Review on Synthesis of Silica-Based Hybrid Sorbents and Their Application in Radionuclide Separation and Removal via Chromatographic Technique
Abstract
1. Introduction
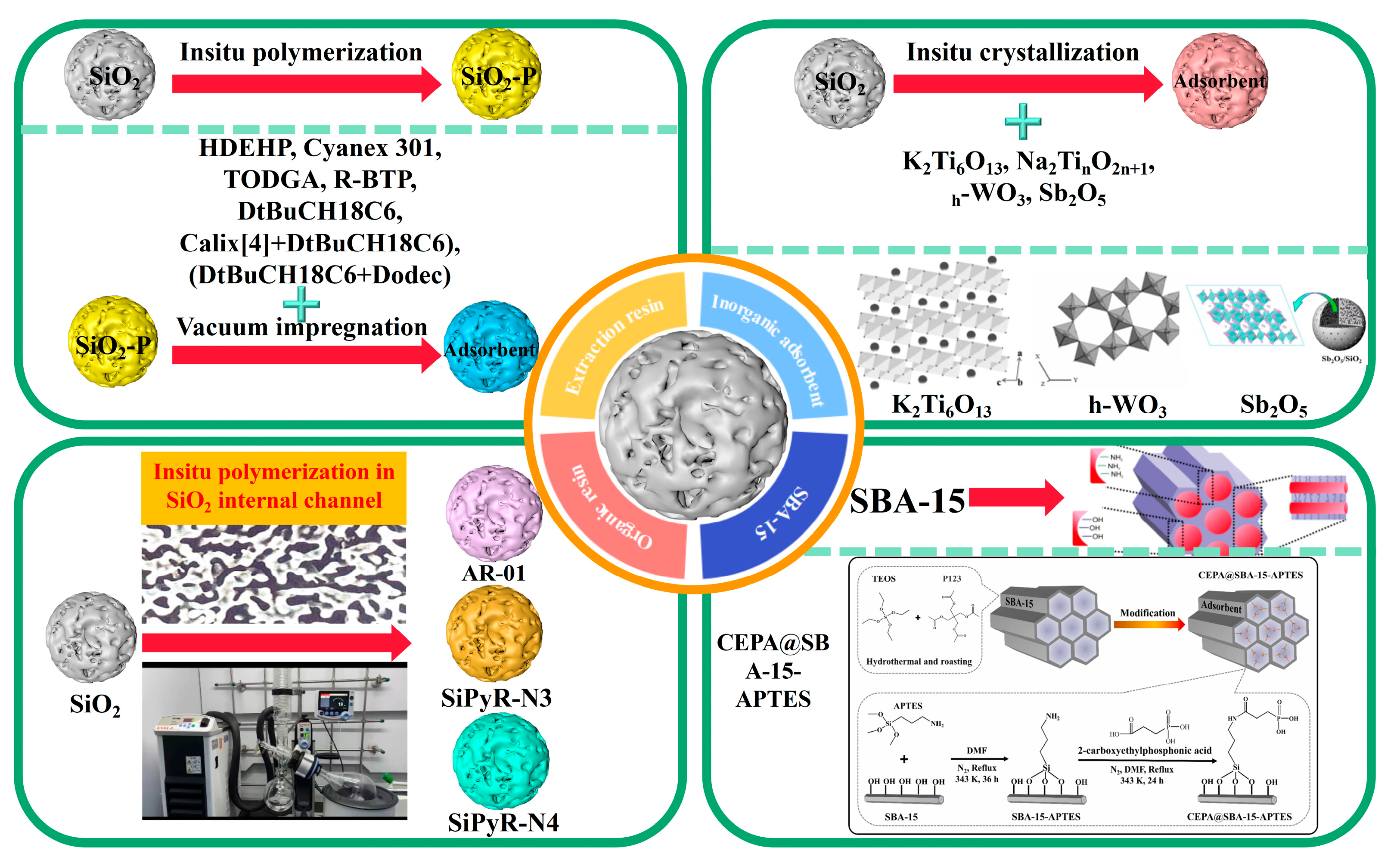
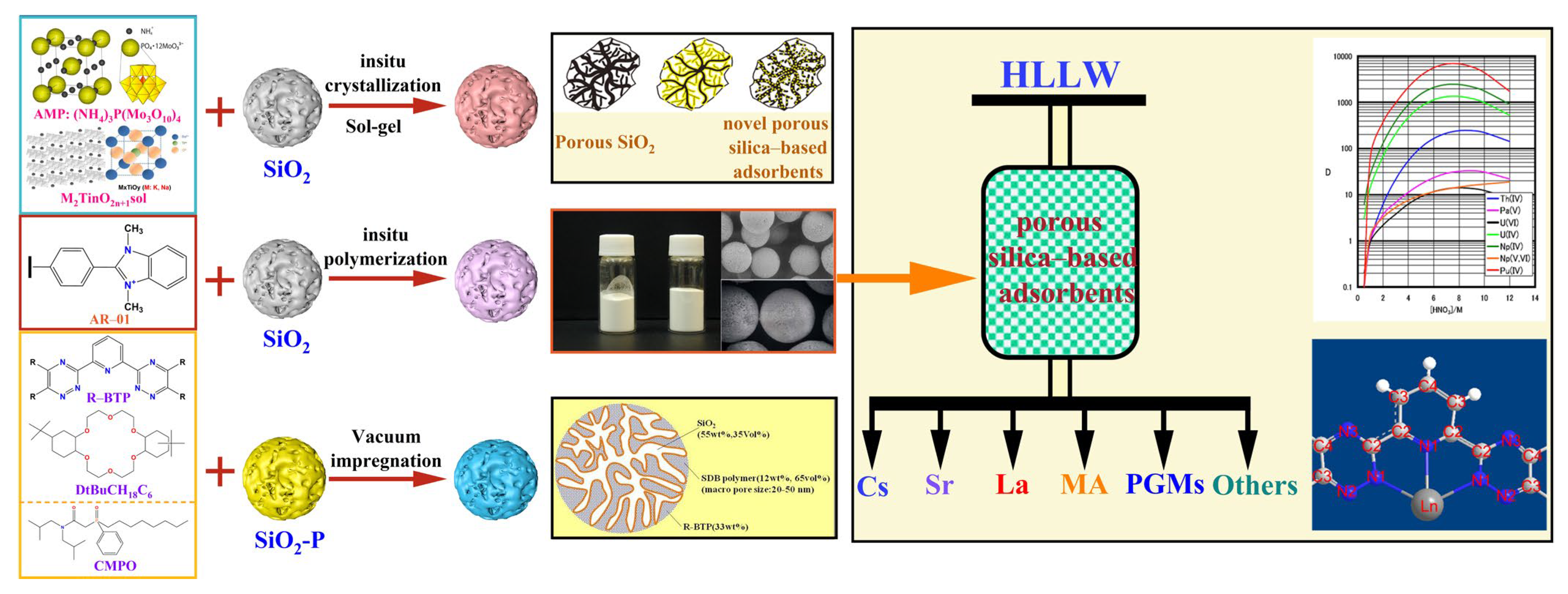
2. Preparation of SiO2- or SiO2-P-Based Adsorbents
3. Separation and Removal of Key Radioactive Nuclides
3.1. Actinide Separation
3.2. Lanthanide Separation
3.3. Sr and Cs Separation
3.3.1. Sr Separation
3.3.2. Cs Separation
3.4. Separation of Platinum Group Metal Fission Products
3.5. I Separation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Y.-M.; Chen, K.-Y.; Kang, J.-N. Policy and Management of Carbon Peaking and Carbon Neutrality: A Literature Review. Engineering 2022, 14, 52–63. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Z.; Chai, J. Progress and prospects of international carbon peaking and carbon neutral research-based on bibliometric analysis (1991–2022). Front. Energy Res. 2023, 11, 1121639. [Google Scholar] [CrossRef]
- Castro, L.G.; Mahieu, N. Giving actinide chemistry a new start. Nat. Rev. Chem. 2025, 9, 86–87. [Google Scholar] [CrossRef] [PubMed]
- Dognon, J.-P. Theoretical insights into the chemical bonding in actinide complexes. Coord. Chem. Rev. 2014, 266–267, 110–122. [Google Scholar] [CrossRef]
- Li, Z.-J.; Liu, J.; Zhang, G.; Benmore, C.; Zhang, L.; Guo, X.; Lin, J. Optimizing iodine adsorption in functionalized metal-organic frameworks via an unprecedented positional isomerism strategy. Chem. Eng. J. 2024, 499, 156586. [Google Scholar] [CrossRef]
- Mostatabi, T.; Hassani, A.H.; Janitabar Darzi, S.; Nilchib, A. Kinetics, isotherms and thermodynamics: Iodine ion adsorption on Ag2O–titanate nanostructures. Int. J. Environ. Sci. Technol. 2025, 22, 1133–1148. [Google Scholar] [CrossRef]
- Guo, X. Perspective Chapter: Safe Disposal and Storage of Nuclear Waste. In Nuclear Fission Energy—Carbon Net Zero, Sustainability and Energy Availability; Tsvetkov, P., Sabharwall, P., Eds.; IntechOpen: Rijeka, Croatia, 2025. [Google Scholar] [CrossRef]
- Harithra, V.; Lakshmi Priya, S.; Vivek, P.; Ivo Romauld, S.; Meenambiga, S.S.; Rajakumari, K. Long-Term Storage and Safety of Radioactive Waste Storage Facilities. In Radioactive Pollutant: Sources, Issues and Remediation; Kumar, N., Ed.; Springer Nature: Cham, Switzerland, 2025; pp. 219–238. [Google Scholar] [CrossRef]
- Tang, H.; Peng, Z.; Tian, R.; Ye, L.; Zhang, J.; Rao, M.; Li, G. Platinum-group metals: Demand, supply, applications and their recycling from spent automotive catalysts. J. Environ. Chem. Eng. 2023, 11, 110237. [Google Scholar] [CrossRef]
- Zhang, S.; He, X.; Ding, Y.; Shi, Z.; Wu, B. Supply and demand of platinum group metals and strategies for sustainable management. Renew. Sustain. Energy Rev. 2024, 204, 114821. [Google Scholar] [CrossRef]
- Chen, B.; Liu, J.; Wei, H.; Yang, Y.; Li, X.; Peng, S.; Yang, Y. Complexation between uranyl(VI) and CMPO in a hydroxyl-functionalized ionic liquid: An extraction, spectrophotography, and calorimetry study. Chin. Chem. Lett. 2022, 33, 3451–3455. [Google Scholar] [CrossRef]
- Milani, S.A.; Zahakifar, F. Stoichiometry and thermodynamics of cerium(IV) solvent extraction from sulfuric acid solutions by CYANEX 301. Braz. J. Chem. Eng. 2022, 39, 553–560. [Google Scholar] [CrossRef]
- Wei, M.; Liu, X.; Chen, J. Agents and processes design for transuranium elements back extraction in TRPO process. J. Radioanal. Nucl. Chem. 2012, 291, 717–723. [Google Scholar] [CrossRef]
- Chu, Z.-Y.; Xu, J.-J.; Li, C.-F.; Yang, Y.-H.; Guo, J.-H. A Chromatographic Method for Separation of Tungsten (W) from Silicate Samples for High-Precision Isotope Analysis Using Negative Thermal Ionization Mass Spectrometry. Anal. Chem. 2020, 92, 11987–11993. [Google Scholar] [CrossRef]
- Badawy, M.E.I.; El-Nouby, M.A.M.; Kimani, P.K.; Lim, L.W.; Rabea, E.I. A review of the modern principles and applications of solid-phase extraction techniques in chromatographic analysis. Anal. Sci. 2022, 38, 1457–1487. [Google Scholar] [CrossRef]
- Debnath, S.; Manosi, D.; Susmita, M.; Sarkar, B.K.; Babu, G. Advances in chromatography: Contemporary techniques and applications. Essent. Chem. 2025, 2, 1–27. [Google Scholar] [CrossRef]
- Kifle, D. Efficient extraction chromatography method for the separation of heavy rare earth elements from various sources. J. Chromatogr. A 2025, 1745, 465751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef]
- Sanku, M.; Forsberg, K.; Svärd, M. Extraction Chromatography for Separation of Rare Earth Elements. In Proceedings of Rare Metal Technology 2021; Springer: Cham, Switzerland, 2021; pp. 155–161. [Google Scholar]
- Zhang, A.; Xiao, C.; Kuraoka, E.; Kumagai, M. Preparation of a Novel Silica-Based DtBuCH18C6 Impregnated Polymeric Composite Modified by Tri-n-butyl Phosphate and Its Application in Chromatographic Partitioning of Strontium from High Level Liquid Waste. Ind. Eng. Chem. Res. 2007, 46, 2164–2171. [Google Scholar] [CrossRef]
- Yuksel, E.; Eksik, O.; Acma, H.; Yaman, S. Mechanical properties of a carbon fiber reinforced epoxy resin composite improved by integrating multi-walled carbon nanotubes and graphene nanoplatelets. J. Compos. Mater. 2024, 58, 911–921. [Google Scholar] [CrossRef]
- Parsons-Moss, T.; Jones, S.; Wang, J.; Wu, Z.; Uribe, E.; Zhao, D.; Nitsche, H. Reduction of plutonium in acidic solutions by mesoporous carbons. J. Radioanal. Nucl. Chem. 2016, 307, 2593–2601. [Google Scholar] [CrossRef]
- Kordala, N.; Wyszkowski, M. Zeolite Properties, Methods of Synthesis, and Selected Applications. Molecules 2024, 29, 1069. [Google Scholar] [CrossRef]
- Minh, D.Q.; Yen, P.N.N.; Nhi, N.V.U.; Hieu, L.N.G.; Bui, T.K. Synthesis and characterization of lightweight geopolymer materials from rich-silica diatomaceous earth curing under hydrothermal conditions. J. Porous Mater. 2025. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, D.; Su, Y.-H.; Hsiao, Y.-L.; You, B.; Wu, L. Synthesis and film performances of SiO2/P(MMA-BA) core–shell structural latex. Prog. Org. Coat. 2014, 77, 1015–1022. [Google Scholar] [CrossRef]
- Hoshi, H.; Wei, Y.Z.; Kumagai, M.; Asakura, T.; Morita, Y. Group separation of trivalent minor actinides and lanthanides by TODGA extraction chromatography for radioactive waste management. J. Alloys Compd. 2004, 374, 451–455. [Google Scholar] [CrossRef]
- Wei, Y.; Mikio, K.; Yoichi, T.; Giuseppe, M.; Odoj, R. Studies on the Separation of Minor Actinides from High-Level Wastes by Extraction Chromatography Using Novel Silica-Based Extraction Resins. Nucl. Technol. 2000, 132, 413–423. [Google Scholar] [CrossRef]
- Wu, Y.; Kim, S.Y.; Tozawa, D.; Ito, T.; Tada, T.; Hitomi, K.; Kuraoka, E.; Yamazaki, H.; Ishii, K. Study on selective separation of cesium from high level liquid waste using a macroporous silica-based supramolecular recognition absorbent. J. Radioanal. Nucl. Chem. 2012, 293, 13–20. [Google Scholar] [CrossRef]
- Xu, S.; Ning, S.; Wang, Y.; Wang, X.; Dong, H.; Chen, L.; Yin, X.; Fujita, T.; Wei, Y. Precise separation and efficient enrichment of palladium from wastewater by amino-functionalized silica adsorbent. J. Clean. Prod. 2023, 396, 136479. [Google Scholar] [CrossRef]
- Zhang, A.; Chen, C.; Kuraoka, E.; Kumagai, M. Impregnation synthesis of a novel macroporous silica-based crown ether polymeric material modified by 1-dodecanol and its adsorption for strontium and some coexistent metals. Sep. Purif. Technol. 2008, 62, 407–414. [Google Scholar] [CrossRef]
- Zhang, A.; Hu, Q. Adsorption of cesium and some typical coexistent elements onto a modified macroporous silica-based supramolecular recognition material. Chem. Eng. J. 2010, 159, 58–66. [Google Scholar] [CrossRef]
- Zhang, A.; Kuraoka, E.; Kumagai, M. Development of the chromatographic partitioning of cesium and strontium utilizing two macroporous silica-based calix[4]arene-crown and amide impregnated polymeric composites: PREC partitioning process. J. Chromatogr. A 2007, 1157, 85–95. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Wu, H.; Wei, Y. Adsorption behaviors of strontium using macroporous silica based hexagonal tungsten oxide. Sci. China Chem. 2016, 59, 601–608. [Google Scholar] [CrossRef]
- Ito, T.; Xu, Y.; Kim, S.-Y.; Nagaishi, R.; Kimura, T. Adsorption behavior and radiation effects of a silica-based (Calix[4]+Dodecanol)/SiO2-P adsorbent for selective separation of Cs(I) from high level liquid waste. Sep. Sci. Technol. 2016, 51, 22–31. [Google Scholar] [CrossRef]
- Saleena, P.; Jayashree, E.; Anees, K. A Comprehensive Review on Vacuum Impregnation: Mechanism, Applications and Prospects. Food Bioprocess Technol. 2024, 17, 1434–1447. [Google Scholar] [CrossRef]
- Higuchi, R.; Lilak, S.; Sillin, H.O.; Tsuruoka, T.; Kunitake, M.; Nakayama, T.; Gimzewski, J.K.; Stieg, A.Z. Metal doped polyaniline as neuromorphic circuit elements for in-materia computing. Sci. Technol. Adv. Mater. 2023, 24, 2178815. [Google Scholar] [CrossRef]
- Navas, D.; Fuentes, S.; Castro-Alvarez, A.; Chavez-Angel, E. Review on Sol-Gel Synthesis of Perovskite and Oxide Nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, T. Advances in Hydrothermal Carbonization for Biomass Wastewater Valorization: Optimizing Nitrogen and Phosphorus Nutrient Management to Enhance Agricultural and Ecological Outcomes. Water 2025, 17, 800. [Google Scholar] [CrossRef]
- Robshaw, T.J.; Kearney, S.; Turner, J.; Simoni, M.; Baidak, A.; Sharrad, C.A.; Walkley, B.; Ogden, M.D. Radioiodine abatement—Development of radioiodine targeting strategies in the light of zero emission. Prog. Nucl. Energy 2023, 165, 104918. [Google Scholar] [CrossRef]
- Wei, Y.; Hosid, H.; Kumagai, M.; Asakura, T.; Uchiyama, G. Preparation of Novel Silica-Based R-BTP Extraction-Resins and Their Application to Trivalent Actinides and Lanthanides Separation. J. Nucl. Sci. Technol. 2002, 39, 761–764. [Google Scholar] [CrossRef]
- Xu, S.; Ning, S.; Wang, X.; Gao, F.; Chen, L.; Yin, X.; Fujita, T.; Wei, Y. Silica-based covalent organic framework composite for efficient separation and enrichment of palladium and its heterogeneous catalysis application. Sep. Purif. Technol. 2023, 327, 124977. [Google Scholar] [CrossRef]
- Zhang, A.; Wei, Y.; Arai, T.; Kumagai, M. Palladium Removal from the Simulated Nuclear Spent Fuel Solution Using a Silica-Based SiPyR-N3 Anion Exchanger. Solvent Extr. Ion Exch. 2006, 24, 447–462. [Google Scholar] [CrossRef]
- Baumgärtner, F.; Ertel, D. The modern purex process and its analytical requirements. J. Radioanal. Chem. 1980, 58, 11–28. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, R.; Chen, X.; Ren, C.; Lv, Y.; Mo, D.; Liu, M.; Yan, S. The development status of PUREX process for nuclear fuel reprocessing from an insight from PATENTS. J. Radioanal. Nucl. Chem. 2019, 322, 1657–1662. [Google Scholar] [CrossRef]
- Kvashnina, K.; Claret, F.; Clavier, N.; Levitskaia, T.G.; Wainwright, H.; Yao, T. Long-term, sustainable solutions to radioactive waste management. Sci. Rep. 2024, 14, 5907. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Shen, Z.; Li, B.; Tan, X.; Han, B.; Ji, Z.; Wang, J.; Zhao, G.; Wang, X. State-of-the-art progress for the selective crystallization of actinides, synthesis of actinide compounds and their functionalization. J. Hazard. Mater. 2022, 426, 127838. [Google Scholar] [CrossRef]
- Peterson, A.; Wacker, J.N. Six degrees of actinide separation. Nat. Rev. Chem. 2024, 8, 408–409. [Google Scholar] [CrossRef]
- Sreenivasulu, B.; Brahmananda Rao, C.V.S.; Suresh, A.; Sivaraman, N. Recovery of actinides from acidic waste solutions generated in research facilities using adsorption and extraction techniques. J. Radioanal. Nucl. Chem. 2022, 331, 3623–3632. [Google Scholar] [CrossRef]
- Zhang, A.; Hu, Q.; Wang, W.; Kuraoka, E. Application of a Macroporous Silica-Based CMPO-Impregnated Polymeric Composite in Group Partitioning of Long-Lived Minor Actinides from Highly Active Liquid by Extraction Chromatography. Ind. Eng. Chem. Res. 2008, 47, 6158–6169. [Google Scholar] [CrossRef]
- Kolarik, Z.; Müllich, U.; Gassner, F. Selective Extraction of Am(III) over Eu(III) by 2,6-Ditriazolyl- and 2,6-Ditriazinylpyridines. Solvent Extr. Ion Exc. 1999, 17, 23–32. [Google Scholar] [CrossRef]
- Kolarik, Z.; Mullich, U.; Gassner, F. Extraction of Am(lll) and Eu(lll) Nitrates by 2-6-di-(5,6-dipropyl-1,2,4-triazin-3-yl)pyridines 1. Solvent Extr. Ion Exch. 1999, 17, 1155–1170. [Google Scholar] [CrossRef]
- Barbette, F.; Rascalou, F.; Chollet, H.; Babouhot, J.L.; Denat, F.; Guilard, R. Extraction of uranyl ions from aqueous solutions using silica-gel-bound macrocycles for alpha contaminated waste water treatment. Anal. Chim. Acta 2004, 502, 179–187. [Google Scholar] [CrossRef]
- Meyer, D.; Bourg, S.; Conocar, O.; Broudic, J.C.; Moreau, J.J.E.; Man, M.W.C. Extraction of plutonium and americium using silica hybrid materials. Comptes Rendus Chim. 2007, 10, 1001–1009. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Watanabe, S.; Sano, Y.; Takeuchi, M.; Kida, F.; Arai, T. Development of MA separation process with TEHDGA/SiO2-P for an advanced reprocessing. J. Radioanal. Nucl. Chem. 2021, 330, 237–244. [Google Scholar] [CrossRef]
- Hao, H.; Zheng, Q.; Zhang, Y.; He, Q.; Feng, X.; Wang, Z.; Chen, J. Highly efficient group separation of hexavalent actinides from lanthanides through a biphasic cooperative extraction system. J. Mol. Liq. 2023, 389, 122909. [Google Scholar] [CrossRef]
- Labb, S.A.; Kmak, K.N.; Despotopulos, J.D.; Kerlin, W.M.; Sudowe, R. Group hexavalent actinide separation from lanthanides using sodium bismuthate chromatography. J. Chromatogr. A 2024, 1736, 465400. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, L.; Dong, X.; Wu, T.; Qing, Q.; Chen, J.; Lu, Y.; Xu, C. Ion sieving in graphene oxide membrane enables efficient actinides/lanthanides separation. Nat. Commun. 2023, 14, 261. [Google Scholar] [CrossRef]
- Liu, B.; Han, J.; Liu, F.; Sheng, J.; Li, Z. Minor actinide transmutation in the lead-cooled fast reactor. Prog. Nucl. Energy 2020, 119, 103148. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, F.; Sun, J.; Hamza, M.F.; Wu, Q.; Wu, Y.; Zheng, N.; Ning, S.; Jiang, T.; Zeng, D.; et al. Highly efficient separation of yttrium from concentrated strontium aqueous solution by novel silica-based HDEHP-impregnating adsorbent. Sep. Purif. Technol. 2025, 361, 131450. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Deng, B.; Huang, Y.; Liu, X.; Ning, S.; Kuang, S.; Liao, W. Separation and purification of heavy rare earth elements by a silica/polymer-based β-aminophosphonic acid resin from chloride media. Sep. Purif. Technol. 2025, 354, 129342. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Xu, X.; Xu, L.; Yang, X.; Guan, H.; Li, Z.; Xiao, C. A negatively-charged supramolecular trap for precisely catching strontium ion. Nat. Commun. 2025, 16, 2606. [Google Scholar] [CrossRef]
- Stojković, I.; Todorović, N.; Nikolov, J.; Papović, S.; Gadžurić, S.; Vraneš, M. Simultaneous 137Cs and 90Sr/90Y detection in water on an LS counter: A quick response in a case of radiation emergency. Radiat. Phys. Chem. 2024, 221, 111766. [Google Scholar] [CrossRef]
- Cao, Y.; Zhou, L.; Ren, H.; Zou, H. Determination, Separation and Application of 137Cs: A Review. Int. J. Environ. Res. Public Health 2022, 19, 10183. [Google Scholar] [CrossRef]
- Grover, J.R. Management of high level radioactive wastes. Ann. Nucl. Sci. Eng. 1974, 1, 329–331. [Google Scholar] [CrossRef]
- Maksimov, I.; Kindra, V.; Vegera, A.; Rogalev, A.; Rogalev, N. Thermodynamic Analysis and Optimization of Power Cycles for Waste Heat Recovery. Energies 2024, 17, 6375. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Feasibility of Separation and Utilization of Caesium and Strontium from High Level Liquid Waste; International Atomic Energy Agency: Vienna, Austria, 1993. [Google Scholar]
- Li, L.; Xu, Z.; Li, H.; Li, J.; Hu, D.; Xiang, Y.; Han, L.; Peng, X. Immobilization of strontium and cesium by aluminosilicate ceramics derived from metakaolin geopolymer-zeolite A composites via 1100 °C heating treatment. Ceram. Int. 2022, 48, 15236–15242. [Google Scholar] [CrossRef]
- Abbasi, A.; Avanes, A.; Davarkhah, R.; Yadollahi, A.; Sepehrian, H. Efficient sorption and secure immobilization of strontium ions onto nanoporous alumino-borosilicate as a new matrix. Sci. Rep. 2024, 14, 21617. [Google Scholar] [CrossRef]
- Pepper, S.E.; Robshaw, T.J.; Amphlett, J.T.M.; Ruder, L.R.; Harwood, L.M.; Lee, T.S.; Whittle, K.R.; Ogden, M.D. Adsorption of strontium from aqueous solution using ethyl butyl phosphonate (EBP) silica. Prog. Nucl. Energy 2024, 177, 105458. [Google Scholar] [CrossRef]
- Pittet, P.-A.; Bochud, F.; Froidevaux, P. Determination of 89Sr and 90Sr in fresh cow milk and raw urine using crystalline synthetic tunnel manganese oxides and layered metal sulfides. Anal. Chim. Acta 2019, 1047, 267–274. [Google Scholar] [CrossRef]
- Ryu, J.; Hong, J.; Park, I.-S.; Ryu, T.; Hong, H.-J. Recovery of strontium (Sr2+) from seawater using a hierarchically structured MnO2/C/Fe3O4 magnetic nanocomposite. Hydrometallurgy 2020, 191, 105224. [Google Scholar] [CrossRef]
- Smičiklas, I.; Coha, I.; Jović, M.; Nodilo, M.; Šljivić-Ivanović, M.; Smiljanić, S.; Grahek, Ž. Efficient separation of strontium radionuclides from high-salinity wastewater by zeolite 4A synthesized from Bayer process liquids. Sci. Rep. 2021, 11, 1738. [Google Scholar] [CrossRef]
- Horwitz, E.P.; Dietz, M.L.; Fisher, D.E. Srex: A Newprocess for the Extraction and Recovery of Strontium from Acidic Nuclear Waste Streams. Solvent Extr. Ion Exch. 1991, 9, 1–25. [Google Scholar] [CrossRef]
- Zhang, A.; Xiao, C.; Liu, Y.; Hu, Q.; Chen, C.; Kuraoka, E. Preparation of macroporous silica-based crown ether materials for strontium separation. J. Porous Mater. 2009, 17, 153–161. [Google Scholar] [CrossRef]
- Xu, Y.; Kim, S.-Y.; Ito, T.; Nakazawa, K.; Funaki, Y.; Tada, T.; Hitomi, K.; Ishii, K. Adsorption and separation behavior of yttrium and strontium in nitric acid solution by extraction chromatography using a macroporous silica-based adsorbent. J. Chromatogr. A 2012, 1263, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Ito, T. Adsorption and Separation of Sr(II) and Y(III) by Extraction Chromatography using HDEHP-impregnated Adsorbent. J. Ion Exch. 2018, 29, 110–115. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Ito, T. Separation of Y(III) and Sr(II) from acid solutions by extraction chromatography using HDEHP-impregnated adsorbent and its medical application. Prog. Nucl. Sci. Technol. 2018, 5, 78–81. [Google Scholar] [CrossRef]
- Kawamura, T.; Wu, H.; Kim, S.-Y. Adsorption and separation behavior of strontium and yttrium using a silica-based bis(2-ethylhexyl) hydrogen phosphate adsorbent. J. Radioanal. Nucl. Chem. 2021, 329, 1001–1009. [Google Scholar] [CrossRef]
- Kawamura, T.; Ito, T.; Kim, S.-Y. Adsorption and separation behavior of strontium and yttrium using a silica-based CMPO adsorbent. J. Radioanal. Nucl. Chem. 2019, 320, 9–14. [Google Scholar] [CrossRef]
- He, J.; Mao, L.; Ma, X.; Hua, J.; Cui, Z.; He, B.; Pei, H.; Li, J. Highly-Efficient adsorptive separation of Cs+ from aqueous solutions by porous polyimide membrane containing Dibenzo-18-Crown-6. Sep. Purif. Technol. 2022, 299, 121757. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Men, J.; Zhu, M.; Liang, C.; Ding, H.; Du, Z.; Bao, P.; Hu, Z. Selective Adsorption of Sr(II) from Aqueous Solution by Na3FePO4CO3: Experimental and DFT Studies. Molecules 2024, 29, 2908. [Google Scholar] [CrossRef] [PubMed]
- Authen, T.L.; Tekikachew, B.E.; Foreman, M.R.S.J.; Wilden, A.; Ekberg, C. A comparison on the use of DEHBA or TBP as extracting agent for tetra- and hexavalent actinides in the CHALMEX Process. J. Radioanal. Nucl. Chem. 2022, 331, 5137–5145. [Google Scholar] [CrossRef]
- Geng, L.; Wang, S.; Wang, T.; Luo, R. Facile Synthesis and Thermal Properties of Nanoencapsulated n-Dodecanol with SiO2 Shell as Shape-Formed Thermal Energy Storage Material. Energy Fuels 2016, 30, 6153–6160. [Google Scholar] [CrossRef]
- Sobhani, A.; Salavati-Niasari, M. Sodium dodecyl benzene sulfonate-assisted synthesis through a hydrothermal reaction. Mater. Res. Bull. 2012, 47, 1905–1911. [Google Scholar] [CrossRef]
- Takahashi, T.; Ito, T.; Kim, S.-Y. Selective extraction of Cs(I) using 1,3-[(2,4-diethylheptylethoxy)oxy]-2,4-crown-6-calix[4]arene in ionic liquid solvents and its application to the treatment of high-level liquid waste. J. Radioanal. Nucl. Chem. 2018, 316, 1067–1073. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, A.; Chai, Z. Synthesis and characterization of novel macroporous silica-polymer-calixcrown hybrid supramolecular recognition materials for effective separation of cesium. J. Hazard. Mater. 2014, 267, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zhang, W.; Wang, Y.; Ding, X. Effective separation of cesium with a new silica-calix(4)biscrown material by extraction chromatography. Sep. Purif. Technol. 2016, 171, 17–25. [Google Scholar] [CrossRef]
- Weng, H.; Wang, Y.; Li, F.; Muroya, Y.; Yamashita, S.; Cheng, S. Recovery of platinum group metal resources from high-level radioactive liquid wastes by non-contact photoreduction. J. Hazard. Mater. 2023, 458, 131852. [Google Scholar] [CrossRef]
- Zhang, M.; Lv, Y.; Xu, Z.; Wang, S.; Wang, J. The Removal of Platinum Group Metals, Cs, Se, and Te from Nuclear Waste Glass Using Liquid Sb Extraction and Phase Separation Methods. Materials 2020, 13, 5305. [Google Scholar] [CrossRef]
- Xia, J.; Ghahreman, A. Platinum group metals recycling from spent automotive catalysts: Metallurgical extraction and recovery technologies. Sep. Purif. Technol. 2023, 311, 123357. [Google Scholar] [CrossRef]
- Ozawa, M.; Koyama, S.; Suzuki, T. Nuclear Rare Metals and their Separation in Advanced ORIENT Cycle Strategy. Energy Procedia 2011, 7, 421–424. [Google Scholar] [CrossRef]
- Mabon, L. Treated water releases from the Fukushima Dai’ichi nuclear power plant: An overview of the decision-making process and governing institutions. Mar. Policy 2024, 163, 106120. [Google Scholar] [CrossRef]
- Onda, Y.; Taniguchi, K.; Yoshimura, K.; Kato, H.; Takahashi, J.; Wakiyama, Y.; Coppin, F.; Smith, H. Radionuclides from the Fukushima Daiichi Nuclear Power Plant in terrestrial systems. Nat. Rev. Earth Environ. 2020, 1, 644–660. [Google Scholar] [CrossRef]
- Sylvester, P.; Milner, T.; Jensen, J. Radioactive Liquid Waste Treatment at Fukushima Dai-Ichi. J. Chem. Technol. Biotechnol. 2013, 88, 1592–1596. [Google Scholar] [CrossRef]
- Ueda, S.; Hasegawa, H.; Ohtsuka, Y.; Ochiai, S. Nuclear accident-derived 129I in several river water, eastern Fukushima, Japan, 2016–2020. J. Environ. Radioact. 2025, 282, 107617. [Google Scholar] [CrossRef] [PubMed]
- Fuge, R.; Johnson, C.C. Iodine and human health, the role of environmental geochemistry and diet, a review. Appl. Geochem. 2015, 63, 282–302. [Google Scholar] [CrossRef]
- Ten Hoeve, J.E.; Jacobson, M.Z. Worldwide health effects of the Fukushima Daiichi nuclear accident. Energy Environ. Sci. 2012, 5, 8743–8757. [Google Scholar] [CrossRef]
- Sanyaolu, O.M.; Mouri, H.; Selinus, O.; Odukoya, A. Sources, Pathways, and Health Effects of Iodine in the Environment. In Practical Applications of Medical Geology; Siegel, M., Selinus, O., Finkelman, R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 565–613. [Google Scholar] [CrossRef]
- Song, M.; Huang, S.; Mo, C.; Chai, G.; Yang, Z. Uncertainty and sensitivity analysis of iodine release in severe accidents of advanced pressurized water reactors based on the Latin Hypercube method and Grey Correlation Coefficients. Nucl. Eng. Des. 2023, 412, 112450. [Google Scholar] [CrossRef]
- Hao, W.; Yan, W.; Zi, C.; Yue-Zhou, W. Adsorption behaviors of iodide anion on silver loaded macroporous silicas. Nucl. Sci. Tech. 2015, 26, 030301. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, X.; Wang, F.; Ning, X.; Wen, Y.; Song, B.; Yang, C.; Wu, D.; Ke, X.; Peng, L. Insights into memory effect mechanisms of layered double hydroxides with solid-state NMR spectroscopy. Nat. Commun. 2022, 13, 6093. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cui, R.; Dai, J.; Wang, Y.; Pan, J. Fast and efficient capture of iodide ions by silver-modified GO nanosheets: From adsorption to membrane filtration. Sep. Purif. Technol. 2024, 333, 125955. [Google Scholar] [CrossRef]
- Riley, B.J.; Kroll, J.O.; Peterson, J.A.; Matyáš, J.; Olszta, M.J.; Li, X.; Vienna, J.D. Silver-Loaded Aluminosilicate Aerogels as Iodine Sorbents. ACS Appl. Mater. Interfaces 2017, 9, 32907–32919. [Google Scholar] [CrossRef]
| Name of Adsor. | Full Name | Chemical Structure | Target Element |
|---|---|---|---|
| HDEHP | Di(2-ethylhexyl)phosphoric acid |  | Gd, Eu, Ce, Am, Sr, and Y |
| Cyanex301 | Bis(2,4,4-trimethylpentyl) dithiophosphinic acid |  | Gd, Eu, Ce, and Am |
| CMPO | Octyl(phenyl)-N, N-diisobutyl-carbamoylmethylphosphine oxide | 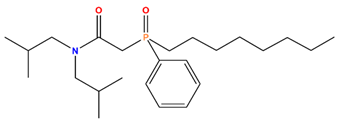 | Mo, MA, Cs, Sr, and Ru |
| TODGA | N,N,N′,N′-tetraoctyl-3-oxapentane-1,5-diamide | 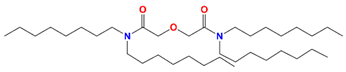 | Am, Nd, Sm, Y, and Sc |
| R-BTP | 2,6-bis-(5,6-dialkyl-l,2,4-triazine-3-yl)-pyridine |  | Am and Dy |
| Isobutyl-BTP | (2,6-di(5,6-diisobutyl-1,2,4-triazin-3-yl) pyridine |  | Am, Pu, and Dy |
| Isohexyl-BTP | 2,6-bis(5,6-dii-sohexyl)-1,2,4-triazin-3-yl) pyridine | 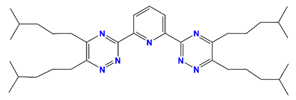 | Am, Pu, Dy, and Eu |
| Me2-CA-BTP | 2,6-bis(5,6,7,8-tetrahydro-5,8,9,9-tetramethyl-5,8-methano-1,2,4-benzotriazin-3-yl) pyridine |  | Am, Dy, Gd, and Eu |
| CA-BTP/SiO2-P | bis-2,6-(5,6,7,8-tetrahydro-5,9,9-trimethyl-5,8-methano-1,2,4-benzotriazin-3-yl) pyridine | 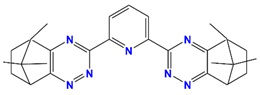 | Am and Pu |
| AR-01 | N-methylbenzimidazole and N, N9-dimethylbenzimidazolium groups as exchange sites |  | U, Np, and Pu |
| Calix[4]arene-R14 | 1,3-[(2,4-diethyl-heptylethoxy) oxy]-2,4-crown-6-calix[4]arene | 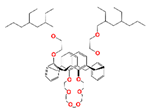 | Cs, Rb, K, Na, and Sr |
| DtBuCH18C6 | 4,4′,(5′)-di-(tert-butylcyclohexano)-18-crown-6 | 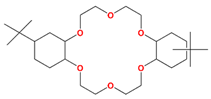 | Sr, Ba, K, Cs, La, and Y |
| MOTDGA | N,N′-dimethyl-N,N′-di-n-octyl-thiodiglycolamide | 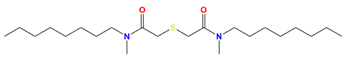 | Pd, Zr, Mo, Ru, and Rh |
| Crea | N′,N′-di-n-hexyl-thiodiglycolamide |  | Ru, Rh, and Pd |
| DAMIA-EH | 2,2′-[(2-ethyl-hexyl) imino]bis[N,Nbis(2ethylhexyl)acetamide] | 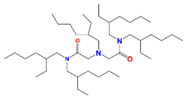 | Pd and Ru |
| TOA | Tri-n-octylamine |  | Pd |
| DTPA | diethylenetriaminepentaacetic acid | 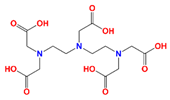 | Sr |
| SiO2-P | P: styrene-divinylbenzenecopolymer |  | / |
| Sorbent | Equilibrium Time | SF | Good (Best) Adsorption Acidity | Kd Order | Column Results | Leakage Rate |
|---|---|---|---|---|---|---|
| HDEHP/SiO2-P | 0.5 h | / | 0.1–0.2 M | Gd(III) > Eu(III) > Ce(III) > Am(III) | / | <1% |
| Cyanex301/SiO2-P | 1 h | SFAm/Eu: 500–5000 | pH 4 | Am(III) >> Gd(III), Eu(III), Ce(III) | Am(III) was separated from Gd(III), Eu(III), and Ce(III). | / |
| CMPO/SiO2-P | 0.5–1 h | / | / | Zr(IV) > Mo(VI) > RE(III) ~ MA(III) > Pd(II) > Cs(I), Sr(II), Ru(III) | MA(III) and Ln(III) are expected co-separated. | 0.4−0.8% |
| TODGA/SiO2-P | 0.5 h | / | / | / | Am(III), Nd(III), Sm(III), and Y(III) were separated from Rh(III), Mo(IV), Sr(II), Ce(III), and Pr(III). | <0.02% |
| nBu-BTP/SiO2-P | 1 h | Increase with NaNO3 concentration | Am(III), Cm(III) >> Gd(III), Eu(III) >> Ce(III), Cs(I), Sr(II), Zr(IV), Mo(IV), Ru(III), Rh(II) | Am(III) was separated from Y(III), Ce(III), Eu(III), and Gd(III). | ||
| isoBu-BTP/SiO2-P | 3 h for stable elements and 0.5 h for trace amount of Am | SFAm/Ln(Ln = Ce, Nd, Gd, Eu) about 100 | pH = 1 + 1–4 M NaNO3 | Am(III) >> Dy(III) > Gd(III) > Nd(III), Ce(III) | Am was separated from Dy and the other light Ln(III). | 0.15% in 0.01 M HNO3 and 1.2% under γ irradiation for 5 months in 0.01 M HNO3 |
| isoHexyl-BTP/SiO2-P | 15–24 h | SFAm/Eu > 100 | 2–4 M HNO3 | Am, Pu >> Dy, Pd > Gd, Eu >> La, Ce, Nd, Sm, Sr, Y, Zr, Mo, Tc, Ru in 3 M HNO3 | Am was separated from Cs, Sr, Y, and Ce in 3 M HNO3. | 0.1–4 M HNO3: TOC < 35 ppm |
| Me2-CA-BTP/ SiO2-P | 12 h | SFAm/Eu about 100 in 0.1–4 M HNO3 | 0.1–4 M HNO3 or 1–3 M NaNO3 | Am > Dy > Gd, Eu > Pd, Sm, Mo, Zr, Cs, La, Ce, Y, Nd in 1 M HNO3 | Am was directly separated from Sr, Y, Zr, Mo, Ru, Pd, and Ln in 3 M HNO3. | Stable in 1–3 M HNO3 under γ irradiation |
| CA-BTP/SiO2–P | >24 h | no adsorption toward Eu | 0.5 M HNO3 | Am > Pu, Zr, Tc, Ru > Ln(III), Sr, Y, Cs | / | Stable in dry state under γ irradiation |
| CyMe4-BTPhen/SiO2-P | 0.5 h for trace amount of Am | SFAm/Eu = 88.6 ± 0.1 | 4 M HNO3 | Am > Pu > Pd, Mo, Ru > Zr, Tc, Y, Cs, Sr | / | 0.1–4 M HNO3: TOC < 75 ppm |
| AR-01 | 10 min | / | 6 M HNO3 | U(IV), Np(IV), Pu(IV) >> FP in 6 M HNO3 | U(IV), Np(IV), and Pu(IV) were separated from most other fission products. | / |
| Sorbent | Equilibrium Time | SF | Good (Best) Adsorption Acidity | Kd Order | Column Results | Stability |
|---|---|---|---|---|---|---|
| HDEHP/SiO2-P | 5 h | SFSr/Y: 1.93 × 103 | 0.1 M | Y(III) > Sr(II) | Y(III) was separated from Sr(II) | <1% |
| HDEHP/SiO2-P | 0.5 h | / | 0.1–0.2 M | Gd(III) >Eu(III) > Ce(III) > Am(III) | / | <1% |
| HEHAEP/SiO2-P | 12 h | SFEr/Ho, Tm/Er, Yb/Tm, Lu/Yb: 2.35, 3.62, 3.14, 1.23 | pH = 2.0 | / | Tm(III) was separated from Lu(III), La(III), and Am(III) | <0.1% |
| TODGA/SiO2-P | 3 h | SFZr/Sc: 3694 | 1 M HNO3 | / | Am(III), Y(III), and Sc(III) were separated from Zr(II) | <0.02% |
| TRPO/SiO2-P | 1 h for Sc | SFZr/Sc: 380 and 977 | 0.2 M H2 SO4 and 5 M HCl | / | Sc(III) was separated from Zr(II) | / |
| Me2-CA-BTP/SiO2-P | 12 h | SFAm/Eu about 100 in 0.1–4 M HNO3 | 0.1–4 M HNO3 or 1–3 M NaNO3 | Am > Dy > Gd, Eu > Pd, Sm, Mo, Zr, Cs, La, Ce, Y, Nd in 1 M HNO3 | Am was directly separated from Sr, Y, Zr, Mo, Ru, Pd, and Ln in 3 M HNO3 | Stable in 1–3 M HNO3 under γ irradiation |
| Sorbent | Kd Order | Capacity mg Sr(II)/g | Equilibrium Time | Good (Best) Adsorption Acidity | Column Results | Stability (TOC) | Sr(II) Desorber |
|---|---|---|---|---|---|---|---|
| DtBuCH18C6/Si-polymer | Sr2+ >> Ba2+ >> K+, Cs+, La3+, Y3+ | 104.6 | >5 h | 1–3 (2) M HNO3 | / | 424.8–634.6 ppm | / |
| (DtBuCH18C6+TBP)/SiO2-P | Sr2+ >> Ba2+ >> K+, Cs+, Na+, Pd2+, Ru3+, Y3+, Mo4+, La3+ | / | About 2 h | 0.5–5 (2) M HNO3 | Sr(II) was separated from 2 M HNO3 | 251.2–352.7 ppm | water |
| DtDo/SiO2-P or (DtBuCH18C6+Dodec)/SiO2-P | Sr2+ >> Ba2+ >> K+, Cs+, Na+, Pd2+, Ru3+, Y3+, Mo4+, La3+ | 27–32 | >5 h | 1–5 (2) M HNO3 | / | 165.1–222.8 ppm | water |
| (DtBuCH18C6+Oct)/ SiO2-P | Sr2+ >> Ba2+ >> K+, Cs+, Na+, Pd2+, Ru3+, Y3+, Mo4+, La3+ | / | About 60 min | / | Sr(II) was separated from 2 M HNO3 | 41 ppm | water |
| TODGA/SiO2-P | Sr2+ >> Ba2+ >> Na+, K+, Cs+, Rb+, Ba2+, Ru3+ | / | About 10 min | 0.5–4 (2) M HNO3 | Sr(II) was separated from 2 M HNO3 | TOC: 40 pm, 0.25% | water |
| (DtBuCH18C6+DBS+dodec)/SiO2-P | Sr2+ > Ba2+ > Zr > Na+ > Re4+ > Pd2+ > Mo4+, Ru3+, Nd3+, Dy3+ | / | 5 h | 2 M HNO3 | Sr(II) was separated from 3 M HNO3 | / | Na-DTPA |
| (DtBuCH18C6+Dodec)/SiAaC-g-ABSA | Sr2+ > Ba2+ > Y3+ > Pd2+ > Ru3+ > Nd3+ > Mo4+, La3+ | 36.9 | 1 h | 2 M HNO3 | Sr(II) was separated from 2 M HNO3 | TOC: 0.56% | / |
| CEPA@SBA-15-APTES | Sr2+ >> Ba2+, K+, Cs+, Na+, Pd2+, Ru3+, Y3+, Mo4+, La3+ | 112.6 | 5 min | 3(4) M HNO3 | Sr(II) was separated from 4 M HNO3 | / | / |
| HEMAP/SiO2-P | Sr2+>Y3+ >> Nd3+> Mo4+, La3+, Ru3+, Dy3+ | 61.2 | 1 min | 3 M HNO3 | Sr(II) was separated from 3 M HNO3 | / | / |
| Sorbent | Synthetic Method | Specific Surface Area (m2/g) | Kd Order | Capacity mg Sr(II)/g | Equilibrium Time | Good Adsorption Acidity | Treatment Bed Volume |
|---|---|---|---|---|---|---|---|
| K2Ti6O13/SiO2 | sol–gel method | / | / | 15 | ≥8 h | pH: 4.2–6.4 | 80 |
| Na2TinO2n+1/SiO2 | sol–gel method | 44.83 | Sr2+ > Ba2+ >> Mg2+, Ca2+, Cs+, K+ | 66.37 | <10 min | pH: 3–10 | 950 |
| h-WO3/SiO2 | hydrothermal method | / | Sr2+ > La3+ > Mg2+ > Dy3+, Ca2+ | 9 | 15 min | pH: 4–7 | / |
| ZrP/MSP | one-pot liquid-phase grafting method | 293.73 | Sr2+ > Ba2+ > Ca2+ > Cu2+ > Mg2+ | 100.77 | 1.5 h | pH: 4–7 | / |
| Sb2O5/SiO2 | vacuum impregnation method | / | Sr2+ > Zr2+ > Mo4+ > Ba2+ > La3+, Mg2+, Dy3+, Ca2+ (1 M HNO3) | 160.6 | 5 min | pH: 6 | 316 |
| SiMaC | in situ polymerization method | 20.8 | / | 142.5 | 45 min | pH: 10 | / |
| Sorbent | PGMs | Kd Order | Capacity mg | Equilibrium Time | Good (Best) Adsorption Acidity | Column Results | Desorber |
|---|---|---|---|---|---|---|---|
| (MOTDGA-TOA)/SiO2-P | Pd, Ru, and Rh | Pd > Zr > Mo > Ru > Rh > La, Ce, Nd, Sm, Gd | About 0.73, 0.31, and 0.63 mmol/g for Ru, Rh, and Pd | 2 h for Pd and over 24 h for Ru and Rh/ | Best for Pd in 0.1 M HNO3, but still kept well in 1–5 M HNO3 solution for Pd and Ru | Pd and Ru were separated from Rh, Zr, Mo Re, La, Ce, Nd, Sm, and Gd in 3 M HNO3 solution at 323 K. | / |
| (MOTDGA-Dodecanol)/SiO2-P | Pd | Pd > Zr > Mo > Ru >> Rh, La, Ce, Nd, Sm, Gd | / | 8 h | 0.1 M HNO3 | / | / |
| (TOA-Dodecanol)/SiO2-P | Pd | Pd >> Zr, Mo, Ru, Rh, La, Ce, Nd, Sm, Gd | / | 8 h | 0.1 M HNO3 | / | / |
| (Crea+Dodec)/SiO2-P | Ru, Rh, and Pd | Pd > Ru > Mo > Rh > Zr > Re >> La, Ce, Nd, Sm, Gd | About 0.7 mmol/g for Pd and over 0.35 mmol/g for Ru and Rh | Within 30 min for Pd, about 15 h for Ru, and over 72 h for Rh at 298 K | 0.1–5 M HNO3 for Pd | Pd and Mo were separated from Ru, Rh, Zr, Re, La, Ce, Nd, Sm, and Gd in 3 M HNO3 solution. | / |
| (Crea+TOA)/SiO2-P | Ru, Rh, and Pd | Pd > Ru > Rh > Mo > Zr > La, Ce, Nd, Sm, Gd | / | About 24 h | 0.1–5 M HNO3 for Pd | Pd and partial Ru were separated from Rh, Zr, Mo Re, La, Ce, Nd, Sm, and Gd in 3 M HNO3 solution at 323 K. | / |
| (DAMIA-EH+TOA)/SiO2-P | Pd and Ru | Pd >> Re >> Ru > Rh, Zr, Mo, Cs, Sr, Ba, La, Ce, Nd, Sm, Eu, Gd | About 0.57 mmol/g for Pd and > 0.3 mmol/g for Ru | 10 min for Pd and 5 h for Ru at 298 K | 0.1 M HNO3 for Pd and 0.5–4 M HNO3 for Ru | Pd was separated from Ru, Rh, Zr, Mo Re, Sr, Cs, Ba, La, Ce, Nd, Sm, Eu, and Gd in 3 M HNO3 solution at 298 K. | 0.01 M SC(NH2)2 (pH = 2) for Pd |
| (DAMIA-EH+1-dodecanol)/SiO2-P | Pd | Pd >> Re > Mo > Zr > Ru | About 0.57 mmol/g for Pd | Within 1 h for Pd | 1 M HNO3 for Pd | / | / |
| Me2-CA-BTP/SiO2-P | Pd | Am >> Pd >> Sr, Y, Zr, Ru, Cs, La, Ce, Nd, Sm, Eu, Gd | 0.76 mmol/g for Pd | About 12 h | 2–4 M HNO3 for Pd | / | / |
| isoHex-BTP/SiO2-P | Pd | Pd >> Pu, Am >> Sr, Y. Zr, Mo, Tc, Ru, La, Ce, Nd, Sm, Eu, Gd, U | 0.85 mmol/g for Pd | About 72 h | 2–4 M HNO3 for Pd | / | 0.5 M SC(NH2)2 (pH = 1) for Pd |
| isoBu-BTP/SiO2-P | Pd, Ru, and Rh | Pd > Ru > Rh <Sr, Y, Zr, Mo, La,Ce, Pr, Nd, Sm, Eu, Gd at 328 K | 0.34, 0.33, and 1.06 mmol/g for Ru, Rh, and Pd at 313 K | About 48 and 24 h at 328 K for Pd | 0.5–5 M HNO3 for Ru, Rh, and Pd | Pd was separated from other fission products during the separation of MA. | 0.1 mol/L SC(NH2)2 (pH = 2) for Pd |
| TpPa-1/SiO2-A600 | Pd | Pd >> Sr, Cs, Ba, Ru, Rh, Zr, Mo, Re, La, Ce, Nd, Sm, Eu, Gd | 0.12 mmol/g for Pd | About 0.5 h | 0.6–5 M HNO3 | / | 0.2 mol/L SC(NH2)2 (pH = 2) for Pd |
| dNbpy/SiO2-P | Pd, Ru, and Rh | Pd > Ru > Rh > Y, La, Ce, Nd, Sm, Eu, Gd | 93.0, 46.0, and 14.9 mg/g for Pd, Ru, and Rh | 10 min for Pd and 24 h for Ru and Rh | 3 M HNO3 | Pd was separated from Ru, Rh, Y, Sr, Cs, Ba, La, Ce, Nd, Sm, Eu, and Gd in 3 M HNO3 solution. | 0.1 M HNO3 and thiourea |
| Tp-Azo-COF/SiO2 | Pd | Pd > Y > Ru > Rh > Pr > La > Nd > Ce > Gd > Sm > Eu | 85.4 mg/g for Pd | 60 min for Pd | 3 M HNO3 | Pd was separated from Ru, Rh, Ba, Y, Sr, Cs, La, Ce, Nd, Sm, Eu, and Gd in 3 M HNO3 solution. | 0.1 M HNO3 and thiourea |
| SiAcyl/SiO2 | Pd | Pd > Mg, Sr, Ni, Co, Ca, Cr, K | 81.8 mg/g for Pd | 60 min for Pd | 1.95–3 M HNO3 | Pd was separated from Ni, Na, Ca, Mg, and K. | 0.1 M HNO3 and thiourea |
| SiVpC/SiO2 | Pd | Pd > Ru, Rh, Y, La, Ce, Pr, Nd, Sm, Eu, Mo | 22.2 mg/g for Pd | 2 h for Pd | 0.5 M HNO3 | Pd was separated from Ru, Rh, Ba, Y, Sr, Cs, La, Ce, Nd, Sm, Eu, and Gd in 0.5 M HNO3 solution. | 0.5 M HNO3–0.5 M THU |
| SBA-15-TEPA | Pd | Pd > Ba > K > Cu > Sr > Na > Zn, Ni, Al, Mg, Ca | 84.21 mg/g for Pd | 2 h for Pd | 1.5 M HNO3 | Pd was separated from Ni, Ca, Na, Mg, and K. | 0.1 M HNO3 and thiourea |
| 2AT-SiAaC | Pd | Pd >> Rh, Y, Sr, Ba, Cs, La, Ce, Pr, Nd, Sm, Eu, Gd | 62.1 mg/g for Pd | 60 min for Pd | 0.5 M HNO3 | Pd was separated from Rh, Y, Sr, Ba, Cs, La, Ce, Pr, Nd, Sm, Eu, and Gd. | 0.5 M HNO3–0.5 M THU |
| SiPS-TU | Pd | Pd > Rh, Ru | 75.93 mg/g for Pd | 1 h | 0.1 M HNO3 | / | / |
| KNiHC/SiO2 | Pd | Pd >> Rh, Ru | 48.5 mg/g for Pd | About 1 h | 1 M NaNO3–3 M HNO3 | / | / |
| Sorbent | Kd Order | Capacity mg Cs(I)/g | Equilibrium Time | Acidity | Column Results | Stability (TOC) | Cs Desorber |
|---|---|---|---|---|---|---|---|
| (Calix[4] R14+TBP)/SiO2-P | Cs >> Rb >> K, Na, Sr | / | Within 30 min | 4.0 M HNO3 | Cs and Rb were separated from Ba, Sr, Ru, Fe, K, Na, Mo, Zr, and Pd in 4.0 M HNO3. | / | H2O |
| (Calix[4]+MODB)/SiO2-P | Cs >> Pd, Ru >> La, Y, Mo, Rh, Zr Kd (Cs) < 50 | / | About 30 min | 3.0 M HNO3 | Cs was separated from Pd, La, Y, Mo, Zr, Ru, and Rh in 3.0 M HNO3. | / | H2O |
| (Calix[4]+Dodecanol)/SiO2-P | Cs >> Zr > Mo, Sr, Pd >> La, Nd, Sm, Ga | 0.4 mmol Cs/g | 5 h | 2.0 M HNO3 | / | ≤180 ppm; γ radiation stability was evaluated | / |
| (Calix[4]+dodecanol+DBS)/SiO2-P | Cs >> Na, K, Sr, Ru, Rh, Zr, Mo, Y, La, Ce, Eu, Pd | 0.12–0.16 mmol Cs/g | More than 60 min | 0.5–5 M (0.5.0 best) HNO3 | / | 1 wt% (75ppm) at 318 K | / |
| BnOCalix[4]C6/SiO2-P | Cs >> Pd > Rb >> Na, K, Ba, Cs, Y, La, Ru, Mo, Zr | / | More than 60 min | 3.0 M HNO3 | / | about 0.29% (110 ppm) | / |
| (CalixBNaphC)@SiO2-P | Cs >> Rb >> K, Fe, Pd, Sr, Fe, Ba | / | About 60 min | 3.0 M HNO3 | Cs and Rb were separated from Li, Na, K, Fe, Sr, Ba, and Pd in 3 M HNO3. | / | H2O |
| (Calix[4]+DtBuCH18C6)/SiO2-P | / | 0.15 and 0.24 mmol/g for Cs and Sr | 1–2 h for Cs and 1–3 h for Sr | 2 and 4 M HNO3 for Sr and Cs | Both Sr and Cs can be adsorbed in 3 M HNO3 and desorbed by H2O. | TOC: 150 ppm | / |
| AMP/SiO2 | / | 0.36 mmol Cs/g | Within 30 min | over 300 mL/g in 3.0 M HNO3 | / | good | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, X.; Wang, F.; Zheng, Q.; Ning, S.; Chen, L.; Wei, Y. Review on Synthesis of Silica-Based Hybrid Sorbents and Their Application in Radionuclide Separation and Removal via Chromatographic Technique. Toxics 2025, 13, 319. https://doi.org/10.3390/toxics13040319
Yin X, Wang F, Zheng Q, Ning S, Chen L, Wei Y. Review on Synthesis of Silica-Based Hybrid Sorbents and Their Application in Radionuclide Separation and Removal via Chromatographic Technique. Toxics. 2025; 13(4):319. https://doi.org/10.3390/toxics13040319
Chicago/Turabian StyleYin, Xiangbiao, Fan Wang, Qi Zheng, Shunyan Ning, Lifeng Chen, and Yuezhou Wei. 2025. "Review on Synthesis of Silica-Based Hybrid Sorbents and Their Application in Radionuclide Separation and Removal via Chromatographic Technique" Toxics 13, no. 4: 319. https://doi.org/10.3390/toxics13040319
APA StyleYin, X., Wang, F., Zheng, Q., Ning, S., Chen, L., & Wei, Y. (2025). Review on Synthesis of Silica-Based Hybrid Sorbents and Their Application in Radionuclide Separation and Removal via Chromatographic Technique. Toxics, 13(4), 319. https://doi.org/10.3390/toxics13040319








