Artificial Intelligence Transforming Post-Translational Modification Research
Abstract
1. Introduction of Post-Translational Modification
1.1. Definition of Post-Translational Modification

1.2. Importance of Post-Translational Modification
1.3. Significance of Artificial Intelligence on Post-Translational Modification Research
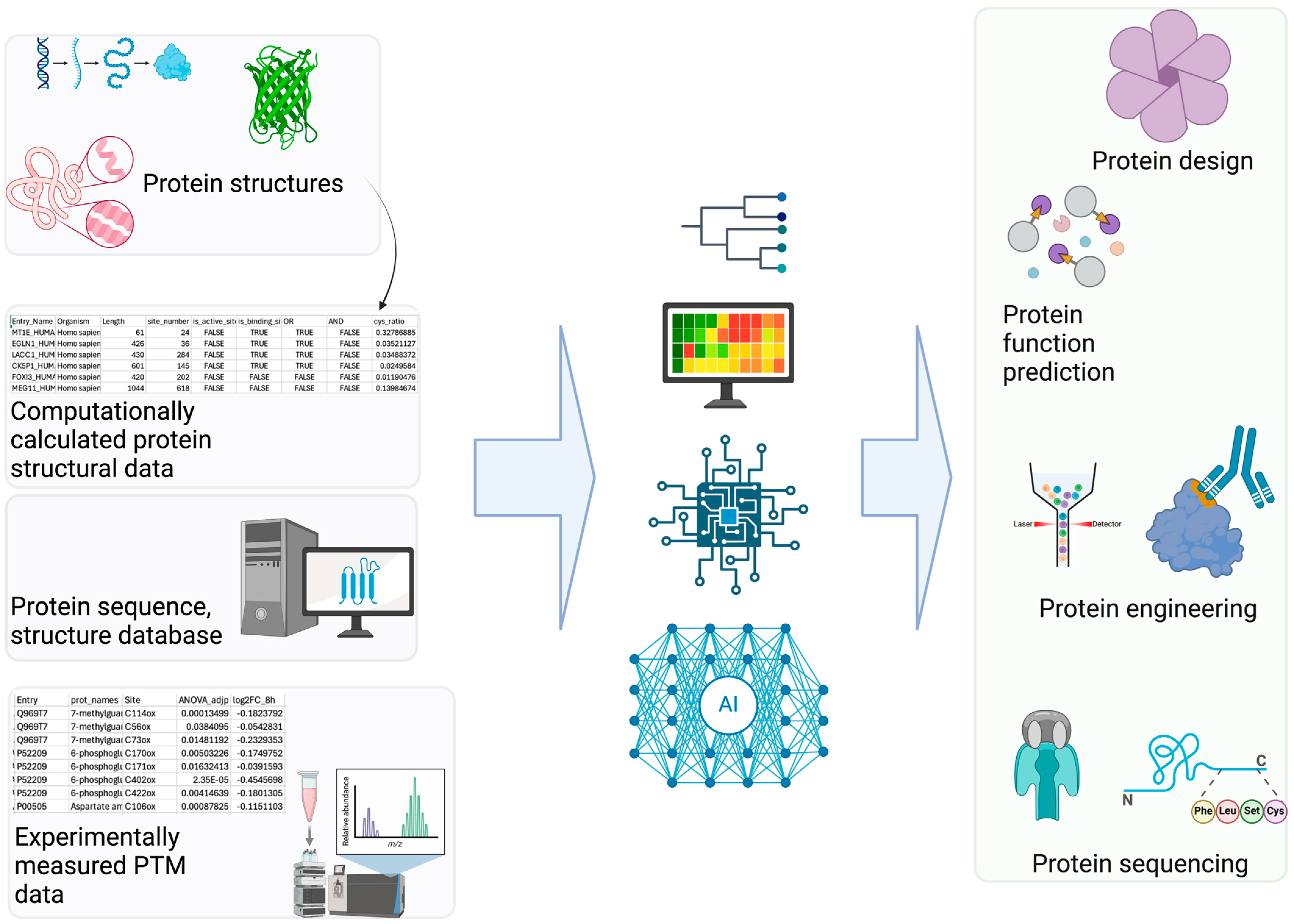
2. Computational Modeling for PTM Research
2.1. Structural Modeling for PTM Prediction
2.1.1. PTM Structural Map
2.1.2. Structural Simulation to Study Non-Canonical Amino Acid Effects
2.2. Deep Learning Approaches for PTM
2.2.1. Language Models for PTM
2.2.2. Comparison of Deep Learning Approaches for PTM
3. Experimental Data for PTM ML
3.1. Mass Spectrometry-Based PTM Proteomics for ML
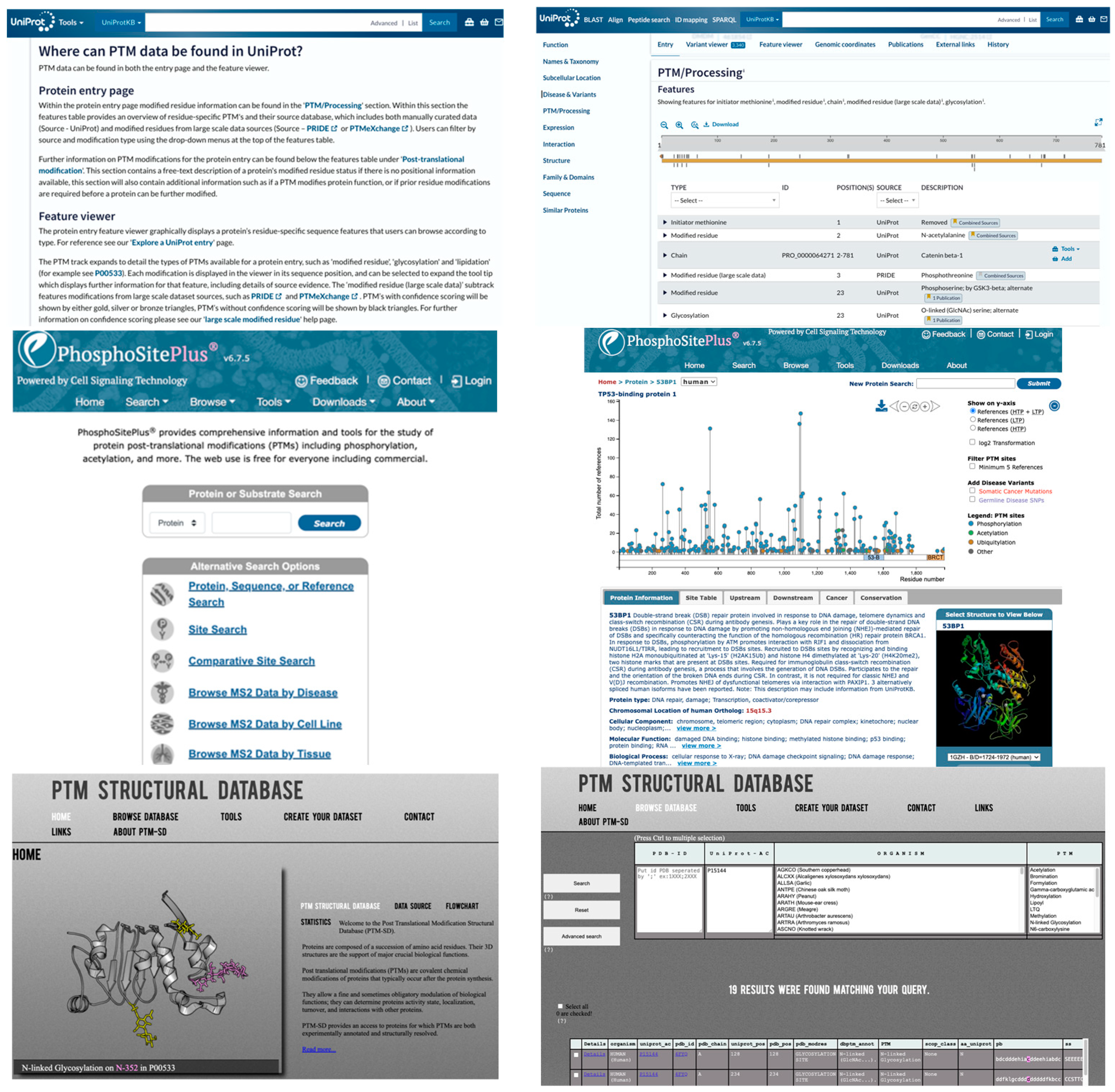
3.2. Public PTM Databases
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Ramazi, S.; Zahiri, J. Post-translational modifications in proteins: Resources, tools and prediction methods. Database J. Biol. Databases Curation 2021, 2021, baab012. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Liu, J.; Inuzuka, H.; Wei, W. Targeted protein posttranslational modifications by chemically induced proximity for cancer therapy. J. Biol. Chem. 2023, 299, 104572. [Google Scholar] [CrossRef]
- Marx, V. Inside the chase after those elusive proteoforms. Nat. Methods 2024, 21, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Leutert, M.; Entwisle, S.W.; Villén, J. Decoding Post-Translational Modification Crosstalk With Proteomics. Mol. Cell. Proteom. MCP 2021, 20, 100129. [Google Scholar] [CrossRef]
- Bobalova, J.; Strouhalova, D.; Bobal, P. Common Post-translational Modifications (PTMs) of Proteins: Analysis by Up-to-Date Analytical Techniques with an Emphasis on Barley. J. Agric. Food Chem. 2023, 71, 14825–14837. [Google Scholar] [CrossRef]
- Chung, H.S.; Wang, S.-B.; Venkatraman, V.; Murray, C.I.; Van Eyk, J.E. Cysteine Oxidative Posttranslational Modifications. Circ. Res. 2013, 112, 382–392. [Google Scholar] [CrossRef]
- ThermoFisher Scientitic Overview of Post-Translational Modifications (PTMs). Available online: https://www.thermofisher.com/ie/en/home/life-science/protein-biology/protein-biology-learning-center/protein-biology-resource-library/pierce-protein-methods/overview-post-translational-modification.html (accessed on 28 December 2024).
- Qian, M.; Yan, F.; Yuan, T.; Yang, B.; He, Q.; Zhu, H. Targeting post-translational modification of transcription factors as cancer therapy. Drug Discov. Today 2020, 25, 1502–1512. [Google Scholar] [CrossRef]
- Dunphy, K.; Dowling, P.; Bazou, D.; O’Gorman, P. Current Methods of Post-Translational Modification Analysis and Their Applications in Blood Cancers. Cancers 2021, 13, 1930. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Lindner, A.B. Protein Posttranslational Modifications: Roles in Aging and Age-Related Disease. Oxid. Med. Cell. Longev. 2017, 2017, 5716409. [Google Scholar] [CrossRef]
- Leonard, B.; Danna, V.; Gorham, L.; Davison, M.; Chrisler, W.; Kim, D.N.; Gerbasi, V.R. Shaping Nanobodies and Intrabodies against Proteoforms. Anal. Chem. 2023, 95, 8747–8751. [Google Scholar] [CrossRef] [PubMed]
- hamster prion disease with brain. Illustration from NIH BIOART Source.
- Correa Marrero, M.; Mello, V.H.; Sartori, P.; Beltrao, P. Global comparative structural analysis of responses to protein phosphorylation. bioRxiv 2024. bioRxiv:2024.10.18.617420. [Google Scholar] [CrossRef]
- Pieroni, S.; Castelli, M.; Piobbico, D.; Ferracchiato, S.; Scopetti, D.; Di-Iacovo, N.; Della-Fazia, M.A.; Servillo, G. The Four Homeostasis Knights: In Balance upon Post-Translational Modifications. Int. J. Mol. Sci. 2022, 23, 14480. [Google Scholar] [CrossRef] [PubMed]
- Chrestia, J.F.; Turani, O.; Araujo, N.R.; Hernando, G.; Esandi, M.d.C.; Bouzat, C. Regulation of nicotinic acetylcholine receptors by post-translational modifications. Pharmacol. Res. 2023, 190, 106712. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, H.; Liu, J.; Long, J. Post-translational modifications on mitochondrial metabolic enzymes in cancer. Free Radic. Biol. Med. 2022, 179, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Gupta, R.; Ambasta, R.K.; Kumar, P. Emerging trends in post-translational modification: Shedding light on Glioblastoma multiforme. Biochim. Biophys. Acta BBA—Rev. Cancer 2023, 1878, 188999. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Muzio, L.L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- Smith, L.E.; Rogowska-Wrzesinska, A. The challenge of detecting modifications on proteins. Essays Biochem. 2020, 64, 135–153. [Google Scholar] [CrossRef]
- Pakhrin, S.C.; Pokharel, S.; Aoki-Kinoshita, K.F.; Beck, M.R.; Dam, T.K.; Caragea, D.; KC, D.B. LMNglyPred: Prediction of human N-linked glycosylation sites using embeddings from a pre-trained protein language model. Glycobiology 2023, 33, 411–422. [Google Scholar] [CrossRef]
- Yu, Z.; Yu, J.; Wang, H.; Zhang, S.; Zhao, L.; Shi, S. PhosAF: An integrated deep learning architecture for predicting protein phosphorylation sites with AlphaFold2 predicted structures. Anal. Biochem. 2024, 690, 115510. [Google Scholar] [CrossRef]
- Bludau, I.; Willems, S.; Zeng, W.-F.; Strauss, M.T.; Hansen, F.M.; Tanzer, M.C.; Karayel, O.; Schulman, B.A.; Mann, M. The structural context of posttranslational modifications at a proteome-wide scale. PLOS Biol. 2022, 20, e3001636. [Google Scholar] [CrossRef] [PubMed]
- Kamacioglu, A.; Tuncbag, N.; Ozlu, N. Structural analysis of mammalian protein phosphorylation at a proteome level. Structure 2021, 29, 1219–1229.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, C.; Cai, M.; Luo, C.; Zhu, F.; Liang, Z. FuncPhos-STR: An integrated deep neural network for functional phosphosite prediction based on AlphaFold protein structure and dynamics. Int. J. Biol. Macromol. 2024, 266, 131180. [Google Scholar] [CrossRef]
- Wang, D.; Cui, P.; Zhu, W. Structural Deep Network Embedding. In Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining; Association for Computing Machinery, New York, NY, USA, 13–17 August 2016; pp. 1225–1234. [Google Scholar]
- Holden, J.K.; Pavlovicz, R.; Gobbi, A.; Song, Y.; Cunningham, C.N. Computational Site Saturation Mutagenesis of Canonical and Non-Canonical Amino Acids to Probe Protein-Peptide Interactions. Front. Mol. Biosci. 2022, 9, 848689. [Google Scholar] [CrossRef] [PubMed]
- Aphicho, K.; Kittipanukul, N.; Uttamapinant, C. Visualizing the complexity of proteins in living cells with genetic code expansion. Curr. Opin. Chem. Biol. 2022, 66, 102108. [Google Scholar] [CrossRef] [PubMed]
- Baumann, T.; Nickling, J.H.; Bartholomae, M.; Buivydas, A.; Kuipers, O.P.; Budisa, N. Prospects of In vivo Incorporation of Non-canonical Amino Acids for the Chemical Diversification of Antimicrobial Peptides. Front. Microbiol. 2017, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Walport, L.J.; Obexer, R.; Suga, H. Strategies for transitioning macrocyclic peptides to cell-pxermeable drug leads. Curr. Opin. Biotechnol. 2017, 48, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lin, Z.; Zhou, Z.; Shen, H.C.; Yan, S.F.; Mayweg, A.V.; Xu, Z.; Qin, N.; Wong, J.C.; Zhang, Z.; et al. Structure-Based Design and Synthesis of Potent Cyclic Peptides Inhibiting the YAP-TEAD Protein-Protein Interaction. ACS Med. Chem. Lett. 2014, 5, 993–998. [Google Scholar] [CrossRef]
- Renfrew, P.D.; Choi, E.J.; Bonneau, R.; Kuhlman, B. Incorporation of Noncanonical Amino Acids into Rosetta and Use in Computational Protein-Peptide Interface Design. PLoS ONE 2012, 7, e32637. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, V.K.; Kang, C.S.; Sawaya, M.R.; Rettie, S.; Li, X.; Antselovich, I.; Craven, T.W.; Watkins, A.M.; Labonte, J.W.; DiMaio, F.; et al. Computational design of mixed chirality peptide macrocycles with internal symmetry. Protein Sci. 2020, 29, 2433–2445. [Google Scholar] [CrossRef]
- Beyer, J.N.; Hosseinzadeh, P.; Gottfried-Lee, I.; Van Fossen, E.M.; Zhu, P.; Bednar, R.M.; Karplus, P.A.; Mehl, R.A.; Cooley, R.B. Overcoming Near-Cognate Suppression in a Release Factor 1-Deficient Host with an Improved Nitro-Tyrosine tRNA Synthetase. J. Mol. Biol. 2020, 432, 4690–4704. [Google Scholar] [CrossRef] [PubMed]
- Baumann, T.; Hauf, M.; Richter, F.; Albers, S.; Möglich, A.; Ignatova, Z.; Budisa, N. Computational Aminoacyl-tRNA Synthetase Library Design for Photocaged Tyrosine. Int. J. Mol. Sci. 2019, 20, 2343. [Google Scholar] [CrossRef] [PubMed]
- Karami, Y.; Murail, S.; Giribaldi, J.; Lefranc, B.; Leprince, J.; de Vries, S.J.; Tufféry, P. A novel computational method for head-to-tail peptide cyclization: Application to urotensin II. bioRxiv 2022. [Google Scholar] [CrossRef]
- Khoury, G.A.; Smadbeck, J.; Tamamis, P.; Vandris, A.C.; Kieslich, C.A.; Floudas, C.A. Forcefield_NCAA: Ab Initio Charge Parameters to Aid in the Discovery and Design of Therapeutic Proteins and Peptides with Unnatural Amino Acids and Their Application to Complement Inhibitors of the Compstatin Family. ACS Synth. Biol. 2014, 3, 855–869. [Google Scholar] [CrossRef] [PubMed]
- Buer, B.C.; Meagher, J.L.; Stuckey, J.A.; Marsh, E.N. Structural basis for the enhanced stability of highly fluorinated proteins. Proc. Natl. Acad. Sci. USA 2012, 109, 4810–4815. [Google Scholar] [CrossRef]
- Jia, F.; Wang, J.; Peng, J.; Zhao, P.; Kong, Z.; Wang, K.; Yan, W.; Wang, R. D-amino acid substitution enhances the stability of antimicrobial peptide polybia-CP. Acta Biochim. Biophys. Sin. 2017, 49, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Regina, T.; Katalin, U.; Dóra, I.; Erzsébet, F.; Alan, P.; Ferenc, H. Partial d-amino acid substitution: Improved enzymatic stability and preserved Ab recognition of a MUC2 epitope peptide. Proc. Natl. Acad. Sci. USA 2005, 102, 413–418. [Google Scholar] [CrossRef]
- Hong, S.Y.; Oh, J.E.; Lee, K.-H. Effect of d-amino acid substitution on the stability, the secondary structure, and the activity of membrane-active peptide. Biochem. Pharmacol. 1999, 58, 1775–1780. [Google Scholar] [CrossRef]
- Burley, S.K.; Bhikadiya, C.; Bi, C.; Bittrich, S.; Chen, L.; Crichlow, G.V.; Christie, C.H.; Dalenberg, K.; Di Costanzo, L.; Duarte, J.M.; et al. RCSB Protein Data Bank: Powerful new tools for exploring 3D structures of biological macromolecules for basic and applied research and education in fundamental biology, biomedicine, biotechnology, bioengineering and energy sciences. Nucleic Acids Res. 2021, 49, D437–D451. [Google Scholar] [CrossRef] [PubMed]
- Garton, M.; Nim, S.; Stone, T.A.; Wang, K.E.; Deber, C.M.; Kim, P.M. Method to generate highly stable D-amino acid analogs of bioactive helical peptides using a mirror image of the entire PDB. Proc. Natl. Acad. Sci. USA 2018, 115, 1505–1510. [Google Scholar] [CrossRef]
- Bannwarth, C.; Caldeweyher, E.; Ehlert, S.; Hansen, A.; Pracht, P.; Seibert, J.; Spicher, S.; Grimme, S. Extended tight-binding quantum chemistry methods. WIREs Comput. Mol. Sci. 2021, 11, e1493. [Google Scholar] [CrossRef]
- Kesharwani, M.K.; Karton, A.; Martin, J.M.L. Benchmark ab Initio Conformational Energies for the Proteinogenic Amino Acids through Explicitly Correlated Methods. Assessment of Density Functional Methods. J. Chem. Theory Comput. 2016, 12, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Freedberg, D.I.; Venable, R.M.; Rossi, A.; Bull, T.E.; Pastor, R.W. Discriminating the Helical Forms of Peptides by NMR and Molecular Dynamics Simulation. J. Am. Chem. Soc. 2004, 126, 10478–10484. [Google Scholar] [CrossRef]
- Buck, M.; Bouguet-Bonnet, S.; Pastor, R.W.; MacKerell Jr, A.D. Importance of the CMAP correction to the CHARMM22 protein force field: Dynamics of hen lysozyme. Biophys. J. 2006, 90, L36–L38. [Google Scholar] [CrossRef] [PubMed]
- Pagar, A.D.; Patil, M.D.; Flood, D.T.; Yoo, T.H.; Dawson, P.E.; Yun, H. Recent Advances in Biocatalysis with Chemical Modification and Expanded Amino Acid Alphabet. Chem. Rev. 2021, 121, 6173–6245. [Google Scholar] [CrossRef]
- Drew, K.; Renfrew, P.D.; Craven, T.W.; Butterfoss, G.L.; Chou, F.-C.; Lyskov, S.; Bullock, B.N.; Watkins, A.; Labonte, J.W.; Pacella, M.; et al. Adding Diverse Noncanonical Backbones to Rosetta: Enabling Peptidomimetic Design. PLoS ONE 2013, 8, e67051. [Google Scholar] [CrossRef] [PubMed]
- Renfrew, P.D.; Craven, T.W.; Butterfoss, G.L.; Kirshenbaum, K.; Bonneau, R. A Rotamer Library to Enable Modeling and Design of Peptoid Foldamers. J. Am. Chem. Soc. 2014, 136, 8772–8782. [Google Scholar] [CrossRef]
- Schneider, J.A.; Craven, T.W.; Kasper, A.C.; Yun, C.; Haugbro, M.; Briggs, E.M.; Svetlov, V.; Nudler, E.; Knaut, H.; Bonneau, R.; et al. Design of Peptoid-peptide Macrocycles to Inhibit the β-catenin TCF Interaction in Prostate Cancer. Nat. Commun. 2018, 9, 4396. [Google Scholar] [CrossRef] [PubMed]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminformatics 2011, 3, 33. [Google Scholar] [CrossRef]
- Watkins, A.; Renfrew, D. Working with Noncanonical Amino Acids in Rosetta. Available online: https://new.rosettacommons.org/docs/latest/rosetta_basics/non_protein_residues/Noncanonical-Amino-Acids (accessed on 5 May 2022).
- Brown, B.P.; Vu, O.; Geanes, A.R.; Kothiwale, S.; Butkiewicz, M.; Lowe, E.W.; Mueller, R.; Pape, R.; Mendenhall, J.; Meiler, J. Introduction to the BioChemical Library (BCL): An Application-Based Open-Source Toolkit for Integrated Cheminformatics and Machine Learning in Computer-Aided Drug Discovery. Front. Pharmacol. 2022, 13, 833099. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.N.; McNaughton, A.D.; Kumar, N. Leveraging Artificial Intelligence to Expedite Antibody Design and Enhance Antibody–Antigen Interactions. Bioengineering 2024, 11, 185. [Google Scholar] [CrossRef]
- Li, X.; Hou, C.; Yang, M.; Luo, B.; Mao, N.; Chen, K.; Chen, Z.; Bai, Y. The effect of phosphorylation on the conformational dynamics and allostery of the association of death-associated protein kinase with calmodulin. J. Biomol. Struct. Dyn. 2024. [Google Scholar] [CrossRef]
- Mejia-Rodriguez, D.; Kim, H.; Sadler, N.; Li, X.; Bohutskyi, P.; Valiev, M.; Qian, W.-J.; Cheung, M.S. PTM-Psi: A python package to facilitate the computational investigation of ost-ranslational odification on rotein tructures and their mpacts on dynamics and functions. Protein Sci. 2023, 32, e4822. [Google Scholar] [CrossRef] [PubMed]
- Tivon, B.; Gabizon, R.; Somsen, B.A.; Cossar, P.J.; Ottmann, C.; London, N. Covalent flexible peptide docking in Rosetta. Chem. Sci. 2021, 12, 10836–10847. [Google Scholar] [CrossRef] [PubMed]
- Drake, Z.C.; Seffernick, J.T.; Lindert, S. Protein complex prediction using Rosetta, AlphaFold, and mass spectrometry covalent labeling. Nat. Commun. 2022, 13, 7846. [Google Scholar] [CrossRef]
- Holcomb, M.; Santos-Martins, D.; Tillack, A.F.; Forli, S. Performance evaluation of flexible macrocycle docking in AutoDock. QRB Discov. 2022, 3, e18. [Google Scholar] [CrossRef] [PubMed]
- Meeko: Preparation of Small Molecules for AutoDock. Docking Covalent Ligands as Flexible Sidechains. Available online: https://github.com/forlilab/Meeko?tab=readme-ov-file#docking-covalent-ligands-as-flexible-sidechains (accessed on 28 December 2024).
- Santos-Martins, D.; Solis-Vasquez, L.; Tillack, A.F.; Sanner, M.F.; Koch, A.; Forli, S. Accelerating AutoDock4 with GPUs and Gradient-Based Local Search. J. Chem. Theory Comput. 2021, 17, 1060–1073. [Google Scholar] [CrossRef]
- Yousef, M.; Allmer, J. Deep learning in bioinformatics. Turk. J. Biol. 2023, 47, 366–382. [Google Scholar] [CrossRef]
- Lee, K.; Famiglietti, M.L.; McMahon, A.; Wei, C.-H.; MacArthur, J.A.L.; Poux, S.; Breuza, L.; Bridge, A.; Cunningham, F.; Xenarios, I.; et al. Scaling up data curation using deep learning: An application to literature triage in genomic variation resources. PLoS Comput. Biol. 2018, 14, e1006390. [Google Scholar] [CrossRef]
- Krishna, R.; Wang, J.; Ahern, W.; Sturmfels, P.; Venkatesh, P.; Kalvet, I.; Lee, G.R.; Morey-Burrows, F.S.; Anishchenko, I.; Humphreys, I.R.; et al. Generalized biomolecular modeling and design with RoseTTAFold All-Atom. Science 2024, 384, eadl2528. [Google Scholar] [CrossRef]
- Abramson, J.; Adler, J.; Dunger, J.; Evans, R.; Green, T.; Pritzel, A.; Ronneberger, O.; Willmore, L.; Ballard, A.J.; Bambrick, J.; et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature 2024, 630, 493–500. [Google Scholar] [CrossRef]
- Ruidong Wu; Fan Ding; Rui Wang; Rui Shen; Xiwen Zhang; Shitong Luo; Chenpeng Su; Zuofan Wu; Qi Xie; Bonnie Berger; et al. High-resolution de novo structure prediction from primary sequence. bioRxiv 2022. bioRxiv:2022.07.21.500999.
- chai-1. Available online: https://chaiassets.com/chai-1/paper/technical_report_v1.pdf (accessed on 28 December 2024).
- Lin, Z.; Akin, H.; Rao, R.; Hie, B.; Zhu, Z.; Lu, W.; Smetanin, N.; Verkuil, R.; Kabeli, O.; Shmueli, Y.; et al. Evolutionary-scale prediction of atomic-level protein structure with a language model. Science 2023, 379, 1123–1130. [Google Scholar] [CrossRef]
- Ertelt, M.; Mulligan, V.K.; Maguire, J.B.; Lyskov, S.; Moretti, R.; Schiffner, T.; Meiler, J.; Schoeder, C.T. Combining machine learning with structure-based protein design to predict and engineer post-translational modifications of proteins. PLoS Comput. Biol. 2024, 20, e1011939. [Google Scholar] [CrossRef] [PubMed]
- Leman, J.K.; Weitzner, B.D.; Renfrew, P.D.; Lewis, S.M.; Moretti, R.; Watkins, A.M.; Mulligan, V.K.; Lyskov, S.; Adolf-Bryfogle, J.; Labonte, J.W.; et al. Better together: Elements of successful scientific software development in a distributed collaborative community. PLoS Comput. Biol. 2020, 16, e1007507. [Google Scholar] [CrossRef]
- Glukhov, E.; Averkava, V.; Kotelnikov, S.; Stepanenko, D.; Nguyen, T.; Mitchell, J.C.; Simmerling, C.; Vajda, S.; Emili, A.; Padhorny, D.; et al. Phospho-Tune: Enhanced Structural Modeling of Phosphorylated Protein Interactions. bioRxiv 2024. bioRxiv:2024.02.29.582580. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, J.-Y.; Fu, M.; Wang, D.; Pelletier, A.R.; Sigdel, D.; Ng, D.C.M.; Wang, W.; Ping, P. MIND-S is a deep-learning prediction model for elucidating protein post-translational modifications in human diseases. Cell Rep. Methods 2023, 3, 100430. [Google Scholar] [CrossRef]
- Cao, C.; Magalhães, P.; Krapp, L.F.; Bada Juarez, J.F.; Mayer, S.F.; Rukes, V.; Chiki, A.; Lashuel, H.A.; Dal Peraro, M. Deep Learning-Assisted Single-Molecule Detection of Protein Post-translational Modifications with a Biological Nanopore. ACS Nano 2023, 18, 1504–1515. [Google Scholar] [CrossRef] [PubMed]
- AI Applications in PTM Research. Created in BioRender. Kim, D. 2024. Available online: https://app.biorender.com/citation/670d390a8a4a644fef1a948a (accessed on 28 December 2024).
- Brandes, N.; Ofer, D.; Peleg, Y.; Rappoport, N.; Linial, M. ProteinBERT: A universal deep-learning model of protein sequence and function. Bioinformatics 2022, 38, 2102–2110. [Google Scholar] [CrossRef]
- Elnaggar, A.; Heinzinger, M.; Dallago, C.; Rehawi, G.; Wang, Y.; Jones, L.; Gibbs, T.; Feher, T.; Angerer, C.; Steinegger, M.; et al. ProtTrans: Toward Understanding the Language of Life Through Self-Supervised Learning. IEEE Trans. Pattern Anal. Mach. Intell. 2022, 44, 7112–7127. [Google Scholar] [CrossRef]
- Rives, A.; Meier, J.; Sercu, T.; Goyal, S.; Lin, Z.; Liu, J.; Guo, D.; Ott, M.; Zitnick, C.L.; Ma, J.; et al. Biological structure and function emerge from scaling unsupervised learning to 250 million protein sequences. Proc. Natl. Acad. Sci. USA 2021, 118, e2016239118. [Google Scholar] [CrossRef] [PubMed]
- Madani, A.; Krause, B.; Greene, E.R.; Subramanian, S.; Mohr, B.P.; Holton, J.M.; Olmos, J.L.; Xiong, C.; Sun, Z.Z.; Socher, R.; et al. Large language models generate functional protein sequences across diverse families. Nat. Biotechnol. 2023, 41, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Ferruz, N.; Schmidt, S.; Höcker, B. ProtGPT2 is a deep unsupervised language model for protein design. Nat. Commun. 2022, 13, 4348. [Google Scholar] [CrossRef]
- Pakhrin, S.C.; Pokharel, S.; Pratyush, P.; Chaudhari, M.; Ismail, H.D.; KC, D.B. LMPhosSite: A Deep Learning-Based Approach for General Protein Phosphorylation Site Prediction Using Embeddings from the Local Window Sequence and Pretrained Protein Language Model. J. Proteome Res. 2023, 22, 2548–2557. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Schussheim, B.; Chatterjee, P. PTM-Mamba: A PTM-Aware Protein Language Model with Bidirectional Gated Mamba Blocks. bioRxiv 2024. bioRxiv:2024.02.28.581983. [Google Scholar] [CrossRef]
- Pokharel, S.; Pratyush, P.; Heinzinger, M.; Newman, R.H.; Kc, D.B. Improving protein succinylation sites prediction using embeddings from protein language model. Sci. Rep. 2022, 12, 16933. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Kandel, J.; Tayara, H.; Chong, K.T. Post-translational modification prediction via prompt-based fine-tuning of a GPT-2 model. Nat. Commun. 2024, 15, 6699. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Chan, W.-S.; Huang, L.; Liu, L.; Chen, X.; Zhang, W.; Wang, F.; Cheng, K.; Sun, H.; Wong, K.-C. Mini-review: Recent advances in post-translational modification site prediction based on deep learning. Comput. Struct. Biotechnol. J. 2022, 20, 3522–3532. [Google Scholar] [CrossRef]
- Wang, D.; Liu, D.; Yuchi, J.; He, F.; Jiang, Y.; Cai, S.; Li, J.; Xu, D. MusiteDeep: A deep-learning based webserver for protein post-translational modification site prediction and visualization. Nucleic Acids Res. 2020, 48, W140–W146. [Google Scholar] [CrossRef]
- Henikoff, J.G.; Henikoff, S. Using substitution probabilities to improve position-specific scoring matrices. Comput. Appl. Biosci. CABIOS 1996, 12, 135–143. [Google Scholar] [CrossRef]
- Wu, M.; Yang, Y.; Wang, H.; Xu, Y. A deep learning method to more accurately recall known lysine acetylation sites. BMC Bioinform. 2019, 20, 49. [Google Scholar] [CrossRef]
- Yang, J.; Anishchenko, I.; Park, H.; Peng, Z.; Ovchinnikov, S.; Baker, D. Improved protein structure prediction using predicted interresidue orientations. Proc. Natl. Acad. Sci. USA 2020, 117, 1496–1503. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Zhang, Q.; Liu, Z.; Du, Y.; Gao, X.; Zhao, Q.; Cheng, H.; Li, X.; Liu, Z.-X. Deep learning based prediction of reversible HAT/HDAC-specific lysine acetylation. Brief. Bioinform. 2020, 21, 1798–1805. [Google Scholar] [CrossRef] [PubMed]
- Histone-Net: A Multi-Paradigm Computational Framework for Histone Occupancy and Modification Prediction|Complex & Intelligent Systems. Available online: https://link.springer.com/article/10.1007/s40747-022-00802-w (accessed on 11 June 2024).
- Luo, F.; Wang, M.; Liu, Y.; Zhao, X.-M.; Li, A. DeepPhos: Prediction of protein phosphorylation sites with deep learning. Bioinformatics 2019, 35, 2766–2773. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Chen, X.; Cheng, K.; Chen, N.; Zheng, Z.; Wang, F.; Sun, H.; Wong, K.-C. TransPTM: A Transformer-Based Model for Non-Histone Acetylation Site Prediction. Brief. Bioinform. 2024, 25, bbae219. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-B.; Karpova, A.; Gritsenko, M.A.; Kyle, J.E.; Cao, S.; Li, Y.; Rykunov, D.; Colaprico, A.; Rothstein, J.H.; Hong, R.; et al. Proteogenomic and metabolomic characterization of human glioblastoma. Cancer Cell 2021, 39, 509–528.e20. [Google Scholar] [CrossRef] [PubMed]
- Day, N.J.; Zhang, T.; Gaffrey, M.J.; Zhao, R.; Fillmore, T.L.; Moore, R.J.; Rodney, G.G.; Qian, W.-J. A deep redox proteome profiling workflow and its application to skeletal muscle of a Duchenne Muscular Dystrophy model. Free Radic. Biol. Med. 2022, 193, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Skowronek, P.; Thielert, M.; Voytik, E.; Tanzer, M.C.; Hansen, F.M.; Willems, S.; Karayel, O.; Brunner, A.-D.; Meier, F.; Mann, M. Rapid and In-Depth Coverage of the (Phospho-)Proteome With Deep Libraries and Optimal Window Design for dia-PASEF. Mol. Cell. Proteom. 2022, 21, 100279. [Google Scholar] [CrossRef] [PubMed]
- Joyce, A.W.; Searle, B.C. Computational approaches to identify sites of phosphorylation. Proteomics 2024, 24, 2300088. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Teo, G.C.; Kong, A.T.; Haynes, S.E.; Avtonomov, D.M.; Geiszler, D.J.; Nesvizhskii, A.I. Identification of modified peptides using localization-aware open search. Nat. Commun. 2020, 11, 4065. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Wang, Y.; Yang, Y.; Zhao, D.; Wang, X.; Shen, C.; Qiao, L. DeepFLR facilitates false localization rate control in phosphoproteomics. Nat. Commun. 2023, 14, 2269. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Wang, Y.; Zheng, Y.; Liu, Z.; Zhang, Q.; Wang, S.; Zhao, Q.; Zhang, X.; Li, X.; Xu, R.-H.; et al. qPTM: An updated database for PTM dynamics in human, mouse, rat and yeast. Nucleic Acids Res. 2023, 51, D479–D487. [Google Scholar] [CrossRef]
- Boatner, L.M.; Palafox, M.F.; Schweppe, D.K.; Backus, K.M. CysDB: A human cysteine database based on experimental quantitative chemoproteomics. Cell Chem. Biol. 2023, 30, 683–698.e3. [Google Scholar] [CrossRef] [PubMed]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res. 2015, 43, D512–D520. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [CrossRef]
- Craveur, P.; Rebehmed, J.; de Brevern, A.G. PTM-SD: A database of structurally resolved and annotated posttranslational modifications in proteins. Database 2014, 2014, bau041. [Google Scholar] [CrossRef] [PubMed]
- Borowiec, M.L.; Dikow, R.B.; Frandsen, P.B.; McKeeken, A.; Valentini, G.; White, A.E. Deep learning as a tool for ecology and evolution. Methods Ecol. Evol. 2022, 13, 1640–1660. [Google Scholar] [CrossRef]
- Bradley, D. The evolution of post-translational modifications. Curr. Opin. Genet. Dev. 2022, 76, 101956. [Google Scholar] [CrossRef]
- Yin, Q.; Wu, M.; Liu, Q.; Lv, H.; Jiang, R. DeepHistone: A deep learning approach to predicting histone modifications. BMC Genom. 2019, 20, 193. [Google Scholar] [CrossRef]
- Nussinov, R.; Tsai, C.-J.; Xin, F.; Radivojac, P. Allosteric post-translational modification codes. Trends Biochem. Sci. 2012, 37, 447–455. [Google Scholar] [CrossRef]
- Ochoa, D.; Jarnuczak, A.F.; Viéitez, C.; Gehre, M.; Soucheray, M.; Mateus, A.; Kleefeldt, A.A.; Hill, A.; Garcia-Alonso, L.; Stein, F.; et al. The functional landscape of the human phosphoproteome. Nat. Biotechnol. 2020, 38, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Jung, Y.; Hwang, G.-S.; Han, H.; Cho, M. Phosphorylation alters backbone conformational preferences of serine and threonine peptides. Proteins Struct. Funct. Bioinform. 2011, 79, 3155–3165. [Google Scholar] [CrossRef]
- Tholey, A.; Lindemann, A.; Kinzel, V.; Reed, J. Direct Effects of Phosphorylation on the Preferred Backbone Conformation of Peptides: A Nuclear Magnetic Resonance Study. Biophys. J. 1999, 76, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Needham, E.J.; Parker, B.L.; Burykin, T.; James, D.E.; Humphrey, S.J. Illuminating the dark phosphoproteome. Sci. Signal. 2019, 12, eaau8645. [Google Scholar] [CrossRef] [PubMed]
| Year | Program Name | PTM Type | Model | Website |
|---|---|---|---|---|
| 2024 | LMNglyPred | Glycosylation | pLM | https://github.com/KCLabMTU/LMNglyPred (accessed on 28 December 2024) |
| 2024 | PTM-Mamba | Multiple | pLM | https://github.com/programmablebio/ptm-mamba (accessed on 28 December 2024) |
| 2024 | Sitetack | Multiple | CNN | https://sitetack.net (accessed on 28 December 2024) |
| 2024 | TransPTM | Acetylation | Transformer | https://github.com/TransPTM/TransPTM (accessed on 28 December 2024) |
| 2023 | MIND-S | Multiple | GNN | https://zenodo.org/records/7659116 (accessed on 28 December 2024) |
| 2022 | LMPhosSite | Phosphorylation | pLM, CNN | https://github.com/KCLabMTU/LMPhosSite (accessed on 28 December 2024) |
| 2021 | ScanSite 4.0 | Phosphorylation | - | https://scansite4.mit.edu (accessed on 28 December 2024) |
| 2020 | MusiteDeep | Multiple | CNN | https://www.musite.net (accessed on 28 December 2024) |
| 2019 | DeepAcet | Acetylation | MLP | https://github.com/Lab-Xu/DeepAcet (accessed on 28 December 2024) |
| 2019 | DeepHistone | Multiple | CNN | https://github.com/QijinYin/DeepHistone (accessed on 28 December 2024) |
| 2019 | DeepPhos | Phosphorylation | CNN | https://github.com/USTC-HIlab/DeepPhos (accessed on 28 December 2024) |
| 2019 | Deep-PLA | Acetylation | MLP | http://deeppla.cancerbio.info (accessed on 28 December 2024) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.N.; Yin, T.; Zhang, T.; Im, A.K.; Cort, J.R.; Rozum, J.C.; Pollock, D.; Qian, W.-J.; Feng, S. Artificial Intelligence Transforming Post-Translational Modification Research. Bioengineering 2025, 12, 26. https://doi.org/10.3390/bioengineering12010026
Kim DN, Yin T, Zhang T, Im AK, Cort JR, Rozum JC, Pollock D, Qian W-J, Feng S. Artificial Intelligence Transforming Post-Translational Modification Research. Bioengineering. 2025; 12(1):26. https://doi.org/10.3390/bioengineering12010026
Chicago/Turabian StyleKim, Doo Nam, Tianzhixi Yin, Tong Zhang, Alexandria K. Im, John R. Cort, Jordan C. Rozum, David Pollock, Wei-Jun Qian, and Song Feng. 2025. "Artificial Intelligence Transforming Post-Translational Modification Research" Bioengineering 12, no. 1: 26. https://doi.org/10.3390/bioengineering12010026
APA StyleKim, D. N., Yin, T., Zhang, T., Im, A. K., Cort, J. R., Rozum, J. C., Pollock, D., Qian, W.-J., & Feng, S. (2025). Artificial Intelligence Transforming Post-Translational Modification Research. Bioengineering, 12(1), 26. https://doi.org/10.3390/bioengineering12010026







