Impact of Different Surface Treatments on Shear Bond Strength between Two Zirconia Ceramics and a Composite Material
Abstract
1. Introduction
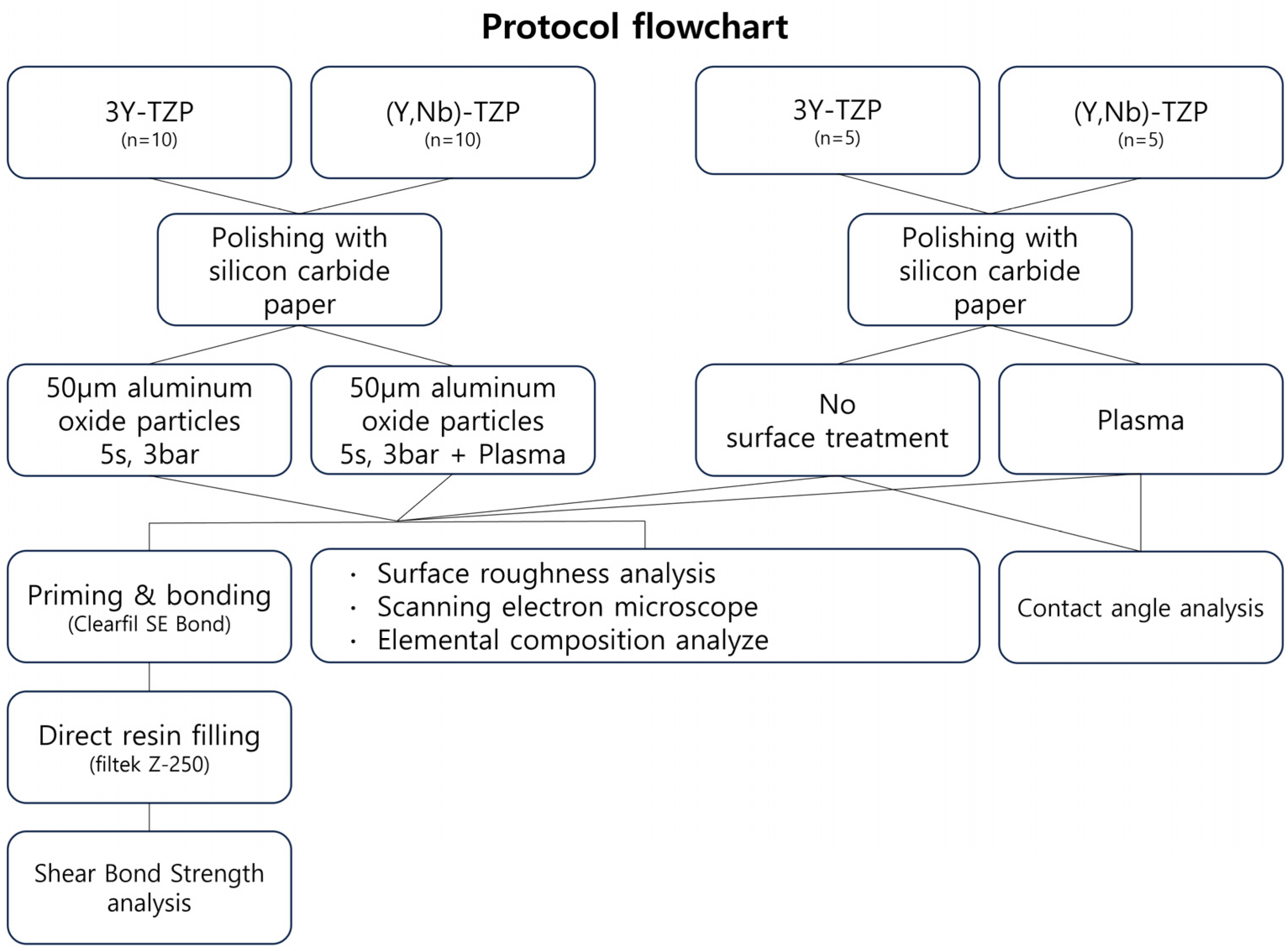
2. Materials and Methods
2.1. Specimen Preparation
2.2. Plasma Treatment
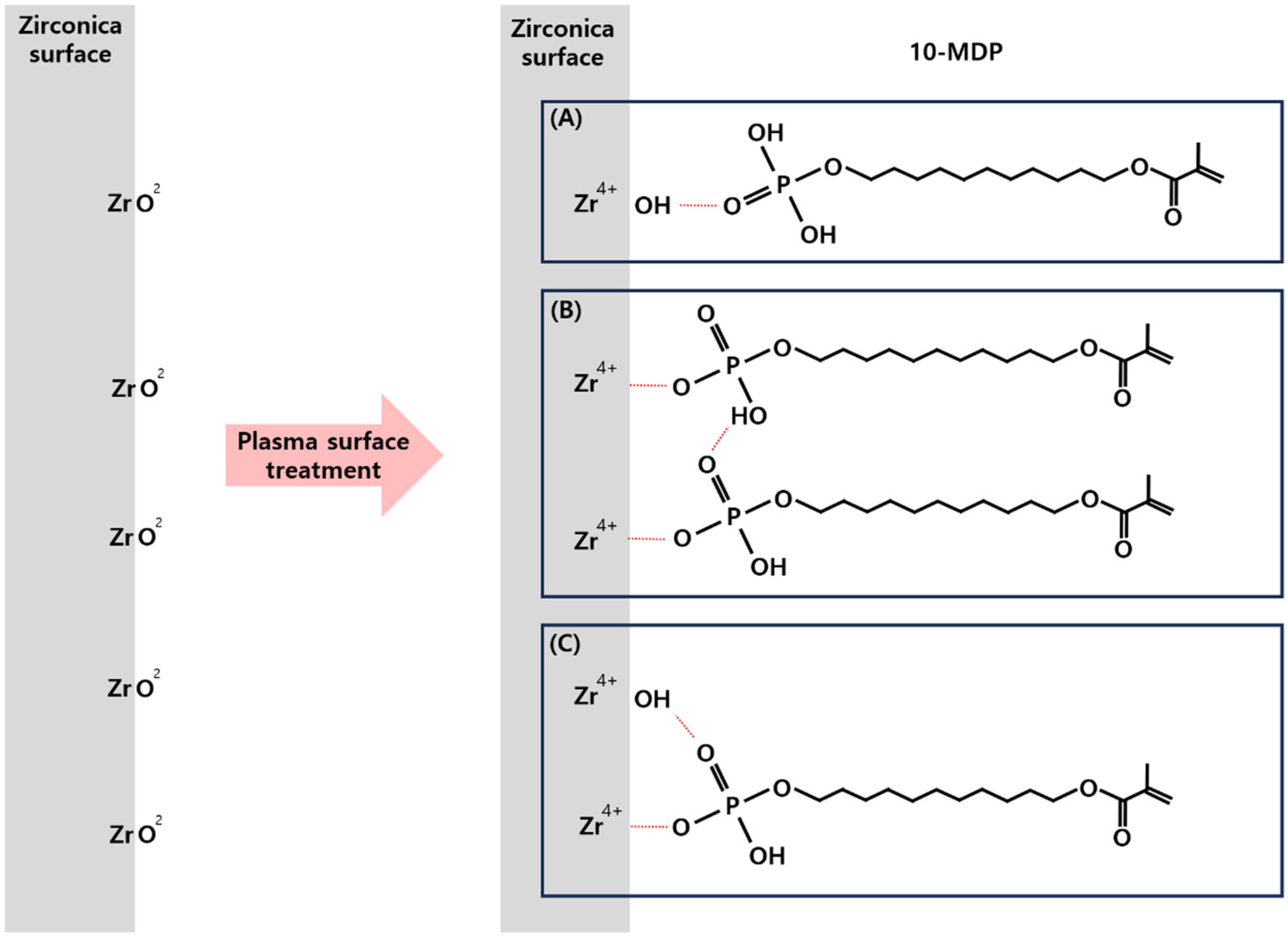
2.3. Priming
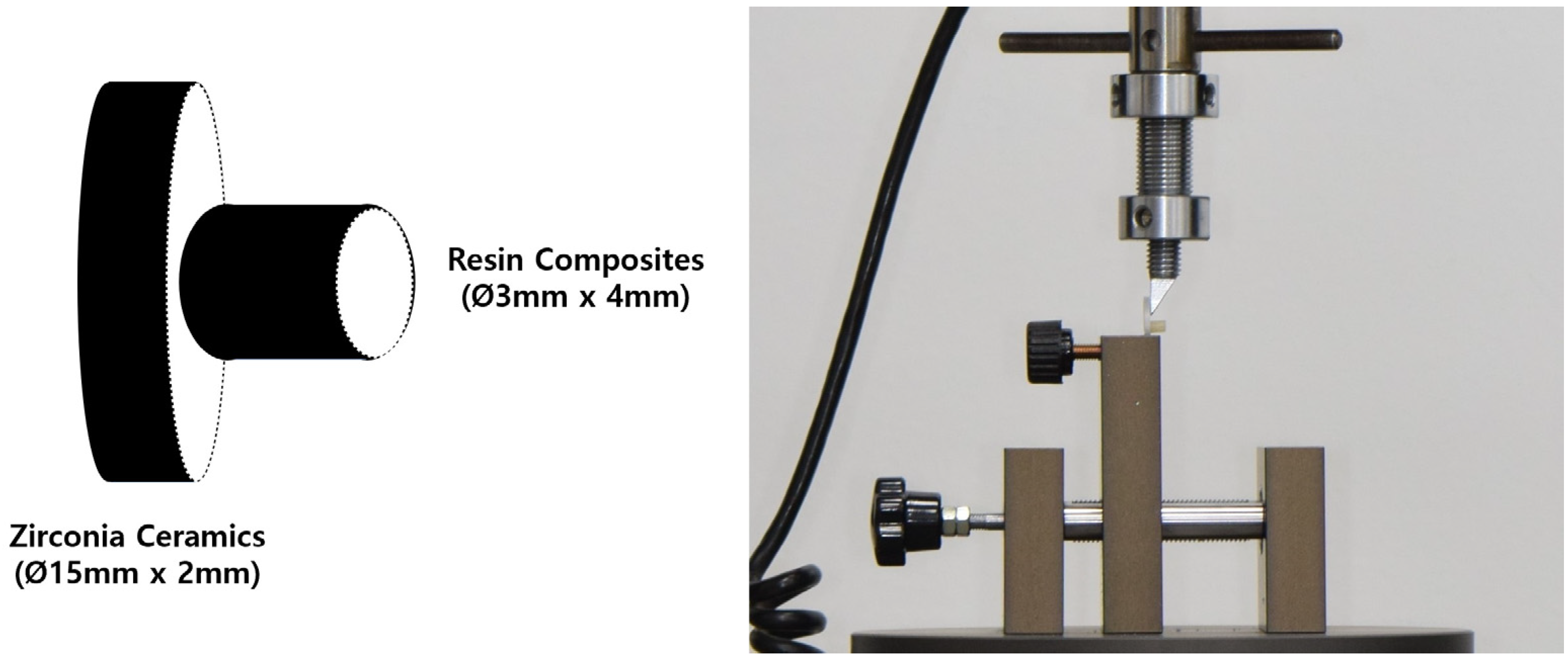
2.4. Resin Composite Application
2.5. Analysis
2.6. Statistical Analysis
3. Results
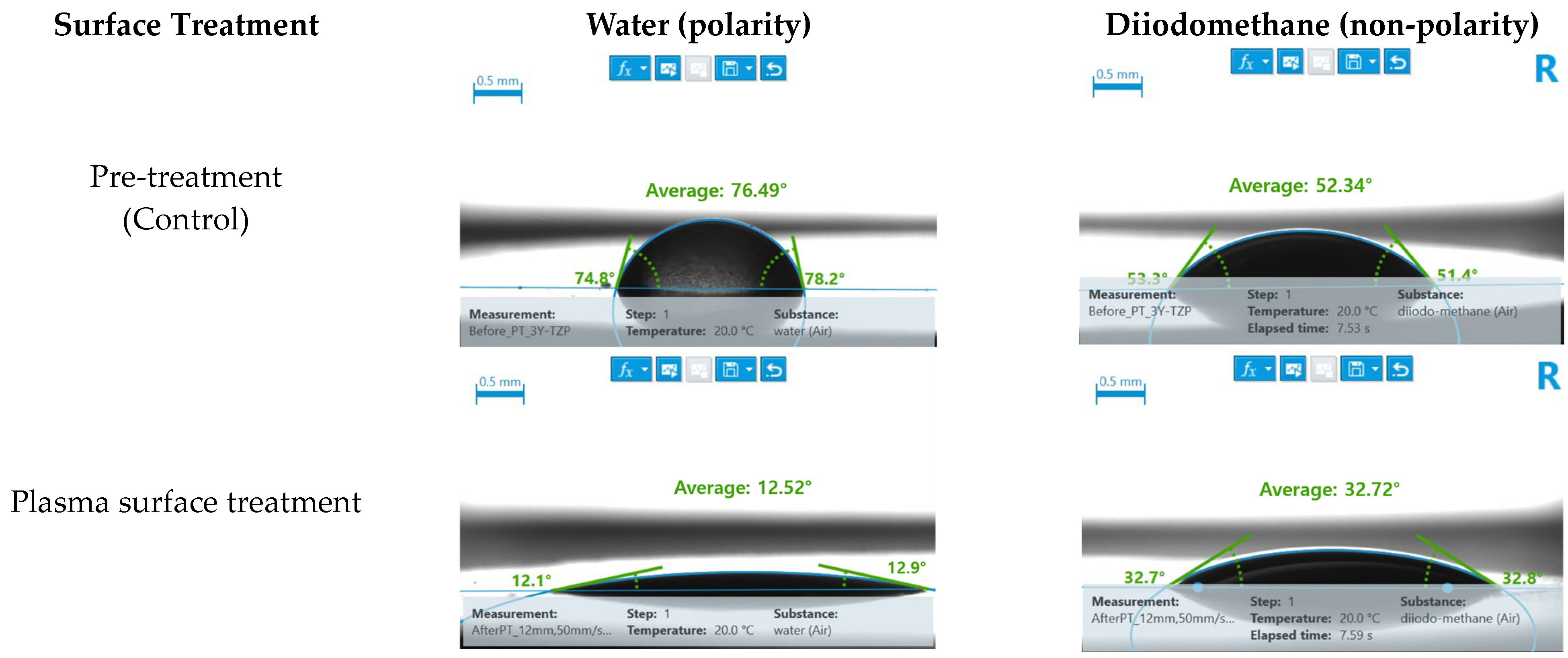
3.1. Contact Angle
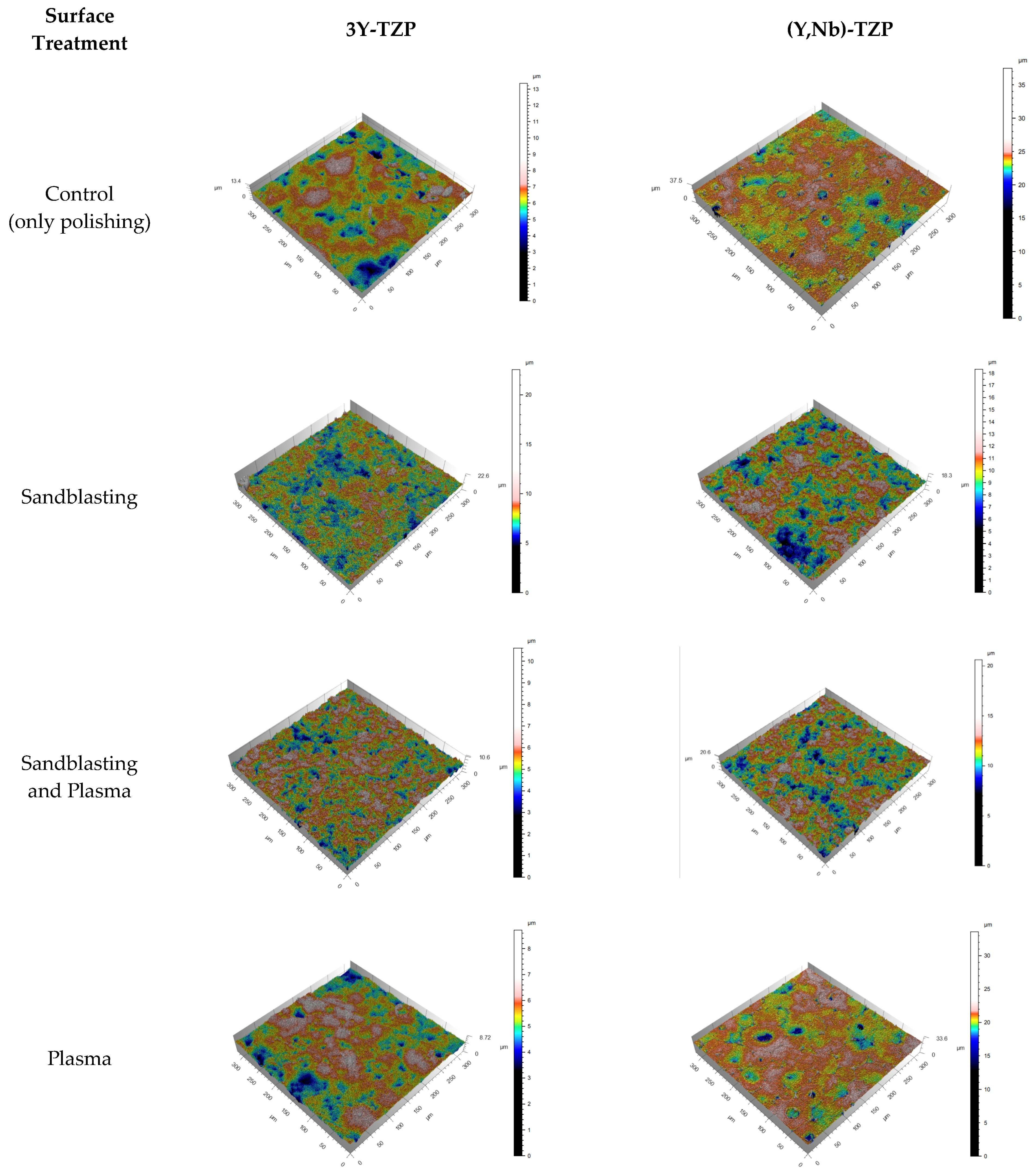
3.2. Surface Roughness
3.3. Scanning Electron Microscope (SEM)
3.4. Shear Bond Strength (SBS)
4. Discussion
5. Conclusions
- When sandblasting and plasma treatments were combined in the (Y,Nb)-TZP specimens, the shear bond strength with the resin composite significantly increased compared to the control group.
- Similarly, for the 3Y-TZP specimens, the shear bond strength increased with both plasma and sandblasting treatments, although no statistically significant change was observed.
- Without sandblasting, when zirconia ceramic specimens were treated with plasma and primers, both (Y,Nb)-TZP and 3Y-TZP showed a significant decrease in the shear bond strength with the resin composite compared to the control group.
- Without mechanical pre-treatment, adhesive bonding with the resin composite using the Clearfil SE Bond is more advantageous for 3Y-TZP than for the (Y,Nb)-TZP.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denry, I.; Kelly, J.R. State of the art of zirconia for dental applications. Dent. Mater. 2008, 24, 299–307. [Google Scholar] [CrossRef]
- Kumchai, H.; Juntavee, P.; Sun, A.F.; Nathanson, D. Comparing the repair of veneered zirconia crowns with ceramic or composite resin: An in vitro study. Dent. J. 2020, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Pang, N.S.; Suh, C.S.; Kim, K.D.; Park, W.; Jung, B.Y. Prevalence of proximal contact loss between implant-supported fixed prostheses and adjacent natural teeth and its associated factors: A 7-year prospective study. Clin. Oral Implant. Res. 2017, 28, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Kaimal, A.; Ramdev, P.; Shruthi, C.S. Evaluation of effect of zirconia surface treatment, using plasma of argon and silane, on the shear bond strength of two composite resin cements. J. Clin. Diagn. Res. 2017, 11, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Prasad, H.A.; Pasha, N.; Hilal, M.; Amarnath, G.S.; Kundapur, V.; Anand, M.; Singh, S. To evaluate effect of airborne particle abrasion using different abrasives particles and compare two commercial available zirconia on flexural strength on heat treatment. Int. J. Biomed. Sci. 2017, 13, 93–112. [Google Scholar] [CrossRef] [PubMed]
- Blatz, M.B.; Richter, C.; Sadan, A.; Chiche, G.J. Critical appraisal. Resin bond to dental ceramics, Part II: High-strength ceramics. J. Esthet. Restor. Dent. 2004, 16, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.F.; Kang, L.L.; Liu, Y.C.; Lin, J.C.; Wang, C.C.; Chen, H.M.; Tai, C.K. Effects of silane- and MDP-based primers application orders on zirconia-resin adhesion—A ToF-SIMS study. Dent. Mater. 2017, 33, 923–933. [Google Scholar] [CrossRef]
- Bagheri, H.; Hooshmand, T.; Aghajani, F. Effect of ceramic surface treatments after machine grinding on the biaxial flexural strength of different CAD-CAM dental ceramic. J. Dent. 2015, 12, 621–629. [Google Scholar]
- Özcan, M.; Bernasconi, M. Adhesion to zirconia used for dental restorations: A systematic review and meta-analysis. J. Adhes. Dent. 2015, 17, 7–26. [Google Scholar]
- Scaminaci Russo, D.; Cinelli, F.; Sarti, C.; Giachetti, L. Adhesion to zirconia: A systematic review of current conditioning methods and bonding materials. Dent. J. 2019, 7, 74. [Google Scholar] [CrossRef]
- Abi-Rached, F.O.; Martins, S.B.; Campos, J.A.; Fonseca, R.G. Evaluation of roughness, wettability, and morphology of an yttria-stabilized tetragonal zirconia polycrystal ceramic after different airborne-particle abrasion protocols. J. Prosthet. Dent. 2014, 112, 1385–1391. [Google Scholar] [CrossRef]
- Mirmohammadi, H.; Aboushelib, M.N.; Salameh, Z.; Feilzer, A.J.; Kleverlaan, C.J. Innovations in bonding to zirconia based ceramics: Part III. Phosphate monomer resin cements. Dent. Mater. 2010, 26, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Aboushelib, M.N.; Mirmohamadi, H.; Matinlinna, J.P.; Kukk, E.; Ounsi, H.F.; Salameh, Z. Innovations in bonding to zirconia-based materials. Part II: Focusing on chemical interactions. Dent. Mater. 2009, 25, 989–993. [Google Scholar] [CrossRef] [PubMed]
- Ritts, A.C.; Li, H.; Yu, Q.; Xu, C.; Yao, X.; Hong, L.; Wang, Y. Dentin surface treatment using a non thermal argon plasma brush for interfacial bonding improvement in composite restoration. Eur. J. Oral Sci. 2010, 118, 510–516. [Google Scholar] [CrossRef]
- Noro, A.; Kameyama, A.; Haruyama, A.; Takahashi, T. Influence of hydrophilic pretreatment on resin bonding to zirconia ceramics. Bull. Tokyo Dent. Coll. 2015, 56, 33–39. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, Y.; Yao, X.; Li, H.; Yu, Q.; Wang, Y. Effect of a non thermal, atmospheric pressure, plasma brush on conversion of model self-etch adhesive formulations compared to conventional photo-polymerization. Dent. Mat. 2012, 28, 1232–1239. [Google Scholar] [CrossRef][Green Version]
- Park, C.; Park, S.W.; Yun, K.D.; Ji, M.K.; Kim, S.; Yang, Y.P.; Lim, H.P. Effect of plasma treatment and its post process duration on shear bonding strength and antibacterial effect of dental zirconia. Materials 2018, 11, 2233. [Google Scholar] [CrossRef]
- Valverde, G.B.; Coelho, P.G.; Janal, M.N.; Lorenzoni, F.C.; Carvalho, R.M.; Thompson, V.P.; Weltemann, K.D.; Nelson, R.F.A.S. Surface characterisation and bonding of Y-TZP following non-thermal plasma treatment. J. Dent. 2013, 41, 51–59. [Google Scholar] [CrossRef]
- Yoshihisa, K.; Yoshimura, A.; Shibamori, Y.; Fuchigami, K.; Kubota, N. Hydrophilic modification of plastic surface by using microwave plasma irradiation. IHI Eng. Rev. 2013, 46, 29–33. [Google Scholar]
- Fronza, B.M.; Noronha, M.D.S.; Price, R.B.; Pecorari, V.G.A.; Giannini, M. Influence of Adhesion Promoter Primers on Polymerization Kinetics and Long-term Bond Strength of Composite Cements to Zirconia. J. Adhes. Dent. 2022, 24, 259–268. [Google Scholar]
- Da Silva, B.T.F.; Trevelin, L.T.; Teixeira, F.D.S.; Salvadori, M.C.; Cesar, P.F.; Bona Matos, A. Non-thermal plasma increase bond strength of zirconia to a resin cement. Braz. Dent. Sci. 2018, 21, 210–219. [Google Scholar] [CrossRef]
- Lopes, B.B.; Ayres, A.P.A.; Lopes, L.B.; Negreiros, W.M.; Giannini, M. The effect of atmospheric plasma treatment of dental zirconia ceramics on the contact angle of water. Appl. Adhes. Sci. 2014, 2, 17. [Google Scholar] [CrossRef]
- Yan, M.; Yang, C.C.; Chen, Y.H.; Ding, S.J. Oxygen plasma improved shear strength of bonding between zirconia and composite resin. Coatings 2020, 10, 635. [Google Scholar] [CrossRef]
- Yoda, N.; Abe, Y.; Suenaga, Y.; Matsudate, Y.; Hoshino, T.; Sugano, T.; Sasaki, K. Resin Cement–Zirconia Bond Strengthening by Exposure to Low-Temperature Atmospheric Pressure Multi-Gas Plasma. Materials 2022, 15, 631. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.C.; Hsieh, J.P.; Chen, Y.C.; Kang, L.L.; Hwang, C.S.; Chuang, S.F. Promoting porcelain–zirconia bonding using different atmospheric pressure gas plasmas. Dent. Mater. 2018, 34, 1188–1198. [Google Scholar] [CrossRef]
- Silva, N.R.; Coelho, P.G.; Valverde, G.B.; Becker, K.; Ihrke, R.; Quade, A.; Thompson, V.P. Surface characterization of Ti and Y-TZP following non-thermal plasma exposure. J. Biomed. Mater. Res. B 2011, 99, 199–206. [Google Scholar] [CrossRef]
- Miura, S.; Fujisawa, M.; Vallittu, P.; Lassila, L. Effects of plasma surface treatment on the bond strength of zirconia with an adhesive resin luting agent. Dent. Mater. J. 2024, 43, 582–590. [Google Scholar] [CrossRef]
- Ito, Y.; Okawa, T.; Fukumoto, T.; Tsurumi, A.; Tatsuta, M.; Fujii, T.; Tanaka, M. Influence of atmospheric pressure low-temperature plasma treatment on the shear bond strength between zirconia and resin cement. J. Prosthodont. Res. 2016, 60, 289–293. [Google Scholar] [CrossRef]
- Ahn, J.J.; Kim, D.S.; Bae, E.B.; Kim, G.C.; Jeong, C.M.; Huh, J.B.; Lee, S.H. Effect of non-thermal atmospheric pressure plasma (NTP) and zirconia primer treatment on shear bond strength between Y-TZP and resin cement. Materials 2020, 13, 3934. [Google Scholar] [CrossRef]
- Ye, S.; Chuang, S.F.; Hou, S.S.; Lin, J.C.; Kang, L.L.; Chen, Y.C. Interaction of silane with 10-MDP on affecting surface chemistry and resin bonding of zirconia. Dent. Mater. 2022, 38, 715–724. [Google Scholar] [CrossRef]
- Kim, S.; Nam, Y.; Kim, S.E. Effects of forming gas plasma treatment on low-temperature Cu–Cu direct bonding. Jpn. J. Appl. Phys. 2016, 55, 06JC02. [Google Scholar] [CrossRef]
- Choi, D.; Han, S.; Chu, H.J.; Kim, I.; Kim, S. H2 Plasma Pre-treatment for Low Temperature Cu-Cu Bonding. J. Microelectron. Packag. Soc. 2021, 28, 109–114. [Google Scholar]
- Kim, D.J.; Jung, H.J.; Jang, J.W.; Lee, H.L. Fracture toughness, ionic conductivity, and low-temperature phase stability of tetragonal zirconia codoped with yttria and niobium oxide. J. Am. Ceram. Soc. 1998, 81, 2309–2314. [Google Scholar] [CrossRef]
- Fathpour, K.; Ahmadabadi, M.N.; Atash, R.; Fathi, A.H. Effect of different surface treatment methods on the shear bond strength of resin composite/zirconia for intra-oral repair of zirconia restorations. Eur. J. Dent. 2022, 17, 809–817. [Google Scholar] [CrossRef]
- Wennerberg, A. The importance of surface roughness for implant incorporation. Int. J. Mach. Tools Manuf. 1998, 38, 657–662. [Google Scholar] [CrossRef]
- Júnior, V.V.B.F.; Dantas, D.C.B.; Bresciani, E.; Huhtala, M.F.R.L. Evaluation of the bond strength and characteristics of zirconia after different surface treatments. J. Prosthet. Dent. 2018, 120, 955–959. [Google Scholar] [CrossRef]
- Tzanakakis, E.G.C.; Tzoutzas, I.G.; Koidis, T.P. Is there a potential for durable adhesion to zirconia restorations? A systematic review. J. Prosthet. Dent. 2016, 115, 9–19. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, W.; Jiang, F.; Wang, Z.; Zhao, J.; Zhou, C.; Wu, J. Effects of air-abrasion pressure on mechanical and bonding properties of translucent zirconia. Clin. Oral Investig. 2021, 25, 1979–1988. [Google Scholar] [CrossRef]
- Nagaoka, N.; Yoshihara, K.; Feitosa, V.P.; Tamada, Y.; Irie, M.; Yoshida, Y.; Hayakawa, S. Chemical interaction mechanism of 10-MDP with zirconia. Sci. Rep. 2017, 7, 45563. [Google Scholar] [CrossRef]
- Kim, D.J. Effect of Ta2O5, Nb2O5, and HfO2 alloying on the transformability of Y2O3-stabilized tetragonal ZrO2. J. Am. Ceram. Soc. 1990, 73, 115–120. [Google Scholar] [CrossRef]
- Ozer, F.; Naden, A.; Turp, V.; Mante, F.; Sen, D.; Blatz, M.B. Effect of thickness and surface modifications on flexural strength of monolithic zirconia. J. Prosthet. Dent. 2018, 119, 987–993. [Google Scholar] [CrossRef]
- Bhargava, S.; Kondo, R.; Aoki, H.; Hanawa, T.; Kasugai, S. Effect of sandblasting on the mechanical properties of Y-TZP zirconia. Bio-Med. Mater. Eng. 2012, 22, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.K.; Jo, Y.H.; Yeo, I.S.L.; Yoon, H.I.; Lee, J.H.; Ahn, J.S.; Han, J.S. Analysis of surface characteristics of (Y, Nb)-TZP after finishing and polishing. J. Adv. Prosthodont. 2022, 14, 335–345. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Zhang, Z.; Zheng, D.; Ding, N.; Liu, Y. Effect of sandblasting on surface roughness of zirconia-based ceramics and shear bond strength of veneering porcelain. Dent. Mater. J. 2014, 33, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Attia, A.; Lehmann, F.; Kern, M. Influence of surface conditioning and cleaning methods on resin bonding to zirconia ceramic. Dent. Mater. 2011, 27, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Matinlinna, J.P.; Vallittu, P.K. Silane based concepts on bonding resin composite to metals. J. Contemp. Dent. Pract. 2007, 8, 1–8. [Google Scholar]
- Lümkemann, N.; Eichberger, M.; Stawarczyk, B. Different surface modifications combined with universal adhesives: The impact on the bonding properties of zirconia to composite resin cement. Clin. Oral Investig. 2019, 23, 3941–3950. [Google Scholar] [CrossRef]
- De Mendonça, B.C.; Negreiros, W.M.; Ambrosano, G.M.B.; Giannini, M. Study of the effectiveness of primers, plasma and their combination on bond strength of a resin cement to zirconia. J. Clin. Dent. Res. 2016, 13, 7. [Google Scholar]
- Lima, W.; Bezerra, R.; Fedoce Silva, A.; Luiz de Oliveira da Rosa, W.; Piva, E.; Marques Duarte, R.; Mendonça De Souza, G. Bonding Efficacy of Universal Resin Adhesives to Zirconia Substrates: Systematic Review and Meta-Analysis. J. Adhes. Dent. 2023, 25, 51–62. [Google Scholar]
- Lai, J.; Sunderland, B.; Xue, J.; Yan, S.; Zhao, W.; Folkard, M.; Wang, Y. Study on hydrophilicity of polymer surfaces improved by plasma treatment. Appl. Surf. Sci. 2006, 252, 3375–3379. [Google Scholar] [CrossRef]




| Description | Parameter Unit |
|---|---|
| P (Power) | 782 W |
| U (Voltage) | 300 V |
| I (Ampere) | 13.8 A |
| Frequency | 23 kHz |
| Q (Air Quantity) | 40 L/m |
| P (Pressure) | 37 mBar |
| Ra | Rq | Rv | Rz | Sa | |
|---|---|---|---|---|---|
| 3Y-TZP–C | 0.35 (±0.04) | 1.21 (±0.40) | 1.61 (±0.46) | 2.82 (±0.86) | 0.56 (±0.02) |
| 3Y-TZP–SB | 0.64 (±0.04) | 2.83 (±0.29) | 2.13 (±0.13) | 4.96 (±0.41) | 0.76 (±0.02) |
| 3Y-TZP–SB + P | 0.46 (±0.02) | 1.56 (±0.13) | 1.70 (±0.17) | 3.26 (±0.14) | 0.59 (±0.00) |
| 3Y-TZP–P | 0.30 (±0.02) | 1.03 (±0.07) | 1.08 (±0.09) | 2.11 (±0.10) | 0.52 (±0.03) |
| (Y,Nb)-TZP–C | 0.54 (±0.05) | 2.02 (±0.33) | 3.41 (±0.82) | 5.43 (±0.90) | 0.82 (±0.02) |
| (Y,Nb)-TZP–SB | 0.88 (±0.12) | 3.20 (±0.59) | 2.83 (±0.29) | 6.03 (±0.88) | 1.32 (±0.12) |
| (Y,Nb)-TZP–SB + P | 0.72 (±0.01) | 2.56 (±0.16) | 2.84 (±0.08) | 5.40 (±0.08) | 1.17 (±0.05) |
| (Y,Nb)-TZP–P | 0.62 (±0.11) | 3.56 (±0.59) | 5.29 (±0.90) | 7.85 (±1.20) | 0.89 (±0.04) |
| (Unit: MPa) | Control | Plasma | Sandblast | Sandblast + Plasma |
|---|---|---|---|---|
| 3Y-TZP | 24.66 (±2.53) A | 8.87 (±2.67) ABC | 22.68 (±3.96) B | 23.14 (±4.09) C |
| (Y,Nb)-TZP | 17.89 (±4.56) ad | 9.17 (±1.79) abc | 21.75 (±4.13) b | 25.34 (±4.23) cd |
| p-value | 0.095 | 0.529 | 0.631 | 0.470 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Lim, Y.-J.; Kim, D.-J.; Kim, M.-J.; Kwon, H.-B.; Baek, Y.-W. Impact of Different Surface Treatments on Shear Bond Strength between Two Zirconia Ceramics and a Composite Material. Bioengineering 2024, 11, 1003. https://doi.org/10.3390/bioengineering11101003
Kim S-H, Lim Y-J, Kim D-J, Kim M-J, Kwon H-B, Baek Y-W. Impact of Different Surface Treatments on Shear Bond Strength between Two Zirconia Ceramics and a Composite Material. Bioengineering. 2024; 11(10):1003. https://doi.org/10.3390/bioengineering11101003
Chicago/Turabian StyleKim, Se-Hyoun, Young-Jun Lim, Dae-Joon Kim, Myung-Joo Kim, Ho-Boem Kwon, and Yeon-Wha Baek. 2024. "Impact of Different Surface Treatments on Shear Bond Strength between Two Zirconia Ceramics and a Composite Material" Bioengineering 11, no. 10: 1003. https://doi.org/10.3390/bioengineering11101003
APA StyleKim, S.-H., Lim, Y.-J., Kim, D.-J., Kim, M.-J., Kwon, H.-B., & Baek, Y.-W. (2024). Impact of Different Surface Treatments on Shear Bond Strength between Two Zirconia Ceramics and a Composite Material. Bioengineering, 11(10), 1003. https://doi.org/10.3390/bioengineering11101003









