Heparin and Gelatin Co-Functionalized Polyurethane Artificial Blood Vessel for Improving Anticoagulation and Biocompatibility
Abstract
1. Introduction
2. Materials and Methods
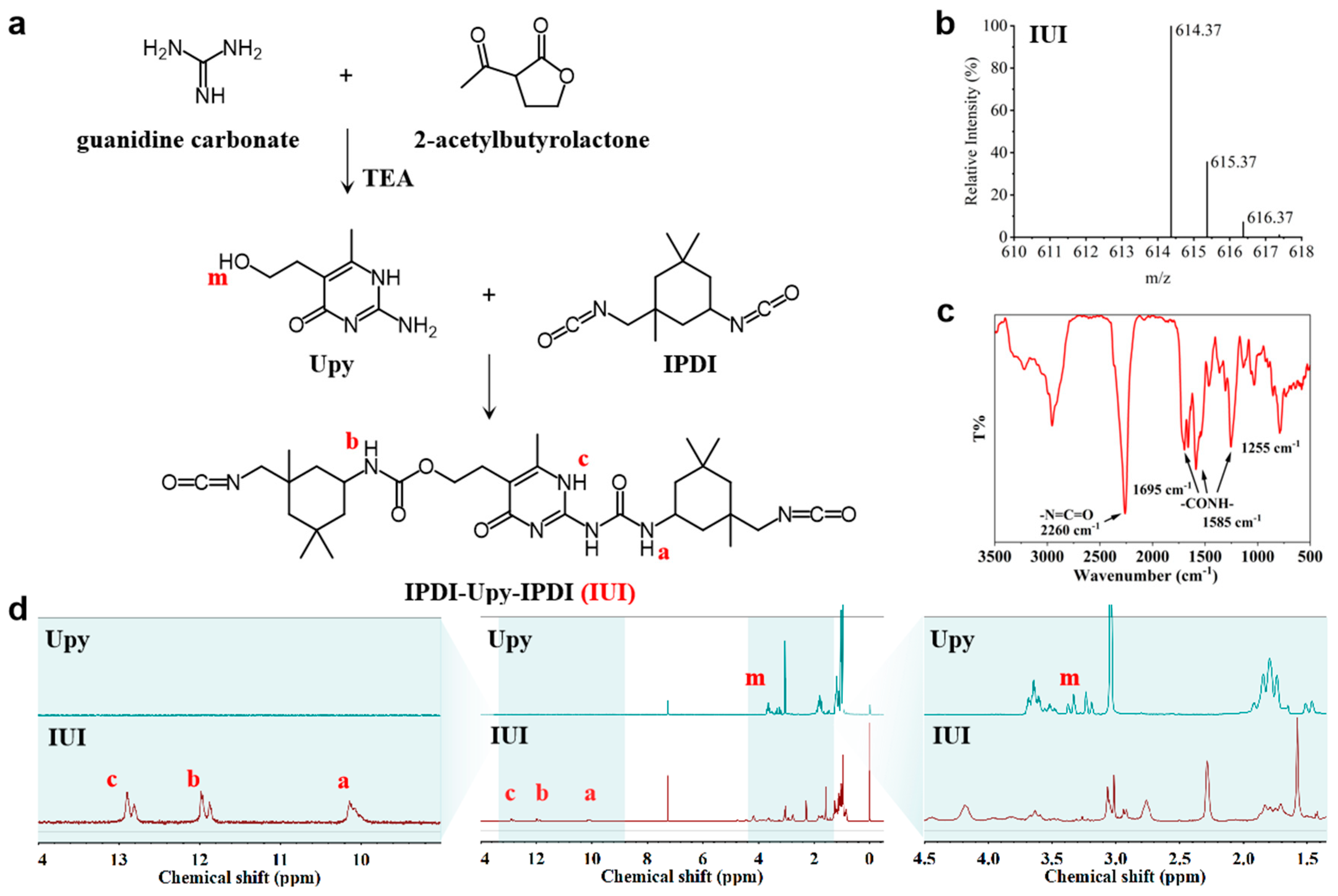
2.1. Synthesis and Characterization of IUI
2.2. Synthesis of PIIU-B Polyurethane Elastomers
2.3. Preparation and Characterization of PIIU-B Artificial Blood Vessels
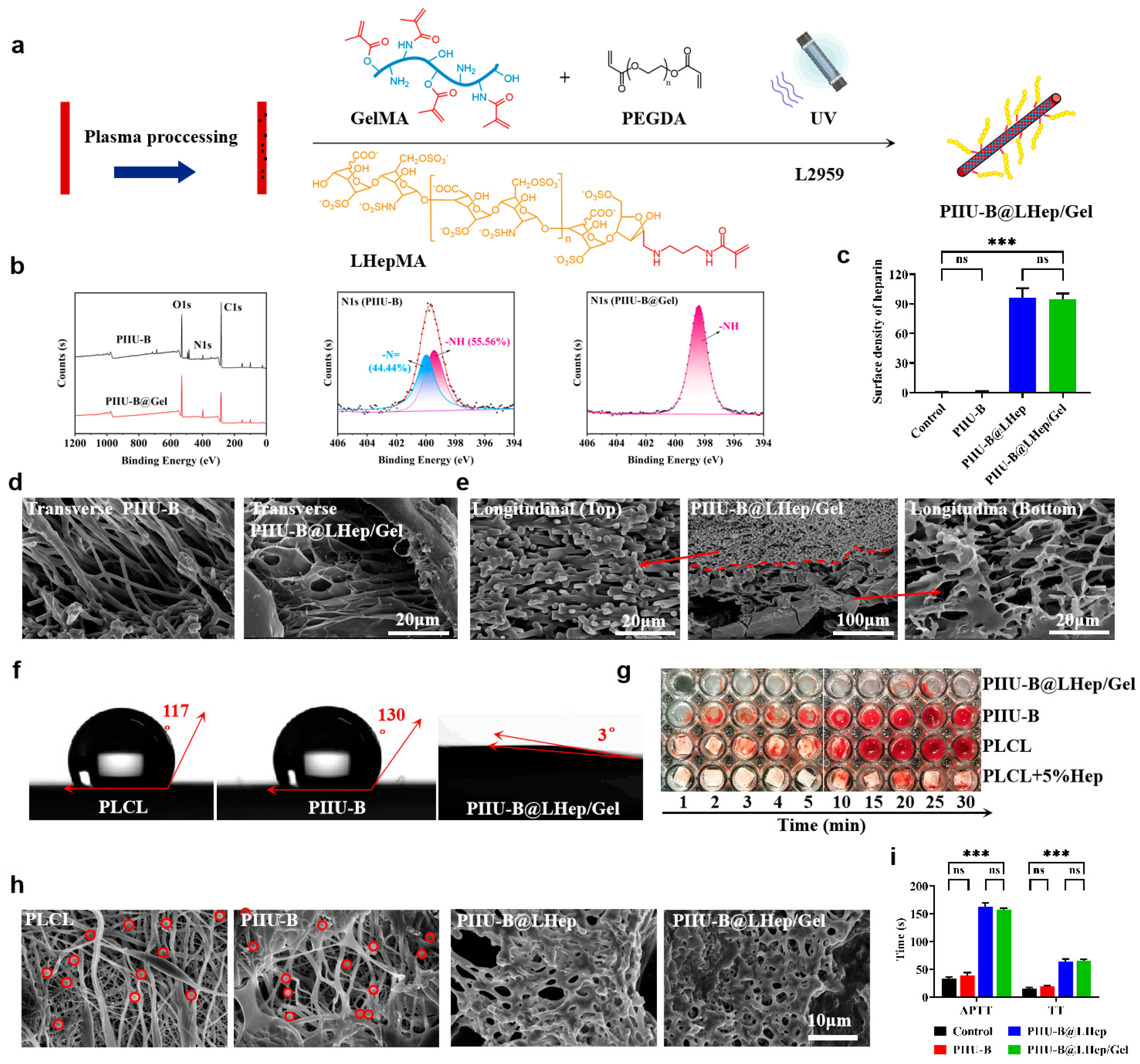
2.4. Synthesis and Characterization of LHepMA and GelMA
2.5. Functionalization of LHepMA and GelMA onto PIIU-B Vessels
2.6. Anticoagulant Activity of PIIU-B@LHep/Gel Vessels
2.7. In Vitro Biocompatibility of PIIU-B@LHep/Gel Vessels
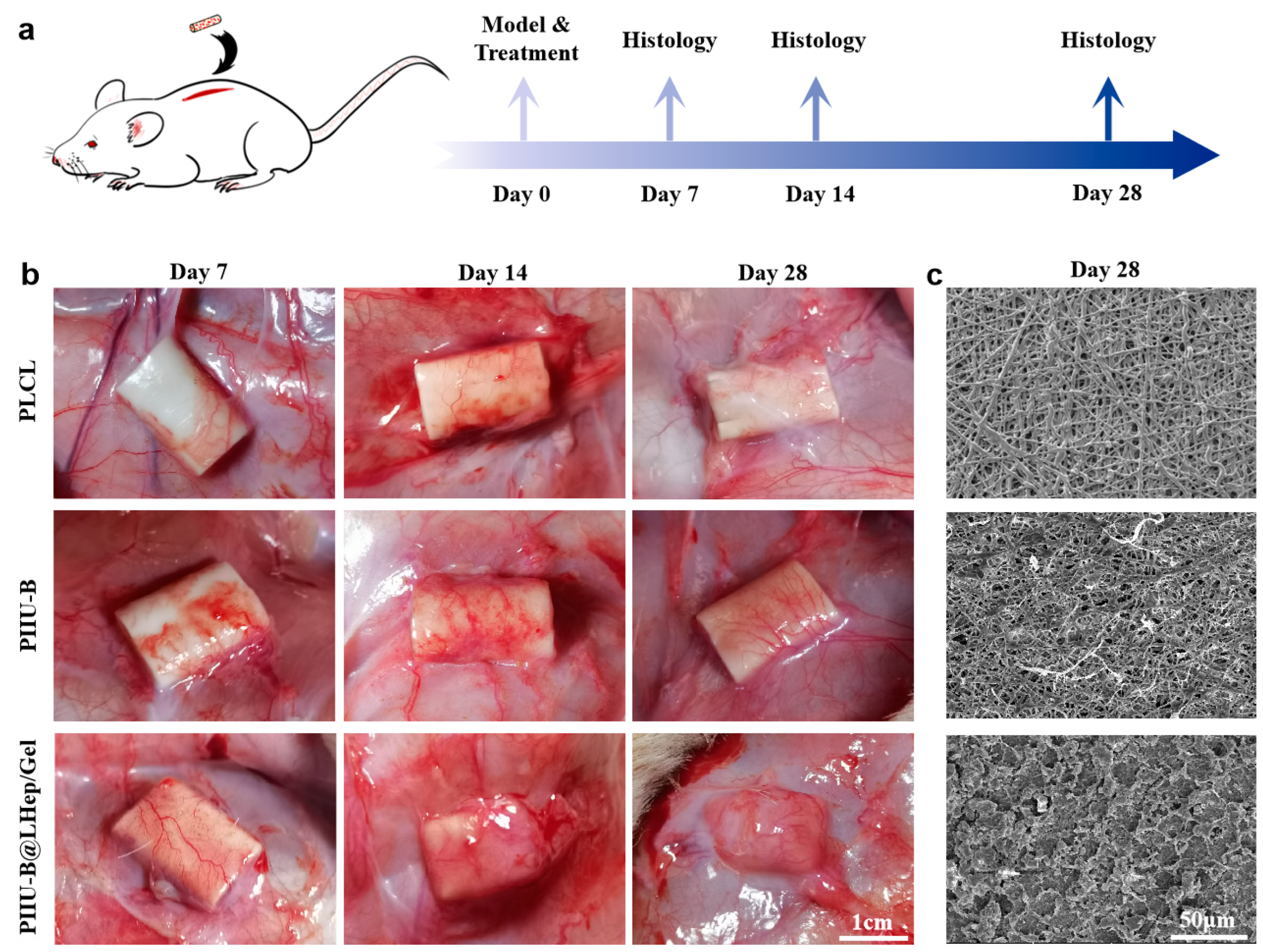
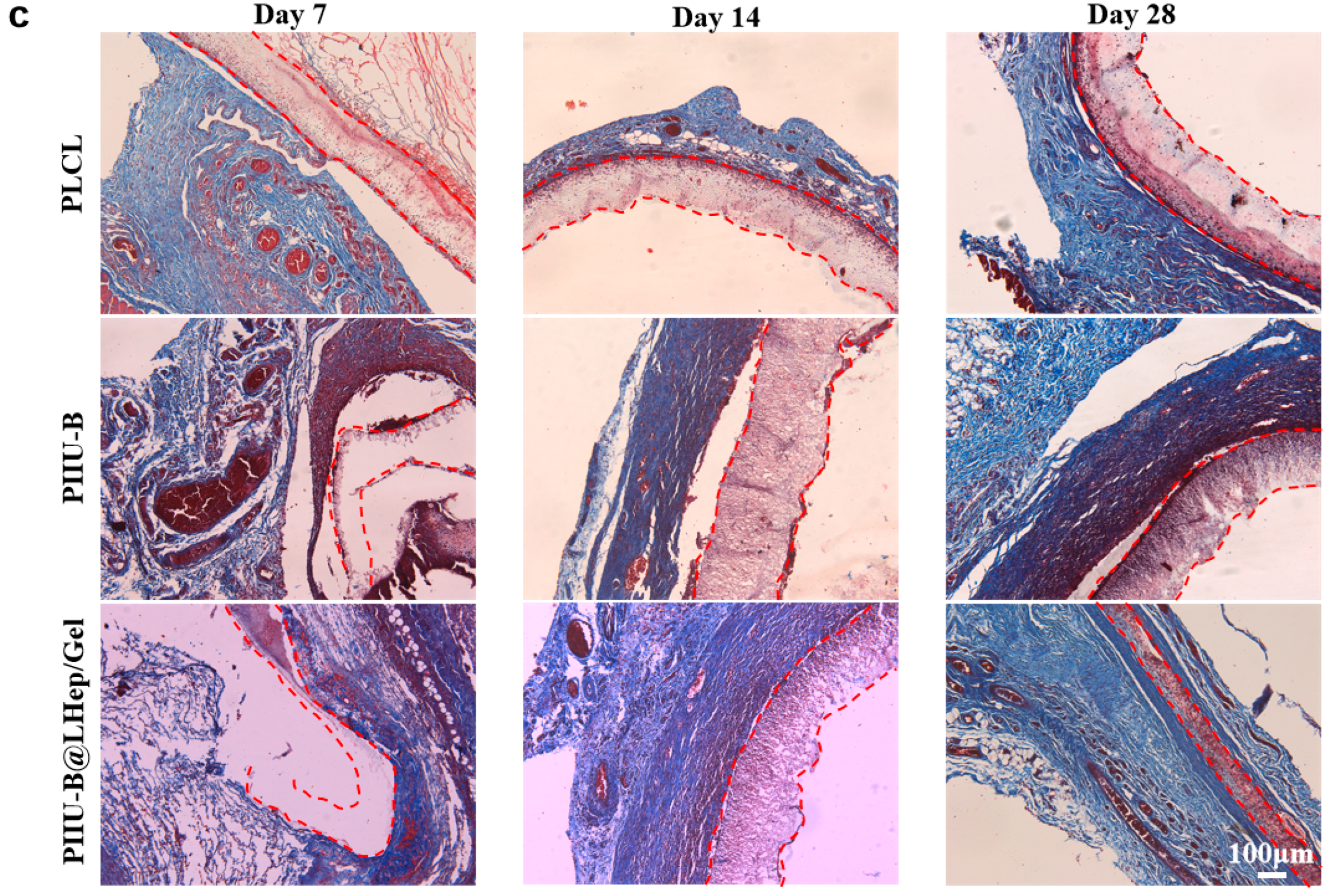
2.8. Subcutaneous Implantation Experiments
2.9. Data Analysis
3. Results
3.1. The Analysis of Synthesis and Characterization of IUI
3.2. The Analysis of Synthesis of PIIU-B Polyurethane Elastomers
3.3. Preparation and Optimization of PIIU-B Artificial Blood Vessels
3.4. The Analysis of Synthesis and Characterization of LHepMA and GelMA
3.5. The Analysis of Functionalization of LHepMA and GelMA onto PIIU-B Vessels
3.6. The Analysis of Anticoagulant Activity of PIIU-B@LHep/Gel Vessels
3.7. The Analysis of PIIU-B@LHep/Gel Vessels In Vitro Biocompatibility
3.8. In Vivo Histocompatibility of PIIU-B@LHep/Gel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PLCL | Poly (L-lactation-ε-caprolactone) |
| IPDI | Isophorone diisocyanate |
| Upy | Urea-pyrimidone |
| IUI | di-(Isophorone diisocyanate)-Ureido pyrimidinone |
| PIIU-B | Urea-pyrimidone -based polyurethane elastomers |
References
- Wang, Y.; Hu, J.; Jiao, J.; Liu, Z.; Zhou, Z.; Zhao, C.; Chang, L.-J.; Chen, Y.E.; Ma, P.X.; Yang, B. Engineering Vascular Tissue with Functional Smooth Muscle Cells Derived from Human iPS Cells and Nanofibrous Scaffolds. Biomaterials 2014, 35, 8960–8969. [Google Scholar] [CrossRef] [PubMed]
- Tzoulaki, I.; Castagné, R.; Boulangé, C.L.; Karaman, I.; Chekmeneva, E.; Evangelou, E.; Ebbels, T.M.D.; Kaluarachchi, M.R.; Chadeau-Hyam, M.; Mosen, D.; et al. Serum Metabolic Signatures of Coronary and Carotid Atherosclerosis and Subsequent Cardiovascular Disease. Eur. Heart J. 2019, 40, 2883–2896. [Google Scholar] [CrossRef]
- Moore, M.J.; Tan, R.P.; Yang, N.; Rnjak-Kovacina, J.; Wise, S.G. Bioengineering Artificial Blood Vessels from Natural Materials. Trends Biotechnol. 2022, 40, 693–707. [Google Scholar] [CrossRef] [PubMed]
- Geelhoed, W.J.; van der Bogt, K.E.A.; Rothuizen, T.C.; Damanik, F.F.R.; Hamming, J.F.; Mota, C.D.; van Agen, M.S.; de Boer, H.C.; Restrepo, M.T.; Hinz, B.; et al. A Novel Method for Engineering Autologous Non-Thrombogenic in Situ Tissue-Engineered Blood Vessels for Arteriovenous Grafting. Biomaterials 2020, 229, 119577. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, F.; Tang, Y.; Zhang, Y.; Rong, H.; Liu, L.; Gao, R.; Liu, X.; Huangfu, Y.; Bai, Y.; et al. Bioinspired, Anticoagulative, 19F MRI-Visualizable Bilayer Hydrogel Tubes as High Patency Small-Diameter Vascular Grafts. Small 2023, 19, 2302621. [Google Scholar] [CrossRef]
- Hess, C.N.; Lopes, R.D.; Gibson, C.M.; Hager, R.; Wojdyla, D.M.; Englum, B.R.; Mack, M.J.; Califf, R.M.; Kouchoukos, N.T.; Peterson, E.D.; et al. Saphenous Vein Graft Failure after Coronary Artery Bypass Surgery: Insights from PREVENT IV. Circulation 2014, 130, 1445–1451. [Google Scholar] [CrossRef]
- Kawecki, F.; L’Heureux, N. Current Biofabrication Methods for Vascular Tissue Engineering and an Introduction to Biological Textiles. Biofabrication 2023, 15, 022004. [Google Scholar] [CrossRef]
- BaoLin, G.; MA, P.X. Synthetic Biodegradable Functional Polymers for Tissue Engineering: A Brief Review. Sci. China Chem. 2014, 57, 490–500. [Google Scholar] [CrossRef]
- Xu, C.; Hong, Y. Rational Design of Biodegradable Thermoplastic Polyurethanes for Tissue Repair. Bioact. Mater. 2021, 15, 250–271. [Google Scholar] [CrossRef]
- Soletti, L.; Hong, Y.; Guan, J.; Stankus, J.J.; El-Kurdi, M.S.; Wagner, W.R.; Vorp, D.A. A Bilayered Elastomeric Scaffold for Tissue Engineering of Small Diameter Vascular Grafts. Acta Biomater. 2010, 6, 110–122. [Google Scholar] [CrossRef]
- Ahmed, F.; Saleemi, S.; Khatri, Z.; Abro, M.I.; Kim, I.-S. Co-Electrospun Poly(ɛ-Caprolactone)/Cellulose Nanofibers-Fabrication and Characterization. Carbohydr. Polym. 2015, 115, 388–393. [Google Scholar] [CrossRef]
- Shi, X.; Jing, Z.; Zhang, G.; Xu, Y.; Yao, Y. Fully Bio-Based Poly(ɛ-Capolactone)/Poly(Lactide) Alternating Multiblock Supramolecular Polymers: Synthesis, Crystallization Behavior, and Properties. J. Appl. Polym. Sci. 2017, 134, 45575. [Google Scholar] [CrossRef]
- Lamboni, L.; Gauthier, M.; Yang, G.; Wang, Q. Silk Sericin: A Versatile Material for Tissue Engineering and Drug Delivery. Biotechnol. Adv. 2015, 33, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Rohringer, S.; Grasl, C.; Ehrmann, K.; Hager, P.; Hahn, C.; Specht, S.J.; Walter, I.; Schneider, K.H.; Zopf, L.M.; Baudis, S.; et al. Biodegradable, Self-Reinforcing Vascular Grafts for In Situ Tissue Engineering Approaches. Adv. Healthc. Mater. 2023, 12, 2300520. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, F.; Guo, Z.; Zhao, Q. Tissue-Engineered Vascular Grafts and Regeneration Mechanisms. J. Mol. Cell. Cardiol. 2022, 165, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Mandal, B.B. Tissue-Engineered Vascular Grafts: Emerging Trends and Technologies. Adv. Funct. Mater. 2021, 31, 2100027. [Google Scholar] [CrossRef]
- Song, H.-H.G.; Rumma, R.T.; Ozaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell 2018, 22, 340–354. [Google Scholar] [CrossRef]
- Im, S.H.; Im, D.H.; Park, S.J.; Jung, Y.; Kim, D.-H.; Kim, S.H. Current status and future direction of metallic and polymeric materials for advanced vascular stents. Prog. Mater. Sci. 2022, 126, 100922. [Google Scholar] [CrossRef]
- Zheng, M.; Guo, J.; Li, Q.; Yang, J.; Han, Y.; Yang, H.; Yu, M.; Zhong, L.; Lu, D.; Li, L.; et al. Syntheses and characterization of anti-thrombotic and anti-oxidative Gastrodin-modified polyurethane for vascular tissue engineering. Bioact. Mater. 2021, 6, 404–419. [Google Scholar] [CrossRef]
- Quicken, S.; de Bruin, Y.; Mees, B.; Tordoir, J.; Delhaas, T.; Huberts, W. Computational study on the haemodynamic and mechanical performance of electrospun polyurethane dialysis grafts. Biomech. Model. Mechanobiol. 2020, 19, 713–722. [Google Scholar] [CrossRef]
- Wan, Y.; Yang, S.; Peng, M.; Gama, M.; Yang, Z.; Deng, X.; Zhou, J.; Ouyang, C.; Luo, H. Controllable Synthesis of Biomimetic Nano/Submicro-Fibrous Tubes for Potential Small-Diameter Vascular Grafts. J. Mater. Chem. B 2020, 8, 5694–5706. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Mizuno, H.; Okada, K.; Katoh, H.; Hitomi, S.; Teramatsu, T. Experimental Application of Polyvinyl Alcohol-Silica for Small Artificial Vessels. Biomater. Med. Devices Artif. Organs 1985, 13, 133–152. [Google Scholar] [CrossRef] [PubMed]
- Gostev, A.A.; Chernonosova, V.S.; Murashov, I.S.; Sergeevichev, D.S.; A Korobeinikov, A.; Karaskov, A.M.; Karpenko, A.A.; Laktionov, P.P. Electrospun polyurethane-based vascular grafts: Physicochemical properties and functioning in vivo. Biomed. Mater. 2020, 15, 015010. [Google Scholar] [CrossRef]
- Kuang, H.; Wang, Y.; Shi, Y.; Yao, W.; He, X.; Liu, X.; Mo, X.; Lu, S.; Zhang, P. Construction and Performance Evaluation of Hep/Silk-PLCL Composite Nanofiber Small-Caliber Artificial Blood Vessel Graft. Biomaterials 2020, 259, 120288. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, H.; Guan, Y.; Li, S.; Yuan, Z.; Lu, L.; Zheng, X. High-precision 3D printing of multi-branch vascular scaffold with plasticized PLCL thermoplastic elastomer. Biomed. Mater. 2024, 19, 035042. [Google Scholar] [CrossRef]
- Ehrmann, K.; Potzmann, P.; Dworak, C.; Bergmeister, H.; Eilenberg, M.; Grasl, C.; Koch, T.; Schima, H.; Liska, R.; Baudis, S. Hard Block Degradable Polycarbonate Urethanes: Promising Biomaterials for Electrospun Vascular Prostheses. Biomacromolecules 2020, 21, 376–387. [Google Scholar] [CrossRef]
- Dankers, P.Y.W.; van Leeuwen, E.N.M.; van Gemert, G.M.L.; Spiering, A.J.H.; Harmsen, M.C.; Brouwer, L.A.; Janssen, H.M.; Bosman, A.W.; van Luyn, M.J.A.; Meijer, E.W. Chemical and Biological Properties of Supramolecular Polymer Systems Based on Oligocaprolactones. Biomaterials 2006, 27, 5490–5501. [Google Scholar] [CrossRef]
- Freire, F.; Quiñoá, E.; Riguera, R. Supramolecular Assemblies from Poly(Phenylacetylene)s. Chem. Rev. 2016, 116, 1242–1271. [Google Scholar] [CrossRef]
- Duan, J.; Jiang, G. Synthesis, Characterization and Properties of Antibacterial Polyurethanes. Polymers 2022, 14, 213. [Google Scholar] [CrossRef]
- Mes, T.; Serrero, A.; Bauer, H.S.; Cox, M.A.J.; Bosman, A.W.; Dankers, P.Y.W.; Meijer, E.W. Supramolecular Polymer Materials Bring Restorative Heart Valve Therapy to Patients. Mater. Today 2022, 52, 175–187. [Google Scholar] [CrossRef]
- Jing, Z.; Shi, X.; Zhang, G.; Gu, J. Synthesis and Properties of Poly(Lactide)/Poly(ε-Caprolactone) Multiblock Supramolecular Polymers Bonded by the Self-Complementary Quadruple Hydrogen Bonding. Polymer 2017, 121, 124–136. [Google Scholar] [CrossRef]
- Sijbesma, R.P.; Beijer, F.H.; Brunsveld, L.; Folmer, B.J.B.; Hirschberg, J.H.K.K.; Lange, R.F.M.; Lowe, J.K.L.; Meijer, E.W. Reversible Polymers Formed from Self-Complementary Monomers Using Quadruple Hydrogen Bonding. Science 1997, 278, 1601–1604. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Wu, Y.; Li, W.; Dong, X.; Chang, H.; Wang, K.; Wu, P.; Zhang, J.; Fan, G.; Wang, L.; et al. Biodegradable and Elastomeric Vascular Grafts Enable Vascular Remodeling. Biomaterials 2018, 183, 306–318. [Google Scholar] [CrossRef]
- Yuan, X.; Li, W.; Yao, B.; Li, Z.; Kong, D.; Huang, S.; Zhu, M. Tri-Layered Vascular Grafts Guide Vascular Cells’ Native-like Arrangement. Polymers 2022, 14, 1370. [Google Scholar] [CrossRef]
- Gao, R.; Kong, P.; Yang, C.; Liu, X.; Wang, J.; Ouyang, W.; Huang, P.; Zhang, C.; Feng, Z.; Wang, W. Gelatinized PLCL Electrospun Membrane for the Prevention of Postoperative Abdominal Adhesion Through Fibrinolysis Activation. Adv. Mater. Interfaces 2022, 9, 2200063. [Google Scholar] [CrossRef]
- Gorbet, M.; Sperling, C.; Maitz, M.F.; Siedlecki, C.A.; Werner, C.; Sefton, M.V. The Blood Compatibility Challenge. Part 3: Material Associated Activation of Blood Cascades and Cells. Acta Biomater. 2019, 94, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Suflita, M.; Linhardt, R.J. Bioengineered Heparins and Heparan Sulfates. Adv. Drug Deliv. Rev. 2016, 97, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yao, Y.; Xu, T.; Wang, W.; Zhou, Y.; He, J.; Jiang, R. Application of Low Molecular Weight Heparins in Umbilical Artery Thrombosis: A Case Series and Review of the Literature. Medicine 2023, 102, e33501. [Google Scholar] [CrossRef]
- Baytas, S.N.; Varghese, S.S.; Jin, W.; Yu, Y.; He, P.; Douaisi, M.; Zhang, F.; Brodfuehrer, P.; Xia, K.; Dordick, J.S.; et al. Preparation of Low Molecular Weight Heparin from a Remodeled Bovine Intestinal Heparin. J. Med. Chem. 2021, 64, 2242–2253. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Y.; Fang, Z.; Xiao, R.; Yuan, W.; Khan, M. Fabrication and Characterization of Electrospun Gelatin-Heparin Nanofibers as Vascular Tissue Engineering. Macromol. Res. 2013, 21, 860–869. [Google Scholar] [CrossRef]
- Wang, B.; Xin, T.; Shen, L.; Zhang, K.; Zhang, D.; Zhang, H.; Liu, J.; Chen, B.; Cui, W.; Shu, Y. Acoustic Transmitted Electrospun Fibrous Membranes for Tympanic Membrane Regeneration. Chem. Eng. J. 2021, 419, 129536. [Google Scholar] [CrossRef]
- Su, J.; Satchell, S.C.; Wertheim, J.A.; Shah, R.N. Poly(Ethylene Glycol)-Crosslinked Gelatin Hydrogel Substrates with Conjugated Bioactive Peptides Influence Endothelial Cell Behavior. Biomaterials 2019, 201, 99–112. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Chen, Y.; Zhao, J.; Xu, H.; Weng, J.; Yu, F.; Xiong, A.; Udduttula, A.; Wang, D.; et al. An Injectable Liposome-Anchored Teriparatide Incorporated Gallic Acid-Grafted Gelatin Hydrogel for Osteoarthritis Treatment. Nat. Commun. 2023, 14, 3159. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun Nanofibers: New Concepts, Materials, and Applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Hoorne-Van Gemert, G.M.L.; Chodorowski-Kimmès, S.; Janssen, H.M.; Meijer, E.W.; Bosman, A.W. High Flow Supramolecular Compounds. U.S. Patent EP WO2010002262A1, 7 January 2010. [Google Scholar]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A Fascinating Fiber Fabrication Technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- GB/T 16886.4-2022; Biological Evaluation of Medical Devices—Part 4: Selection of Tests for Interactions with Blood. The National Standards of the People’s Republic of China: Beijing, China, 2022.
- Gu, Y.; Wang, F.; Wang, R.; Li, J.; Wang, C.; Li, L.; Xu, Z.; Zhang, J. Preparation and evaluation of decellularized porcine carotid arteries cross-linked by genipin: The preliminary results. Cell Tissue Bank 2018, 19, 311–321. [Google Scholar] [CrossRef]
- Meng, J.; Yang, G.; Liu, L.; Song, Y.; Jiang, L.; Wang, S. Cell adhesive spectra along surface wettability gradient from superhydrophilicity to superhydrophobicity. Sci. China Chem. 2017, 60, 614–620. [Google Scholar] [CrossRef]
- Xie, J.; Liu, W.; MacEwan, M.R.; Bridgman, P.C.; Xia, Y. Neurite Outgrowth on Electrospun Nanofibers with Uniaxial Alignment: The Effects of Fiber Density, Surface Coating, and Supporting Substrate. ACS Nano 2014, 8, 1878–1885. [Google Scholar] [CrossRef]
- Wang, K.; Liu, L.; Xie, J.; Shen, L.; Tao, J.; Zhu, J. Facile Strategy to Generate Aligned Polymer Nanofibers: Effects on Cell Adhesion. ACS Appl. Mater. Interfaces 2018, 10, 1566–1574. [Google Scholar] [CrossRef]
- Gugutkov, D.; Gustavsson, J.; Ginebra, M.P.; Altankov, G. Fibrinogen nanofibers for guiding endothelial cell behavior. Biomater. Sci. 2013, 1, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell Shape, Cytoskeletal Tension, and RhoA Regulate Stem Cell Lineage Commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Viswanathan, P.; Ondeck, M.G.; Chirasatitsin, S.; Ngamkham, K.; Reilly, G.C.; Engler, A.J.; Battaglia, G. 3D surface topology guides stem cell adhesion and differentiation. Biomaterials 2015, 52, 140–147. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, Y.; Liu, C. The Horizon of Materiobiology: A Perspective on Material-Guided Cell Behaviors and Tissue Engineering. Chem. Rev. 2017, 117, 4376–4421. [Google Scholar] [CrossRef]
- Tan, Z.; Gao, X.; Liu, T.; Yang, Y.; Zhong, J.; Tong, C.; Tan, Y. Electrospun vein grafts with high cell infiltration for vascular tissue engineering. Mater. Sci. Eng. C 2017, 81, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Tornello, P.R.C.; Caracciolo, P.C.; Roselló, J.I.I.; Abraham, G.A. Electrospun scaffolds with enlarged pore size: Porosimetry analysis. Mater. Lett. 2018, 227, 191–193. [Google Scholar] [CrossRef]
- Awad, N.K.; Niu, H.; Ali, U.; Morsi, Y.S.; Lin, T. Electrospun Fibrous Scaffolds for Small-Diameter Blood Vessels: A Review. Membranes 2018, 8, 15. [Google Scholar] [CrossRef]
- Li, S.; Yang, L.; Zhao, Z.; Yang, X.; Lv, H. A polyurethane-based hydrophilic elastomer with multi-biological functions for small-diameter vascular grafts. Acta Biomater. 2024, 176, 234–249. [Google Scholar] [CrossRef]
- Pramanik, S.; Alhomrani, M.; Alamri, A.; Alsanie, W.; Nainwal, P. Unveiling the versatility of gelatin methacryloyl hydrogels: A comprehensive journey into biomedical applications. Biomed. Mater. 2024, 19, 042008. [Google Scholar] [CrossRef]
- Xiao, Y.; Jin, X.; Jia, L.; Li, J.; Zhang, B. Long-term observation of polycaprolactone small-diameter vascular grafts with thickened outer layer and heparinized inner layer in rabbit carotid arteries. Biomed. Mater. 2024, 19, 035018. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, X.; Feng, B.; Liang, C.; Wan, X. Fiber configuration determines foreign body response of electrospun scaffolds: In vitro and in vivo assessments. Biomed. Mater. 2024, 19, 025007. [Google Scholar] [CrossRef]
- Xie, Z.; Zhang, P.; Zhang, Z.; Chen, C.; Wang, X. The choice of antimicrobial polymers: Hydrophilic or hydrophobic? Chin. Chem. Lett. 2024, 35, 109768. [Google Scholar] [CrossRef]
- Yao, Y.; Mu, J.; Liao, J.; Dong, J.; Luo, B.; Ruan, H.; Shen, Z.; Shen, J. Imparting antibacterial adhesion property to anion exchange membrane by constructing negatively charged functional layer. Sep. Purif. Technol. 2022, 288, 120628. [Google Scholar] [CrossRef]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020, 283, 102228. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Qian, Y.; Jiang, C.; Lv, Y.; Liu, W.; Zhong, L.; Cai, K.; Li, S.; Yang, L. The use of hyaluronan to regulate protein adsorption and cell infiltration in nanofibrous scaffolds. Biomaterials 2012, 33, 3428–3445. [Google Scholar] [CrossRef]
- Horakova, J.; Mikes, P.; Lukas, D.; Saman, A.; Jencova, V.; Klapstova, A.; Svarcova, T.; Ackermann, M.; Novotny, V.; Kalab, M.; et al. Electrospun vascular grafts fabricated from poly(L-lactide-co-ε-caprolactone) used as a bypass for the rabbit carotid artery. Biomed. Mater. 2018, 13, 065009. [Google Scholar] [CrossRef] [PubMed]
- Joy, J.; Aid-Launais, R.; Pereira, J.; Pavon-Djavid, G.; Ray, A.R.; Letourneur, D.; Meddahi-Pellé, A.; Gupta, B. Gelatin-polytrimethylene carbonate blend based electrospun tubular construct as a potential vascular biomaterial. Mater. Sci. Eng. C 2020, 106, 110178. [Google Scholar] [CrossRef]
- Boodagh, P.; Johnson, R.; Maly, C.; Ding, Y.; Tan, W. Soft-sheath, stiff-core microfiber hydrogel for coating vascular implants. Colloids Surf. B Biointerfaces 2019, 183, 110395. [Google Scholar] [CrossRef]
- Ratner, B.D.; Bryant, S.J. Biomaterials: Where We Have Been and Where We Are Going. Annu. Rev. Biomed. Eng. 2004, 6, 41–75. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound repair and regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Guo, J.; Zhang, J.; Li, D.; Zhong, M.; Gu, Y.; Yan, X.; Huang, P. Heparin and Gelatin Co-Functionalized Polyurethane Artificial Blood Vessel for Improving Anticoagulation and Biocompatibility. Bioengineering 2025, 12, 304. https://doi.org/10.3390/bioengineering12030304
Zhang J, Guo J, Zhang J, Li D, Zhong M, Gu Y, Yan X, Huang P. Heparin and Gelatin Co-Functionalized Polyurethane Artificial Blood Vessel for Improving Anticoagulation and Biocompatibility. Bioengineering. 2025; 12(3):304. https://doi.org/10.3390/bioengineering12030304
Chicago/Turabian StyleZhang, Jimin, Jingzhe Guo, Junxian Zhang, Danting Li, Meihui Zhong, Yuxuan Gu, Xiaozhe Yan, and Pingsheng Huang. 2025. "Heparin and Gelatin Co-Functionalized Polyurethane Artificial Blood Vessel for Improving Anticoagulation and Biocompatibility" Bioengineering 12, no. 3: 304. https://doi.org/10.3390/bioengineering12030304
APA StyleZhang, J., Guo, J., Zhang, J., Li, D., Zhong, M., Gu, Y., Yan, X., & Huang, P. (2025). Heparin and Gelatin Co-Functionalized Polyurethane Artificial Blood Vessel for Improving Anticoagulation and Biocompatibility. Bioengineering, 12(3), 304. https://doi.org/10.3390/bioengineering12030304





