Endocrine-Disrupting Chemicals Exposure and Neurocognitive Function in the General Population: A Community-Based Study
Abstract
:1. Introduction
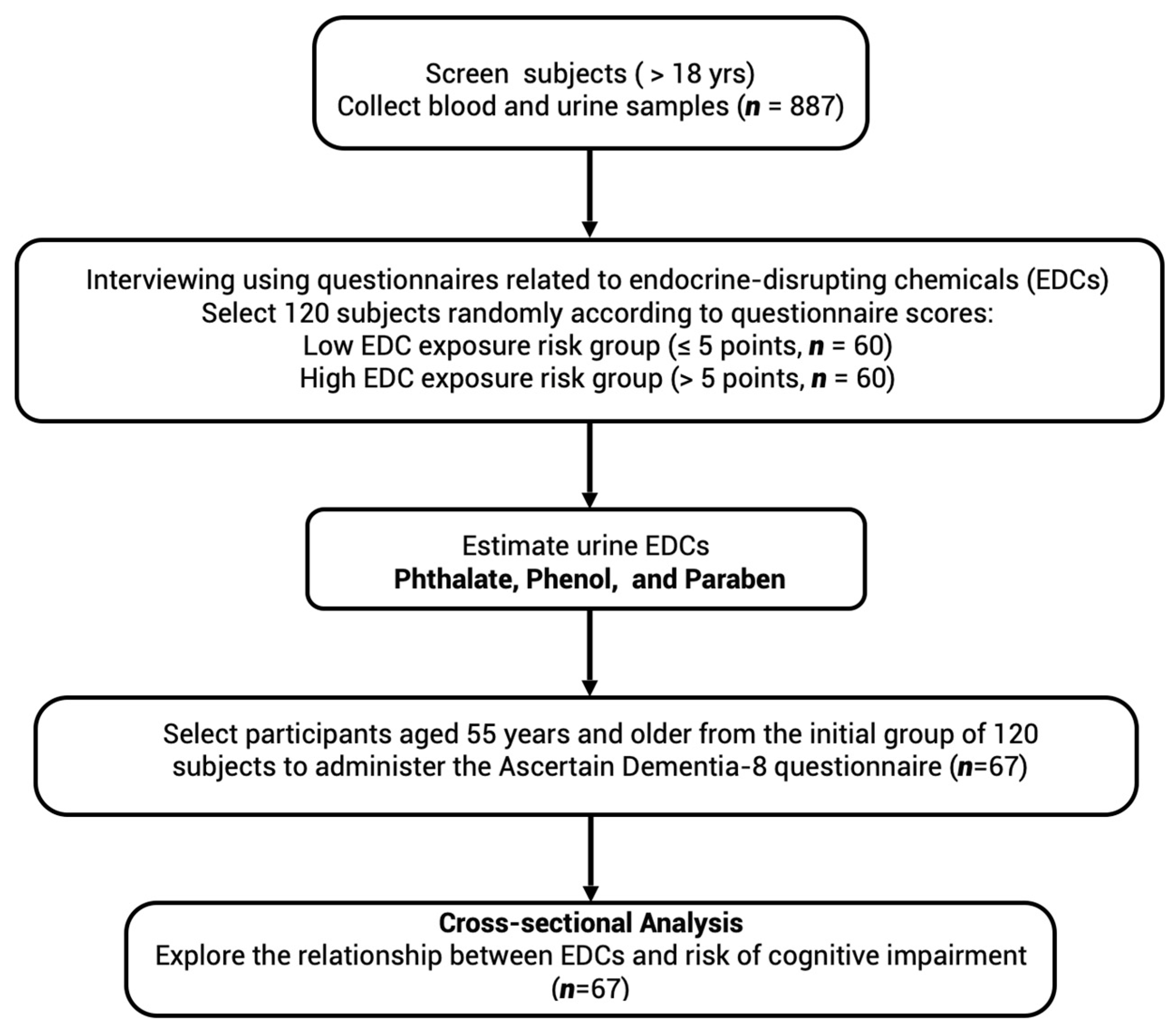
2. Materials and Methods
2.1. Study Design
2.2. Patient and Public Involvement
2.3. Questionnaires Concerning EDC Exposure and Potential Cognitive Impairment
2.4. Collection and Processing of Serum and Urine Samples
2.5. Analysis of EDCs
2.6. Validation of the Method for Measuring EDCs
2.7. Outcomes
2.8. Statistical Analysis
3. Results
3.1. Study Characteristics
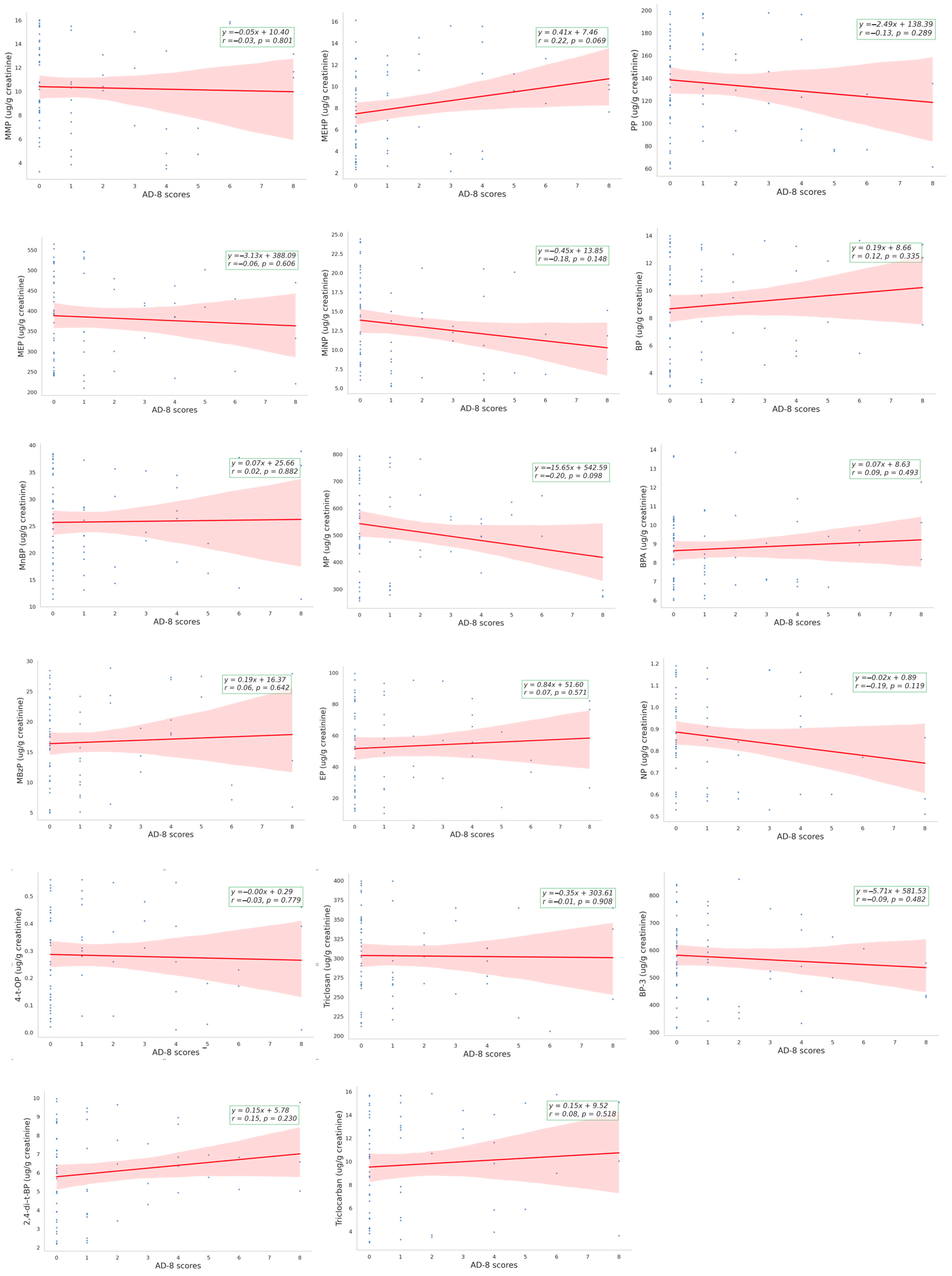
3.2. The Correlation between EDCs and AD-8 Assessment
3.3. EDCs Level, EDC Exposure and Risk of Cognitive Impairment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, G.X.; Lian, Q.Q.; Ge, R.S.; Hardy, D.O.; Li, X.K. Phthalate-induced testicular dysgenesis syndrome: Leydig cell influence. Trends Endocrinol. Metab. 2009, 20, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Terekeci, H.; Sandal, S.; Kelestimur, F. Endocrine disrupting chemicals: Exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord. 2020, 21, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, J.; Wang, Z.T.; Fan, Y.X. An estimation of the daily intake of di(2-ethlhexyl) phthalate (DEHP) among workers in flavoring factories. Biomed. Environ. Sci. 2014, 27, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Lim, S. The associations between personal care products use and urinary concentrations of phthalates, parabens, and triclosan in various age groups: The Korean National Environmental Health Survey Cycle 3 2015–2017. Sci. Total Environ. 2020, 742, 140640. [Google Scholar] [CrossRef] [PubMed]
- Duty, S.M.; Singh, N.P.; Silva, M.J.; Barr, D.B.; Brock, J.W.; Ryan, L.; Herrick, R.F.; Christiani, D.C.; Hauser, R. The relationship between environmental exposures to phthalates and DNA damage in human sperm using the neutral comet assay. Environ. Health Perspect. 2003, 111, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Yang, J.; Liu, Y.; Bi, Y.; Wang, H. Association between Phthalate Metabolites and Risk of Endometriosis: A Meta-Analysis. Int. J. Environ. Res. Public. Health 2019, 16, 3678. [Google Scholar] [CrossRef]
- Bornehag, C.G.; Sundell, J.; Weschler, C.J.; Sigsgaard, T.; Lundgren, B.; Hasselgren, M.; Hagerhed-Engman, L. The association between asthma and allergic symptoms in children and phthalates in house dust: A nested case-control study. Environ. Health Perspect. 2004, 112, 1393–1397. [Google Scholar] [CrossRef]
- Colon, I.; Caro, D.; Bourdony, C.J.; Rosario, O. Identification of phthalate esters in the serum of young Puerto Rican girls with premature breast development. Environ. Health Perspect. 2000, 108, 895–900. [Google Scholar] [CrossRef]
- Wei, Z.; Song, L.; Wei, J.; Chen, T.; Chen, J.; Lin, Y.; Xia, W.; Xu, B.; Li, X.; Chen, X.; et al. Maternal exposure to di-(2-ethylhexyl)phthalate alters kidney development through the renin-angiotensin system in offspring. Toxicol. Lett. 2012, 212, 212–221. [Google Scholar] [CrossRef]
- Tsai, H.J.; Chen, B.H.; Wu, C.F.; Wang, S.L.; Huang, P.C.; Tsai, Y.C.; Chen, M.L.; Ho, C.K.; Hsiung, C.A.; Wu, M.T. Intake of phthalate-tainted foods and microalbuminuria in children: The 2011 Taiwan food scandal. Environ. Int. 2016, 89–90, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Sun, C.Y.; Hsu, H.J.; Wu, I.W.; Chen, Y.C.; Lee, C.C. Xenoestrogen exposure and kidney function in the general population: Results of a community-based study by laboratory tests and questionnaire-based interviewing. Environ. Int. 2021, 155, 106585. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Lee, C.-C.; Hsu, H.-J.; Wu, I.W.; Chen, Y.-C.; Pan, H.-C.; Chen, Y.-T.; Hsu, C.-K.; Sun, C.-Y. Long-term Impacts of Endocrine-Disrupting Chemicals Exposure on Kidney Function: A Community-Based Cohort Study. Environ. Toxicol. Pharmacol. 2024, 106, 104379. [Google Scholar] [CrossRef]
- Schumacher, M.; Weill-Engerer, S.; Liere, P.; Robert, F.; Franklin, R.J.; Garcia-Segura, L.M.; Lambert, J.J.; Mayo, W.; Melcangi, R.C.; Parducz, A.; et al. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog. Neurobiol. 2003, 71, 3–29. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B. Can endocrine disruptors influence neuroplasticity in the aging brain? Neurotoxicology 2007, 28, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Janowsky, J.S. The role of androgens in cognition and brain aging in men. Neuroscience 2006, 138, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Weiss, B. Endocrine disruptors as a threat to neurological function. J. Neurol. Sci. 2011, 305, 11–21. [Google Scholar] [CrossRef]
- Salazar, P.; Villaseca, P.; Cisternas, P.; Inestrosa, N.C. Neurodevelopmental impact of the offspring by thyroid hormone system-disrupting environmental chemicals during pregnancy. Environ. Res. 2021, 200, 111345. [Google Scholar] [CrossRef]
- Knight, B.A.; Shields, B.M.; He, X.; Pearce, E.N.; Braverman, L.E.; Sturley, R.; Vaidya, B. Effect of perchlorate and thiocyanate exposure on thyroid function of pregnant women from South-West England: A cohort study. Thyroid. Res. 2018, 11, 9. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.M.; Kim, J.W.; Cheong, J.H.; Yun, H.J.; Hong, Y.C.; Kim, Y.; Han, D.H.; Yoo, H.J.; Shin, M.S.; et al. Association between phthalates and externalizing behaviors and cortical thickness in children with attention deficit hyperactivity disorder. Psychol. Med. 2015, 45, 1601–1612. [Google Scholar] [CrossRef]
- Chen, M.H.; Ha, E.H.; Liao, H.F.; Jeng, S.F.; Su, Y.N.; Wen, T.W.; Lien, G.W.; Chen, C.Y.; Hsieh, W.S.; Chen, P.C. Perfluorinated compound levels in cord blood and neurodevelopment at 2 years of age. Epidemiology 2013, 24, 800–808. [Google Scholar] [CrossRef] [PubMed]
- de Cock, M.; Maas, Y.G.; van de Bor, M. Does perinatal exposure to endocrine disruptors induce autism spectrum and attention deficit hyperactivity disorders? Review. Acta Paediatr. 2012, 101, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Ko, Y.C. Plasticizer incident and its health effects in Taiwan. Kaohsiung J. Med. Sci. 2012, 28, S17–S21. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.L.; Chen, J.S.; Tang, C.L.; Mao, I.F. The internal exposure of Taiwanese to phthalate--an evidence of intensive use of plastic materials. Environ. Int. 2008, 34, 79–85. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, T.P.; van Faassen, M.; Frederiksen, H.; van Beek, A.P.; Wolffenbuttel, B.H.R.; Kema, I.P.; van Vliet-Ostaptchouk, J.V. Development and Interlaboratory Validation of Two Fast UPLC-MS-MS Methods Determining Urinary Bisphenols, Parabens and Phthalates. J. Anal. Toxicol. 2019, 43, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.C. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef] [PubMed]
- Galvin, J.E.; Roe, C.M.; Powlishta, K.K.; Coats, M.A.; Muich, S.J.; Grant, E.; Miller, J.P.; Storandt, M.; Morris, J.C. The AD8: A brief informant interview to detect dementia. Neurology 2005, 65, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, T.; Cai, Y.; Wu, Y.; Nie, Y.; Kuang, W.; Qiu, P.; Wan, Y. The Chinese version of informant AD8 for mild cognitive impairment and dementia screening in community-dwelling older adults. Public. Health Nurs. 2023, 40, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Usarel, C.; Dokuzlar, O.; Aydin, A.E.; Soysal, P.; Isik, A.T. The AD8 (Dementia Screening Interview) is a valid and reliable screening scale not only for dementia but also for mild cognitive impairment in the Turkish geriatric outpatients. Int. Psychogeriatr. 2019, 31, 223–229. [Google Scholar] [CrossRef]
- Tanwani, R.; Danquah, M.O.; Butris, N.; Saripella, A.; Yan, E.; Kapoor, P.; Englesakis, M.; Tang-Wai, D.F.; Tartaglia, M.C.; He, D.; et al. Diagnostic accuracy of Ascertain Dementia 8-item Questionnaire by participant and informant-A systematic review and meta-analysis. PLoS ONE 2023, 18, e0291291. [Google Scholar] [CrossRef]
- Safe, S. Endocrine disruptors and falling sperm counts: Lessons learned or not! Asian J. Androl. 2013, 15, 191–194. [Google Scholar] [CrossRef]
- Newbold, R.R.; Padilla-Banks, E.; Jefferson, W.N. Environmental estrogens and obesity. Mol. Cell. Endocrinol. 2009, 304, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Williams, G. Aromatase up-regulation, insulin and raised intracellular oestrogens in men, induce adiposity, metabolic syndrome and prostate disease, via aberrant ER-alpha and GPER signalling. Mol. Cell. Endocrinol. 2012, 351, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Preau, L.; Fini, J.B.; Morvan-Dubois, G.; Demeneix, B. Thyroid hormone signaling during early neurogenesis and its significance as a vulnerable window for endocrine disruption. Biochim. Biophys. Acta 2015, 1849, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.W. Adrenocortical endocrine disruption. J. Steroid Biochem. Mol. Biol. 2016, 155, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, S.A.; Hoogendijk, W.J.; van Pelt, J.; Derijk, R.H.; Verhagen, J.C.; van Dyck, R.; Smit, J.H.; Zitman, F.G.; Penninx, B.W. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: Results from a large cohort study. Arch. Gen. Psychiatry 2009, 66, 617–626. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Hof, P.R.; Morrison, J.H. The aging brain: Morphomolecular senescence of cortical circuits. Trends Neurosci. 2004, 27, 607–613. [Google Scholar] [CrossRef]
- Boss, L.; Kang, D.H.; Bergstrom, N.; Leasure, J.L. Endogenous sex hormones and cognitive function in the elderly. Aging Clin. Exp. Res. 2015, 27, 515–521. [Google Scholar] [CrossRef]
- Holland, J.; Bandelow, S.; Hogervorst, E. Testosterone levels and cognition in elderly men: A review. Maturitas 2011, 69, 322–337. [Google Scholar] [CrossRef]
- Hogervorst, E.; Bandelow, S.; Combrinck, M.; Smith, A.D. Low free testosterone is an independent risk factor for Alzheimer’s disease. Exp. Gerontol. 2004, 39, 1633–1639. [Google Scholar] [CrossRef]
- Hara, Y.; Yuk, F.; Puri, R.; Janssen, W.G.; Rapp, P.R.; Morrison, J.H. Presynaptic mitochondrial morphology in monkey prefrontal cortex correlates with working memory and is improved with estrogen treatment. Proc. Natl. Acad. Sci. USA 2014, 111, 486–491. [Google Scholar] [CrossRef]
- Kohama, S.G.; Renner, L.; Landauer, N.; Weiss, A.R.; Urbanski, H.F.; Park, B.; Voytko, M.L.; Neuringer, M. Effect of Ovarian Hormone Therapy on Cognition in the Aged Female Rhesus Macaque. J. Neurosci. 2016, 36, 10416–10424. [Google Scholar] [CrossRef]
- Liu, J.; Lin, H.; Huang, Y.; Liu, Y.; Wang, B.; Su, F. Cognitive effects of long-term dydrogesterone treatment used alone or with estrogen on rat menopausal models of different ages. Neuroscience 2015, 290, 103–114. [Google Scholar] [CrossRef]
- Guarnotta, V.; Amodei, R.; Frasca, F.; Aversa, A.; Giordano, C. Impact of Chemical Endocrine Disruptors and Hormone Modulators on the Endocrine System. Int. J. Mol. Sci. 2022, 23, 5710. [Google Scholar] [CrossRef]
- Ulleras, E.; Ohlsson, A.; Oskarsson, A. Secretion of cortisol and aldosterone as a vulnerable target for adrenal endocrine disruption-screening of 30 selected chemicals in the human H295R cell model. J. Appl. Toxicol. 2008, 28, 1045–1053. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Upson, K.; Cook, N.R.; Weinberg, C.R. Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. Environ. Health Perspect. 2016, 124, 220–227. [Google Scholar] [CrossRef]

| AD-8 Score ≥ 2 (n = 19) | AD-8 Score < 2 (n = 48) | p Value | |
|---|---|---|---|
| Age (years) | 63.89 ± 5.772 | 64.83 ± 5.994 | 0.866 |
| Male, n, (%) | 5 (26.3) | 17 (35.4) | 0.475 |
| Diabetes, n, (%) | 3 (15.8) | 12 (25) | 0.415 |
| Dyslipidemia, n, (%) | 16 (84.2) | 42 (87.5) | 0.722 |
| CKD, n, (%) | 5 (26.3) | 11 (22.9) | 0.769 |
| Hypertension, n, (%) | 13 (68.4) | 38 (79.2) | 0.352 |
| White blood cell (1000/μL) | 5.38 ± 0.92 | 6.00 ± 1.77 | 0.029 * |
| Hemoglobin(g/dL) | 13.86 ± 1.07 | 14.45 ± 1.18 | 0.595 |
| Ferritin (ng/mL) | 254.47 ± 155.54 | 246.93 ± 203.33 | 0.682 |
| Folate (ng/mL) | 11.59 ± 5.59 | 13.62 ± 8.47 | 0.116 |
| AST (U/L) | 24.05 ± 6.74 | 23.04 ± 5.83 | 0.964 |
| ALT (U/L) | 30.26 ± 18.32 | 28.13 ± 12.64 | 0.531 |
| Total bilirubin (mg/dL) | 0.62 ± 0.21 | 0.68 ± 0.31 | 0.407 |
| Insulin (uU/mL) | 12.39 ± 8.44 | 12.39 ± 7.5 | 0.507 |
| Cholesterol (mg/dL) | 197.11 ± 29.814 | 199.33 ± 35.19 | 0.615 |
| HS-CRP (mg/L) | 1.38 ± 1.18 | 1.94 ± 2.98 | 0.518 |
| Bun (mg/dL) | 14.78 ± 4.79 | 15.56 ± 5.95 | 0.603 |
| Creatinine (mg/dL) | 0.77 ± 0.20 | 0.81 ± 0.26 | 0.628 |
| eGFR (mL/min/1.73 m2) | 86.02 ± 16.19 | 84.81 ± 17.83 | 0.794 |
| Na (mEq/L) | 142.63 ± 1.50 | 141.88 ± 1.53 | 0.954 |
| K (mEq/L) | 4.48 ± 0.35 | 4.49 ± 0.33 | 0.908 |
| Ca (mg/dL) | 9.55 ± 0.31 | 9.51 ± 0.26 | 0.267 |
| P (mg/dL) | 3.71 ± 0.54 | 3.60 ± 0.54 | 0.683 |
| Albumin (g/dL) | 4.69 ± 0.29 | 4.69 ± 0.24 | 0.533 |
| Vitamin D (ng/mL) | 28.06 ± 7.76 | 31.08 ± 9.53 | 0.846 |
| Vitamin B12 (pg/mL) | 684.86 ± 408.42 | 753.83 ± 536.09 | 0.532 |
| Folate (ng/mL) | 11.58 ± 5.59 | 13.62 ± 8.47 | 0.116 |
| Body-mass index | 25.76 ± 5.12 | 26.21 ± 3.87 | 0.406 |
| UACR (mg/g) | 6.80 (3.55–18.90) | 7.95 (4.73–23.78) | 0.526 |
| UPCR (mg/g) | 63.27 (50.42–78.17) | 72.55 (59.03–93.19) | 0.117 |
| HOMA-IR | 3.73 ± 4.30 | 3.52 ± 2.91 | 0.343 |
| Leptin (ng/mL) | 9.25 ± 6.18 | 11.49 ± 6.90 | 0.740 |
| AD-8 Score ≥ 2 (n = 19) | AD-8 Score < 2 (n = 48) | p Value | |
|---|---|---|---|
| Urinary XEs (ug/g creatinine) | |||
| BPA | 8.759 (7.873–9.745) | 8.473 (7.995–8.980) | 0.646 |
| NP | 0.787 (0.686–0.902) | 0.857 (0.802–0.915) | 0.284 |
| 4-t-OP | 0.179 (0.097–0.327) | 0.223 (0.176–0.282) | 0.856 |
| 2,4-di–t-BP | 6.411 (5.607–7.331) | 5.214 (4.555–5.968) | 0.160 |
| Triclosan | 299.7 (275.5–326.0) | 297.8 (282.3–314.2) | 0.878 |
| Triclocarban | 8.952 (6.866–11.67) | 8.601 (7.443–9.939) | 0.559 |
| MP | 480.5 (416.2–554.7) | 496.3 (445.7–552.6) | 0.487 |
| EP | 51.46 (40.61–65.21) | 42.98 (35.65–51.83) | 0.396 |
| PP | 119.6 (100.5–142.3) | 131.4 (119.4–144.6) | 0.381 |
| BP | 8.837 (7.366–10.60) | 7.871 (6.828–9.074) | 0.436 |
| MMP | 9.09 (7.191–11.49) | 9.746 (8.687–10.93) | 0.760 |
| MEP | 365.0 (321.9–413.9) | 369.7 (338.2–404.2) | 0.666 |
| MnBP | 24.33 (20.18–29.33) | 24.37 (22.12–26.86) | 0.900 |
| MBzP | 16.64 (12.93–21.43) | 14.27 (12.38–16.46) | 0.137 |
| MEHP | 8.511 (6.436–11.26) | 6.432 (5.485–7.544) | 0.038 * |
| MiNP | 11.47 (9.437–13.95) | 12.32 (10.80–14.05) | 0.461 |
| BP-3 | 528.1 (460.4–605.7) | 566.3 (526.4–609.3) | 0.323 |
| Simple Linear Regression | Multiple Regression Analysis | Multiple Regression Analysis | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| β ± SE | p | β ± SE | p | β ± SE | p | |
| Age | 0.014 ± 0.046 | 0.769 | — | — | 0.006 ± 0.055 | 0.907 |
| Gender | −0.555 ± 0.568 | 0.332 | — | — | −0.231 ± 0.689 | 0.739 |
| Diabetes | −0.710 ± 0.638 | 0.270 | −0.692 ± 0.645 | 0.287 | −1.244 ± 0.740 | 0.099 |
| eGFR | 0.001 ± 0.016 | 0.948 | 0.003 ± 0.016 | 0.876 | 0.011 ± 0.019 | 0.562 |
| BPA | 0.101 ± 0.146 | 0.491 | 0.088 ± 0.147 | 0.552 | −0.029 ± 0.178 | 0.870 |
| NP | −2.030 ± 1.311 | 0.126 | −1.796 ± 1.386 | 0.200 | −2.638 ± 1.612 | 0.109 |
| 4-t-OP | −0.427 ± 1.626 | 0.794 | −0.336 ± 1.678 | 0.842 | 0.875 ± 2.135 | 0.684 |
| 2,4-di–t-BP | 0.144 ± 0.119 | 0.230 | 0.151 ± 0.121 | 0.219 | 0.158 ± 0.143 | 0.273 |
| Triclosan | −0.001 ± 0.005 | 0.908 | −0.001 ± 0.005 | 0.897 | −0.001 ± 0.006 | 0.856 |
| Triclocarban | 0.042 ± 0.065 | 0.518 | 0.031 ± 0.067 | 0.641 | 0.055 ± 0.075 | 0.463 |
| MP | −0.003 ± 0.002 | 0.098 | −0.002 ± 0.002 | 0.133 | −0.005 ± 0.002 | 0.020 * |
| EP | 0.006 ± 0.010 | 0.571 | 0.007 ± 0.011 | 0.502 | 0.015 ± 0.013 | 0.250 |
| PP | −0.007 ± 0.006 | 0.289 | −0.007 ± 0.007 | 0.274 | −0.009 ± 0.008 | 0.228 |
| BP | 0.074 ± 0.076 | 0.335 | 0.083 ± 0.077 | 0.287 | 0.067 ± 0.092 | 0.471 |
| MMP | −0.018 ± 0.073 | 0.801 | −0.005 ± 0.079 | 0.945 | −0.025 ± 0.094 | 0.794 |
| MEP | −0.001 ± 0.003 | 0.606 | −0.002 ± 0.003 | 0.539 | −0.001 ± 0.003 | 0.703 |
| MnBP | 0.005 ± 0.033 | 0.882 | 0.007 ± 0.033 | 0.837 | 0.000 ± 0.038 | 0.993 |
| MBzP | 0.018 ± 0.038 | 0.642 | 0.022 ± 0.039 | 0.581 | 0.018 ± 0.043 | 0.677 |
| MEHP | 0.123 ± 0.067 | 0.069 | 0.133 ± 0.067 | 0.053 | 0.160 ± 0.076 | 0.042 * |
| MiNP | −0.071 ± 0.049 | 0.148 | −0.061 ± 0.054 | 0.262 | −0.026 ± 0.063 | 0.681 |
| BP-3 | −0.001 ± 0.002 | 0.482 | −0.001 ± 0.002 | 0.561 | 0.002 ± 0.002 | 0.446 |
| Univariate (n = 67) | Multivariable, Model 1 | Multivariable, Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Crude OR | 95% CI | p Value | Adjusted OR | 95% CI | p Value | Adjusted OR | 95% CI | p Value | |
| MEHP | 1.168 | 1.011–1.349 | 0.035 * | 1.175 | 1.014–1.362 | 0.032 * | 1.217 | 1.011–1.464 | 0.038 * |
| MBzP | 1.059 | 0.979–1.146 | 0.150 | 1.071 | 0.985–1.163 | 0.107 | 1.083 | 0.978–1.200 | 0.127 |
| NP | 0.222 | 0.015–3.290 | 0.274 | 0.220 | 0.013–3.596 | 0.288 | 0.025 | 0.001–1.214 | 0.063 |
| BPA | 1.100 | 0.828–1.462 | 0.512 | 1.095 | 0.821–1.460 | 0.538 | 0.949 | 0.665–1.353 | 0.771 |
| MP | 0.999 | 0.996–1.002 | 0.524 | 0.999 | 0.996–1.002 | 0.615 | 0.998 | 0.994–1.003 | 0.522 |
| EP | 1.009 | 0.988–1.030 | 0.422 | 1.009 | 0.988–1.031 | 0.386 | 1.025 | 0.994–1.057 | 0.111 |
| BP-3 | 0.998 | 0.994–1.002 | 0.370 | 0.998 | 0.994–1.002 | 0.355 | 0.999 | 0.994–1.004 | 0.804 |
| Age | 0.972 | 0.884–1.068 | 0.556 | — | — | — | 0.907 | 0.786–1.046 | 0.179 |
| Gender | 0.651 | 0.200–2.120 | 0.476 | — | — | — | 1.087 | 0.195–6.056 | 0.924 |
| eGFR | 1.001 | 0.973–1.036 | 0.795 | 1.003 | 0.970–1.036 | 0.870 | 1.018 | 0.973–1.065 | 0.440 |
| Diabetes | 0.563 | 0.139–2.271 | 0.419 | 0.549 | 0.135–2.232 | 0.402 | 0.230 | 0.027–1.966 | 0.180 |
| Vitamin D | 0.959 | 0.898–1.026 | 0.224 | 0.962 | 0.899–1.029 | 0.261 | 0.993 | 0.908–1.086 | 0.880 |
| Hemoglobin | 0.626 | 0.378–1.037 | 0.069 | 0.526 | 0.289–0.957 | 0.036 * | 0.521 | 0.237–1.145 | 0.104 |
| AD-8 Scores ≥ 2 (n = 19) | AD-8 Score < 2 (n = 48) | p Value | |
|---|---|---|---|
| EDC exp score > 5 (n = 18), n (%) | 9 (47.4) | 9 (18.8) | 0.017 * |
| EDC exposure scores | 5.21 ± 5.493 | 2.33 ± 3.772 | 0.060 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, F.-C.; Wei, Y.-C.; Sun, C.-Y.; Hsu, H.-J.; Lee, C.-C.; Chen, Y.-T.; Pan, H.-C.; Hsu, C.-K.; Liu, Y.-A.; Chen, C.-Y. Endocrine-Disrupting Chemicals Exposure and Neurocognitive Function in the General Population: A Community-Based Study. Toxics 2024, 12, 514. https://doi.org/10.3390/toxics12070514
Su F-C, Wei Y-C, Sun C-Y, Hsu H-J, Lee C-C, Chen Y-T, Pan H-C, Hsu C-K, Liu Y-A, Chen C-Y. Endocrine-Disrupting Chemicals Exposure and Neurocognitive Function in the General Population: A Community-Based Study. Toxics. 2024; 12(7):514. https://doi.org/10.3390/toxics12070514
Chicago/Turabian StyleSu, Feng-Chieh, Yi-Chia Wei, Chiao-Yin Sun, Heng-Jung Hsu, Chin-Chan Lee, Yih-Ting Chen, Heng-Chih Pan, Cheng-Kai Hsu, Yun-An Liu, and Chun-Yu Chen. 2024. "Endocrine-Disrupting Chemicals Exposure and Neurocognitive Function in the General Population: A Community-Based Study" Toxics 12, no. 7: 514. https://doi.org/10.3390/toxics12070514







