Recovery of Ag(I) from Wastewater by Adsorption: Status and Challenges
Abstract
:1. Introduction
2. Types of Adsorbents
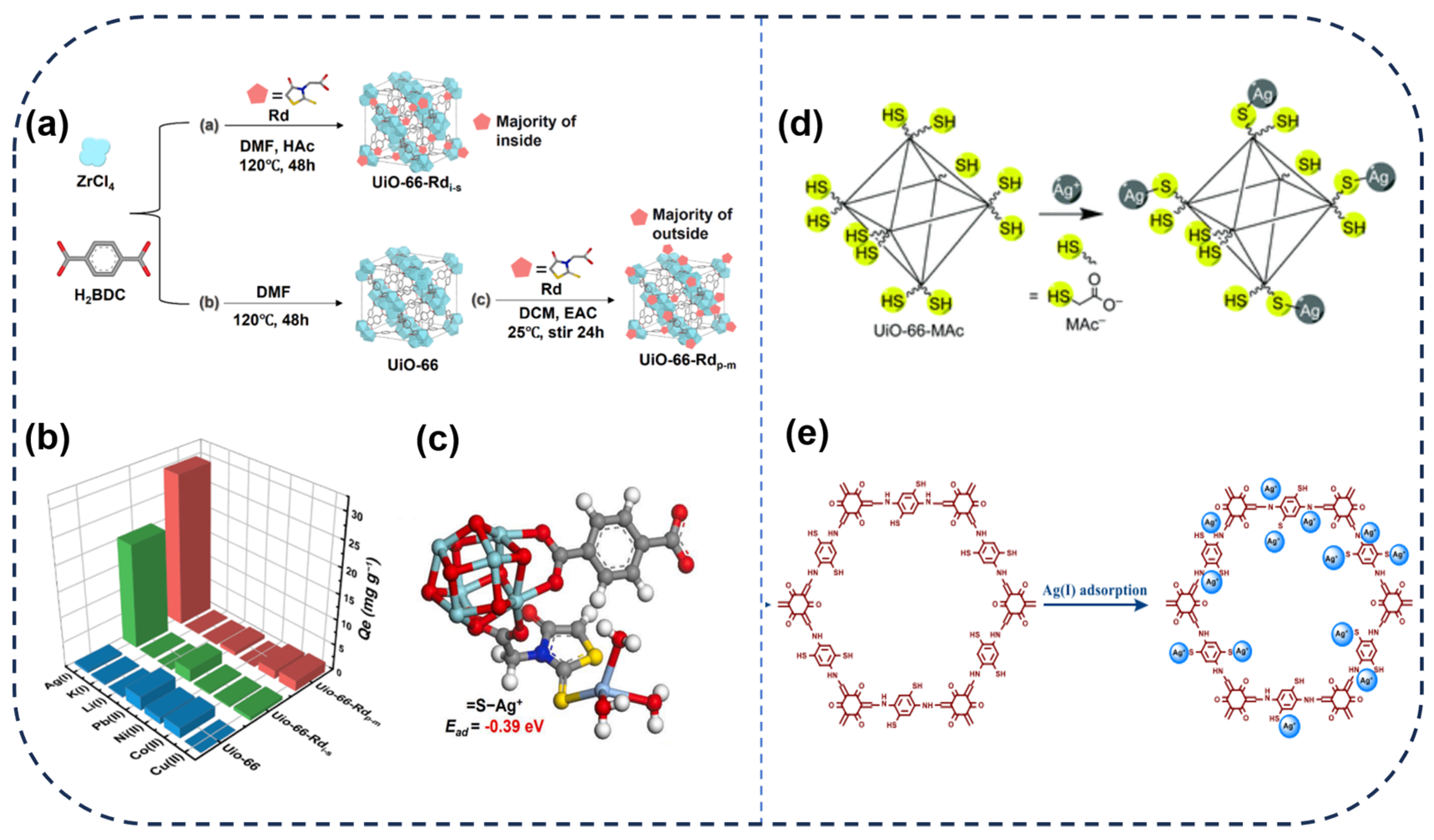
2.1. MOFs and COFs
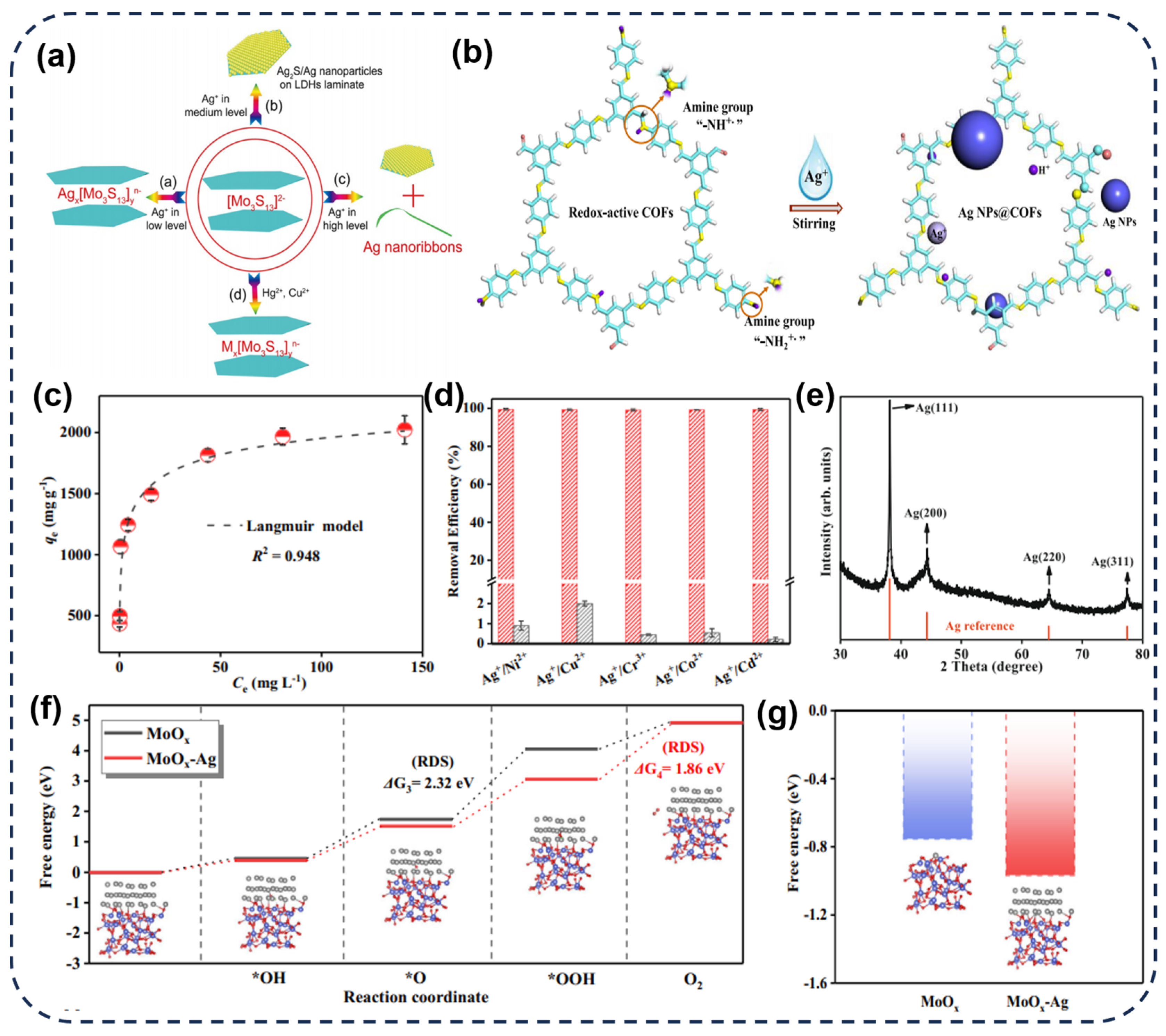
2.2. Transition Metal Dichalcogenides (TMDs) and Metal Oxides
2.3. Biomass Adsorbents
2.4. Other Macromolecule Polymer
3. Adsorption Mechanisms
3.1. Coordination Complexation
3.2. Redox Reaction
3.3. Ionic Exchange
3.4. Physical Adsorption
4. Defects and Challenges of Adsorbents
5. Adsorbent Optimization
5.1. Modification of Adsorbents
5.2. Regeneration and Recycling of Adsorbents
5.3. Selectivity of Adsorbents
| Type | Adsorbent | Adsorption Capacity/(mg·g−1) | Equilibrim Adsorption/(min) | Adsorption Mechanism | Ref. |
|---|---|---|---|---|---|
| MOFs | (fcu) UiO−66 (hcp) UiO−66 | 84 36 | 120 | Coordination −complexation | [66] |
| NH2−MIL−125 | 192.5 | 60 | Electrostatic −interaction/Coordination −complexation | [98] | |
| UiO−66−Rd UiO−66−NH2−Rd | 112 163 | 15 | Coordination −complexation | [99] | |
| Fe3O4@UiO−66−NH2/CTS−PEI | 226.88 | 120 | Coordination −complexation | [92] | |
| UiO−66−Rdp−m UiO−66−Rdi−s | 120 109 | 10 30 | Coordination −complexation | [20] | |
| MIL−127/26PoPD | 560 | 5 | Redox −reaction/Coordination −complexation | [26] | |
| COFs | COF−SH | 609.89 | 16 | Ionic −exchange/Coordination −complexation | [4] |
| COF−HNU27 | 349.6 | 2 | Coordination −complexation | [28] | |
| TAPA−BTDC | 398.61 | 30 | Coordination− complexation | [29] | |
| COF−LZU1 | 175 | 720 | Redox −reaction | [70] | |
| TMDs | MoS2–HNR | 1350 | 60 | Redox −reaction/Coordination −complexation | [101] |
| MoS4−Ppy | 480 (pH ≈ 5)/725 (pH ≈ 1) | 5 | Coordination− complexation/Redox −reaction | [82] | |
| SA@MoS2/rGO/MF | 1012.51 | 1440 | Redox −reaction/Coordination −complexation | [94] | |
| Mo3S13− LDH | 1073 | 60 | Redox− reaction/Coordination −complexation | [21] | |
| MoS2/MWCNTs | 601.97 | 1440 | Electrostatic −interaction/Coordination− complexation | [32] | |
| Metal Oxide | SNFO | 223.68 | 120 | Redox− reaction Electrostatic interaction | [100] |
| Fe3O4@SiO2@CaSiO3 | 127.84 | 150 | Ionic− exchange/Coordination −complexation | [74] | |
| SiO2@TF | 1356 | 20 | Coordination −complexation | [90] | |
| ASPSS | 416.37 | 240 | Coordination− complexation | [89] | |
| MI−SiNSs MBI−SiNSs MTT−SiNSs | 70.3 103.2 139.5 | 30 | Coordination −complexation | [36] | |
| FO@SD@CS | 116.28 | 200 | Electrostatic −interaction/Coordination −complexation | [76] | |
| HMDNC | 81.97 | 30 | Electrostatic −interaction | [37] | |
| MoOx | 2605.91 | 120/ | Redox −reaction | [71] | |
| Biomass Adsorbent | ZMC−MAH−TEPA | 70.12 | 60 | Electrostatic −interaction/Hydrogen −bonding/Coordination −complexation | [81] |
| CB−G3 | 105.62 | 210 | Electrostatic −interaction/Hydrogen −bonding/Coordination −complexation | [41] | |
| Chitosan supported on porous glass beads | 555.52 | 90 | Coordination− complexation | [91] | |
| DMC | 1175.8 | 60 | Coordination− complexation | [43] | |
| CuS/Dt−P | 822.7 | 10 | Coordination −complexation | [93] | |
| Eco−friendly adsorbent | 23.92 | 90 | Coordination− complexation | [102] |
| Adsorbent | Adsorption Capacity/(mg·g−1) | Equilibrim Adsorption/(min) | Adsorption Mechanism | Ref. |
|---|---|---|---|---|
| CAT | 42.12 | 2160 | Electrostatic −interaction/Coordination −complexation | [103] |
| P(Penta3MP4/PEGDA/AAc) | 92.8 | 960 | Coordination −complexation | [47] |
| P(Penta3MP4/PEG−DA/HEMA) | 102 | 1440 | Coordination− complexation | [104] |
| ATU−PEGDA | 83.33 | 240 | Electrostatic −interaction/Coordination− complexation | [48] |
| TSC−CC | 872.63 | 500 | Ionic −exchange/Coordination −complexation | [50] |
| PS−TMT | 187.1 | 360 | Coordination −complexation | [105] |
| BA−PGMA | 157.05 | 300 | Electrostatic −interaction/Coordination− complexation | [106] |
| T−PGMA | 217.17 | 840 | Coordination −complexation | [107] |
| Ag+−imprinted PHEMAC cryogel | 49.27 | 90 | Cavity match | [54] |
| Ag(I)−MGOIIP | 77.6 | 10 | Cavity match | [108] |
| ITG−OCMC | 156.32 | 2880 | Coordination− complexation | [87] |
| Mag−IIP | 28.2 | 5 | Cavity match | [109] |
| Ag+−imprinted poly (HEMA−MAC) polymeric nanoparticles | 196.9 | 40 | Cavity match/Coordination −complexation | [110] |
| Fe3O4@SiO2@TiO2−IIP | 35.475 | 80 | Redox −reaction/Cavity match | [111] |
| Ag−TCM | 566 | 50 | Cavity match | [78] |
| IIM | 212 | 120 | Coordination −complexation | [112] |
| Ag(I)−MIIP | 62.5 | 15 | Cavity match | [113] |
| Fe3O4@SiO2@Ag−IIP | 149 | 30 | Cavity match | [114] |
| MPHCP P−MPHCP | 321 353 | 15 | Coordination−complexation | [115] |
| L−PRL | 325.8 | 20 | Coordination −complexation | [56] |
| PSDT | 303 | 300 | Coordination −complexation | [96] |
| TCP | 556 | 300 | Coordination− complexation | [68] |
| P−2AT | 336.98 | 90 | Coordination −complexation | [116] |
| HOM−Si−MTHL | 179.23 | 20 | Coordination −complexation | [117] |
| Atrz | 94 | 240 | Coordination −complexation | [118] |
5.4. Large−Scale Application of Adsorbents
6. Outlook
- (1)
- Addressing the existing deficiencies and issues of current adsorbents through systematic engineering optimization efforts aimed at enhancing their stability and sustainability. Additionally, successful translation of laboratory research outcomes into large−scale synthesis of adsorbents is crucial for advancing adsorption technology in wastewater treatment.
- (2)
- Considering the pursuit of environmental friendliness, future adsorbent design should prioritize renewability and biocompatibility. Exploration of natural biological materials or biomimetic materials could lead to more environmentally friendly and sustainable adsorption technologies.
- (3)
- On the theoretical front, the application of machine learning and artificial intelligence techniques holds promise in expediting the adsorbent design process. Analysis of extensive experimental data and simulation results can unveil more subtle adsorption patterns and optimization pathways, thereby providing deeper guidance for the precise design of adsorbents.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birloaga, I.; Vegliò, F. Overview on hydrometallurgical procedures for silver recovery from various wastes. J. Environ. Chem. Eng. 2018, 6, 2932–2938. [Google Scholar] [CrossRef]
- Yazdani-Ahmadabadi, H.; Felix, D.F.; Yu, K.; Yeh, H.H.; Luo, H.D.; Khoddami, S.; Takeuchi, L.E.; Alzahrani, A.; Abbina, S.; Mei, Y.; et al. Durable Surfaces from Film-Forming Silver Assemblies for Long-Term Zero Bacterial Adhesion without Toxicity. Acs Cent. Sci. 2022, 8, 546–561. [Google Scholar] [CrossRef]
- Jarchum, I. Turning silver dust into electronics. Nat. Biotechnol. 2017, 35, 723. [Google Scholar] [CrossRef]
- Pan, X.H.; Zu, J.H.; Diao, J.J.; Xue, Y.; Liu, S.Y. Rapid and selective recovery of Ag(I) from simulative electroplating effluents by sulfydryl-rich covalent organic framework (COF-SH) with high adsorption capacity. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129156. [Google Scholar] [CrossRef]
- Abeyweera, S.C.; Rasamani, K.D.; Sun, Y.G. Ternary Silver Halide Nanocrystals. Acc. Chem. Res. 2017, 50, 1754–1761. [Google Scholar] [CrossRef]
- The Silver Institute. World Silver Survey 2023; The Silver Institute and Metals Focus: Washington, DC, USA; Available online: https://www.silverinstitute.org/ (accessed on 1 March 2024).
- Xu, Q.S.; Hu, J.Z.; Xie, K.B.; Yang, H.Y.; Du, K.H.; Shi, G.X. Accumulation and acute toxicity of silver in Potamogeton crispus L. J. Hazard. Mater. 2010, 173, 186–193. [Google Scholar] [CrossRef]
- Newton, K.M.; Puppala, H.L.; Kitchens, C.L.; Colvin, V.L.; Klaine, S.J. Silver nanoparticle toxicity to Daphnia magna is a function of dissolved silver concentration. Environ. Toxicol. Chem. 2013, 32, 2356–2364. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhou, Y.; Yoon, J.; Kim, J.S. Recent progress in fluorescent and colorimetric chemosensors for detection of precious metal ions (silver, gold and platinum ions). Chem. Soc. Rev. 2011, 40, 3416–3429. [Google Scholar] [CrossRef]
- Padhye, L.P.; Jasemizad, T.; Bolan, S.; Tsyusko, O.V.; Unrine, J.M.; Biswal, B.K.; Balasubramanian, R.; Zhang, Y.Y.; Zhang, T.; Zhao, J.; et al. Silver contamination and its toxicity and risk management in terrestrial and aquatic ecosystems. Sci. Total Environ. 2023, 871, 161926. [Google Scholar] [CrossRef]
- dos Santos, A.B.; Cervantes, F.J.; van Lier, J.B. Review paper on current technologies for decolourisation of textile wastewaters: Perspectives for anaerobic biotechnology. Bioresour. Technol. 2007, 98, 2369–2385. [Google Scholar] [CrossRef]
- Amin, G.; Asif, M.H.; Zainelabdin, A.; Zaman, S.; Nur, O.; Willander, M. CuO Nanopetals Based Electrochemical Sensor for Selective Ag+ Measurements. Sens. Lett. 2012, 10, 754–759. [Google Scholar] [CrossRef]
- Breza, M.; Kortisová, I.; Cibulková, Z. DFT study of the reaction sites of N,N′-substituted p-phenylenediamine antioxidants. Polym. Degrad. Stab. 2006, 91, 2848–2852. [Google Scholar] [CrossRef]
- Demirbas, A. Heavy metal adsorption onto agro-based waste materials: A review. J. Hazard. Mater. 2008, 157, 220–229. [Google Scholar] [CrossRef]
- Hanzlík, J.; Jehlicka, J.; Sebek, O.; Weishauptová, Z.; Machovic, V. Multi-component adsorption of Ag(I), Cd(II) and Cu(II) by natural carbonaceous materials. Water Res. 2004, 38, 2178–2184. [Google Scholar] [CrossRef]
- Ma, L.J.; Wang, Q.; Islam, S.M.; Liu, Y.C.; Ma, S.L.; Kanatzidis, M.G. Highly Selective and Efficient Removal of Heavy Metals by Layered Double Hydroxide Intercalated with the MoS42- Ion. J. Am. Chem. Soc. 2016, 138, 2858–2866. [Google Scholar] [CrossRef]
- Song, X.T.; Niu, Y.Z.; Qiu, Z.M.; Zhang, Z.X.; Zhou, Y.Z.; Zhao, J.J.; Chen, H. Adsorption of Hg(II) and Ag(I) from fuel ethanol by silica gel supported sulfur-containing PAMAM dendrimers: Kinetics, equilibrium and thermodynamics. Fuel 2017, 206, 80–88. [Google Scholar] [CrossRef]
- Wu, Y.H.; Pang, H.W.; Liu, Y.; Wang, X.X.; Yu, S.J.; Fu, D.; Chen, J.R.; Wang, X.K. Environmental remediation of heavy metal ions by novel-nanomaterials: A review. Environ. Pollut. 2019, 246, 608–620. [Google Scholar] [CrossRef]
- Yen, C.H.; Lien, H.L.; Chung, J.S.; Yeh, H.D. Adsorption of precious metals in water by dendrimer modified magnetic nanoparticles. J. Hazard. Mater. 2017, 322, 215–222. [Google Scholar] [CrossRef]
- Ding, L.; Xiong, M.P.; Yin, X.C.; Fang, L.L.; Liu, L.L.; Ren, W.; Deng, F.; Luo, X.B. Post-modified ligand exchange of UiO-66 with high-exposure rhodanine sites for enhancing the rapid and selective capture of Ag(I) from wastewater. Sep. Purif. Technol. 2023, 314, 123541. [Google Scholar] [CrossRef]
- Yang, L.X.; Xie, L.X.; Chu, M.L.; Wang, H.; Yuan, M.W.; Yu, Z.H.; Wang, C.N.; Yao, H.Q.; Islam, S.M.; Shi, K.R.; et al. Mo3S132- Intercalated Layered Double Hydroxide: Highly Selective Removal of Heavy Metals and Simultaneous Reduction of Ag+ Ions to Metallic Ag0 Ribbons. Angew. Chem. Int. Ed. 2022, 61, e202112511. [Google Scholar] [CrossRef]
- Lin, G.; Zeng, B.; Li, J.; Wang, Z.Y.; Wang, S.X.; Hu, T.; Zhang, L.B. A systematic review of metal organic frameworks materials for heavy metal removal: Synthesis, applications and mechanism. Chem. Eng. J. 2023, 460, 141710. [Google Scholar] [CrossRef]
- Lin, Z.J.; Liu, T.F.; Huang, Y.B.; Lu, J.; Cao, R. A Guest-Dependent Approach to Retain Permanent Pores in Flexible Metal-Organic Frameworks by Cation Exchange. Chem. A Eur. J. 2012, 18, 7896–7902. [Google Scholar] [CrossRef] [PubMed]
- Maranescu, B.; Lupa, L.; Visa, A. Heavy metal removal from waste waters by phosphonate metal organic frameworks. Pure Appl. Chem. 2018, 90, 35–47. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.B.; Xie, L.H.; Rutledge, W.; Li, J.R.; Zhou, H.C. Zr-based metal-organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef]
- Xue, T.W.; Peng, L.; Syzgantseva, O.A.; Syzgantseva, M.A.; Guo, P.W.; Lai, H.Y.; Li, R.Q.; Chen, J.W.; Li, S.M.; Yan, X.M.; et al. Rapid, Selective Extraction of Silver from Complex Water Matrices with a Metal-Organic Framework/Oligomer Composite Constructed via Supercritical CO2. Angew. Chem. Int. Ed. 2023, 62, e202309737. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Feng, J.; Wang, W. Covalent Organic Frameworks in Separation. Annu. Rev. Chem. Biomol. Eng. 2020, 11, 131–153. [Google Scholar] [CrossRef]
- Qiu, J.K.; Xu, C.; Xu, X.H.; Li, Z.Y.; Wang, H.Y.; Zhao, Y.; Zhao, Y.L. An ultrastable cationic covalent organic framework for selective capture of silver from aqueous solution. J. Mol. Struct. 2023, 1292, 136173. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Wang, Q.; Li, Y.Q.; Hu, R.G. Bithiophene-based COFs for silver ions detection and removal. Microporous Mesoporous Mater. 2022, 346, 112289. [Google Scholar] [CrossRef]
- Kajana, T.; Pirashanthan, A.; Velauthapillai, D.; Yuvapragasam, A.; Yohi, S.; Ravirajan, P.; Senthilnanthanan, M. Potential transition and post-transition metal sulfides as efficient electrodes for energy storage applications: Review. Rsc Adv. 2022, 12, 18041–18062. [Google Scholar] [CrossRef]
- Yang, H.J.; Ma, Y.X.; Shi, X.F.; Li, X.H.; Wang, J.W.; Meng, W.L.; Xue, J.G. Fabrication of multiwall carbon nanotubes decorated with MoS2 nanoflowers for adsorption of Ag(I) from aqueous solution. Diam. Relat. Mater. 2022, 127, 109147. [Google Scholar] [CrossRef]
- Tan, L.; Liu, Y.S.; Meng, F.L.; Wu, P.F.; Xia, Y.X.; Tang, Y.Y. 3D hierarchical defect-rich C@MoS2 nanosheet arrays developed on montmorillonite with enhanced performance in Pb(II) removal. Environ. Sci. Nano 2020, 7, 3088–3099. [Google Scholar] [CrossRef]
- Alhalili, Z. Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment. Molecules 2023, 28, 3086. [Google Scholar] [CrossRef] [PubMed]
- Deliyanni, E.A.; Peleka, E.N.; Matis, K.A. Modeling the sorption of metal ions from aqueous solution by iron-based adsorbents. J. Hazard. Mater. 2009, 172, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Soltani, R.; Marjani, A.; Shirazian, S. Shell-in-shell monodispersed triamine-functionalized SiO2 hollow microspheres with micro-mesostructured shells for highly efficient removal of heavy metals from aqueous solutions. J. Environ. Chem. Eng. 2019, 7, 102832. [Google Scholar] [CrossRef]
- Mao, S.S.; Shen, T.; Zhao, Q.; Han, T.; Ding, F.; Jin, X.; Gao, M.L. Selective capture of silver ions from aqueous solution by series of azole derivatives-functionalized silica nanosheets. Chin. J. Chem. Eng. 2023, 57, 319–328. [Google Scholar] [CrossRef]
- Karami, H.; Najafi, B.A. Manganese dioxide nanoclusters as a potential nano-sorbent for the removal of silver ion from water. Iran. J. Anal. Chem. 2015, 2, 127–137. [Google Scholar]
- Wang, P.Y.; Li, L.; Pang, X.P.; Zhang, Y.; Zhang, Y.; Dong, W.F.; Yan, R.H. Chitosan-based carbon nanoparticles as a heavy metal indicator and for wastewater treatment. Rsc. Adv. 2021, 11, 12015–12021. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Y.J.; Xu, J.H.; Luo, G.S. Chitosan supported on porous glass beads as a new green adsorbent for heavy metal recovery. Chem. Eng. J. 2013, 229, 217–224. [Google Scholar] [CrossRef]
- Liu, X.Q.; Zhao, X.X.; Liu, Y.; Zhang, T.A. Review on preparation and adsorption properties of chitosan and chitosan composites. Polym. Bull. 2022, 79, 2633–2665. [Google Scholar] [CrossRef]
- Liu, X.Q.; Zhang, Y.Y.; Liu, Y.; Zhang, T.A. Preparation of polyamidoamine dendrimer-functionalized chitosan beads for the removal of Ag(I), Cu(II), and Pb(II). Int. J. Biol. Macromol. 2023, 242, 124543. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A review on modification methods to cellulose-based adsorbents to improve adsorption capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Biswas, F.B.; Rahman, I.M.M.; Nakakubo, K.; Yunoshita, K.; Endo, M.; Nagai, K.; Mashio, A.S.; Taniguchi, T.; Nishimura, T.; Maeda, K.; et al. Selective recovery of silver and palladium from acidic waste solutions using dithiocarbamate-functionalized cellulose. Chem. Eng. J. 2021, 407, 127225. [Google Scholar] [CrossRef]
- Fan, J.L.; Duan, L.; Zhang, X.B.; Li, Z.M.; Qiu, P.J.; He, Y.J.; Shang, P. Selective Adsorption and Recovery of Silver from Acidic Solution Using Biomass-Derived Sulfur-Doped Porous Carbon. Acs Appl. Mater. Interfaces 2023, 15, 40088–40099. [Google Scholar] [CrossRef]
- Bao, Y.; Luo, Z.L.; Cui, S.X. Environment-dependent single-chain mechanics of synthetic polymers and biomacromolecules by atomic force microscopy-based single-molecule force spectroscopy and the implications for advanced polymer materials. Chem. Soc. Rev. 2020, 49, 2799–2827. [Google Scholar] [CrossRef]
- Kloxin, A.M.; Kasko, A.M.; Salinas, C.N.; Anseth, K.S. Photodegradable Hydrogels for Dynamic Tuning of Physical and Chemical Properties. Science 2009, 324, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Firlak, M.; Kahraman, M.V.; Yetimoglu, E.K. Removal of Ag(I) from Aqueous Solutions by Thiol-ene-Based Hydrogel and Its Application to Radiographic Films. Water Air Soil Pollut. 2014, 225, 1843. [Google Scholar] [CrossRef]
- Dharmapriya, T.N.; Lee, D.Y.; Huang, P.J. Novel reusable hydrogel adsorbents for precious metal recycle. Sci. Rep. 2021, 11, 19577. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.Y.; Feng, X.F.; Liu, C.C.; Wu, H.R.; Liu, X.B. Advances in Chelating Resins for Adsorption of Heavy Metal Ions. Ind. Eng. Chem. Res. 2022, 61, 11309–11328. [Google Scholar] [CrossRef]
- Lin, G.; Wang, S.X.; Zhang, L.B.; Hu, T.; Peng, J.H.; Cheng, S.; Fu, L.K. Selective Adsorption of Ag+ on a New Cyanuric-Thiosemicarbazide Chelating Resin with High Capacity from Acid Solutions. Polymers 2017, 9, 568. [Google Scholar] [CrossRef]
- Wang, C.; Yang, K.K.; Xie, Q.H.; Pan, J.H.; Jiang, Z.H.; Yang, H.; Zhang, Y.; Wu, Y.T.; Han, J. Tandem Efficient Bromine Removal and Silver Recovery by Resorcinol-Formaldehyde Resin Nanoparticles. Nano Lett. 2023, 23, 2239–2246. [Google Scholar] [CrossRef]
- Nawaz, T.; Sengupta, S. Silver Recovery from Laundry Washwater: The Role of Detergent Chemistry. Acs Sustain. Chem. Eng. 2018, 6, 600–608. [Google Scholar] [CrossRef]
- Du, M.; Zhang, Y.Y.; Wang, Z.Y.; Lv, M.R.; Tang, A.Q.; Yu, Y.; Qu, X.; Chen, Z.Q.; Wen, Q.X.; Li, A. Insight into the synthesis and adsorption mechanism of adsorbents for efficient phosphate removal: Exploration from synthesis to modification. Chem. Eng. J. 2022, 442, 136147. [Google Scholar] [CrossRef]
- Sarkaya, K.; Bakhshpour, M.; Denizli, A. Ag+ ions imprinted cryogels for selective removal of silver ions from aqueous solutions. Sep. Sci. Technol. 2019, 54, 2993–3004. [Google Scholar] [CrossRef]
- Huo, H.Y.; Su, H.J.; Tan, T.W. Adsorption of Ag+ by a surface molecular-imprinted biosorbent. Chem. Eng. J. 2009, 150, 139–144. [Google Scholar] [CrossRef]
- Ding, X.; Yu, W.J.; Sheng, X.; Shi, H.; You, D.; Peng, M.M.; Shao, P.H.; Yang, L.M.; Liu, L.L.; Luo, X.B. Feasible fabrication of o -phenanthroline-based polymer adsorbent for selective capture of aqueous Ag(I). Chin. Chem. Lett. 2023, 34, 107485. [Google Scholar] [CrossRef]
- Zhang, J.; Pu, N.; Li, M.L.; Sang, W.H.; He, Q.; Tian, Q.Q.; Zhang, W. High-efficient Ag(I) ion binding, Ag(0) nanoparticle loading, and iodine trapping in ultrastable benzimidazole-linked polymers. Sep. Purif. Technol. 2024, 328, 125052. [Google Scholar] [CrossRef]
- Thommes, M.; Cychosz, K.A. Physical adsorption characterization of nanoporous materials: Progress and challenges. Adsorpt. -J. Int. Adsorpt. Soc. 2014, 20, 233–250. [Google Scholar] [CrossRef]
- Ferre-Vilaplana, A.; Herrero, E. Understanding the chemisorption-based activation mechanism of the oxygen reduction reaction on nitrogen-doped graphitic materials. Electrochim. Acta 2016, 204, 245–254. [Google Scholar] [CrossRef]
- Dhaouadi, F.; Sellaoui, L.; Badawi, M.; Reynel-Avila, H.E.; Mendoza-Castillo, D.I.; Jaime-Leal, J.E.; Bonilla-Petriciolet, A.; Ben Lamine, A. Statistical physics interpretation of the adsorption mechanism of Pb2+, Cd2+and Ni2+ on chicken feathers. J. Mol. Liq. 2020, 319, 114168. [Google Scholar] [CrossRef]
- Hasan, Z.; Jhung, S.H. Removal of hazardous organics from water using metal-organic frameworks (MOFs): Plausible mechanisms for selective adsorptions. J. Hazard. Mater. 2015, 283, 329–339. [Google Scholar] [CrossRef]
- Bulin, C.; Zheng, R.X.; Song, J.L.; Bao, J.X.; Xin, G.X.; Zhang, B.W. Magnetic graphene oxide-chitosan nanohybrid for efficient removal of aqueous Hg(Π) and the interaction mechanism. J. Mol. Liq. 2023, 370, 121050. [Google Scholar] [CrossRef]
- Muscarella, S.M.; Badalucco, L.; Cano, B.; Laudicina, V.A.; Mannina, G. Ammonium adsorption, desorption and recovery by acid and alkaline treated zeolite. Bioresour. Technol. 2021, 341, 125812. [Google Scholar] [CrossRef]
- Wang, H.L.; Wang, Q.; Ding, L.; Wang, P.X.; Luo, X.B. Evaluating the Role of Functional Groups in the Selective Capture of Ag(I) onto UiO-66-Type Metal-Organic Frameworks. Langmuir 2024, 40, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Moll, B.; Müller, T.; Schlüsener, C.; Schmitz, A.; Brandt, P.; Öztürk, S.; Janiak, C. Modulated synthesis of thiol-functionalized fcu and hcp UiO-66(Zr) for the removal of silver(I) ions from water. Mater. Adv. 2021, 2, 804–812. [Google Scholar] [CrossRef]
- Ye, H.; Zhao, B.Y.; Zhou, Y.H.; Du, J.Y.; Huang, M.Q. Recent advances in adsorbents for the removal of phthalate esters from water: Material, modification, and application. Chem. Eng. J. 2021, 409, 128127. [Google Scholar] [CrossRef]
- Wu, D.G.; Shi, W.; Ding, S.; Xie, Z.F. Diverse functional groups decorated, bifunctional polyesteramide as efficient Pb(II) electrochemical probe and methylene blue adsorbent. Eur. Polym. J. 2021, 160, 110810. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, W.; Zhang, X.; Peng, H.; Gong, Y. Thiol-ene synthesis of thioether/carboxyl-functionalized polymers for selective adsorption of silver (I) ions. Chem. Eng. J. 2019, 375, 121935. [Google Scholar] [CrossRef]
- Liu, F.L.; Peng, G.L.; Li, T.; Yu, G.; Deng, S.B. Au(III) adsorption and reduction to gold particles on cost-effective tannin acid immobilized dialdehyde corn starch. Chem. Eng. J. 2019, 370, 228–236. [Google Scholar] [CrossRef]
- Wang, L.L.; Deng, M.; Xu, H.M.; Li, W.W.; Huang, W.J.; Yan, N.Q.; Zhou, Y.X.; Chen, J.S.; Qu, Z. Selective Reductive Removal of Silver Ions from Acidic Solutions by Redox-Active Covalent Organic Frameworks. ACS Appl. Mater. Interfaces 2020, 12, 37619–37627. [Google Scholar] [CrossRef]
- Shao, P.H.; Chang, Z.W.; Li, M.; Lu, X.; Jiang, W.L.; Zhang, K.; Luo, X.B.; Yang, L.M. Mixed-valence molybdenum oxide as a recyclable sorbent for silver removal and recovery from wastewater. Nat. Commun. 2023, 14, 1365. [Google Scholar] [CrossRef]
- Amphlett, J.T.M.; Choi, S.; Parry, S.A.; Moon, E.M.; Sharrad, C.A.; Ogden, M.D. Insights on uranium uptake mechanisms by ion exchange resins with chelating functionalities: Chelation vs. anion exchange. Chem. Eng. J. 2020, 392, 123712. [Google Scholar] [CrossRef]
- Atia, A.A.; Donia, A.M.; Yousif, A.M. Comparative study of the recovery of silver(I) from aqueous solutions with different chelating resins derived from glycidyl methacrylate. J. Appl. Polym. Sci. 2005, 97, 806–812. [Google Scholar] [CrossRef]
- Liu, L.H.; Zhao, L.; Liu, J.Y.; Yang, Z.C.; Su, G.; Song, H.S.; Xue, J.R.; Tang, A.P. Preparation of magnetic Fe3O4@SiO2@CaSiO3 composite for removal of Ag+ from aqueous solution. J. Mol. Liq. 2020, 299, 112222. [Google Scholar] [CrossRef]
- Solís-Rodríguez, R.; Pérez-Garibay, R.; Alonso-González, O.; Mendieta-George, D. Enhancing the arsenic adsorption by controlling the zeta potential of Zn (OH)2 flocs. J. Environ. Chem. Eng. 2021, 9, 106300. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Wu, Y.Y.; Ding, W.; Sun, Q.; Hu, C.; Liu, B.Z.; Liu, H.X.; Zheng, H.L. Anion-synergistic adsorption enhances the selective removal of silver ions from complex wastewater by chitosan-coated magnetic silica core-shell nanoparticles. J. Clean. Prod. 2022, 339, 130777. [Google Scholar] [CrossRef]
- Kang, J.Y.; Ha, W.; Zhang, H.X.; Shi, Y.P. 4′-Aminobenzo-18-crown-6 functionalized magnetic nanoparticles as a solid-phase extraction adsorbent for the determination of Pb2+. Anal. Methods 2019, 11, 1735–1742. [Google Scholar] [CrossRef]
- Fan, L.L.; Luo, C.N.; Lv, Z.; Lu, F.G.; Qiu, H.M. Removal of Ag+ from water environment using a novel magnetic thiourea-chitosan imprinted Ag+. J. Hazard. Mater. 2011, 194, 193–201. [Google Scholar] [CrossRef]
- Y Yin, X.; Zhang, Q.; Yang, L.; Geng, Z.; Luo, X.; Ren, W. Rapid and selective recycling of Ag(I) from wastewater through an allylrhodanine functionalized micro-filtration membrane. Chem. Eng. J. 2022, 443, 136376. [Google Scholar] [CrossRef]
- Li, N.A.; Liu, Y.F.; Du, C.; Wang, Y.; Wang, L.J.; Li, X.Y. A novel role of various hydrogen bonds in adsorption, desorption and co- adsorption of PPCPs on corn straw-derived biochars. Sci. Total Environ. 2023, 861, 160623. [Google Scholar] [CrossRef]
- Liu, X.Q.; Zhang, Y.Y.; Liu, Y.; Zhang, T.A. Removal of Cr(VI) and Ag(I) by grafted magnetic zeolite/chitosan for water purification: Synthesis and adsorption mechanism. Int. J. Biol. Macromol. 2022, 222, 2615–2627. [Google Scholar] [CrossRef]
- Xie, L.X.; Yu, Z.H.; Islam, S.M.; Shi, K.R.; Cheng, Y.H.; Yuan, M.W.; Zhao, J.; Sun, G.B.; Li, H.F.; Ma, S.L.; et al. Remarkable Acid Stability of Polypyrrole-MoS4: A Highly Selective and Efficient Scavenger of Heavy Metals Over a Wide pH Range. Adv. Funct. Mater. 2018, 28, 1800502. [Google Scholar] [CrossRef]
- Wei, Y.F.; Wang, L.; Li, H.B.; Yan, W.; Feng, J.T. Synergistic Fluoride Adsorption by Composite Adsorbents Synthesized From Different Types of Materials—A Review. Front. Chem. 2022, 10, 900660. [Google Scholar] [CrossRef]
- Yadav, S.; Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Adsorptive potential of modified plant-based adsorbents for sequestration of dyes and heavy metals from wastewater-A review. J. Water Process Eng. 2021, 42, 102148. [Google Scholar] [CrossRef]
- Zhou, Y.B.; Lu, J.; Zhou, Y.; Liu, Y.D. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.T.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membr. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Othman, N.; Mat, H.; Goto, M. Separation of silver from photographic wastes by emulsion liquid membrane system. J. Membr. Sci. 2006, 282, 171–177. [Google Scholar] [CrossRef]
- Li, R.F.; Lan, G.H.; Liu, Y.Q.; Sun, Q.; Luo, B.; Zhang, M.; Qiu, H.Y.; Xu, B.; Deng, C.P. Polyurethane sponge loading improves the suspension of magnetic materials without affecting the Pb(II) adsorption. J. Environ. Chem. Eng. 2023, 11, 110475. [Google Scholar] [CrossRef]
- Wang, B.X.; Wu, K.Y.; Liu, T.H.; Cheng, Z.K.; Liu, Y.; Liu, Y.F.; Niu, Y.Z. Feasible synthesis of bifunctional polysilsesquioxane microspheres for robust adsorption of Hg(II) and Ag(I): Behavior and mechanism. J. Hazard. Mater. 2023, 442, 130121. [Google Scholar] [CrossRef] [PubMed]
- Alsulami, Q.A.; Hussein, M.A.; Alsheheri, S.Z.; Elshehy, E.A.; El-Said, W.A. Unexpected ultrafast and high adsorption performance of Ag(I) and Hg(II) ions from multiple aqueous solutions using microporous functional silica-polymer sponge-like composite. J. Mater. Res. Technol. Jmrt 2022, 17, 2000–2013. [Google Scholar] [CrossRef]
- Zheng, Q.; Li, Q.; Tao, Y.; Gong, J.; Shi, J.; Yan, Y.; Guo, X.; Yang, H. Efficient removal of copper and silver ions in electroplating wastewater by magnetic-MOF-based hydrogel and a reuse case for photocatalytic application. Chemosphere 2023, 340, 139885. [Google Scholar] [CrossRef]
- Yuan, W.; yuan Li, M.; Chen, H.; Liu, G.; Liu, D.; Chen, X.; Song, W.; Su, Y. Preparation of porous CuS/modified-diatomite composite via a facile in situ loading process for efficient recovery of silver ion from aqueous solution. Appl. Surf. Sci. 2023, 644, 158753. [Google Scholar] [CrossRef]
- Yang, H.J.; Ma, Y.X.; Meng, W.L.; Li, T.Z.; Wang, J.W.; Li, X.H.; Liu, J.Y.; Zhang, Y.W. Fabrication of 3D hierarchical networks adsorbent immobilized MoS2 for adsorption of Ag(I) from aqueous solution. Appl. Surf. Sci. 2023, 637, 157932. [Google Scholar] [CrossRef]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef]
- Jain, A.; Kumari, S.; Agarwal, S.; Khan, S. Water purification via novel nano-adsorbents and their regeneration strategies. Process Saf. Environ. Prot. 2021, 152, 441–454. [Google Scholar] [CrossRef]
- Yao, Z.W.; Shao, P.H.; Fang, D.F.; Shao, J.C.; Li, D.W.; Liu, L.L.; Huang, Y.; Yu, Z.; Yang, L.M.; Yu, K.; et al. Thiol-rich, porous carbon for the efficient capture of silver: Understanding the relationship between the surface groups and transformation pathways of silver. Chem. Eng. J. 2022, 427, 131470. [Google Scholar] [CrossRef]
- Zhao, W.C.; Huang, Y.J.; Chen, R.; Peng, H.; Liao, Y.G.; Wang, Q. Facile preparation of thioether/hydroxyl functionalized polyhedral oligomeric silsesquioxanes hybrid polymer for ultrahigh selective adsorption of silver(I) ions. React. Funct. Polym. 2021, 163, 104899. [Google Scholar] [CrossRef]
- Ren, X.Y.; Wang, C.C.; Li, Y.; Wang, C.Y.; Wang, P.; Gao, S.J. Ag(I) removal and recovery from wastewater adopting NH2-MIL-125 as efficient adsorbent: A 3Rs (reduce, recycle and reuse) approach and practice. Chem. Eng. J. 2022, 442, 136306. [Google Scholar] [CrossRef]
- Ding, L.; Shao, P.H.; Luo, Y.; Yin, X.C.; Yu, S.P.; Fang, L.L.; Yang, L.M.; Yang, J.K.; Luo, X.B. Functionalization of UiO-66-NH2 with rhodanine via amidation: Towarding a robust adsorbent with dual coordination sites for selective capture of Ag(I) from wastewater. Chem. Eng. J. 2020, 382, 123009. [Google Scholar] [CrossRef]
- Zeng, Q.; Sun, W.; Zhong, H.; He, Z.G. Efficient and selective removal of Ag+ as nano silver particles by the composite of SiO2 supported nano ferrous oxalate. Environ. Res. 2021, 202, 111696. [Google Scholar] [CrossRef]
- Jayadharan Salini, A.N.; Ramachandran, A.; Sadasivakurup, S.; Yesodha, S.K. Versatile MoS2 hollow nanoroses for a quick-witted removal of Hg (II), Pb (II) and Ag (I) from water and the mechanism: Affinity or Electrochemistry? Appl. Mater. Today 2020, 20, 100642. [Google Scholar] [CrossRef]
- Cigil, A.B.; Urucu, O.A.; Birtane, H.; Kahraman, M.V. Cellulose/cysteine based thiol-ene UV cured adsorbent: Removal of silver (I) ions from aqueous solution. Cellulose 2021, 28, 6439–6448. [Google Scholar] [CrossRef]
- Liu, Y.F.; Pei, R.H.; Lv, Y.C.; Lin, C.X.; Huang, J.H.; Liu, M.H. Removal behavior and mechanism of silver from low concentration wastewater using cellulose aerogel modified by thiosemicarbazide. J. Appl. Polym. Sci. 2021, 138, 51226. [Google Scholar] [CrossRef]
- Firlak, M.; Kahraman, M.V.; Yetimoglu, E.K.; Zeytuncu, B. Adsorption of Ag (I) Ions from Aqueous Solutions Using Photocured Thiol-Ene Hydrogel. Sep. Sci. Technol. 2013, 48, 2860–2870. [Google Scholar] [CrossRef]
- Wang, S.M.; Li, H.L.; Chen, X.Y.; Yang, M.; Qi, Y.X. Selective adsorption of silver ions from aqueous solution using polystyrene-supported trimercaptotriazine resin. J. Environ. Sci. 2012, 24, 2166–2172. [Google Scholar] [CrossRef]
- Zhao, J.L.; Wang, S.X.; Zhang, L.B.; Wang, C.; Zhang, B. Kinetic, Isotherm, and Thermodynamic Studies for Ag(I) Adsorption Using Carboxymethyl Functionalized Poly(glycidyl methacrylate). Polymers 2018, 10, 1090. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, S.X.; Zhang, L.B.; Li, Y.; Zhou, Y.; Peng, J.H. Selective recovery of silver from aqueous solutions by poly (glycidyl methacrylate) microsphere modified with trithiocyanuric acid. J. Mol. Liq. 2018, 254, 340–348. [Google Scholar] [CrossRef]
- Taghizadeh, M.; Shafizadeh, F. Fabrication of a novel hydrophilic ion-imprinted polymer based on silica-modified magnetic graphene oxide as a sensitive sorbent for the fast separation of silver ions from aqueous media. J. Dispers. Sci. Technol. 2023, 1–17. [Google Scholar] [CrossRef]
- Kazemi, E.; Shabani, A.M.H.; Dadfarnia, S. Synthesis and characterization of a nanomagnetic ion imprinted polymer for selective extraction of silver ions from aqueous samples. Microchim. Acta 2015, 182, 1025–1033. [Google Scholar] [CrossRef]
- KarakoÇ, V. Adsorption of silver from aqueous solution with high capacity and selectively by using Ag(I) imprinted polymeric nanoadsorbent. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Yin, X.C.; Long, J.; Xi, Y.; Luo, X.B. Recovery of Silver from Wastewater Using a New Magnetic Photocatalytic Ion-Imprinted Polymer. Acs Sustain. Chem. Eng. 2017, 5, 2090–2097. [Google Scholar] [CrossRef]
- Jin, K.; Huang, X.; Yang, H.; Li, Y.; Zeng, J.; Zhou, H.; Liu, Y.; Zhang, R. A acylthiourea based ion-imprinted membrane for selective removal of Ag+ from aqueous solution. Colloids Surf. A: Physicochem. Eng. Asp. 2024, 684, 133162. [Google Scholar] [CrossRef]
- Jalilian, R.; Taheri, A. Synthesis and application of a novel core-shell-shell magnetic ion imprinted polymer as a selective adsorbent of trace amounts of silver ions. Polymers 2018, 18, 123–134. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, H.; Wu, Y.W.; Yu, X.X. Silver ion-imprinted magnetic adsorbent hyphenated to single particle-ICP-MS for separation and analysis of dissolved silver and silver nanoparticles in antibacterial gel extracts. Anal. Chim. Acta 2023, 1279, 341846. [Google Scholar] [CrossRef]
- Peng, Q.; Zhao, H.W.; Chen, G.; Yang, Q.L.; Cao, X.X.; Xiong, S.H.; Xiao, A.G.; Li, G.; Liu, B.; Liu, Q.Q. Synthesis of novel magnetic pitch-based hypercrosslinked polymers as adsorbents for effective recovery of Ag plus with high selectivity. J. Environ. Manag. 2023, 339, 117763. [Google Scholar] [CrossRef]
- Biyikoglu, M.; Çiftçi, H. Adsorption of Ag(I) ions from wastewaters using poly(2-aminothiazole): Kinetic and isotherm studies. Polym. Bull. 2020, 77, 6161–6174. [Google Scholar] [CrossRef]
- Elshehy, E.A.; Shenashen, M.A.; El-Magied, M.O.A.; Tolan, D.A.; El-Nahas, A.M.; Halada, K.; Atia, A.A.; El-Safty, S.A. Selective Recovery of Silver(I) Ions from E-Waste using Cubically Multithiolated Cage Mesoporous Monoliths. Eur. J. Inorg. Chem. 2017, 2017, 4823–4833. [Google Scholar] [CrossRef]
- Peng, P.P.; Zhao, C.F.; Ji, J.; Chen, W.X.; Ding, N.; Li, S.H.; Pang, S.P. Simple and selective method for simultaneous removal of mercury(II) and recovery of silver(I) from aqueous media by organic ligand 4,4′-azo-1,2,4-triazole. Environ. Sci. Water Res. Technol. 2022, 8, 534–542. [Google Scholar] [CrossRef]
- Wu, T.; Lin, Z.; Wu, H.; Wang, M.; Zhu, C.; Kanazawa, N.; Shi, J.; Liang, R. Adsorption Studies on Ag(I) Using Poly(N-phenylglycine) Membrane and Application in Practical Silver Recycling. ACS Appl. Polym. Mater. 2022, 4, 3333–3342. [Google Scholar] [CrossRef]
- Huang, X.; Jin, K.; Yang, S.; Zeng, J.; Zhou, H.; Zhang, R.; Xue, J.; Liu, Y.; Liu, G.; Peng, H. Fabrication of polyvinylidene fluoride and acylthiourea composite membrane and its adsorption performance and mechanism on silver ions. Sep. Purif. Technol. 2023, 315, 123675. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Li, M.; Xi, M.; Zhao, M.; Wang, X.; Chen, X.; Ding, L. Recovery of Ag(I) from Wastewater by Adsorption: Status and Challenges. Toxics 2024, 12, 351. https://doi.org/10.3390/toxics12050351
Wang Q, Li M, Xi M, Zhao M, Wang X, Chen X, Ding L. Recovery of Ag(I) from Wastewater by Adsorption: Status and Challenges. Toxics. 2024; 12(5):351. https://doi.org/10.3390/toxics12050351
Chicago/Turabian StyleWang, Qiang, Mengling Li, Meng Xi, Mengyuan Zhao, Xiaotong Wang, Xiaoyu Chen, and Lin Ding. 2024. "Recovery of Ag(I) from Wastewater by Adsorption: Status and Challenges" Toxics 12, no. 5: 351. https://doi.org/10.3390/toxics12050351
APA StyleWang, Q., Li, M., Xi, M., Zhao, M., Wang, X., Chen, X., & Ding, L. (2024). Recovery of Ag(I) from Wastewater by Adsorption: Status and Challenges. Toxics, 12(5), 351. https://doi.org/10.3390/toxics12050351






