The Flavoring Agent Ethyl Vanillin Induces Cellular Stress Responses in HK-2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Culture and Treatment
2.3. Trypan Blue Exclusion
2.4. MTT Assay
2.5. Western Blot Analysis
2.6. Seahorse XFp Analysis
2.7. Cell Normalization
2.8. Statistical Analysis
3. Results
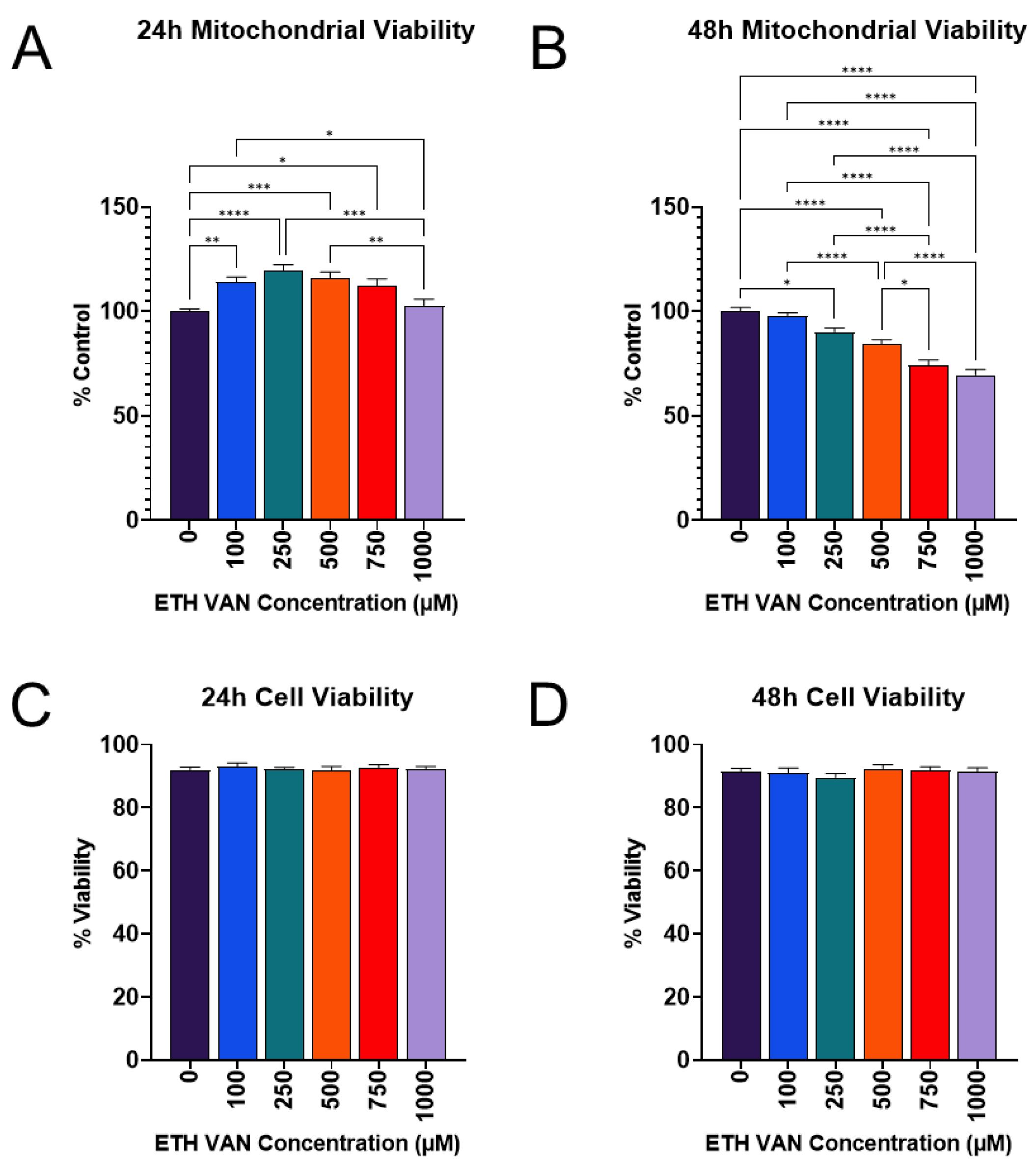
3.1. ETH VAN Alters Mitochondrial Activity
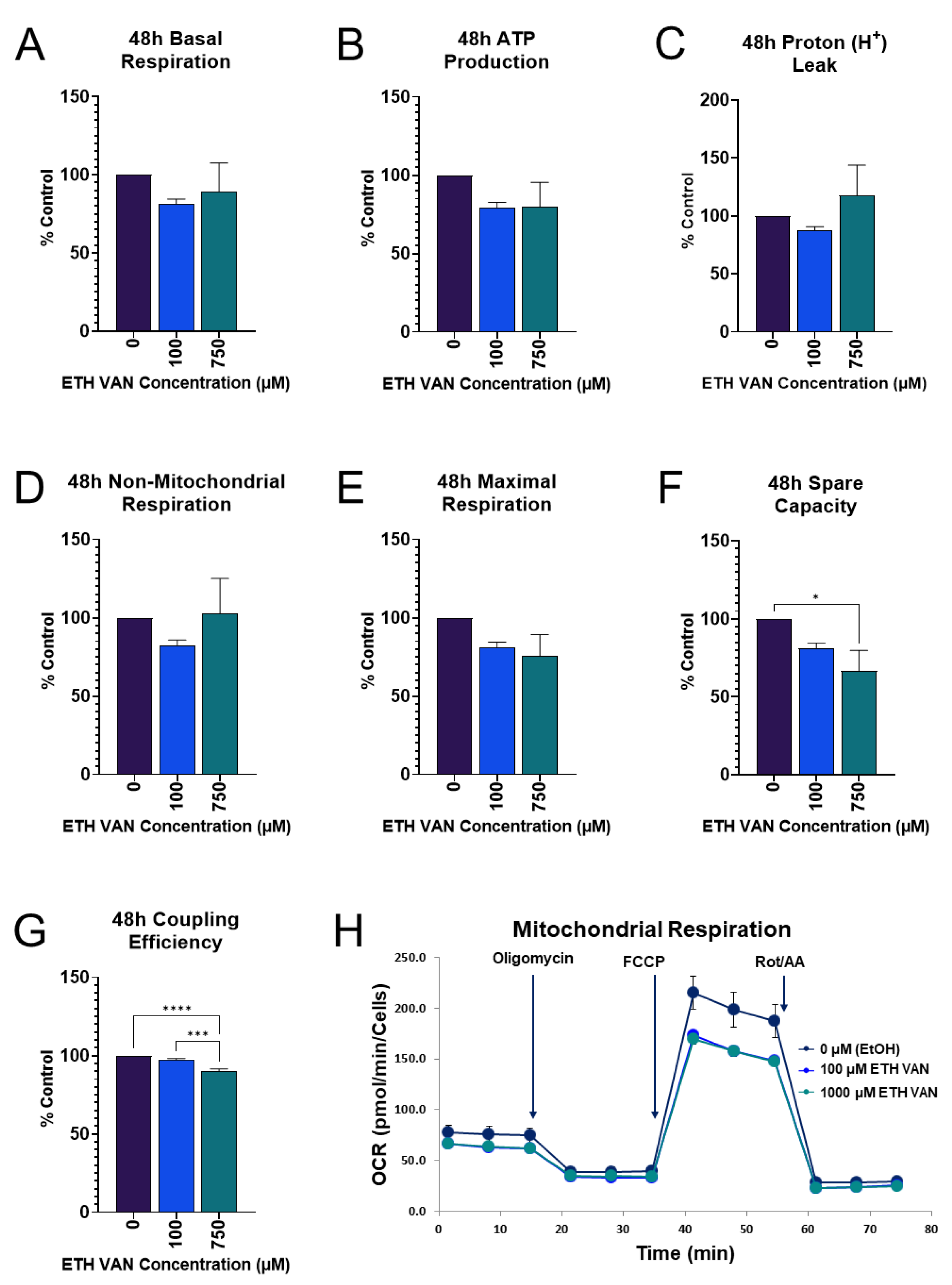
3.2. ETH VAN Alters Mitochondrial Energy Pathways
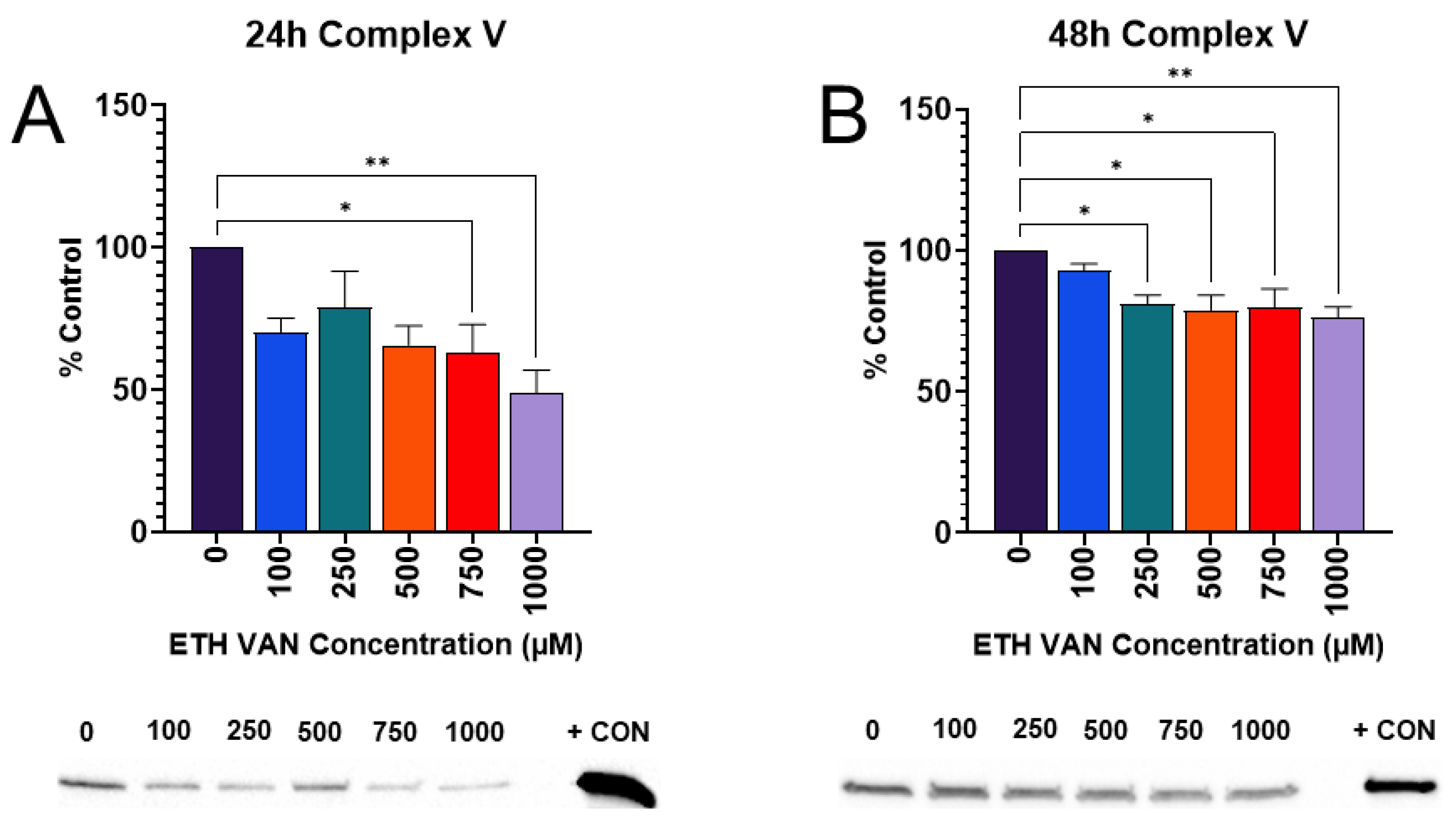
3.3. ETH VAN Decreases Expression of ATP Synthase
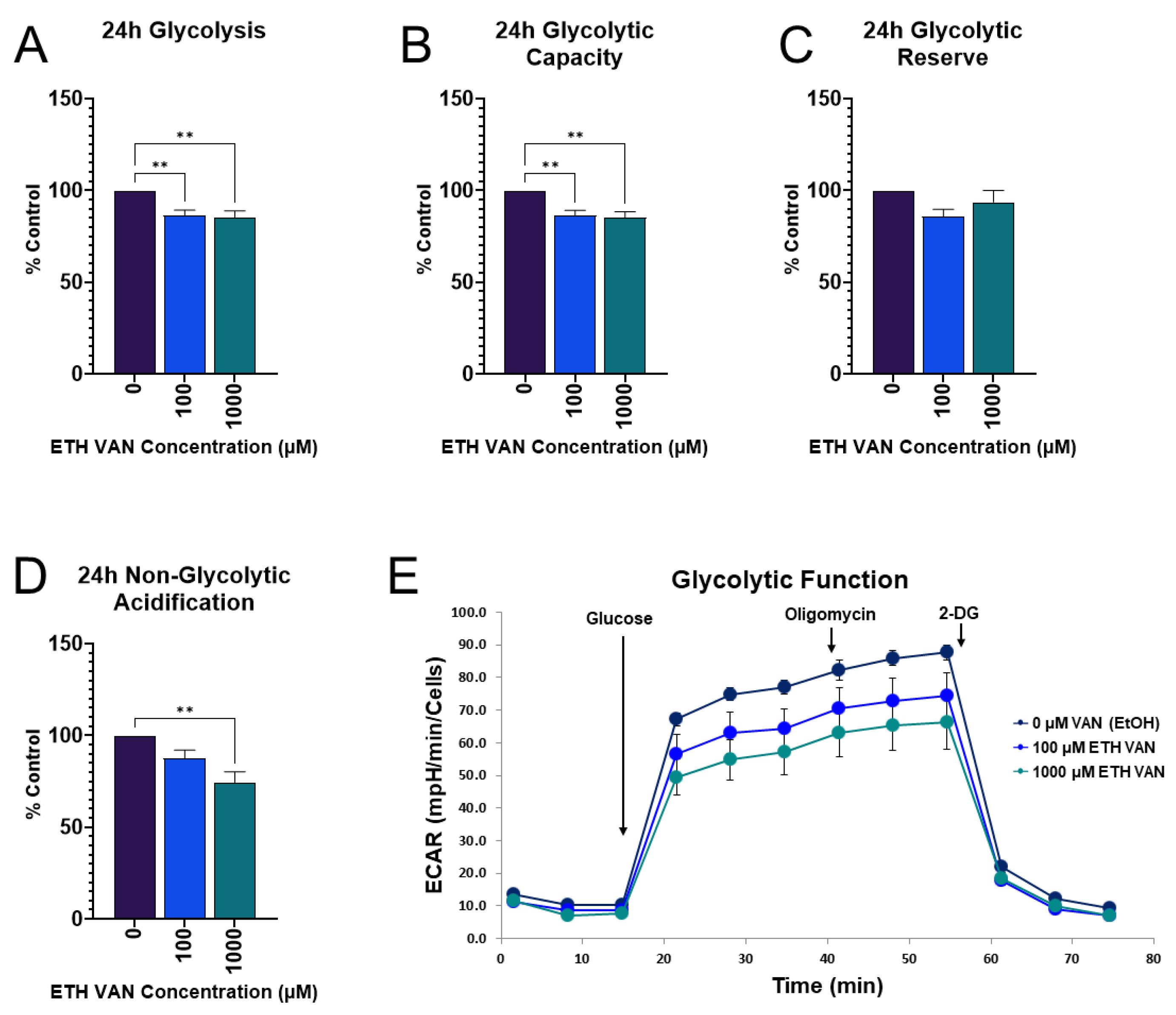
3.4. ETH VAN Decreases Glycolytic Function
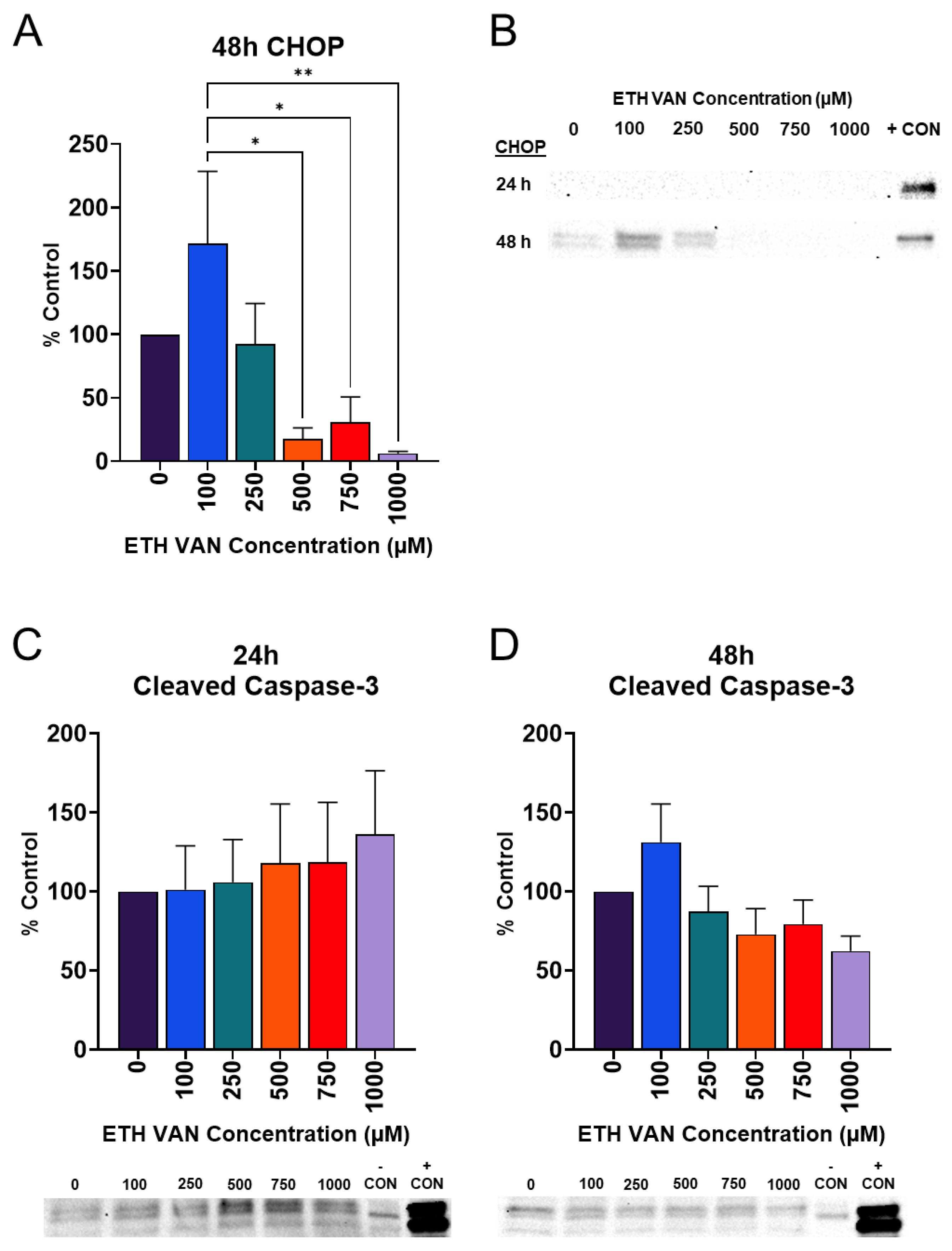
3.5. ETH VAN Alters Cleaved Caspase-3 and Induces Loss of CHOP Expression
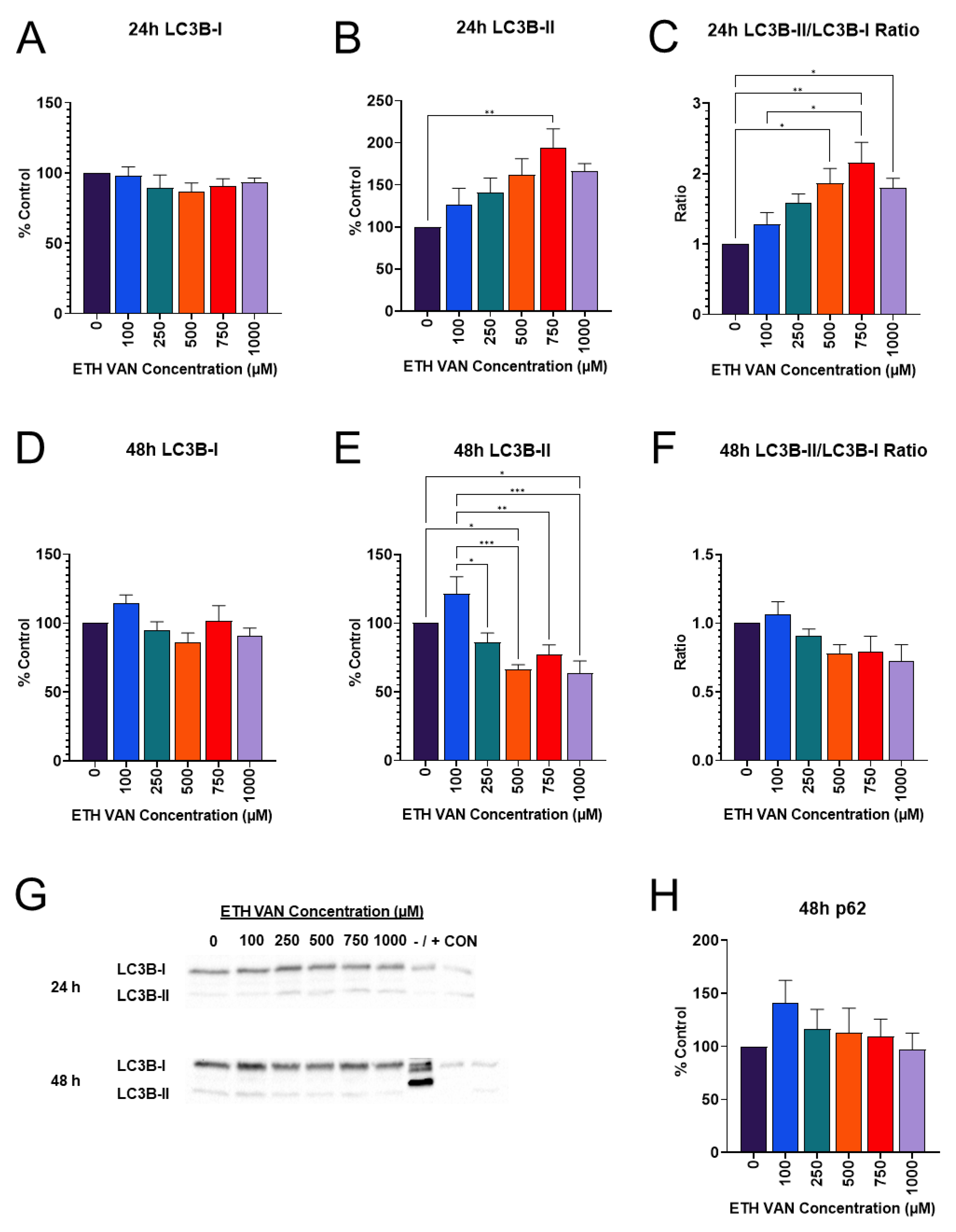
3.6. ETH VAN Alters Autophagy Markers
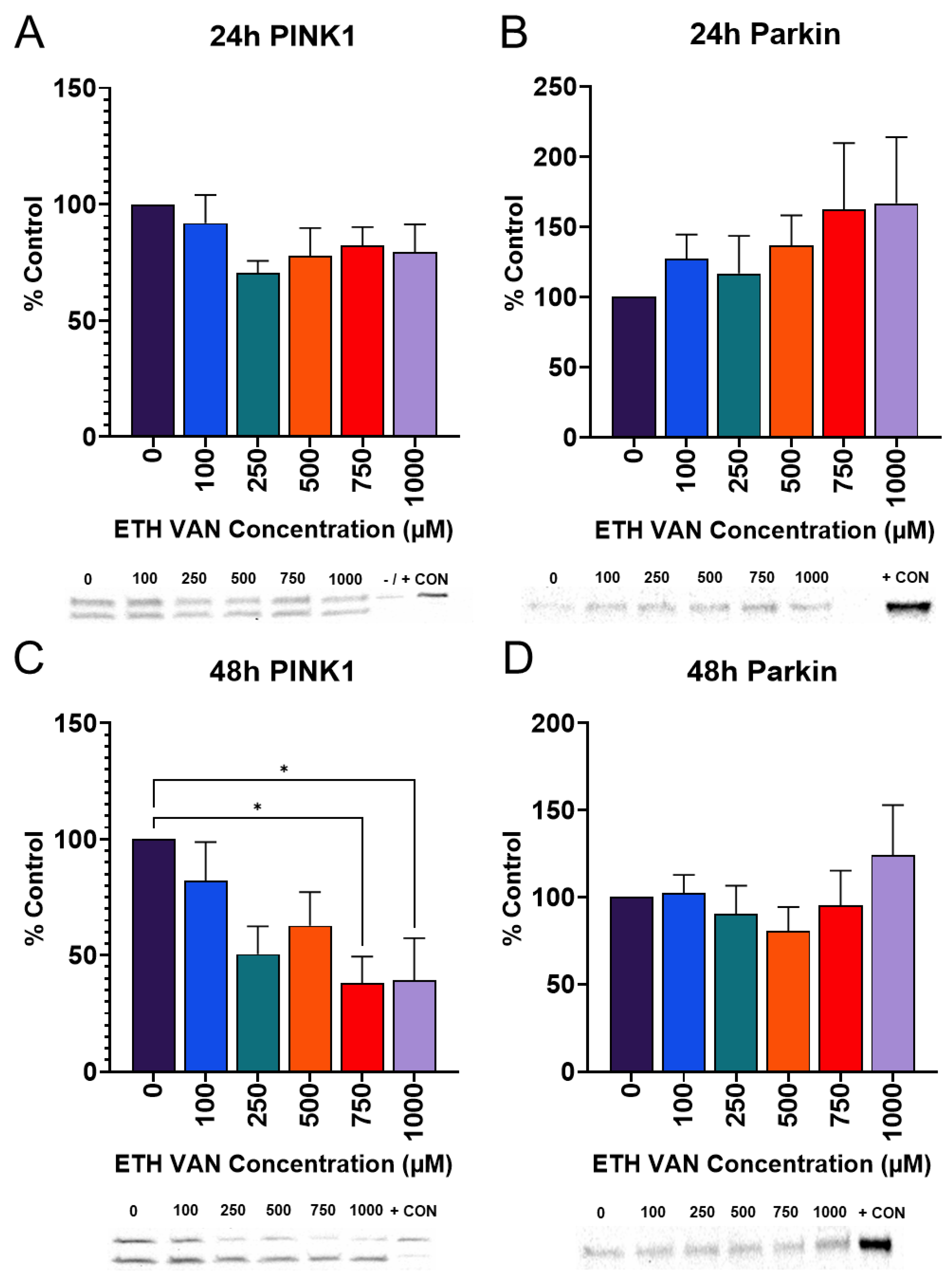
3.7. ETH VAN Alters Mitophagy Pathway Markers
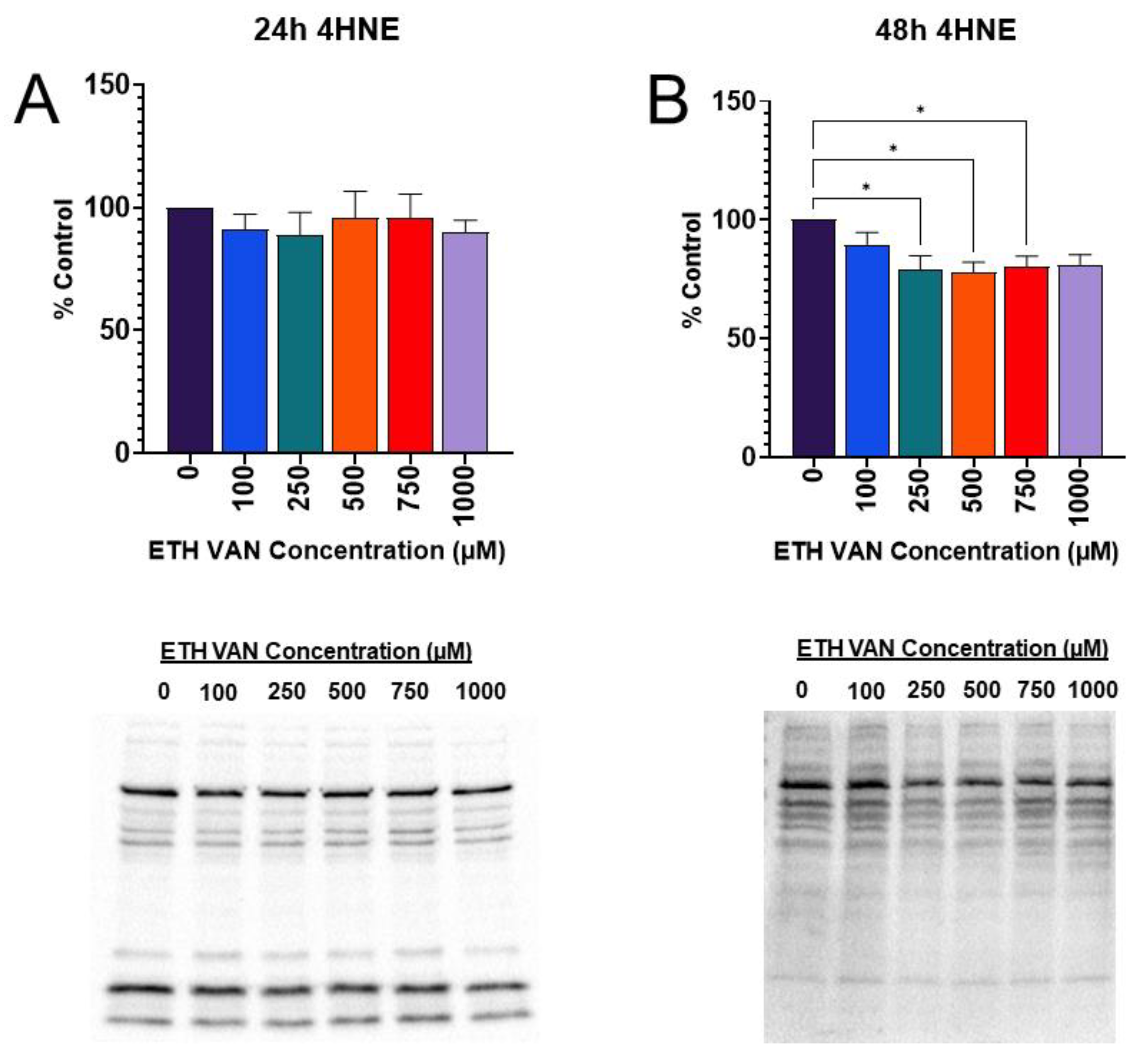
3.8. ETH VAN Mediates Lipid Peroxidation-Induced Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Miech, R.A.; Johnston, L.D.; Patrick, M.E.; O’Malley, P.M.; Bachman, J.G.; Schulenberg, J.E. Monitoring the Future National Survey Results on Drug Use 1975–2022—Secondary School Students; Monitoring the Future Monograph Series; Institute for Social Research, University of Michigan: Ann Arbor, MI, USA, 2023; Available online: https://monitoringthefuture.org/results/publications/monographs/ (accessed on 24 June 2024).
- Patrick, M.E.; Miech, R.A.; Johnston, L.D.; O’Malley, P.M. Monitoring the Future Panel Study Annual Report—National Data on Substance Use among Adults Ages 19 to 60—1976–2022; Monitoring the Future Monograph Series; Institute for Social Research, University of Michigan: Ann Arbor, MI, USA, 2023. [Google Scholar] [CrossRef]
- Cooper, S.Y.; Akers, A.T.; Henderson, B.J. Flavors Enhance Nicotine Vapor Self-administration in Male Mice. Nicotine Tob. Res. 2021, 23, 566–572. [Google Scholar] [CrossRef]
- Landry, R.L.; Groom, A.L.; Vu, T.-H.T.; Stokes, A.C.; Berry, K.M.; Kesh, A.; Hart, J.L.; Walker, K.L.; Giachello, A.L.; Sears, C.G.; et al. The role of flavors in vaping initiation and satisfaction among U.S. adults. Addict. Behav. 2019, 99, 106077. [Google Scholar] [CrossRef]
- Leventhal, A.M.; Goldenson, N.I.; Cho, J.; Kirkpatrick, M.G.; McConnell, R.S.; Stone, M.D.; Pang, R.D.; Audrain-McGovern, J.; Barrington-Trimis, J.L. Flavored E-cigarette Use and Progression of Vaping in Adolescents. Pediatrics 2019, 144, e20190789. [Google Scholar] [CrossRef]
- Villanti, A.C.; Johnson, A.L.; Ambrose, B.K.; Cummings, K.M.; Stanton, C.A.; Rose, S.W.; Feirman, S.P.; Tworek, C.; Glasser, A.M.; Pearson, J.L.; et al. Flavored Tobacco Product Use in Youth and Adults: Findings From the First Wave of the PATH Study (2013–2014). Am. J. Prev. Med. 2017, 53, 139–151. [Google Scholar] [CrossRef]
- Zhu, S.-H.; Sun, J.Y.; Bonnevie, E.; Cummins, S.E.; Gamst, A.; Yin, L.; Lee, M. Four hundred and sixty brands of e-cigarettes and counting: Implications for product regulation. Tob. Control 2014, 23 (Suppl. S3), iii3–iii9. [Google Scholar] [CrossRef]
- Omaiye, E.E.; McWhirter, K.J.; Luo, W.; Tierney, P.A.; Pankow, J.F.; Talbot, P. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci. Rep. 2019, 9, 2468. [Google Scholar] [CrossRef]
- Tierney, P.A.; Karpinski, C.D.; Brown, J.E.; Luo, W.; Pankow, J.F. Flavour chemicals in electronic cigarette fluids. Tob. Control 2015, 25, e10–e15. [Google Scholar] [CrossRef]
- Larcombe, A.; Allard, S.; Pringle, P.; Anderson, N.; Mullins, B.; Mead-Hunter, R. Chemical analysis of fresh and aged Australian e-cigarette liquids. Med. J. Aust. 2022, 216, 27–32. [Google Scholar] [CrossRef]
- Omaiye, E.E.; Luo, W.; McWhirter, K.J.; Pankow, J.F.; Talbot, P. Electronic Cigarette Refill Fluids Sold Worldwide: Flavor Chemical Composition, Toxicity, and Hazard Analysis. Chem. Res. Toxicol. 2020, 33, 2972–2987. [Google Scholar] [CrossRef]
- Rickard, B.P.; Ho, H.; Tiley, J.B.; Jaspers, I.; Brouwer, K.L.R. E-Cigarette Flavoring Chemicals Induce Cytotoxicity in HepG2 Cells. ACS Omega 2021, 6, 6708–6713. [Google Scholar] [CrossRef]
- Bessac, B.F.; Jordt, S.E.; Meents, J.E.; Ciotu, C.I.; Fischer, M.J.M.; Xu, F.; Rowell, T.R.; Tarran, R.; Lazrak, A.; Creighton, J.; et al. Breathtaking TRP Channels: TRPA1 and TRPV1 in Airway Chemosensation and Reflex Control. Physiology 2008, 23, 360–370. [Google Scholar] [CrossRef]
- Clapp, P.W.; Jaspers, I. Electronic Cigarettes: Their Constituents and Potential Links to Asthma. Curr. Allergy Asthma Rep. 2017, 17, 79. [Google Scholar] [CrossRef]
- Winters, B.R.; Kochar, T.K.; Clapp, P.W.; Jaspers, I.; Madden, M.C. Impact of E-Cigarette Liquid Flavoring Agents on Activity of Microsomal Recombinant CYP2A6, the Primary Nicotine-Metabolizing Enzyme. Chem. Res. Toxicol. 2020, 33, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Winters, B.R.; Clapp, P.W.; Simmons, S.O.; Kochar, T.K.; Jaspers, I.; Madden, M.C. E-Cigarette Liquids and Aldehyde Flavoring Agents Inhibit CYP2A6 Activity in Lung Epithelial Cells. ACS Omega 2023, 8, 11261–11266. [Google Scholar] [CrossRef]
- Golli, N.E.; Jrad-Lamine, A.; Neffati, H.; Dkhili, H.; Rahali, D.; Dallagi, Y.; El May, M.V.; El Fazaa, S. Impact of e-cigarette refill liquid exposure on rat kidney. Regul. Toxicol. Pharmacol. 2016, 77, 109–116. [Google Scholar] [CrossRef]
- Dong, Y.; Chenna, A. Dilemma of Vaping-Associated Lung Injury in Patient Presenting With Shortness of Breath and Acute Kidney Injury. Chest 2020, 158, 2365A. [Google Scholar] [CrossRef]
- Macias, A.E.; Garcia, F.J.; Saldana, S.G. A patient from Mexico with vaping-associated lung injury, seizures and renal failure. Tob. Induc. Dis. 2019, 17, 91. [Google Scholar] [CrossRef]
- Harada, H.A.; Liu, J.; Shortell, J.D.; Beesley, S.J. A 20-Year-Old Man with e-Cigarette or Vaping Product Use-Associated Lung Injury (EVALI) and Thrombotic Coagulopathy. Am. J. Case Rep. 2021, 22, e929915. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Jabba, S.V.; Diaz, A.N.; Erythropel, H.C.; Zimmerman, J.B.; Jordt, S.-E. Chemical Adducts of Reactive Flavor Aldehydes Formed in E-Cigarette Liquids Are Cytotoxic and Inhibit Mitochondrial Function in Respiratory Epithelial Cells. Nicotine Tob. Res. 2020, 22, S25–S34. [Google Scholar] [CrossRef]
- Wang, Z.; Ying, Z.; Bosy-Westphal, A.; Zhang, J.; Schautz, B.; Later, W.; Heymsfield, S.B.; Müller, M.J. Specific metabolic rates of major organs and tissues across adulthood: Evaluation by mechanistic model of resting energy expenditure. Am. J. Clin. Nutr. 2010, 92, 1369–1377. [Google Scholar] [CrossRef]
- Geisler, S.; Holmström, K.M.; Skujat, D.; Fiesel, F.C.; Rothfuss, O.C.; Kahle, P.J.; Springer, W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat. Cell Biol. 2010, 12, 119–131. [Google Scholar] [CrossRef]
- Ding, W.-X.; Ni, H.-M.; Li, M.; Liao, Y.; Chen, X.; Stolz, D.B.; Dorn, G.W., 2nd; Yin, X.-M. Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Par-kin-ubiquitin-p62-mediated mitochondrial priming. J. Biol. Chem. 2010, 285, 27879–27890. [Google Scholar] [CrossRef]
- Youle, R.J.; Narendra, D.P. Mechanisms of mitophagy. Nat. Rev. Mol. Cell Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef]
- Narendra, D.; Kane, L.A.; Hauser, D.N.; Fearnley, I.M.; Youle, R.J. p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy 2010, 6, 1090–1106. [Google Scholar] [CrossRef]
- Jung, S.; Jeong, H.; Yu, S.-W. Autophagy as a decisive process for cell death. Exp. Mol. Med. 2020, 52, 921–930. [Google Scholar] [CrossRef] [PubMed]
- El-Khattouti, A.; Selimovic, D.; Haikel, Y.; Hassan, M. Crosstalk between apoptosis and autophagy: Molecular mechanisms and therapeutic strategies in cancer. J. Cell Death 2013, 6, 37–55. [Google Scholar] [CrossRef] [PubMed]
- Duann, P.; Lianos, E.A.; Ma, J.; Lin, P.-H. Autophagy, Innate Immunity and Tissue Repair in Acute Kidney Injury. Int. J. Mol. Sci. 2016, 17, 662. [Google Scholar] [CrossRef]
- Duann, P.; Lin, P.H. Mitochondria Damage and Kidney Disease. Adv. Exp. Med. Biol. 2017, 982, 529–551. [Google Scholar] [PubMed]
- Tong, Y.; Liu, S.; Gong, R.; Zhong, L.; Duan, X.; Zhu, Y. Ethyl Vanillin Protects against Kidney Injury in Diabetic Nephropathy by Inhibiting Oxidative Stress and Apoptosis. Oxidative Med. Cell. Longev. 2019, 2019, 2129350. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Sawano, T.; Yazama, F. Antioxidant properties of ethyl vanillin in vitro and in vivo. Biosci. Biotechnol. Biochem. 2011, 75, 2346–2350. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.; Al-Melhim, W. Vanillin mitigates the adverse impact of cisplatin and methotrexate on rat kidneys. Hum. Exp. Toxicol. 2018, 37, 937–943. [Google Scholar] [CrossRef]
- Ben Saad, H.; Kharrat, N.; Driss, D.; Gargouri, M.; Marrakchi, R.; Jammoussi, K.; Magné, C.; Boudawara, T.; Chaabouni, S.E.; Zeghal, K.M.; et al. Effects of vanillin on potassium bromate-induced neurotoxicity in adult mice: Impact on behavior, oxidative stress, genes expression, inflammation and fatty acid composition. Arch. Physiol. Biochem. 2017, 123, 165–174. [Google Scholar] [CrossRef]
- Metwaly, H.A.; El-Eraky, A.M.; Ibrahim, E.E.; Kandil, K.K.; El-Sayed, M.A.; El-Tabakh, N.M.; Motawea, A.M.; Ali, H.A.; Jabban, M.Z.; Mahmoud, M.E.; et al. Vanillin attenuates thioacetamide-induced renal assault by direct and indirect mediation of the TGFβ, ERK and Smad signalling pathways in rats. Cell Biochem. Funct. 2022, 40, 175–188. [Google Scholar] [CrossRef]
- Hawkins, D.R.; Midgley, I.; Proctor, P.; Bennett, S.; Huckstep, M.R.; Cheng, K. Biokinetics and metabolism of ethyl 14C-vanillin following oral (gavage) administration to rats. HRC/EVT 5/921134 of Huntingdon Research Centre. Ltd. Submitted to WHO by Ethyl Vanillin Task Force, International Food. Additives Council (IFAC), Atlanta, Georgia, USA, 1992. Available online: https://www.inchem.org/documents/jecfa/jecmono/v35je07.htm (accessed on 24 June 2024).
- Hua, M.; Omaiye, E.E.; Luo, W.; McWhirter, K.J.; Pankow, J.F.; Talbot, P. Identification of Cytotoxic Flavor Chemicals in Top-Selling Electronic Cigarette Refill Fluids. Sci. Rep. 2019, 9, 2782. [Google Scholar] [CrossRef]
- Page, M.K.; Goniewicz, M.L. New Analytical Method for Quantifying Flavoring Chemicals of Potential Respiratory Health Risk Concerns in e-Cigarette Liquids. Front. Chem. 2021, 9, 763940. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cox, A.J.; Brown, K.C.; Valentovic, M.A. The Flavoring Agent Ethyl Vanillin Induces Cellular Stress Responses in HK-2 Cells. Toxics 2024, 12, 472. https://doi.org/10.3390/toxics12070472
Cox AJ, Brown KC, Valentovic MA. The Flavoring Agent Ethyl Vanillin Induces Cellular Stress Responses in HK-2 Cells. Toxics. 2024; 12(7):472. https://doi.org/10.3390/toxics12070472
Chicago/Turabian StyleCox, Ashley J., Kathleen C. Brown, and Monica A. Valentovic. 2024. "The Flavoring Agent Ethyl Vanillin Induces Cellular Stress Responses in HK-2 Cells" Toxics 12, no. 7: 472. https://doi.org/10.3390/toxics12070472





