus platyurus) and Fish (Oryzias latipes). J. Toxicol. Sci. 2009, 34, 227–232. [Google Scholar] [CrossRef]
- Li, M.-H. Acute Toxicity of 30 Pharmaceutically Active Compounds to Freshwater Planarians, Dugesia japonica. Toxicol. Environ. Chem. 2013, 95, 1157–1170. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, Q.; Song, M.; Jiang, G.; Shao, J. Vitellogenic Responses of 17β-Estradiol and Bisphenol A in Male Chinese Loach (Misgurnus anguillicaudatus). Environ. Toxicol. Pharmacol. 2007, 24, 155–159. [Google Scholar] [CrossRef]
- Qiu, W.; Shen, Y.; Pan, C.; Liu, S.; Wu, M.; Yang, M.; Wang, K.-J. The Potential Immune Modulatory Effect of Chronic Bisphenol A Exposure on Gene Regulation in Male Medaka (Oryzias latipes) Liver. Ecotoxicol. Environ. Saf. 2016, 130, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Haubruge, E.; Petit, F.; Gage, M.J. Reduced Sperm Counts in Guppies (Poecilia reticulata) Following Exposure to Low Levels of Tributyltin and Bisphenol A. Proc. Biol. Sci. 2000, 267, 2333–2337. [Google Scholar] [CrossRef] [PubMed]
- Hatef, A.; Zare, A.; Alavi, S.M.H.; Habibi, H.R.; Linhart, O. Modulations in Androgen and Estrogen Mediating Genes and Testicular Response in Male Goldfish Exposed to Bisphenol A. Environ. Toxicol. Chem. 2012, 31, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Keiter, S.; Baumann, L.; Färber, H.; Holbech, H.; Skutlarek, D.; Engwall, M.; Braunbeck, T. Long-Term Effects of a Binary Mixture of Perfluorooctane Sulfonate (PFOS) and Bisphenol A (BPA) in Zebrafish (Danio rerio). Aquat. Toxicol. 2012, 118–119, 116–129. [Google Scholar] [CrossRef] [PubMed]
- Mochida, K.; Fujii, K.; Kakuno, A.; Matsubara, T.; Ohkubo, N.; Adachi, S.; Yamauchi, K. Expression of Ubiquitin C-Terminal Hydrolase Is Regulated by Estradiol-17β in Testis and Brain of the Japanese Common Goby. Fish. Physiol. Biochem. 2003, 28, 435–436. [Google Scholar] [CrossRef]
- Yang, F.-X.; Xu, Y.; Wen, S. Endocrine-Disrupting Effects of Nonylphenol, Bisphenol A, and p,p’-DDE on Rana Nigromaculata Tadpoles. Bull. Environ. Contam. Toxicol. 2005, 75, 1168–1175. [Google Scholar] [CrossRef]
- Ha, M.-H.; Choi, J. Effects of Environmental Contaminants on Hemoglobin Gene Expression in Daphnia magna: A Potential Biomarker for Freshwater Quality Monitoring. Arch. Environ. Contam. Toxicol. 2009, 57, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Ladewig, V.; Jungmann, D.; Köhler, H.-R.; Licht, O.; Ludwichowski, K.-U.; Schirling, M.; Triebskorn, R.; Nagel, R. Effects of Bisphenol A on Gammarus Fossarum and Lumbriculus Variegatus in Artificial Indoor Streams. Toxicol. Environ. Chem. 2006, 88, 649–664. [Google Scholar] [CrossRef]
- Lahnsteiner, F.; Berger, B.; Kletzl, M.; Weismann, T. Effect of Bisphenol A on Maturation and Quality of Semen and Eggs in the Brown Trout, Salmo trutta f. fario. Aquat. Toxicol. 2005, 75, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Staples, C.A.; Tilghman Hall, A.; Friederich, U.; Caspers, N.; Klecka, G.M. Early Life-Stage and Multigeneration Toxicity Study with Bisphenol A and Fathead Minnows (Pimephales promelas). Ecotoxicol. Environ. Saf. 2011, 74, 1548–1557. [Google Scholar] [CrossRef]
- Van den Belt, K.; Verheyen, R.; Witters, H. Comparison of Vitellogenin Responses in Zebrafish and Rainbow Trout Following Exposure to Environmental Estrogens. Ecotoxicol. Environ. Saf. 2003, 56, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.-W.; Kang, J.-S.; Choi, J.; Park, J.-W. Chronic Toxicity of Endocrine Disrupting Chemicals Used in Plastic Products in Korean Resident Species: Implications for Aquatic Ecological Risk Assessment. Ecotoxicol. Environ. Saf. 2020, 192, 110309. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yuan, C.; Wang, M.; Liu, Y.; Wang, Z.; Seif, M.M. Bisphenol A-Associated Alterations in DNA and Histone Methylation Affects Semen Quality in Rare Minnow Gobiocypris rarus. Aquat. Toxicol. 2020, 226, 105580. [Google Scholar] [CrossRef] [PubMed]
- Plahuta, M.; Tišler, T.; Pintar, A.; Toman, M.J. Adverse Effects of Bisphenol A on Water Louse (Asellus aquaticus). Ecotoxicol. Environ. Saf. 2015, 117, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Mihaich, E.M.; Friederich, U.; Caspers, N.; Hall, A.T.; Klecka, G.M.; Dimond, S.S.; Staples, C.A.; Ortego, L.S.; Hentges, S.G. Acute and Chronic Toxicity Testing of Bisphenol A with Aquatic Invertebrates and Plants. Ecotoxicol. Environ. Saf. 2009, 72, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiong, B.; Sun, W.-F.; An, S.; Lin, K.-F.; Guo, M.-J.; Cui, X.-H. Acute and Chronic Toxic Effects of Bisphenol A on Chlorella pyrenoidosa and Scenedesmus obliquus. Environ. Toxicol. 2014, 29, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, M.; Güngördü, A.; Erdemoglu, S.; Ozmen, N.; Asilturk, M. Toxicological Aspects of Photocatalytic Degradation of Selected Xenobiotics with Nano-Sized Mn-Doped TiO2. Aquat. Toxicol. 2015, 165, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.M.; Pascoe, D.; Carroll, K. Survival and Precopulatory Behaviour of Gammarus pulex (L.). Exposed to Two Xenoestrogens. Water Res. 2001, 35, 2347–2352. [Google Scholar] [CrossRef] [PubMed]
- Wolkowicz, I.R.H.; Herkovits, J.; Pérez Coll, C.S. Stage-Dependent Toxicity of Bisphenol a on Rhinella arenarum (Anura, Bufonidae) Embryos and Larvae. Environ. Toxicol. 2014, 29, 146–154. [Google Scholar] [CrossRef]
- Kashiwada, S.; Ishikawa, H.; Miyamoto, N.; Ohnishi, Y.; Magara, Y. Fish Test for Endocrine-Disruption and Estimation of Water Quality of Japanese Rivers. Water Res. 2002, 36, 2161–2166. [Google Scholar] [CrossRef]
- Debenest, T.; Gagné, F.; Petit, A.-N.; André, C.; Kohli, M.; Blaise, C. Ecotoxicity of a Brominated Flame Retardant (Tetrabromobisphenol A) and Its Derivatives to Aquatic Organisms. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 152, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.K.; Chan, K.M. Disruption of the Hypothalamic-Pituitary-Thyroid Axis in Zebrafish Embryo-Larvae Following Waterborne Exposure to BDE-47, TBBPA and BPA. Aquat. Toxicol. 2012, 108, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Alexander, H.C.; Dill, D.C.; Smith, L.W.; Guiney, P.D.; Dorn, P. Bisphenol a: Acute Aquatic Toxicity. Environ. Toxicol. Chem. 1988, 7, 19–26. [Google Scholar] [CrossRef]
- Jemec, A.; Tišler, T.; Erjavec, B.; Pintar, A. Antioxidant Responses and Whole-Organism Changes in Daphnia magna Acutely and Chronically Exposed to Endocrine Disruptor Bisphenol A. Ecotoxicol. Environ. Saf. 2012, 86, 213–218. [Google Scholar] [CrossRef] [PubMed]


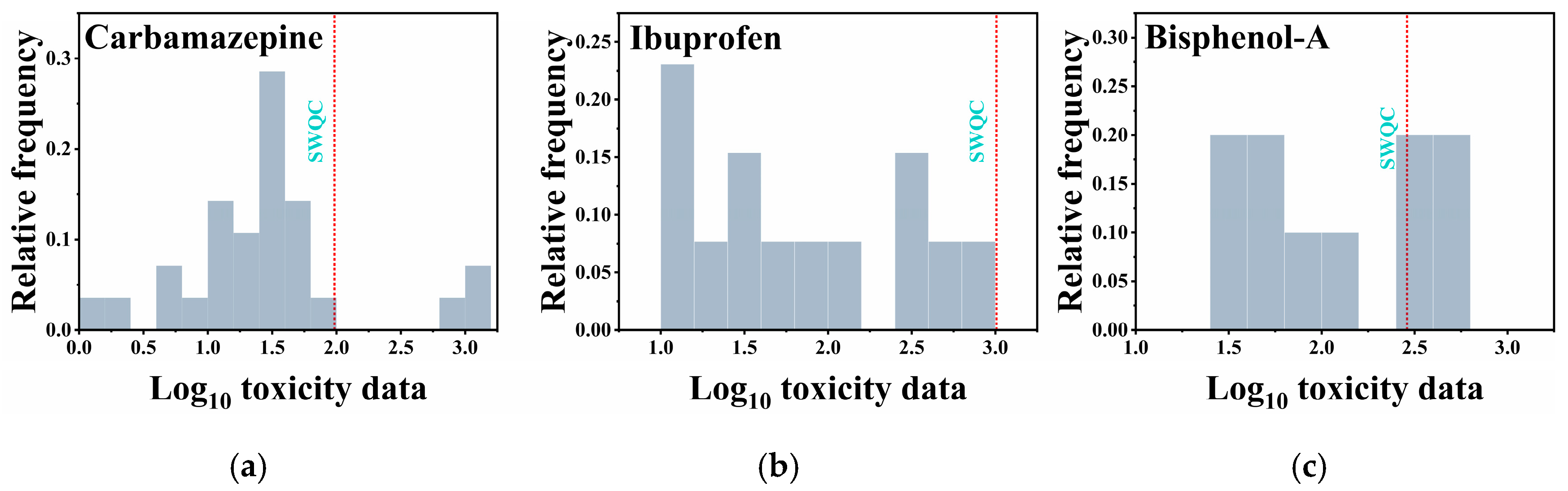
| Chemicals | Acute | Chronic | ||||||
|---|---|---|---|---|---|---|---|---|
| Model | SHC5 (mg/L) | SAF | SWQC (mg/L) | Model | LHC5 (ng/L) | LAF | LWQC (ng/L) | |
| Carbamazepine | Logistic distribution | 10.19 | 3 | 3.40 | Log-normal distribution | 192.8 | 2 | 96.4 |
| Ibuprofen | Logistic distribution | 5.59 | 3 | 1.86 | Logistic distribution | 3039.9 | 3 | 1010 |
| Bisphenol-A | Log-logistic distribution | 2.66 | 3 | 0.89 | Normal distribution | 575.3 | 2 | 288 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Bu, Q.; Shi, Q.; Zhao, R.; Huang, H.; Yang, L.; Tang, J.; Ma, Y. Emerging Contaminants in the Effluent of Wastewater Should Be Regulated: Which and to What Extent? Toxics 2024, 12, 309. https://doi.org/10.3390/toxics12050309
Yang W, Bu Q, Shi Q, Zhao R, Huang H, Yang L, Tang J, Ma Y. Emerging Contaminants in the Effluent of Wastewater Should Be Regulated: Which and to What Extent? Toxics. 2024; 12(5):309. https://doi.org/10.3390/toxics12050309
Chicago/Turabian StyleYang, Weiwei, Qingwei Bu, Qianhui Shi, Ruiqing Zhao, Haitao Huang, Lei Yang, Jianfeng Tang, and Yuning Ma. 2024. "Emerging Contaminants in the Effluent of Wastewater Should Be Regulated: Which and to What Extent?" Toxics 12, no. 5: 309. https://doi.org/10.3390/toxics12050309








