Advances in Diagnostic Approaches and Therapeutic Management in Bovine Mastitis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Pathogen Causing Mastitis
3. Clinical Relevance of Bovine Mastitis
4. Economic Significance
5. Advances in Diagnostic Approaches
5.1. Milk Somatic Cell Count (SCC)
5.2. Polymerase Chain Reaction (PCR)
5.3. Nanotechnology and Biosensor-Based Diagnosis
5.4. Enzyme-Linked ImmunoSorbent Assay (ELISA)
5.5. Proteomic-Based Diagnosis
6. Alternative Therapeutic Approaches for Bovine Mastitis
6.1. Nanoparticle-Based Therapeutic Interventions for Bovine Mastitis
6.2. Herbal Therapeutic Interventions for Bovine Mastitis
6.3. Role of Bioactive Compounds Presents in Herbal Remedies against Mastitis
7. Role of Immunization and Its Constraints
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bari, M.S.; Rahman, M.M.; Persson, Y.; Derks, M.; Sayeed, M.A.; Hossain, D.; Singha, S.; Hoque, M.A.; Sivaraman, S.; Fernando, P.; et al. Subclinical Mastitis in Dairy Cows in South-Asian Countries: A Review of Risk Factors and Etiology to Prioritize Control Measures. Vet. Res. Commun. 2022, 46, 621–640. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, M.; Haine, D.; Kelton, D.F.; Barkema, H.W.; Hogeveen, H.; Keefe, G.P.; Dufour, S. Herd-Level Mastitis-Associated Costs on Canadian Dairy Farms. Front. Vet. Sci. 2018, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, A.C.; Guerra Liera, J.E.; Cervantes, R.E.; Inzunza Castro, J.F.; Villa Mancera, E.A.; Huerta Crispin, R.; Juarez Mosqueda, M.d.L.; Vazquez, A.G.; Olivares Perez, J.; Aparicio, P.S.; et al. Production of Milk and Bovine Mastitis. Adv. Dairy Res. 2017, 5, 174. [Google Scholar] [CrossRef] [Green Version]
- Sinha, M.K.; Thombare, N.N.; Mondal, B. Subclinical Mastitis in Dairy Animals: Incidence, Economics, and Predisposing Factors. Sci. World J. 2014, 2014, 523984. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, D. Estimates of Economic Losses Due to Clinical Mastitis in Organized Dairy Farms. Indian J. Dairy Sci. 2013, 66, 168–172. [Google Scholar]
- Sharma, N.; Singh, N.K.; Bhadwal, M.S. Relationship of Somatic Cell Count and Mastitis: An Overview. Asian-Australas. J. Anim. Sci. 2011, 24, 429–438. [Google Scholar] [CrossRef]
- Seegers, H.; Fourichon, C.; Beaudeau, F. Production Effects Related to Mastitis and Mastitis Economics in Dairy Cattle Herds. Vet. Res. 2003, 34, 475–491. [Google Scholar] [CrossRef] [Green Version]
- Batavani, R.A.; Asri, S.; Naebzadeh, H. The Effect of Subclinical Mastitis on Milk Composition in Dairy Cows. Iran. J. Vet. Res. 2007, 8, 205–211. [Google Scholar]
- Bruckmaier, R.M.; Ontsouka, C.E.; Blum, J.W. Fractionized Milk Composition in Dairy Cows with Subclinical Mastitis. Vet. Med. (Praha) 2004, 49, 283–290. [Google Scholar] [CrossRef]
- Urech, E.; Puhan, Z.; Schällibaum, M. Changes in Milk Protein Fraction as Affected by Subclinical Mastitis. J. Dairy Sci. 1999, 82, 2402–2411. [Google Scholar] [CrossRef]
- Huma, Z.I.; Sharma, N.; Kour, S.; Lee, S.J. Phenotypic and Molecular Characterization of Bovine Mastitis Milk Origin Bacteria and Linkage of Intramammary Infection With Milk Quality. Front. Vet. Sci. 2022, 9, 885134. [Google Scholar] [CrossRef]
- Jánosi, S.; Ratz, F.; Szigeti, G.; Kulcsar, M.; Kerényi, J.; Laukó, T.; Katona, F.; Huszenicza, G. Pathophysiology: Review of the Microbiological, Pathological, and Clinical Aspects of Bovine Mastitis Caused by the Alga Protothecazopfii. Vet. Quart. 2001, 23, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Osumi, T.; Kishimoto, Y.; Kano, R.; Maruyama, H.; Onozaki, M.; Makimura, K.; Ito, T.; Matsubara, K.; Hasegawa, A. Protothecazopfii Genotypes Isolated from Cow Barns and Bovine Mastitis in Japan. Vet. Microbiol. 2008, 131, 419–423. [Google Scholar] [CrossRef]
- Nemeth, J.; Muckle, C.A.; Gyles, C.L. In vitro Comparison of Bovine Mastitis and Fecal Escherichia Coli Isolates. Vet. Microbiol. 1994, 40, 231–238. [Google Scholar] [CrossRef]
- Rajala-Schultz, P.J.; Gröhn, Y.T.; McCulloch, C.E. Effects of Milk Fever, Ketosis, and Lameness on Milk Yield in Dairy Cows. J. Dairy Sci. 1999, 82, 288–294. [Google Scholar] [CrossRef]
- Ruegg, P.L. Management of Mastitis on Organic and Conventional Dairy Farms1. J. Anim. Sci. 2009, 87, 43–55. [Google Scholar] [CrossRef]
- Ndlela, M.; Laing, M.; Basdew, I. Biological Control of Staphylococcus Aureus-Induced Bovine Mastitis in Dairy Cows Using Bacteriophages. In Proceedings of the 6th IDF Mastitis Conference, Nantes, France, 7–9 September 2016; pp. 7–9. [Google Scholar]
- Naqvi, S.A.; De Buck, J.; Dufour, S.; Barkema, H.W. Udder Health in Canadian Dairy Heifers during Early Lactation. J. Dairy Sci. 2018, 101, 3233–3247. [Google Scholar] [CrossRef]
- Hussein, H.A.; Abd El-Razik, K.A.E.-H.; Gomaa, A.M.; Elbayoumy, M.K.; Abdelrahman, K.A.; Hosein, H.I. Milk Amyloid A as a Biomarker for Diagnosis of Subclinical Mastitis in Cattle. Vet. World 2018, 11, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, S.; Dhama, K.; Tiwari, R.; Iqbal Yatoo, M.; Khurana, S.K.; Khandia, R.; Munjal, A.; Munuswamy, P.; Kumar, M.A.; Singh, M.; et al. Technological Interventions and Advances in the Diagnosis of Intramammary Infections in Animals with Emphasis on Bovine Population—A Review. Vet. Q. 2019, 39, 76–94. [Google Scholar] [CrossRef] [Green Version]
- Gomes, F.; Henriques, M. Control of Bovine Mastitis: Old and Recent Therapeutic Approaches. Curr. Microbiol. 2016, 72, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Skowron, K.; Sękowska, A.; Kaczmarek, A.; Grudlewska, K.; Budzyńska, A.; Białucha, A.; Gospodarek-Komkowska, E. Comparison of the Effectiveness of Dipping Agents on Bacteria Causing Mastitis in Cattle. Ann. Agric. Environ. Med. 2019, 26, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Fox, L.K.; Hancock, D.D.; McMahan, W.; Park, Y.H. Prevalence and Antibiotic Resistance of Mastitis Pathogens Isolated from Dairy Herds Transitioning to Organic Management. J. Vet. Sci. 2012, 13, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babra, C.; Tiwari, J.G.; Pier, G.; Thein, T.H.; Sunagar, R.; Sundareshan, S.; Isloor, S.; Hegde, N.R.; de Wet, S.; Deighton, M.; et al. The Persistence of Biofilm-Associated Antibiotic Resistance of Staphylococcus Aureus Isolated from Clinical Bovine Mastitis Cases in Australia. Folia Microbiol. (Praha) 2013, 58, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Collado, R.; Prenafeta, A.; González-González, L.; Pérez-Pons, J.A.; Sitjà, M. Probing Vaccine Antigens against Bovine Mastitis Caused by Streptococcus Uberis. Vaccine 2016, 34, 3848–3854. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Imran, M. Causes, Types, Etiological Agents, Prevalence, Diagnosis, Treatment, Prevention, Effects on Human Health and Future Aspects of Bovine Mastitis. Anim. Health Res. Rev. 2020, 21, 36–49. [Google Scholar] [CrossRef]
- Bradley, A.J.; Breen, J.E.; Payne, B.; White, V.; Green, M.J. An Investigation of the Efficacy of a Polyvalent Mastitis Vaccine Using Different Vaccination Regimens under Field Conditions in the United Kingdom. J. Dairy Sci. 2015, 98, 1706–1720. [Google Scholar] [CrossRef] [Green Version]
- Côté-Gravel, J.; Malouin, F. Symposium Review: Features of Staphylococcus Aureus Mastitis Pathogenesis That Guide Vaccine Development Strategies. J. Dairy Sci. 2019, 102, 4727–4740. [Google Scholar] [CrossRef]
- Castelani, L.; Arcaro, J.R.P.; Braga, J.E.P.; Bosso, A.S.; Moura, Q.; Esposito, F.; Sauter, I.P.; Cortez, M.; Lincopan, N. Short Communication: Activity of Nisin, Lipid Bilayer Fragments and Cationic Nisin-Lipid Nanoparticles against Multidrug-Resistant Staphylococcus spp. Isolated from Bovine Mastitis. J. Dairy Sci. 2019, 102, 678–683. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro Machado, G.T.; Veleirinho, M.B.; Mazzarino, L.; Machado Filho, L.C.P.; Maraschin, M.; Cerri, R.L.A.; Kuhnen, S. Development of Propolis Nanoparticles for the Treatment of Bovine Mastitis: In vitro Studies on Antimicrobial and Cytotoxic Activities. Can. J. Anim. Sci. 2019, 99, 713–723. [Google Scholar] [CrossRef]
- Council, N.R. An Evaluation of the Role of Microbiological Criteria for Foods and Food Ingredients; The National Academies Press: Washington, DC, USA, 1985. [Google Scholar] [CrossRef]
- Abebe, R.; Hatiya, H.; Abera, M.; Megersa, B.; Asmare, K. Bovine Mastitis: Prevalence, Risk Factors and Isolation of Staphylococcus Aureus in Dairy Herds at Hawassa Milk Shed, South Ethiopia. BMC Vet. Res. 2016, 12, 270. [Google Scholar] [CrossRef] [Green Version]
- Klaas, I.C.; Zadoks, R.N. An Update on Environmental Mastitis: Challenging Perceptions. Transbound. Emerg. Dis. 2018, 65, 166–185. [Google Scholar] [CrossRef] [Green Version]
- Levison, L.J.; Miller-Cushon, E.K.; Tucker, A.L.; Bergeron, R.; Leslie, K.E.; Barkema, H.W.; DeVries, T.J. Incidence Rate of Pathogen-Specific Clinical Mastitis on Conventional and Organic Canadian Dairy Farms. J. Dairy Sci. 2016, 99, 1341–1350. [Google Scholar] [CrossRef] [Green Version]
- Shinozuka, Y.; Morita, T.; Watanabe, A.; Kawai, K. Live Bacteria in Clots from Bovine Clinical Mastitis Milk with No Growth in Conventional Culturing. Asian J. Anim. Vet. Adv. 2018, 13, 197–200. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, J.; Barkema, H.W.; Ali, T.; Liu, G.; Deng, Y.; Naushad, S.; Kastelic, J.P.; Han, B. Virulence Gene Profiles: Alpha-Hemolysin and Clonal Diversity in Staphylococcus Aureus Isolates from Bovine Clinical Mastitis in China. BMC Vet. Res. 2018, 14, 63. [Google Scholar] [CrossRef] [Green Version]
- Abdalhamed, A.M.; Ghazy, A.A.; Ibrahim, E.S.; Arafa, A.A.; Zeedan, G.S.G. Therapeutic Effect of Biosynthetic Gold Nanoparticles on Multidrug-Resistant Escherichia Coli and Salmonella Species Isolated from Ruminants. Vet. World 2021, 14, 3200–3210. [Google Scholar] [CrossRef]
- Petersson-Wolfe, C.S.; Mullarky, I.K.; Jones, G.M. Staphylococcus Aureus Mastitis: Cause, Detection, and Control; Communications and Marketing, College of Agriculture and Life Sciences, Virginia Polytechnic Institute and State University: Blacksburg, VA, USA, 2010. [Google Scholar]
- Cortinhas, C.S.; Tomazi, T.; Zoni, M.S.F.; Moro, E.; Veiga dos Santos, M. Randomized Clinical Trial Comparing Ceftiofur Hydrochloride with a Positive Control Protocol for Intramammary Treatment of Nonsevere Clinical Mastitis in Dairy Cows. J. Dairy Sci. 2016, 99, 5619–5628. [Google Scholar] [CrossRef] [Green Version]
- Keefe, G. Update on Control of Staphylococcus Aureus and Streptococcus Agalactiae for Management of Mastitis. Vet. Clin. Food Anim. Pract. 2012, 28, 203–216. [Google Scholar] [CrossRef]
- Malinowski, E.; Lassa, H.; Klossowska, A.; Markiewicz, H.; Kaczmarowski, M.; Smulski, S. Relationship between Mastitis Agents and Somatic Cell Count in Foremilk Samples. Bull.-Vet. Inst. Puławy 2006, 50, 349. [Google Scholar]
- Gröhn, Y.T.; Wilson, D.J.; González, R.N.; Hertl, J.A.; Schulte, H.; Bennett, G.; Schukken, Y.H. Effect of Pathogen-Specific Clinical Mastitis on Milk Yield in Dairy Cows. J. Dairy Sci. 2004, 87, 3358–3374. [Google Scholar] [CrossRef] [Green Version]
- Vakkamäki, J.; Taponen, S.; Heikkilä, A.-M.; Pyörälä, S. Bacteriological Etiology and Treatment of Mastitis in Finnish Dairy Herds. Acta Vet. Scand. 2017, 59, 33. [Google Scholar] [CrossRef] [Green Version]
- Wernicki, A.; Puchalski, A.; Urban-Chmiel, R.; Dec, M.; Stegierska, D.; Dudzic, A.; Wojcik, A. Antimicrobial Properties of Gold, Silver, Copper and Platinum Nanoparticles against Selected Microorganisms Isolated from Cases of Mastitis in Cattle. Med. Weter. 2014, 70, 564–567. [Google Scholar]
- Wilson, D.J.; Gonzalez, R.N.; Das, H.H. Bovine Mastitis Pathogens in New York and Pennsylvania: Prevalence and Effects on Somatic Cell Count and Milk Production. J. Dairy Sci. 1997, 80, 2592–2598. [Google Scholar] [CrossRef] [PubMed]
- Steele, N.; McDougall, S. Effect of Prolonged Duration Therapy of Subclinical Mastitis in Lactating Dairy Cows Using Penethamate Hydriodide. N. Z. Vet. J. 2014, 62, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.C.; Capra, E.; Morandi, S.; Cremonesi, P.; Pantoja, J.C.F.; Langoni, H.; de Vargas, A.P.C.; Da Costa, M.M.; Jagielski, T.; Bolaños, C.A.D. In vitro Algicidal Effect of Guanidine on Protothecazopfii Genotype 2 Strains Isolated from Clinical and Subclinical Bovine Mastitis. Lett. Appl. Microbiol. 2017, 64, 419–423. [Google Scholar] [CrossRef]
- dos Anjos, C.; Sellera, F.P.; Gargano, R.G.; Lincopan, N.; Pogliani, F.C.; Ribeiro, M.G.; Jagielski, T.; Sabino, C.P. Algicidal Effect of Blue Light on Pathogenic Prototheca Species. Photodiagnosis Photodyn. Ther. 2019, 26, 210–213. [Google Scholar] [CrossRef]
- Machtinger, E.T.; Gerry, A.C.; Murillo, A.C.; Talley, J.L. Filth Fly Impacts to Animal Production in the United States and Associated Research and Extension Needs. J. Integr. Pest. Manag. 2021, 12, 41. [Google Scholar] [CrossRef]
- Bruce, W.N.; Decker, G.C. The Relationship of Stable Fly Abundance to Milk Production in Dairy Cattle1. J. Econ. Entomol. 1958, 51, 269–274. [Google Scholar] [CrossRef]
- Harris, J.A.; Hillerton, J.E.; Morant, S.V. Effect on Milk Production of Controlling Muscid Flies, and Reducing Fly-Avoidance Behaviour, by the Use of Fenvalerate Ear Tags during the Dry Period. J. Dairy. Res. 1987, 54, 165–171. [Google Scholar] [CrossRef]
- Basiel, B.L. Genomic Evaluation of Horn Fly Resistance and Phenotypes of Cholesterol Deficiency and Carriers in Holstein Cattle. Master’s Thesis, The Graduate School, The Pennsylvania State University, University Park, PA, USA, 2020. [Google Scholar]
- Owens, W.E.; Oliver, S.P.; Gillespie, B.E.; Ray, C.H.; Nickerson, S.C. Role of Horn Flies (Haematobia Irritans) in Staphylococcus Aureus-Induced Mastitis in Dairy Heifers. Am. J. Vet. Res. 1998, 59, 1122–1124. [Google Scholar]
- Anderson, K.L.; Lyman, R.; Moury, K.; Ray, D.; Watson, D.W.; Correa, M.T. Molecular Epidemiology of Staphylococcus Aureus Mastitis in Dairy Heifers. J. Dairy Sci. 2012, 95, 4921–4930. [Google Scholar] [CrossRef] [Green Version]
- Woudstra, S.; Wente, N.; Zhang, Y.; Leimbach, S.; Kirkeby, C.; Gussmann, M.K.; Krömker, V. Reservoirs of Staphylococcus spp. and Streptococcus spp. Associated with Intramammary Infections of Dairy Cows. Pathogens 2023, 12, 699. [Google Scholar] [CrossRef]
- Bahrndorff, S.; de Jonge, N.; Skovgård, H.; Nielsen, J.L. Bacterial Communities Associated with Houseflies (Musca Domestica L.) Sampled within and between Farms. PLoS ONE 2017, 12, e0169753. [Google Scholar] [CrossRef] [Green Version]
- Ranjbar, R.; Izadi, M.; Hafshejani, T.T.; Khamesipour, F. Molecular Detection and Antimicrobial Resistance of Klebsiella Pneumoniae from House Flies (Musca Domestica) in Kitchens, Farms, Hospitals and Slaughterhouses. J. Infect. Public. Health 2016, 9, 499–505. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.F.; Ward, G.E. Cause, Occurrence, and Clinical Signs of Mastitis and Anorexia in Cows in a Wisconsin Study. J. Am. Vet. Med. Assoc. 1989, 195, 1108–1113. [Google Scholar]
- Workineh, S.; Bayleyegn, M.; Mekonnen, H.; Potgieter, L.N.D. Prevalence and Aetiology of Mastitis in Cows from Two Major Ethiopian Dairies. Trop. Anim. Health Prod. 2002, 34, 19–25. [Google Scholar] [CrossRef]
- Nickerson, S.C.; Owens, W.E.; Boddie, R.L. Mastitis in Dairy Heifers: Initial Studies on Prevalence and Control. J. Dairy Sci. 1995, 78, 1607–1618. [Google Scholar] [CrossRef]
- Garcia, M.L.; Moreno, B.; Bergdoll, M.S. Characterization of Staphylococci Isolated from Mastitic Cows in Spain. Appl. Environ. Microbiol. 1980, 39, 548–553. [Google Scholar] [CrossRef] [Green Version]
- Davis, W.T.; Maplesden, D.C.; Natzke, R.P.; Philpot, W.N. Sodium Cloxacillin for Treatment of Mastitis in Lactating Cows. J. Dairy Sci. 1975, 58, 1822–1827. [Google Scholar] [CrossRef]
- Uzoaga, G.O.; Umeokonkwo, C.D.; Usman, A.B.; Kia, G.S.; Okolocha, E.C. Bacteriological Quality of Nono, a Milk Product Sold at Retail Outlets in Federal Capital Territory, Nigeria. J. Interval Epidemiol. Public Health 1920, 3, 1. [Google Scholar] [CrossRef]
- Minett, F.C. Clinical Forms and Bacteriology of Bovine Mastitis. Proc. R. Soc. Med. 1930, 23, 1344–1346. [Google Scholar] [CrossRef] [Green Version]
- Fazal, M.A.; Rana, E.A.; Akter, S.; Alim, M.A.; Barua, H.; Ahad, A. Molecular Identification, Antimicrobial Resistance and Virulence Gene Profiling of Diverse Staphylococcus spp. Involved in Bovine Sub-Clinical Mastitis, Bangladesh. Vet. Anim. Sci. 2023, 21, 100297. [Google Scholar] [CrossRef] [PubMed]
- Nalband, S.M.; Kolhe, R.P.; Deshpande, P.D.; Jadhav, S.N.; Gandhale, D.G.; Muglikar, D.M.; Kolhe, S.R.; Bhave, S.S.; Jagtap, U.V.; Dhandore, C. V Characterization of Escherichia Coli Isolated from Bovine Subclinical Mastitis for Virulence Genes, Phylogenetic Groups and ESBL Production. Indian J. Anim. Res. 2020, 54, 1265–1271. [Google Scholar] [CrossRef]
- Kurjogi, M.M.; Kaliwal, B.B. Prevalence and Antimicrobial Susceptibility of Bacteria Isolated from Bovine Mastitis. Adv. Appl. Sci. Res. 2011, 2, 229–235. [Google Scholar]
- Nigam, R. Incidence and Pattern of Antibiotic Resistance of Staphylococcus Aureus Isolated from Clinical and Subclinical Mastitis in Cattle and Buffaloes. Asian J. Anim. Sci. 2015, 9, 100–109. [Google Scholar]
- Atyabi, N.; Vodjgani, M.; Gharagozloo, F.; Bahonar, A. Prevalence of Bacterial Mastitis in Cattle from the Farms around Tehran. Iran. J. Vet. Res. 2006, 7, 76–79. [Google Scholar]
- Sivakumar, R.; Pranav, P.S.; Annamanedi, M.; Chandrapriya, S.; Isloor, S.; Rajendhran, J.; Hegde, N.R. Genome Sequencing and Comparative Genomic Analysis of Bovine Mastitis-Associated Staphylococcus Aureus Strains from India. BMC Genom. 2023, 24, 44. [Google Scholar] [CrossRef]
- Exel, C.E.; Gerritsen, K.; Spaninks, M.; Duim, B.; Koop, G.; Benedictus, L. Association of Staphylococcus Aureus Genotypes with Milk or Colonization of Extramammary Sites in Dutch Dairy Cattle Indicates Strain Variation in Reservoirs for Intramammary Infections. Res. Vet. Sci. 2023, 154, 138–144. [Google Scholar] [CrossRef]
- Huma, Z.I.; Sharma, N.; Kour, S.; Tandon, S.; Guttula, P.K.; Kour, S.; Singh, A.K.; Singh, R.; Gupta, M.K. Putative Biomarkers for Early Detection of Mastitis in Cattle. Anim. Prod. Sci. 2020, 60, 1721. [Google Scholar] [CrossRef]
- Campos, B.; Pickering, A.C.; Rocha, L.S.; Aguilar, A.P.; Fabres-Klein, M.H.; de Oliveira Mendes, T.A.; Fitzgerald, J.R.; de Oliveira Barros Ribon, A. Diversity and Pathogenesis of Staphylococcus Aureus from Bovine Mastitis: Current Understanding and Future Perspectives. BMC Vet. Res. 2022, 18, 115. [Google Scholar] [CrossRef]
- Asadpour, R.; Zangiband, P.; Nofouzi, K.; Saberivand, A. Differential Expression of Antioxidant Genes during Clinical Mastitis of Cow Caused by Staphylococcus Aureus and Escherichia Coli. Vet. Arh. 2021, 91, 451–458. [Google Scholar] [CrossRef]
- Greening, S.S.; Zhang, J.; Midwinter, A.C.; Wilkinson, D.A.; McDougall, S.; Gates, M.C.; French, N.P. The Genetic Relatedness and Antimicrobial Resistance Patterns of Mastitis-Causing Staphylococcus Aureus Strains Isolated from New Zealand Dairy Cattle. Vet. Sci. 2021, 8, 287. [Google Scholar] [CrossRef]
- Putz, E.J.; Palmer, M.V.; Ma, H.; Casas, E.; Reinhardt, T.A.; Lippolis, J.D. Case Report: Characterization of a Persistent, Treatment-Resistant, Novel Staphylococcus Aureus Infection Causing Chronic Mastitis in a Holstein Dairy Cow. BMC Vet. Res. 2020, 16, 336. [Google Scholar] [CrossRef]
- Hoekstra, J.; Zomer, A.L.; Rutten, V.P.M.G.; Benedictus, L.; Stegeman, A.; Spaninks, M.P.; Bennedsgaard, T.W.; Biggs, A.; De Vliegher, S.; Mateo, D.H.; et al. Genomic Analysis of European Bovine Staphylococcus Aureus from Clinical versus Subclinical Mastitis. Sci. Rep. 2020, 10, 18172. [Google Scholar] [CrossRef]
- Ewida, R.M.; Al-Hosary, A.A.T. Prevalence of Enterotoxins and Other Virulence Genes of Staphylococcus Aureus Caused Subclinical Mastitis in Dairy Cows. Vet. World 2020, 13, 1193–1198. [Google Scholar] [CrossRef]
- Capurro, A.; Aspán, A.; Ericsson Unnerstad, H.; Persson Waller, K.; Artursson, K. Identification of Potential Sources of Staphylococcus Aureus in Herds with Mastitis Problems. J. Dairy Sci. 2010, 93, 180–191. [Google Scholar] [CrossRef] [Green Version]
- Gentilini, E.; Denamiel, G.; Llorente, P.; Godaly, S.; Rebuelto, M.; DeGregorio, O. Antimicrobial Susceptibility of Staphylococcus Aureus Isolated from Bovine Mastitis in Argentina. J. Dairy Sci. 2000, 83, 1224–1227. [Google Scholar] [CrossRef]
- Buddle, B.M.; Pulford, H.D. Evaluation of Levamisole for Use in Control of Bovine Staphylococcus aureus Mastitis. N. Z. Vet. J. 1985, 33, 177–180. [Google Scholar] [CrossRef]
- Bhatt, V.D.; Patel, M.S.; Joshi, C.G.; Kunjadia, A. Identification and Antibiogram of Microbes Associated with Bovine Mastitis. Anim. Biotechnol. 2011, 22, 163–169. [Google Scholar] [CrossRef]
- Savage, W.G. Domestic Animals as a Factor in the Spread of Infection. J. R. Sanit. Inst. 1920, 41, 285–291. [Google Scholar] [CrossRef]
- Gwatkin, R.; Hadwen, S.; Legard, H.M. Bovine Mastitis: Notes on Incidence, Aetiology and Diagnosis. Can. J. Comp. Med. 1937, 1, 7–16. [Google Scholar]
- Baba, E.; Fukata, T.; Matsumoto, H. Ecological Studies on Coagulase-Negative Staphylococci in and around Bovine Udder. Bull. Osaka Pref. 1980, 32, 69–75. [Google Scholar]
- Trinidad, P.; Nickerson, S.C.; Alley, T.K. Prevalence of Intramammary Infection and Teat Canal Colonization In Unbred and Primigravid Dairy Heifers. J. Dairy Sci. 1990, 73, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Maddox, C.W. Cytotoxic Activity of Coagulase-Negative Staphylococci in Bovine Mastitis. Infect. Immun. 2000, 68, 1102–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björk, S.; Båge, R.; Kanyima, B.M.; André, S.; Nassuna-Musoke, M.G.; Owiny, D.O.; Persson, Y. Characterization of Coagulase Negative Staphylococci from Cases of Subclinical Mastitis in Dairy Cattle in Kampala, Uganda. Ir. Vet. J. 2014, 67, 12. [Google Scholar] [CrossRef] [Green Version]
- Bochniarz, M.; Wawron, W.; Szczubiał, M. Coagulase-Negative Staphylococci (CNS) as an Aetiological Factor of Mastitis in Cows. Pol. J. Vet. Sci. 2013, 16, 487–492. [Google Scholar] [CrossRef]
- Bochniarz, M.; Zdzisińska, B.; Wawron, W.; Szczubiał, M.; Dąbrowski, R. Milk and Serum IL-4, IL-6, IL-10, and Amyloid A Concentrations in Cows with Subclinical Mastitis Caused by Coagulase-Negative Staphylococci. J. Dairy Sci. 2017, 100, 9674–9680. [Google Scholar] [CrossRef] [Green Version]
- Khazandi, M.; Al-Farha, A.A.B.; Coombs, G.W.; O’Dea, M.; Pang, S.; Trott, D.J.; Aviles, R.R.; Hemmatzadeh, F.; Venter, H.; Ogunniyi, A.D.; et al. Genomic Characterization of Coagulase-Negative Staphylococci Including Methicillin-Resistant Staphylococcus Sciuri Causing Bovine Mastitis. Vet. Microbiol. 2018, 219, 17–22. [Google Scholar] [CrossRef]
- Momtaz, H.; Tajbakhsh, E.; Rahimi, E.; Momeni, M. Coagulase Gene Polymorphism of Staphylococcus Aureus Isolated from Clinical and Sub-Clinical Bovine Mastitis in Isfahan and Chaharmahalva Bakhtiari Provinces of Iran. Comp. Clin. Path 2011, 20, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Sağlam, A.G.; Şahin, M.; Çelik, E.; Çelebi, Ö.; Akça, D.; Otlu, S. The Role of Staphylococci in Subclinical Mastitis of Cows and Lytic Phage Isolation against to Staphylococcus Aureus. Vet. World 2017, 10, 1481–1485. [Google Scholar] [CrossRef] [Green Version]
- Brooks, P.B. Bovine Mastitis Involved in Three Milk-Borne Epidemics. Cornell Vet. 1930, 20, 59–62. [Google Scholar]
- Miller, W.T.; Heishman, J.O. Bovine Mastitis Caused by Unusual Types of Streptococci. Cornell Vet. 1940, 30, 310–318. [Google Scholar]
- Bagadi, H.O. The Aetiology of Bovine Mastitis in Three Areas in the Sudan. Trop. Anim. Health Prod. 1970, 2, 28–34. [Google Scholar] [CrossRef]
- Rustamov, R.R.; Khushmanov, B.M.; Sattarov, R.S.; Shatokhin, N.G. Aetiology of Bovine Mastitis in Samarkand Region. Vet. Mosc. USSR 1970, 8, 90–91. [Google Scholar]
- Slanetz, L.W.; Naghski, J. Studies on Streptococci of Bovine Mastitis. J. Infect. Dis. 1940, 66, 80–85. [Google Scholar] [CrossRef]
- Elhaig, M.M.; Selim, A. Molecular and Bacteriological Investigation of Subclinical Mastitis Caused by Staphylococcus Aureus and Streptococcus Agalactiae in Domestic Bovids from Ismailia, Egypt. Trop. Anim. Health Prod. 2015, 47, 271–276. [Google Scholar] [CrossRef]
- Bengtsson, B.; Unnerstad, H.E.; Ekman, T.; Artursson, K.; Nilsson-Öst, M.; Waller, K.P. Antimicrobial Susceptibility of Udder Pathogens from Cases of Acute Clinical Mastitis in Dairy Cows. Vet. Microbiol. 2009, 136, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Haltia, L.; Honkanen-Buzalski, T.; Spiridonova, I.; Olkonen, A.; Myllys, V. A Study of Bovine Mastitis, Milking Procedures and Management Practices on 25 Estonian Dairy Herds. Acta Vet. Scand. 2006, 48, 22. [Google Scholar] [CrossRef] [Green Version]
- Oliver, S.P.; Mitchell, B.A. Prevalence of Mastitis Pathogens in Herds Participating in a Mastitis Control Program. J. Dairy Sci. 1984, 67, 2436–2440. [Google Scholar] [CrossRef]
- Barnum, D.A. A Method for the Control of Staphylococcal and Streptococcal Mastitis in Ontario. Can. Vet. J. 1962, 3, 161–169. [Google Scholar]
- Sumathi, B.R.; Veeregowda, B.M.; Amitha, R.G. Prevalence and Antibiogram Profile of Bacterial Isolates from Clinical Bovine Mastitis. Vet. World 2008, 1, 237–238. [Google Scholar]
- Stark, N. A Bacteriologic Survey of Cows in a Certified Dairy. J. Infect. Dis. 1926, 39, 114–121. [Google Scholar] [CrossRef]
- Oliver, S.P.; Almeida, R.A.; Calvinho, L.F. Virulence Factors of Streptococcus Uberis Isolated From Cows With Mastitis. J. Vet. Med. Ser. B 1998, 45, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.M. Experimental Production of Bovine Mastitis with Streptococci and Other Bacteria. J. Infect. Dis. 1922, 31, 1–9. [Google Scholar] [CrossRef]
- Mathers, G. Different Types of Streptococci and Their Relation to Bovine Mastitis. J. Infect. Dis. 1916, 19, 222–235. [Google Scholar] [CrossRef]
- Rosell, J.M.; Miller, W.T. A Biological and Bacteriological Study of Bovine Mastitis. J. Am. Vet. Med. Assoc. 1933, 82, 587–607. [Google Scholar]
- Ayers, S.H.; Mudge, C.S. The Streptococci of the Bovine Udder: IV. Studies of the Streptococci. J. Infect. Dis. 1922, 31, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Plastridge, W.N.; Anderson, E.O. Detection of Shedders of Streptococci Responsible for Infectious Bovine Mastitis. Am. J. Public. Health Nations Health 1936, 26, 711–715. [Google Scholar] [CrossRef] [Green Version]
- Colque, J.I.C.; Devriese, L.A.; Haesebrouck, F. Streptococci and Enterococci Associated with Tonsils of Cattle. Lett. Appl. Microbiol. 1993, 16, 72–74. [Google Scholar] [CrossRef]
- Davies, P.L.; Leigh, J.A.; Bradley, A.J.; Archer, S.C.; Emes, R.D.; Green, M.J. Molecular Epidemiology of Streptococcus Uberis Clinical Mastitis in Dairy Herds: Strain Heterogeneity and Transmission. J. Clin. Microbiol. 2016, 54, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Samson, O.; Gaudout, N.; Schmitt, E.; Schukken, Y.H.; Zadoks, R. Use of On-Farm Data to Guide Treatment and Control Mastitis Caused by Streptococcus Uberis. J. Dairy Sci. 2016, 99, 7690–7699. [Google Scholar] [CrossRef] [Green Version]
- Tassi, R.; McNeilly, T.N.; Fitzpatrick, J.L.; Fontaine, M.C.; Reddick, D.; Ramage, C.; Lutton, M.; Schukken, Y.H.; Zadoks, R.N. Strain-Specific Pathogenicity of Putative Host-Adapted and Nonadapted Strains of Streptococcus Uberis in Dairy Cattle. J. Dairy Sci. 2013, 96, 5129–5145. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Benavides, M.G.; Williamson, J.H.; Pullinger, G.D.; Lacy-Hulbert, S.J.; Cursons, R.T.; Leigh, J.A. Field Observations on the Variation of Streptococcus Uberis Populations in a Pasture-Based Dairy Farm. J. Dairy Sci. 2007, 90, 5558–5566. [Google Scholar] [CrossRef] [Green Version]
- Zadoks, R.N.; Allore, H.G.; Barkema, H.W.; Sampimon, O.C.; Wellenberg, G.J.; Gröhn, Y.T.; Schukken, Y.H. Cow- and Quarter-Level Risk Factors for Streptococcus Uberis and Staphylococcus Aureus Mastitis. J. Dairy Sci. 2001, 84, 2649–2663. [Google Scholar] [CrossRef]
- Fredebeul-Krein, F.; Schmenger, A.; Wente, N.; Zhang, Y.; Krömker, V. Factors Associated with the Severity of Clinical Mastitis. Pathogens 2022, 11, 1089. [Google Scholar] [CrossRef]
- Watts, J.L. Etiological Agents of Bovine Mastitis. Vet. Microbiol. 1988, 16, 41–66. [Google Scholar] [CrossRef]
- Menzies, F.; Bryson, D.; McCallion, T.; Matthews, D. A Study of Mortality among Suckler and Dairy Cows in Northern Ireland in 1992. Vet. Rec. 1995, 137, 531–536. [Google Scholar] [CrossRef]
- Bradley, A.J.; Green, M.J. A Study of the Incidence and Significance of Intramammary Enterobacterial Infections Acquired During the Dry Period. J. Dairy Sci. 2000, 83, 1957–1965. [Google Scholar] [CrossRef]
- Darang, E.; Pezeshkian, Z.; Mirhoseini, S.Z.; Ghovvati, S. Identification of Key Genes and Potential Pathways Associated with Mastitis Induced by E. Coli. Biochem. Genet. 2023, 61, 202–220. [Google Scholar] [CrossRef]
- Zaatout, N. An Overview on Mastitis-Associated Escherichia Coli: Pathogenicity, Host Immunity and the Use of Alternative Therapies. Microbiol. Res. 2022, 256, 126960. [Google Scholar] [CrossRef]
- Dhaouadi, S.; Romdhani, A.; Bouglita, W.; Chedli, S.; Chaari, S.; Soufi, L.; Cherif, A.; Mnif, W.; Abbassi, M.S.; Elandoulsi, R.B. High Biofilm-Forming Ability and Clonal Dissemination among Colistin-Resistant Escherichia Coli Isolates Recovered from Cows with Mastitis, Diarrheic Calves, and Chickens with Colibacillosis in Tunisia. Life 2023, 13, 299. [Google Scholar] [CrossRef]
- Orsi, H.; Guimarães, F.F.; Leite, D.S.; Guerra, S.T.; Joaquim, S.F.; Pantoja, J.C.F.; Hernandes, R.T.; Lucheis, S.B.; Ribeiro, M.G.; Langoni, H.; et al. Characterization of Mammary Pathogenic Escherichia Coli Reveals the Diversity of Escherichia Coli Isolates Associated with Bovine Clinical Mastitis in Brazil. J. Dairy Sci. 2023, 106, 1403–1413. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Cao, W.; Huang, Y.; Zhao, J.; Wu, X.; Yang, Z. The Prevalence of Escherichia Coli Derived from Bovine Clinical Mastitis and Distribution of Resistance to Antimicrobials in Part of Jiangsu Province, China. Agriculture 2022, 13, 90. [Google Scholar] [CrossRef]
- Bag, M.A.S.; Khan, M.S.R.; Sami, M.D.H.; Begum, F.; Islam, M.S.; Rahman, M.M.; Rahman, M.T.; Hassan, J. Virulence Determinants and Antimicrobial Resistance of E. Coli Isolated from Bovine Clinical Mastitis in Some Selected Dairy Farms of Bangladesh. Saudi J. Biol. Sci. 2021, 28, 6317–6323. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.; Gálvez, F.L.A.; Fukuda, Y.; Tada, C.; Jimenez, I.L.; Valle, W.F.M.; Nakai, Y. Prevalence and Etiology of Mastitis in Dairy Cattle in El Oro Province, Ecuador. J Vet. Med. Sci. 2018, 80, 861–868. [Google Scholar] [CrossRef] [Green Version]
- Etifu, M.; Tilahun, M. Prevalence of Bovine Mastitis, Risk Factors, Isolation and Anti-Bio Gram of Major Pathogens in Mid Rift Valley, Ethiopia. Int. J. Livest. Prod. 2019, 10, 14–23. [Google Scholar]
- Fisseha, D.; Tessema, T.S.; Birhanu, B.T. Aerobic Bacterial Isolates, Incidence Rate and Associated Risk Factors of Heifer and Cow Mastitis in and around Debre-Libanos District, Oromia, Ethiopia. J. Vet. Med. Anim. Health 2020, 12, 7–13. [Google Scholar]
- Saravanan, S.; Palanivel, K.M. Acute Gangrenous and Haemorrhagic Mastitis Due to Bacillus Species in a Preparturient Cow. Rumin. Sci. 2020, 9, 199–201. [Google Scholar]
- Soucie, J.M. Epidemiology of Kidney Stones in the United States; Emory University: Atlanta, GA, USA, 1994; ISBN 9798208369999. [Google Scholar]
- Mbindyo, C.M.; Gitao, G.C.; Mulei, C.M. Prevalence, Etiology, and Risk Factors of Mastitis in Dairy Cattle in Embu and Kajiado Counties, Kenya. Vet. Med. Int. 2020, 2020, 8831172. [Google Scholar] [CrossRef]
- Cone, J.F. Pseudomonas Aeruginosa in Bovine Mastitis. J. Agric. Res. 1939, 58, 141. [Google Scholar]
- Bitew, M.; Tafere, A.; Tolosa, T. Study on Bovine Mastitis in Dairy Farms of Bahir Dar and Its Environs. J. Anim. Vet. Adv. 2010, 9, 2912–2917. [Google Scholar] [CrossRef] [Green Version]
- Loong, S.K.; Lee, H.Y.; Khoo, J.J.; Lim, F.S.; Ahmad-Nasrah, S.N.; Azman, A.S.; Suntharalingam, C.; Panchadcharam, C.; AbuBakar, S. Microbiological Analysis of Raw Milk Unveiled the Presence of a Dairy Contaminant, Corynebacterium Lipophiloflavum. J. Appl. Biol. Biotechnol. 2019, 7, 41–44. [Google Scholar] [CrossRef] [Green Version]
- Kirkeby, C.; Halasa, T.; Farre, M.; Chehabi, G.N.; Græsbøll, K. Transmission Dynamics of Corynebacterium spp. Within Two Danish Dairy Cattle Herds. Front. Vet. Sci. 2021, 8, 735345. [Google Scholar] [CrossRef]
- Lücken, A.; Wente, N.; Zhang, Y.; Woudstra, S.; Krömker, V. Corynebacteria in Bovine Quarter Milk Samples—Species and Somatic Cell Counts. Pathogens 2021, 10, 831. [Google Scholar] [CrossRef]
- Yimana, M.; Bekele, J. Isolation, Identification and Antimicrobial Profile of Corynebacterium Bovis From Selected Dairy Farm In Bishoftu, Central Ethiopia. Arch. Vet. Med. 2022, 15, 69–84. [Google Scholar] [CrossRef]
- Woudstra, S.; Lücken, A.; Wente, N.; Zhang, Y.; Leimbach, S.; Gussmann, M.K.; Kirkeby, C.; Krömker, V. Reservoirs of Corynebacterium spp. in the Environment of Dairy Cows. Pathogens 2023, 12, 139. [Google Scholar] [CrossRef]
- Myllys, V.; Asplund, K.; Brofeldt, E.; Hirvelä-Koski, V.; Honkanen-Buzalski, T.; Junttila, J.; Kulkas, L.; Myllykangas, O.; Niskanen, M.; Saloniemi, H. Bovine Mastitis in Finland in 1988 and 1995-Changes in Prevalence and Antimicrobial Resistance. Acta Vet. Scand. 1998, 39, 119. [Google Scholar] [CrossRef]
- Steeneveld, W.; van Werven, T.; Barkema, H.W.; Hogeveen, H. Cow-Specific Treatment of Clinical Mastitis: An Economic Approach. J. Dairy Sci. 2011, 94, 174–188. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.S.N.; Appannavar, M.M.; Suranagi, M.D.; Kotresh, A.M. Study on Incidence and Economics of Clinical Mastitis. Karnataka J. Agric. Sci. 2010, 23, 407–408. [Google Scholar]
- Akers, R.M.; Nickerson, S.C. Mastitis and Its Impact on Structure and Function in the Ruminant Mammary Gland. J. Mammary Gland. Biol. Neoplasia 2011, 16, 275–289. [Google Scholar] [CrossRef]
- Gurjar, A.; Gioia, G.; Schukken, Y.; Welcome, F.; Zadoks, R.; Moroni, P. Molecular Diagnostics Applied to Mastitis Problems on Dairy Farms. Vet. Clin. N. Am.—Food Anim. Pract. 2012, 28, 565–576. [Google Scholar] [CrossRef]
- Schroder, K.; Sweet, M.J.; Hume, D.A. Signal Integration between IFNγ and TLR Signalling Pathways in Macrophages. Immunobiology 2006, 211, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Rainard, P. Letter to the Editor: Comments on “Mammary Microbial Dysbiosis Leads to the Zoonosis of Bovine Mastitis: A One-Health Perspective” by Maity and Ambatipudi. FEMS Microbiol. Ecol. 2021, 97, fiab077. [Google Scholar] [CrossRef] [PubMed]
- Andrews, T.; Neher, D.A.; Weicht, T.R.; Barlow, J.W. Mammary Microbiome of Lactating Organic Dairy Cows Varies by Time, Tissue Site, and Infection Status. PLoS ONE 2019, 14, e0225001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambabu, K.; Sreenu, M.; Sureshkumar, R.V.; Rao, T.S.C. Incidence of Udder and Teat Affections in Buffaloes. Tamilnadu J. Vet. Anim. Sci. 2011, 7, 309–311. [Google Scholar]
- Falentin, H.; Rault, L.; Nicolas, A.; Bouchard, D.S.; Lassalas, J.; Lamberton, P.; Aubry, J.-M.; Marnet, P.-G.; Le Loir, Y.; Even, S. Bovine Teat Microbiome Analysis Revealed Reduced Alpha Diversity and Significant Changes in Taxonomic Profiles in Quarters with a History of Mastitis. Front. Microbiol. 2016, 7, 480. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, S.N.; Okello, E.; Rossitto, P.V.; Lehenbauer, T.W.; Champagne, J.; Penedo, M.C.T.; Arruda, A.G.; Godden, S.; Rapnicki, P.; Gorden, P.J.; et al. Molecular Epidemiology of Coagulase-Negative Staphylococcus Species Isolated at Different Lactation Stages from Dairy Cattle in the United States. PeerJ 2019, 7, e6749. [Google Scholar] [CrossRef] [Green Version]
- Bhattarai, D.; Worku, T.; Dad, R.; Rehman, Z.U.; Gong, X.; Zhang, S. Mechanism of Pattern Recognition Receptors (PRRs) and Host Pathogen Interplay in Bovine Mastitis. Microb. Pathog. 2018, 120, 64–70. [Google Scholar] [CrossRef]
- De Vliegher, S.; Fox, L.K.; Piepers, S.; McDougall, S.; Barkema, H.W. Invited Review: Mastitis in Dairy Heifers: Nature of the Disease, Potential Impact, Prevention, and Control. J. Dairy Sci. 2012, 95, 1025–1040. [Google Scholar] [CrossRef] [Green Version]
- Jingar, S.C.; Mahendra, S.; Roy, A.K. Economic Losses Due to Clinical Mastitis in Cross-Bred Cows. Dairy. Vet. Sci. J. 2017, 3, 555606. [Google Scholar]
- Krishnamoorthy, P.; Suresh, K.P.; Saha, S.; Govindaraj, G.; Shome, B.R.; Roy, P. Meta-Analysis of Prevalence of Subclinical and Clinical Mastitis, Major Mastitis Pathogens in Dairy Cattle in India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1214–1234. [Google Scholar]
- Rathod, P.; Shivamurty, V.; Desai, A.R. Economic Losses Due to Subclinical Mastitis in Dairy Animals: A Study in Bidar District of Karnataka. Indian J. Vet. Sci. Biotechnol. 2017, 13, 37–41. [Google Scholar] [CrossRef]
- Krishnamoorthy, P.; Goudar, A.L.; Suresh, K.P.; Roy, P. Global and Countrywide Prevalence of Subclinical and Clinical Mastitis in Dairy Cattle and Buffaloes by Systematic Review and Meta-Analysis. Res. Vet. Sci. 2021, 136, 561–586. [Google Scholar] [CrossRef]
- Wani, S.A.; Haq, U.; Parray, O.R.; Ul, Q.; Nazir, A.; Mushtaq, M.; Bhat, R.A.; Parrah, J.U.; Chakraborty, S.; Dhama, K. A Brief Analysis of Economic Losses Due to Mastitis in Dairy Cattle. Indian Vet. J. 2022, 90, 27–31. [Google Scholar]
- Richardet, M.; Solari, H.G.; Cabrera, V.E.; Vissio, C.; Agüero, D.; Bartolomé, J.A.; Bó, G.A.; Bogni, C.I.; Larriestra, A.J. The Economic Evaluation of Mastitis Control Strategies in Holstein-Friesian Dairy Herds. Animals 2023, 13, 1701. [Google Scholar] [CrossRef]
- Puerto, M.A.; Shepley, E.; Cue, R.I.; Warner, D.; Dubuc, J.; Vasseur, E. The Hidden Cost of Disease: I. Impact of the First Incidence of Mastitis on Production and Economic Indicators of Primiparous Dairy Cows. J. Dairy Sci. 2021, 104, 7932–7943. [Google Scholar] [CrossRef]
- Dingwell, R.T.; Kelton, D.F.; Leslie, K.E. Management of the Dry Cow in Control of Peripartum Disease and Mastitis. Vet. Clin. N. Am.-Food Anim. 2003, 19, 235–265. [Google Scholar] [CrossRef]
- Reyher, K.K.; Dohoo, I.R. Diagnosing Intramammary Infections: Evaluation of Composite Milk Samples to Detect Intramammary Infections. J. Dairy Sci. 2011, 94, 3387–3396. [Google Scholar] [CrossRef]
- El-Sayed, A.; Awad, W.; Abdou, N.E.; Castañeda Vázquez, H. Molecular Biological Tools Applied for Identification of Mastitis Causing Pathogens. Int. J. Vet. Sci. Med. 2017, 5, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Adkins, P.R.F.; Middleton, J.R. Methods for Diagnosing Mastitis. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 479–491. [Google Scholar] [CrossRef]
- McDermott, M.P.; Erb, H.N.; Natzke, R.P.; Barnes, F.D.; Bray, D. Cost Benefit Analysis of Lactation Therapy with Somatic Cell Counts as Indications for Treatment. J. Dairy Sci. 1983, 66, 1198–1203. [Google Scholar] [CrossRef]
- Jashari, R.; Piepers, S.; De Vliegher, S. Evaluation of the Composite Milk Somatic Cell Count as a Predictor of Intramammary Infection in Dairy Cattle. J. Dairy Sci. 2016, 99, 9271–9286. [Google Scholar] [CrossRef] [PubMed]
- Schukken, Y.H.; González, R.N.; Tikofsky, L.L.; Schulte, H.F.; Santisteban, C.G.; Welcome, F.L.; Bennett, G.J.; Zurakowski, M.J.; Zadoks, R.N. CNS Mastitis: Nothing to Worry About? Vet. Microbiol. 2009, 134, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Boonyayatra, S.; Fox, L.K.; Besser, T.E.; Sawant, A.; Gay, J.M.; Raviv, Z. A PCR Assay and PCR-Restriction Fragment Length Polymorphism Combination Identifying the 3 Primary Mycoplasma Species Causing Mastitis. J. Dairy Sci. 2012, 95, 196–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almeida, P.; Mezzomo, N.; Ferreira, S. Extraction of Mentha Spicata L. Volatile Compounds: Evaluation of Process Parameters and Extract Composition. Food Bioproc. Technol. 2012, 5, 548–559. [Google Scholar] [CrossRef]
- Shome, B.R.; Das Mitra, S.; Bhuvana, M.; Krithiga, N.; Velu, D.; Shome, R.; Isloor, S.; Barbuddhe, S.B.; Rahman, H. Multiplex PCR Assay for Species Identification of Bovine Mastitis Pathogens. J. Appl. Microbiol. 2011, 111, 1349–1356. [Google Scholar] [CrossRef]
- Ding, Z.; Cao, X. Affinity Precipitation of Human Serum Albumin Using a Thermo-Response Polymer with an L-Thyroxin Ligand. BMC Biotechnol. 2013, 13, 109. [Google Scholar] [CrossRef] [Green Version]
- Gillespie, D.C.; Kim, G.; Kandler, K. Inhibitory Synapses in the Developing Auditory System Are Glutamatergic. Nat. Neurosci. 2005, 8, 332–338. [Google Scholar] [CrossRef]
- Mahmmod, Y.S.; Toft, N.; Katholm, J.; Grønbæk, C.; Klaas, I.C. Bayesian Estimation of Test Characteristics of Real-Time PCR, Bacteriological Culture and California Mastitis Test for Diagnosis of Intramammary Infections with Staphylococcus Aureus in Dairy Cattle at Routine Milk Recordings. Prev. Vet. Med. 2013, 112, 309–317. [Google Scholar] [CrossRef] [Green Version]
- Koskinen, M.T.; Holopainen, J.; Pyörälä, S.; Bredbacka, P.; Pitkälä, A.; Barkema, H.W.; Bexiga, R.; Roberson, J.; Sølverød, L.; Piccinini, R.; et al. Analytical Specificity and Sensitivity of a Real-Time Polymerase Chain Reaction Assay for Identification of Bovine Mastitis Pathogens. J. Dairy Sci. 2009, 92, 952–959. [Google Scholar] [CrossRef]
- Driskell, J.D.; Tripp, R.A. Emerging Technologies in Nanotechnology-Based Pathogen Detection. Clin. Microbiol. Newsl. 2009, 31, 137–144. [Google Scholar] [CrossRef]
- Mujawar, M.A.; Gohel, H.; Bhardwaj, S.K.; Srinivasan, S.; Hickman, N.; Kaushik, A. Nano-Enabled Biosensing Systems for Intelligent Healthcare: Towards COVID-19 Management. Mater. Today Chem. 2020, 17, 100306. [Google Scholar] [CrossRef]
- Chinnappan, R.; Al Attas, S.; Kaman, W.E.; Bikker, F.J.; Zourob, M. Development of Magnetic Nanoparticle Based Calorimetric Assay for the Detection of Bovine Mastitis in Cow Milk. Anal. Biochem. 2017, 523, 58–64. [Google Scholar] [CrossRef]
- Chitra Devi, M.; Pirabaharan, P.; Rajendran, L.; Abukhaled, M. Amperometric Biosensors in an Uncompetitive Inhibition Processes: A Complete Theoretical and Numerical Analysis. React. Kinet. Mech. Catal. 2021, 133, 655–668. [Google Scholar] [CrossRef]
- Ashley, J.; Li, S.F.Y. An Aptamer Based Surface Plasmon Resonance Biosensor for the Detection of Bovine Catalase in Milk. Biosens. Bioelectron. 2013, 48, 126–131. [Google Scholar] [CrossRef]
- Nagasawa, Y.; Kiku, Y.; Sugawara, K.; Yabusaki, N.; Oono, K.; Fujii, K.; Suzuki, T.; Maehana, K.; Hayashi, T. Rapid Staphylococcus Aureus Detection From Clinical Mastitis Milk by Colloidal Gold Nanoparticle-Based Immunochromatographic Strips. Front. Vet. Sci. 2020, 6, 504. [Google Scholar] [CrossRef]
- Engvall, E.; Jonsson, K.; Perlmann, P. Enzyme-Linked Immunosorbent Assay. II. Quantitative Assay of Protein Antigen, Immunoglobulin G, by Means of Enzyme-Labelled Antigen and Antibody-Coated Tubes. Biochim. Et Biophys. Acta (BBA)-Protein Struct. 1971, 251, 427–434. [Google Scholar] [CrossRef]
- Abdel Hafez, S.M.; Yassin, M.H.; Gomaa, A.M. The Use of Latex Agglutination Test as a Rapid Method for Detection of Antibodies to Mycoplasma BovisIn Comparison With ELISA. Assiut Vet. Med. J. 2009, 55, 1–8. [Google Scholar]
- Wawegama, N.K.; Markham, P.F.; Kanci, A.; Schibrowski, M.; Oswin, S.; Barnes, T.S.; Firestone, S.M.; Mahony, T.J.; Browning, G.F. Evaluation of an IgG Enzyme-Linked Immunosorbent Assay as a Serological Assay for Detection of Mycoplasma Bovis Infection in Feedlot Cattle. J. Clin. Microbiol. 2016, 54, 1269–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopinath, S.M.; Joshi, R.R. Biomarker Based Detection of Subclinical Mastitis by Liquid Phase Blocking ELISA. Int. J. Pharma Bio Sci. 2013, 4, 776–786. [Google Scholar]
- Wu, J.; Bu, R.; Wang, J.; Chen, J.; Xilin, G.; Sun, L.; Wang, H.; Wang, X.; Liu, Y.; Chao, L. Establishment of Indirect ELISA for Bovine Mastitis Streptococcus Agalactiae Based on RSip-PGK-FbsA Fusion Protein. ZhongguoYufangShouyiXuebao/Chin. J. Prev. Vet. Med. 2016, 38, 230–234. [Google Scholar]
- Chao, L.; Wang, J.; Bu, R.; Wu, J.; Sun, L.; Xi, L.G.; Chen, J. Establishment of an Indirect ELISA for Detecting the Antibody against Dairy Mastitis Streptococcus Agalactiae with Its RAP1-BP-AP2 Proteins as Coating Antigen. ZhongguoYufangShouyiXuebao/Chin. J. Prev. Vet. Med. 2018, 40, 611–615. [Google Scholar]
- Nakajima, Y.; Mikami, O.; Yoshioka, M.; Motoi, Y.; Ito, T.; Ishikawa, Y.; Fuse, M.; Nakano, K.; Yasukawa, K. Elevated Levels of Tumor Necrosis Factor-α, (TNF-α) and Interleukin-6 (IL-6) Activities in the Sera and Milk of Cows with Naturally Occurring Coliform Mastitis. Res. Vet. Sci. 1997, 62, 297–298. [Google Scholar] [CrossRef]
- Riollet, C.; Rainard, P.; Poutrel, B. Differential Induction of Complement Fragment C5a and Inflammatory Cytokines during Intramammary Infections with Escherichia Coli and Staphylococcus Aureus. Clin Diagn Lab Immunol. 2000, 7, 161–167. [Google Scholar] [CrossRef] [Green Version]
- Sakemi, Y.; Tamura, Y.; Hagiwara, K. Interleukin-6 in Quarter Milk as a Further Prediction Marker for Bovine Subclinical Mastitis. J. Dairy Res. 2011, 78, 118–121. [Google Scholar] [CrossRef]
- Roussel, P.; Cunha, P.; Porcherie, A.; Petzl, W.; Gilbert, F.B.; Riollet, C.; Zerbe, H.; Rainard, P.; Germon, P. Investigating the Contribution of IL-17A and IL-17F to the Host Response during Escherichia Coli Mastitis. Vet. Res. 2015, 46, 56. [Google Scholar] [CrossRef] [Green Version]
- Herry, V.; Gitton, C.; Tabouret, G.; Répérant, M.; Forge, L.; Tasca, C.; Gilbert, F.B.; Guitton, E.; Barc, C.; Staub, C.; et al. Local Immunization Impacts the Response of Dairy Cows to Escherichia Coli Mastitis. Sci. Rep. 2017, 7, 3441. [Google Scholar] [CrossRef]
- El-Deeb, W.; Fayez, M.; Alhumam, N.; Elsohaby, I.; Quadri, S.A.; Mkrtchyan, H. The Effect of Staphylococcal Mastitis Including Resistant Strains on Serum Procalcitonin, Neopterin, Acute Phase Response and Stress Biomarkers in Holstein Dairy Cows. PeerJ 2021, 9, e11511. [Google Scholar] [CrossRef]
- Al-Rasheed, A.A.; Sana’a, S.A.; Al-Jashamy, K.A.; Garba, B. Immunopathological Responses to the Bovine Mastitis Associated with Staphylococcus Species Infection. Iraqi J. Vet. Med. 2022, 46, 7–11. [Google Scholar] [CrossRef]
- Thomas, F.C.; Geraghty, T.; Simões, P.B.A.; Mshelbwala, F.M.; Haining, H.; Eckersall, P.D. A Pilot Study of Acute Phase Proteins as Indicators of Bovine Mastitis Caused by Different Pathogens. Res. Vet. Sci. 2018, 119, 176–181. [Google Scholar] [CrossRef]
- Tabatabaee, N.; Heidarpour, M.; Khoramian, B. Milk Metabolites, Proteins and Oxidative Stress Markers in Dairy Cows Suffering from Staphylococcus Aureus Subclinical Mastitis with or without Spontaneous Cure. J. Dairy. Res. 2021, 88, 326–329. [Google Scholar] [CrossRef]
- Trela, M.; Domańska, D.; Witkowska-Piłaszewicz, O. Diagnostic Use of Serum Amyloid A in Dairy Cattle. Agriculture 2022, 12, 459. [Google Scholar] [CrossRef]
- O’Connell, G.C.; Alder, M.L.; Smothers, C.G.; Still, C.H.; Webel, A.R.; Moore, S.M. Diagnosis of Ischemic Stroke Using Circulating Levels of Brain-Specific Proteins Measured via High-Sensitivity Digital ELISA. Brain Res. 2020, 1739, 146861. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and Unhealthful Plant-Based Diets and the Risk of Coronary Heart Disease in U.S. Adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.; Vázquez-Villegas, P.; Rito-Palomares, M.; Martinez-Chapa, S.O. Advantages, Disadvantages and Modifications of Conventional ELISA. In Enzyme-Linked Immunoabsorbent Assay ELISA; Springer: Singapore, 2018; pp. 67–115. [Google Scholar]
- Vivekananda, J.; Kiel, J.L. Anti-Francisella Tularensis DNA Aptamers Detect Tularemia Antigen from Different Subspecies by Aptamer-Linked Immobilized Sorbent Assay. Lab. Investig. 2006, 86, 610–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.W.; Sun, C.J.; Zhang, F.T.; Xu, L.; Zhou, Y.L.; Zhang, X.X. An Electrochemical Aptasensor Based on Enzyme Linked Aptamer Assay. Biosens. Bioelectron. 2012, 31, 363–368. [Google Scholar] [CrossRef]
- Cheng, D.; Yu, M.; Fu, F.; Han, W.; Li, G.; Xie, J.; Song, Y.; Swihart, M.T.; Song, E. Dual Recognition Strategy for Specific and Sensitive Detection of Bacteria Using Aptamer-Coated Magnetic Beads and Antibiotic-Capped Gold Nanoclusters. Anal. Chem. 2016, 88, 820–825. [Google Scholar] [CrossRef]
- Baumstummler, A.; Lehmann, D.; Janjic, N.; Ochsner, U.A. Specific Capture and Detection of Taphylococcus Aureus with High-affinity Modified Aptamers to Cell Surface Components. Lett. Appl. Microbiol. 2014, 59, 422–431. [Google Scholar] [CrossRef] [Green Version]
- Stoltenburg, R.; Krafčiková, P.; Víglaský, V.; Strehlitz, B. G-Quadruplex Aptamer Targeting Protein A and Its Capability to Detect Staphylococcus Aureus Demonstrated by ELONA. Sci. Rep. 2016, 6, 33812. [Google Scholar] [CrossRef] [Green Version]
- Roushani, M.; Sarabaegi, M.; Pourahmad, F. Impedimetric Aptasensor for Pseudomonas Aeruginosa by Using a Glassy Carbon Electrode Modified with Silver Nanoparticles. Microchimica Acta 2019, 186, 725. [Google Scholar] [CrossRef]
- Citartan, M.; Gopinath, S.C.B.; Tominaga, J.; Tan, S.C.; Tang, T.H. Assays for Aptamer-Based Platforms. Biosens. Bioelectron. 2012, 34, 1–11. [Google Scholar] [CrossRef]
- Shahdordizadeh, M.; Taghdisi, S.M.; Ansari, N.; AlebooyeLangroodi, F.; Abnous, K.; Ramezani, M. Aptamer Based Biosensors for Detection of Staphylococcus Aureus. Sens. Actuators B Chem. 2017, 241, 619–635. [Google Scholar] [CrossRef]
- Balsam, J.; Ossandon, M.; Bruck, H.A.; Lubensky, I.; Rasooly, A. Low-Cost Technologies for Medical Diagnostics in Low-Resource Settings. Expert. Opin. Med. Diagn. 2013, 7, 243–255. [Google Scholar] [CrossRef]
- Randall, L.P.; Lemma, F.; Koylass, M.; Rogers, J.; Ayling, R.D.; Worth, D.; Klita, M.; Steventon, A.; Line, K.; Wragg, P.; et al. Evaluation of MALDI-ToF as a Method for the Identification of Bacteria in the Veterinary Diagnostic Laboratory. Res. Vet. Sci. 2015, 101, 42–49. [Google Scholar] [CrossRef]
- Barreiro, J.R.; Ferreira, C.R.; Sanvido, G.B.; Kostrzewa, M.; Maier, T.; Wegemann, B.; Böttcher, V.; Eberlin, M.N.; dos Santos, M.V. Short Communication: Identification of Subclinical Cow Mastitis Pathogens in Milk by Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. J. Dairy Sci. 2010, 93, 5661–5667. [Google Scholar] [CrossRef]
- Barreiro, J.R.; Gonçalves, J.L.; Braga, P.A.C.; Dibbern, A.G.; Eberlin, M.N.; Veiga dos Santos, M. Non-Culture-Based Identification of Mastitis-Causing Bacteria by MALDI-TOF Mass Spectrometry. J. Dairy Sci. 2017, 100, 2928–2934. [Google Scholar] [CrossRef]
- Nonnemann, B.; Lyhs, U.; Svennesen, L.; Kristensen, K.A.; Klaas, I.C.; Pedersen, K. Bovine Mastitis Bacteria Resolved by MALDI-TOF Mass Spectrometry. J. Dairy Sci. 2019, 102, 2515–2524. [Google Scholar] [CrossRef]
- Fidelis, C.E.; Franke, M.; de Abreu, L.C.R.; Jagielski, T.; Ribeiro, M.G.; Dos Santos, M.V.; Gonçalves, J.L. MALDI-TOF MS Identification of Prototheca Algae Associated with Bovine Mastitis. J. Vet. Diagn. Investig. 2021, 33, 1168–1171. [Google Scholar] [CrossRef]
- Braga, P.A.C.; Gonçalves, J.L.; Barreiro, J.R.; Ferreira, C.R.; Tomazi, T.; Eberlin, M.N.; Santos, M.V. Rapid Identification of Bovine Mastitis Pathogens by MALDI-TOF Mass Spectrometry. Pesqui. Veterinária Bras. 2018, 38, 586–594. [Google Scholar] [CrossRef] [Green Version]
- Alnakip, M.E.A.; Rhouma, N.R.; Abd-Elfatah, E.N.; Quintela-Baluja, M.; Böhme, K.; Fernández-No, I.; Bayoumi, M.A.; Abdelhafez, M.M.; Taboada-Rodríguez, A.; Calo-Mata, P.; et al. Discrimination of Major and Minor Streptococci Incriminated in Bovine Mastitis by MALDI-TOF MS Fingerprinting and 16S RRNA Gene Sequencing. Res. Vet. Sci. 2020, 132, 426–438. [Google Scholar] [CrossRef]
- El-Ashker, M.; Gwida, M.; Monecke, S.; El-Gohary, F.; Ehricht, R.; Elsayed, M.; Akinduti, P.; El-Fateh, M.; Maurischat, S. Antimicrobial Resistance Pattern and Virulence Profile of S. Aureus Isolated from Household Cattle and Buffalo with Mastitis in Egypt. Vet. Microbiol. 2020, 240, 108535. [Google Scholar] [CrossRef]
- Jahan, N.A.; Godden, S.M.; Royster, E.; Schoenfuss, T.C.; Gebhart, C.; Timmerman, J.; Fink, R.C. Evaluation of the Matrix-Assisted Laser Desorption Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS) System in the Detection of Mastitis Pathogens from Bovine Milk Samples. J. Microbiol. Methods 2021, 182, 106168. [Google Scholar] [CrossRef] [PubMed]
- Astrup, L.B.; Pedersen, K.; Farre, M. Microbiological Diagnoses on Clinical Mastitis—Comparison between Diagnoses Made in Veterinary Clinics versus in Laboratory Applying MALDI-TOF MS. Antibiotics 2022, 11, 271. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Everhart Nunn, S.L.; Sarkar, S.; Clayton, B. Diagnostic Screening of Bovine Mastitis Using MALDI-TOF MS Direct-Spotting of Milk and Machine Learning. Vet. Sci. 2023, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus Aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Algharib, S.A.; Dawood, A.; Xie, S. Nanoparticles for Treatment of Bovine Staphylococcus Aureus Mastitis. Drug Deliv. 2020, 27, 292–308. [Google Scholar] [CrossRef] [Green Version]
- Ender, M.; Berger-Bächi, B.; McCallum, N. A Novel DNA-Binding Protein Modulating Methicillin Resistance in Staphylococcus Aureus. BMC Microbiol. 2009, 9, 15. [Google Scholar] [CrossRef] [Green Version]
- Moellering, R.C., Jr. Current Treatment Options for Community-Acquired Methicillin-Resistant Staphylococcus Aureus Infection. Clin. Infect. Dis. 2008, 46, 1032–1037. [Google Scholar] [CrossRef] [Green Version]
- Tacconelli, E.; Cataldo, M.A. Antimicrobial Therapy of Staphylococcus Aureus Bloodstream Infection. Expert. Opin. Pharmacother. 2007, 8, 2505–2518. [Google Scholar] [CrossRef]
- Sanche, S.E.; Blondeau, J.M. Quinupristin/Dalfopristin. Expert. Opin. Pharmacother. 2002, 3, 1341–1364. [Google Scholar] [CrossRef]
- Sader, H.S.; Jones, R.N.; Stilwell, M.G.; Dowzicky, M.J.; Fritsche, T.R. Tigecycline Activity Tested against 26,474 Bloodstream Infection Isolates: A Collection from 6 Continents. Diagn. Microbiol. Infect. Dis. 2005, 52, 181–186. [Google Scholar] [CrossRef]
- Khawcharoenporn, T.; Tice, A. Oral Antibiotic Treatment for Methicillin-Resistant Staphylococcus Aureus Skin and Soft Tissue Infections: Review of the Literatures. Hawaii Med. J. 2006, 65, 290. [Google Scholar] [PubMed]
- Liaqat, F.; Eltem, R. Chitooligosaccharides and Their Biological Activities: A Comprehensive Review. Carbohydr. Polym. 2018, 184, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Huang, K.; Yang, F.; Wang, R.; Han, L.; Yu, H.; Ye, Z.; Wu, F. Chitosan Nanocomposite Films Based on Halloysite Nanotubes Modification for Potential Biomedical Applications. Int. J. Biol. Macromol. 2020, 151, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, Y.; Yang, L.; Zhou, F. Synthesis of Sulfonated Chitosan and Its Antibiofilm Formation Activity against E. Coli and S. Aureus. Int. J. Biol. Macromol. 2019, 129, 980–988. [Google Scholar] [CrossRef] [PubMed]
- Paomephan, P.; Assavanig, A.; Chaturongakul, S.; Cady, N.C.; Bergkvist, M.; Niamsiri, N. Insight into the Antibacterial Property of Chitosan Nanoparticles against Escherichia Coli and Salmonella Typhimurium and Their Application as Vegetable Wash Disinfectant. Food Control 2018, 86, 294–301. [Google Scholar] [CrossRef]
- Rivera Aguayo, P.; Bruna Larenas, T.; Alarcón Godoy, C.; Cayupe Rivas, B.; González-Casanova, J.; Rojas-Gómez, D.; Caro Fuentes, N. Antimicrobial and Antibiofilm Capacity of Chitosan Nanoparticles against Wild Type Strain of Pseudomonas Sp. Isolated from Milk of Cows Diagnosed with Bovine Mastitis. Antibiotics 2020, 9, 551. [Google Scholar] [CrossRef]
- Orellano, M.S.; Isaac, P.; Breser, M.L.; Bohl, L.P.; Conesa, A.; Falcone, R.D.; Porporatto, C. Chitosan Nanoparticles Enhance the Antibacterial Activity of the Native Polymer against Bovine Mastitis Pathogens. Carbohydr. Polym. 2019, 213, 1–9. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Karathanasis, E.; Ayyagari, A.L.; Bhavane, R.; Bellamkonda, R.V.; Annapragada, A.V. Preparation of in vivo Cleavable Agglomerated Liposomes Suitable for Modulated Pulmonary Drug Delivery. J. Control. Release 2005, 103, 159–175. [Google Scholar] [CrossRef]
- Pumerantz, A.; Muppidi, K.; Agnihotri, S.; Guerra, C.; Venketaraman, V.; Wang, J.; Betageri, G. Preparation of Liposomal Vancomycin and Intracellular Killing of Meticillin-Resistant Staphylococcus Aureus (MRSA). Int. J. Antimicrob. Agents 2011, 37, 140–144. [Google Scholar] [CrossRef]
- Zhou, T.-H.; Su, M.; Shang, B.-C.; Ma, T.; Xu, G.-L.; Li, H.-L.; Chen, Q.-H.; Sun, W.; Xu, Y.-Q. Nano-Hydroxyapatite/β-Tricalcium Phosphate Ceramics Scaffolds Loaded with Cationic Liposomal Ceftazidime: Preparation, Release Characteristics in vitro and Inhibition to Staphylococcus Aureus Biofilms. Drug Dev. Ind. Pharm. 2012, 38, 1298–1304. [Google Scholar] [CrossRef]
- Gupta, P.V.; Nirwane, A.M.; Belubbi, T.; Nagarsenker, M.S. Pulmonary Delivery of Synergistic Combination of Fluoroquinolone Antibiotic Complemented with Proteolytic Enzyme: A Novel Antimicrobial and Antibiofilm Strategy. Nanomedicine 2017, 13, 2371–2384. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Yang, S.-C.; Sung, C.T.; Weng, Y.-H.; Fang, J.-Y. Anti-MRSA Malleable Liposomes Carrying Chloramphenicol for Ameliorating Hair Follicle Targeting. Int. J. Nanomed. 2017, 12, 8227–8238. [Google Scholar] [CrossRef] [Green Version]
- Jijie, R.; Barras, A.; Teodorescu, F.; Boukherroub, R.; Szunerits, S. Advancements on the Molecular Design of Nanoantibiotics: Current Level of Development and Future Challenges. Mol. Syst. Des. Eng. 2017, 2, 349–369. [Google Scholar] [CrossRef] [Green Version]
- Allen, T.M.; Martin, F.J. Advantages of Liposomal Delivery Systems for Anthracyclines. Semin. Oncol. 2004, 31, 5–15. [Google Scholar] [CrossRef]
- Wang, X.F.; Zhang, S.L.; Zhu, L.Y.; Xie, S.Y.; Dong, Z.; Wang, Y.; Zhou, W.Z. Enhancement of Antibacterial Activity of Tilmicosin against Staphylococcus Aureus by Solid Lipid Nanoparticles in vitro and in vivo. Vet. J. 2012, 191, 115–120. [Google Scholar] [CrossRef]
- Maya, S.; Kumar, L.G.; Sarmento, B.; SanojRejinold, N.; Menon, D.; Nair, S.V.; Jayakumar, R. Cetuximab Conjugated O-Carboxymethyl Chitosan Nanoparticles for Targeting EGFR Overexpressing Cancer Cells. Carbohydr. Polym. 2013, 93, 661–669. [Google Scholar] [CrossRef]
- Shi, S.-F.; Jia, J.-F.; Guo, X.-K.; Zhao, Y.-P.; Chen, D.-S.; Guo, Y.-Y.; Zhang, X.-L. Reduced Staphylococcus Aureus Biofilm Formation in the Presence of Chitosan-Coated Iron Oxide Nanoparticles. Int. J. Nanomed. 2016, 11, 6499–6506. [Google Scholar] [CrossRef] [Green Version]
- Bastari, K.; Arshath, M.; NG, Z.H.M.; Chia, J.H.; Yow, Z.X.D.; Sana, B.; Tan, M.F.C.; Lim, S.; Loo, S.C.J. A Controlled Release of Antibiotics from Calcium Phosphate-Coated Poly(Lactic-Co-Glycolic Acid) Particles and Their in vitro Efficacy against Staphylococcus Aureus Biofilm. J. Mater. Sci. Mater. Med. 2014, 25, 747–757. [Google Scholar] [CrossRef]
- Sultana, A.; Zare, M.; Thomas, V.; Kumar, T.S.S.; Ramakrishna, S. Nano-Based Drug Delivery Systems: Conventional Drug Delivery Routes, Recent Developments and Future Prospects. Med. Drug Discov. 2022, 15, 100134. [Google Scholar] [CrossRef]
- Xiong, M.-H.; Li, Y.-J.; Bao, Y.; Yang, X.-Z.; Hu, B.; Wang, J. Bacteria-Responsive Multifunctional Nanogel for Targeted Antibiotic Delivery. Adv. Mater. 2012, 24, 6175–6180. [Google Scholar] [CrossRef] [PubMed]
- Coll Ferrer, M.C.; Dastgheyb, S.; Hickok, N.J.; Eckmann, D.M.; Composto, R.J. Designing Nanogel Carriers for Antibacterial Applications. Acta Biomater. 2014, 10, 2105–2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.; Zhi, Z.; Duan, M.; Sun, J.; Jiang, H.; Pang, J. Insights into the Formation of Carboxymethyl Chitosan-Nisin Nanogels for Sustainable Antibacterial Activity. Food Chem. 2023, 402, 134260. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, F.; Ma, X.; Sun, S.; Leng, F.; Zhang, W.; Wang, X. Extraction, Purification, Characterization and Antioxidant Activity of Polysaccharides from Piteguo Fruit. Ind. Crops Prod. 2015, 77, 467–475. [Google Scholar] [CrossRef]
- Mohsenabadi, N.; Rajaei, A.; Tabatabaei, M.; Mohsenifar, A. Physical and Antimicrobial Properties of Starch-Carboxy Methyl Cellulose Film Containing Rosemary Essential Oils Encapsulated in Chitosan Nanogel. Int. J. Biol. Macromol. 2018, 112, 148–155. [Google Scholar] [CrossRef]
- Kazemi, J.; Ahmadi, M.; Dastmalchi, S.H.; Adibhesami, M. Antibacterial Effect of Silver Nanoparticles along with Protein Synthesis-Inhibiting Antibiotics on Staphylococcus Aureus Isolated from Cattle Mastitis. J. Biol. Micro. 2014, 2, 8–12. [Google Scholar]
- Wady, A.F.; Machado, A.L.; Foggi, C.C.; Zamperini, C.A.; Zucolotto, V.; Moffa, E.B.; Vergani, C.E. Effect of a Silver Nanoparticles Solution on Staphylococcus Aureus and Candida spp. J. Nanomater. 2014, 2014, 545279. [Google Scholar] [CrossRef] [Green Version]
- Friedman, A.J.; Han, G.; Navati, M.S.; Chacko, M.; Gunther, L.; Alfieri, A.; Friedman, J.M. Sustained Release Nitric Oxide Releasing Nanoparticles: Characterization of a Novel Delivery Platform Based on Nitrite Containing Hydrogel/Glass Composites. Nitric Oxide 2008, 19, 12–20. [Google Scholar] [CrossRef]
- Cardozo, V.F.; Lancheros, C.A.C.; Narciso, A.M.; Valereto, E.C.S.; Kobayashi, R.K.T.; Seabra, A.B.; Nakazato, G. Evaluation of Antibacterial Activity of Nitric Oxide-Releasing Polymeric Particles against Staphylococcus Aureus and Escherichia Coli from Bovine Mastitis. Int. J. Pharm. 2014, 473, 20–29. [Google Scholar] [CrossRef]
- Zafar, N.; Uzair, B.; Niazi, M.B.K.; Samin, G.; Bano, A.; Jamil, N.; Waqar-Un-Nisa; Sajjad, S.; Menaa, F. Synthesis and Characterization of Potent and Safe Ciprofloxacin-Loaded Ag/TiO2/CS Nanohybrid against Mastitis Causing E. Coli. Crystals 2021, 11, 319. [Google Scholar] [CrossRef]
- Yadav, P.; Yadav, A.B.; Gaur, P.; Mishra, V.; Huma, Z.-I.; Sharma, N.; Son, Y.-O. Bioengineered Ciprofloxacin-Loaded Chitosan Nanoparticles for the Treatment of Bovine Mastitis. Biomedicines 2022, 10, 3282. [Google Scholar] [CrossRef]
- Kour, S. Clinico-Epidemiological Investigation of Heifer Mastitis and In-Vitro Efficacy of Drug-Loaded Chitosan Nano-Particles against the Isolates. Ph.D. Thesis, Sher-E-Kashmir University of Agricultural Sciences and Technology of Jammu, Union Territory of Jammu and Kashmir, India, 2017. [Google Scholar]
- Yu, L.; Shang, F.; Chen, X.; Ni, J.; Yu, L.; Zhang, M.; Sun, D.; Xue, T. The Anti-Biofilm Effect of Silver-Nanoparticle-Decorated Quercetin Nanoparticles on a Multi-Drug Resistant Escherichia Coli Strain Isolated from a Dairy Cow with Mastitis. PeerJ 2018, 6, e5711. [Google Scholar] [CrossRef] [Green Version]
- Hozyen, H.F.; Ibrahim, E.S.; Khairy, E.A.; El-Dek, S.I. Enhanced Antibacterial Activity of Capped Zinc Oxide Nanoparticles: A Step towards the Control of Clinical Bovine Mastitis. Vet. World 2019, 12, 1225–1232. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.A.; Aqib, A.I.; Ashfaq, K.; Deeba, F.; Khan, M.K.; Khan, S.R.; Muzammil, I.; Shoaib, M.; Naseer, M.A.; Riaz, T.; et al. Antimicrobial Resistance Modulation of MDR E. Coli by Antibiotic Coated ZnO Nanoparticles. Microb. Pathog. 2020, 148, 104450. [Google Scholar] [CrossRef]
- Asli, A.; Brouillette, E.; Ster, C.; Ghinet, M.G.; Brzezinski, R.; Lacasse, P.; Jacques, M.; Malouin, F. Antibiofilm and Antibacterial Effects of Specific Chitosan Molecules on Staphylococcus Aureus Isolates Associated with Bovine Mastitis. PLoS ONE 2017, 12, e0176988. [Google Scholar] [CrossRef] [Green Version]
- Breser, M.L.; Felipe, V.; Bohl, L.P.; Orellano, M.S.; Isaac, P.; Conesa, A.; Rivero, V.E.; Correa, S.G.; Bianco, I.D.; Porporatto, C. Chitosan and Cloxacillin Combination Improve Antibiotic Efficacy against Different Lifestyle of Coagulase-Negative Staphylococcus Isolates from Chronic Bovine Mastitis. Sci. Rep. 2018, 8, 5081. [Google Scholar] [CrossRef]
- Felipe, V.; Breser, M.L.; Bohl, L.P.; Rodrigues da Silva, E.; Morgante, C.A.; Correa, S.G.; Porporatto, C. Chitosan Disrupts Biofilm Formation and Promotes Biofilm Eradication in Staphylococcus Species Isolated from Bovine Mastitis. Int. J. Biol. Macromol. 2019, 126, 60–67. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Jiang, H.-R.; Chen, D.-J.; Shen, Z.-L.; Mao, Y.-J.; Liang, Y.-S.; Juan, J.L.; Yang, Z.-P. Evaluation of a Povidone-Iodine and Chitosan-Based Barrier Teat Dip in the Prevention of Mastitis in Dairy Cows. J. Integr. Agric. 2021, 20, 1615–1625. [Google Scholar] [CrossRef]
- Doehring, C.; Sundrum, A. Efficacy of Homeopathy in Livestock According to Peer-Reviewed Publications from 1981 to 2014. Vet. Rec. 2016, 179, 628. [Google Scholar] [CrossRef] [Green Version]
- Galhotra, A.P. Effect of’galog’on Milk Yield in Buffaloes and Crossbred Cows with Agalactia or Hypogalactia. Indian Vet. Med. J. 1990, 14, 245–248. [Google Scholar]
- Berhane, M. Studies on Feeding Some Indigenous Galactopoeitic Feed Supplements on Milk Production in Crossbred Cows. Ph.D. Thesis, JNKVV, Jabalpur, India, 2000. [Google Scholar]
- Wheeler, G.E.; Wait, C. Use of Herbal Medicines in Modern Dairy Farming-a Breeding Efficiency Programme. In Proceedings of the WOCMAP I-Medicinal and Aromatic Plants Conference: Part 1 of 4, Maastricht, The Netherlands, 19–25 July 1992; pp. 299–308. [Google Scholar]
- Fang, W.; Jiang, C.; Liu, H. Epidemiologic Aspects of Bovine Mastitis and Its Control in Several Dairy Herds in Southeastern China. Prev. Vet. Med. 1993, 15, 169–180. [Google Scholar] [CrossRef]
- Hu, S.; Cai, W.; Ye, J.; Qian, Z.; Sun, Z. Influence of Medicinal Herbs on Phagocytosis by Bovine Neutrophils. J. Vet. Med. Ser. A 1992, 39, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Tomanić, D.; Kladar, N.; Radinović, M.; Stančić, I.; Erdeljan, M.; Stanojević, J.; Galić, I.; Bijelić, K.; Kovačević, Z. Intramammary Ethno-Veterinary Formulation in Bovine Mastitis Treatment for Optimization of Antibiotic Use. Pathogens 2023, 12, 259. [Google Scholar] [CrossRef] [PubMed]
- Chishti, M.A.; Afzal, M.; Muneer, R. Effect of Immunopotentiating Agents on Subclinical Mastitis in Cattle and Buffaloes. Asian-Australas. J. Anim. Sci. 1992, 5, 733–736. [Google Scholar] [CrossRef]
- Delves-Broughton, J.; Blackburn, P.; Evans, R.J.; Hugenholtz, J. Applications of the Bacteriocin, Nisin. Antonie Van. Leeuwenhoek 1996, 69, 193–202. [Google Scholar] [CrossRef]
- Hurst, A. Nisin. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 1981; Volume 27, pp. 85–123. ISBN 0065-2164. [Google Scholar]
- Howell, T.H.; Williams, R.C. Nonsteroidal Antiinflammatory Drugs as Inhibitors of Periodontal Disease Progression. Crit. Rev. Oral. Biol. Med. 1993, 4, 177–196. [Google Scholar] [CrossRef]
- Islam, M.M.; Kashem, M.A. Farmers’ Use of Ethno-Veterinary Medicine (EVM) in the Rearing and Management of Livestock: An Empirical Study in Bangladesh. J. Sustain. Agric. 1999, 13, 39–56. [Google Scholar] [CrossRef]
- Rahman, H.; Sharma, K. Efficacy of Mastilep as Supportive Therapy for Clinical Mastitis in Cows. Indian Vet. J. 2000, 77, 50–52. [Google Scholar]
- Mandeep, S.; Mandial, R.K.; Katoch, R.C.; Batta, M.K.; Nagal, K.B. Therapeutic Efficacy of Mastilep in Treating Subclinical Mastitis of Variable Etiology in Lactating Cows. Indian Vet. J. 2000, 77, 261–263. [Google Scholar]
- Deepa, P.M.; Kanagaraj, D.; Raphael, P.A.; Saseendranath, M.R.; Ally, K. Efficacy of Mastilep in Subclinical Mastitis in Dairy Cows. Indian J. Vet. Med. 2000, 20, 101. [Google Scholar]
- Nath, K.; Dutta, J.B. Control of Subclinical Mastitis (Scm) in Cows through Application of an Herbal Gel. Int. J. Cow Sci. 2005, 1, 63–65. [Google Scholar]
- Abaineh, D.; Sintayehu, A. Treatment Trial of Subclinical Mastitis with the Herb Persicaria Senegalense (Polygonaceae). Trop. Anim. Health Prod. 2001, 33, 511–519. [Google Scholar] [CrossRef]
- Takhar, H.K.; Chaudhary, B.L. Folk Herbal Veterinary Medicines of Southern Rajasthan. Folk. Herb. Vet. Med. South. Rajasthan 2004, 3, 407–418. [Google Scholar]
- Mukherjee, R.; Dash, P.K.; Ram, G.C. Immunotherapeutic Potential of Ocimum Sanctum (L) in Bovine Subclinical Mastitis. Res. Vet. Sci. 2005, 79, 37–43. [Google Scholar] [CrossRef]
- Bullitta, S.; Piluzza, G.; Viegi, L. Plant Resources Used for Traditional Ethnoveterinary Phytotherapy in Sardinia (Italy). Genet. Resour. Crop Evol. 2007, 54, 1447–1464. [Google Scholar] [CrossRef]
- Giacinti, G.; Rosati, R.; Boselli, C.; Tammaro, A.; Amatiste, S.; Ronchi, B. Control of Bovine Sub-Clinical Mastitis by Using Herbal Extract during Lactation. In Proceedings of the Cultivating the Future Based on Science: 2nd Conference of the International Society of Organic Agriculture Research ISOFAR, Modena, Italy, 18–20 June 2008; Available online: https://orgprints.org/id/eprint/12239/ (accessed on 15 April 2023).
- Lu, Y.; Hu, Y.L.; Kong, X.F.; Wang, D.Y. Selection of Component Drug in Activating Blood Flow and Removing Blood Stasis of Chinese Herbal Medicinal Formula for Dairy Cow Mastitis by Hemorheological Method. J. Ethnopharmacol. 2008, 116, 313–317. [Google Scholar] [CrossRef]
- De, U.K.; Mukherjee, R. Expression of Cytokines and Respiratory Burst Activity of Milk Cells in Response to Azadirachta Indica during Bovine Mastitis. Trop. Anim. Health Prod. 2009, 41, 189–197. [Google Scholar] [CrossRef]
- Ahmad, S.; Maqbool, A.; Srivastava, A.; Gogoi, S. Biological Detail and Therapeutic Effect OfAzadirachta Indica (Neem Tree) Products- A Review. J. Evid. Based Med. Healthc. 2019, 6, 1607–1612. [Google Scholar] [CrossRef]
- Sharma, K.; Morla, S.; Khaire, K.C.; Thakur, A.; Moholkar, V.S.; Kumar, S.; Goyal, A. Extraction, Characterization of Xylan from Azadirachta Indica (Neem) Sawdust and Production of Antiproliferative Xylooligosaccharides. Int. J. Biol. Macromol. 2020, 163, 1897–1907. [Google Scholar] [CrossRef]
- Hu, S.H.; Du, A.F. Treatment of Bovine Mastitis with Houttuynin Sodium Bisulphate. J. Vet. Med. Ser. B 1997, 44, 365–370. [Google Scholar] [CrossRef]
- Panya, A.; Pundith, H.; Thongyim, S.; Kaewkod, T.; Chitov, T.; Bovonsombut, S.; Tragoolpua, Y. Antibiotic-Antiapoptotic Dual Function of Clinacanthus Nutans (Burm. f.) Lindau Leaf Extracts against Bovine Mastitis. Antibiotics 2020, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Choi, J.H.; Kim, H.J.; Park, S.H.; Go, G.; Kim, W. In vitro Evidence of Anti-Inflammatory and Anti-Obesity Effects of Medium-Chain Fatty Acid-Diacylglycerols. J. Microbiol. Biotechnol. 2017, 27, 1617–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon-Montano, J.M.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A Review on the Dietary Flavonoid Kaempferol. Mini-Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Morales-Ubaldo, A.L.; Gonzalez-Cortazar, M.; Zaragoza-Bastida, A.; Meza-Nieto, M.A.; Valladares-Carranza, B.A.; Alsayegh, A.; El-Saber Batiha, G.; Rivero-Perez, N. Nor 3′-Demethoxyisoguaiacin from Larrea Tridentata Is a Potential Alternative against Multidrug-Resistant Bacteria Associated with Bovine Mastitis. Molecules 2022, 27, 3620. [Google Scholar] [CrossRef]
- Favela-Hernández, J.; Clemente-Soto, A.; Balderas-Rentería, I.; Garza-González, E.; Camacho-Corona, M. Potential Mechanism of Action of 3′-Demethoxy-6-O-Demethyl-Isoguaiacin on Methicillin Resistant Staphylococcus Aureus. Molecules 2015, 20, 12450–12458. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wang, L. Effects of Sodium New Houttuyfonate on Proliferation, Apoptosis and Migration of A2780 Cells. Int. J. Front. Med. 2022, 4, 48–54. [Google Scholar] [CrossRef]
- Forno-Bell, N.; Bucarey, S.A.; García, D.; Iragüen, D.; Chacón, O.; San Martín, B. Antimicrobial Effects Caused by Aloe Barbadensis Miller on Bacteria Associated with Mastitis in Dairy Cattle. Nat. Prod. Commun. 2019, 14, 1934578X1989667. [Google Scholar] [CrossRef] [Green Version]
- Forno-Bell, N.; Munoz, M.A.; Chacón, O.; Pachá, P.; Iragüen, D.; Cornejo, J.; San Martín, B. Efficacy Prediction of Four Pharmaceutical Formulations for Intramammary Administration Containing Aloe Vera (L.) Burm. f. Combined With Ceftiofur or Cloxacillin in Lactating Cows as an Alternative Therapy to Treat Mastitis Caused by Staphylococcus Aureus. Front. Vet. Sci. 2021, 8, 572568. [Google Scholar] [CrossRef]
- Srichok, J.; Yingbun, N.; Kowawisetsut, T.; Kornmatitsuk, S.; Suttisansanee, U.; Temviriyanukul, P.; Chantong, B. Synergistic Antibacterial and Anti-Inflammatory Activities of Ocimum Tenuiflorum Ethanolic Extract against Major Bacterial Mastitis Pathogens. Antibiotics 2022, 11, 510. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Alsahli, M.A.; Almatroudi, A.; Rahmani, A.H. Ocimum Sanctum: Role in Diseases Management Through Modulating Various Biological Activity. Pharmacogn. J. 2020, 12, 1198–1205. [Google Scholar] [CrossRef]
- Ruegg, P.L. A 100-Year Review: Mastitis Detection, Management, and Prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [Green Version]
- El-Sayed, A.; Kamel, M. Bovine Mastitis Prevention and Control in the Post-Antibiotic Era. Trop. Anim. Health Prod. 2021, 53, 236. [Google Scholar] [CrossRef]
- Zhylkaidar, A.; Oryntaev, K.; Altenov, A.; Kylpybai, E.; Chayxmet, E. Prevention of Bovine Mastitis through Vaccination. Arch. Razi Inst. 2021, 76, 1381–1387. [Google Scholar] [CrossRef]
- Tashakkori, N.; Khoramian, B.; Farhoodi Moghadam, M.; Heidarpour, M.; Mashayekhi, K.; Farzaneh, N. Evaluating the Effectiveness of Two Bovine Mastitis Vaccines and Their Influences on Oxidant and Antioxidant Capacities of Milk. Trop. Anim. Health Prod. 2020, 52, 1493–1501. [Google Scholar] [CrossRef]
- Huang, T.; Song, X.; Jing, J.; Zhao, K.; Shen, Y.; Zhang, X.; Yue, B. Chitosan-DNA Nanoparticles Enhanced the Immunogenicity of Multivalent DNA Vaccination on Mice against Trueperella Pyogenes Infection. J. Nanobiotechnol. 2018, 16, 8. [Google Scholar] [CrossRef] [Green Version]
- Quiroga, J.; Vidal, S.; Siel, D.; Caruffo, M.; Valdés, A.; Cabrera, G.; Lapierre, L.; Sáenz, L. Novel Proteoliposome-Based Vaccine against E. Coli: A Potential New Tool for the Control of Bovine Mastitis. Animals 2022, 12, 2533. [Google Scholar] [CrossRef]
- Zeng, X.; Vidlund, J.; Gillespie, B.; Cao, L.; Agga, G.E.; Lin, J.; Dego, O.K. Evaluation of Immunogenicity of Enterobactin Conjugate Vaccine for the Control of E. Coli Mastitis in Dairy Cows. J. Dairy Sci. 2023; in press. [Google Scholar] [CrossRef]
- Wang, H.; Zeng, X.; Mo, Y.; He, B.; Lin, H.; Lin, J. Enterobactin-Specific Antibodies Induced by a Novel Enterobactin Conjugate Vaccine. Appl. Environ. Microbiol. 2019, 85, e00358-19. [Google Scholar] [CrossRef] [Green Version]
- Hogan, J.S.; Todhunter, D.A.; Tomita, G.M.; Smith, K.L.; Schoenberger, P.S. Opsonic Activity of Bovine Serum and Mammary Secretion After Escherichia Coli J5 Vaccination. J. Dairy Sci. 1992, 75, 72–77. [Google Scholar] [CrossRef]
- McClure, A.M.; Christopher, E.E.; Wolff, W.A.; Fales, W.H.; Krause, G.F.; Miramonti, J. Effect of Re-17 Mutant Salmonella Typhimurium Bacterin Toxoid on Clinical Coliform Mastitis. J. Dairy Sci. 1994, 77, 2272–2280. [Google Scholar] [CrossRef]
- Tomazi, T.; Tomazi, A.C.C.H.; Silva, J.C.C.; Bringhenti, L.; Bravo, M.L.M.C.; Rodrigues, M.X.; Bicalho, R.C. Immunization with a Novel Recombinant Protein (YidR) Reduced the Risk of Clinical Mastitis Caused by Klebsiella spp. and Decreased Milk Losses and Culling Risk after Escherichia Coli Infections. J. Dairy Sci. 2021, 104, 4787–4802. [Google Scholar] [CrossRef] [PubMed]
- Pankey, J.W.; Boddie, N.T.; Watts, J.L.; Nickerson, S.C. Evaluation of Protein A and a Commercial Bacterin as Vaccines Against Staphylococcus Aureus Mastitis by Experimental Challenge. J. Dairy Sci. 1985, 68, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.L. Vaccination against Experimental Staphylococcal Mastitis in Dairy Heifers. Res. Vet. Sci. 1992, 53, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Middleton, J.R.; Ma, J.; Rinehart, C.L.; Taylor, V.N.; Luby, C.D.; Steevens, B.J. Efficacy of Different LysiginTM Formulations in the Prevention of Staphylococcus Aureus Intramammary Infection in Dairy Heifers. J. Dairy. Res. 2006, 73, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Ster, C.; Beaudoin, F.; Diarra, M.S.; Jacques, M.; Malouin, F.; Lacasse, P. Evaluation of Some Staphylococcus Aureus Iron-Regulated Proteins as Vaccine Targets. Vet. Immunol. Immunopathol. 2010, 136, 311–318. [Google Scholar] [CrossRef]
- Schukken, Y.H.; Bronzo, V.; Locatelli, C.; Pollera, C.; Rota, N.; Casula, A.; Testa, F.; Scaccabarozzi, L.; March, R.; Zalduendo, D.; et al. Efficacy of Vaccination on Staphylococcus Aureus and Coagulase-Negative Staphylococci Intramammary Infection Dynamics in 2 Dairy Herds. J. Dairy Sci. 2014, 97, 5250–5264. [Google Scholar] [CrossRef] [Green Version]
- COLDITZ, I.G.; WATSON, D.L. The Immunophysiological Basis for Vaccinating Ruminants against Mastitis. Aust. Vet. J. 1985, 62, 145–153. [Google Scholar] [CrossRef]
- Anderson, J. Progressive Pathology of Staphylococcal Mastitis with a Note on Control, Immunisation and Therapy. Vet. Rec. 1982, 110, 372–376. [Google Scholar] [CrossRef]
- Rainard, P. Staphylococcus Aureus LeucotoxinLukM/F’ Is Secreted and Stimulates Neutralising Antibody Response in the Course of Intramammary Infection. Vet. Res. 2007, 38, 685–696. [Google Scholar] [CrossRef] [Green Version]
- Rainard, P.; Gilbert, F.B.; Martins, R.P.; Germon, P.; Foucras, G. Progress towards the Elusive Mastitis Vaccines. Vaccines 2022, 10, 296. [Google Scholar] [CrossRef]
- Tan, L.K.K.; Reglinski, M.; Teo, D.; Reza, N.; Lamb, L.E.M.; Nageshwaran, V.; Turner, C.E.; Wikstrom, M.; Frick, I.-M.; Bjorck, L.; et al. Vaccine-Induced, but Not Natural Immunity, against the Streptococcal Inhibitor of Complement Protects against Invasive Disease. Npj Vaccin. 2021, 6, 62. [Google Scholar] [CrossRef]
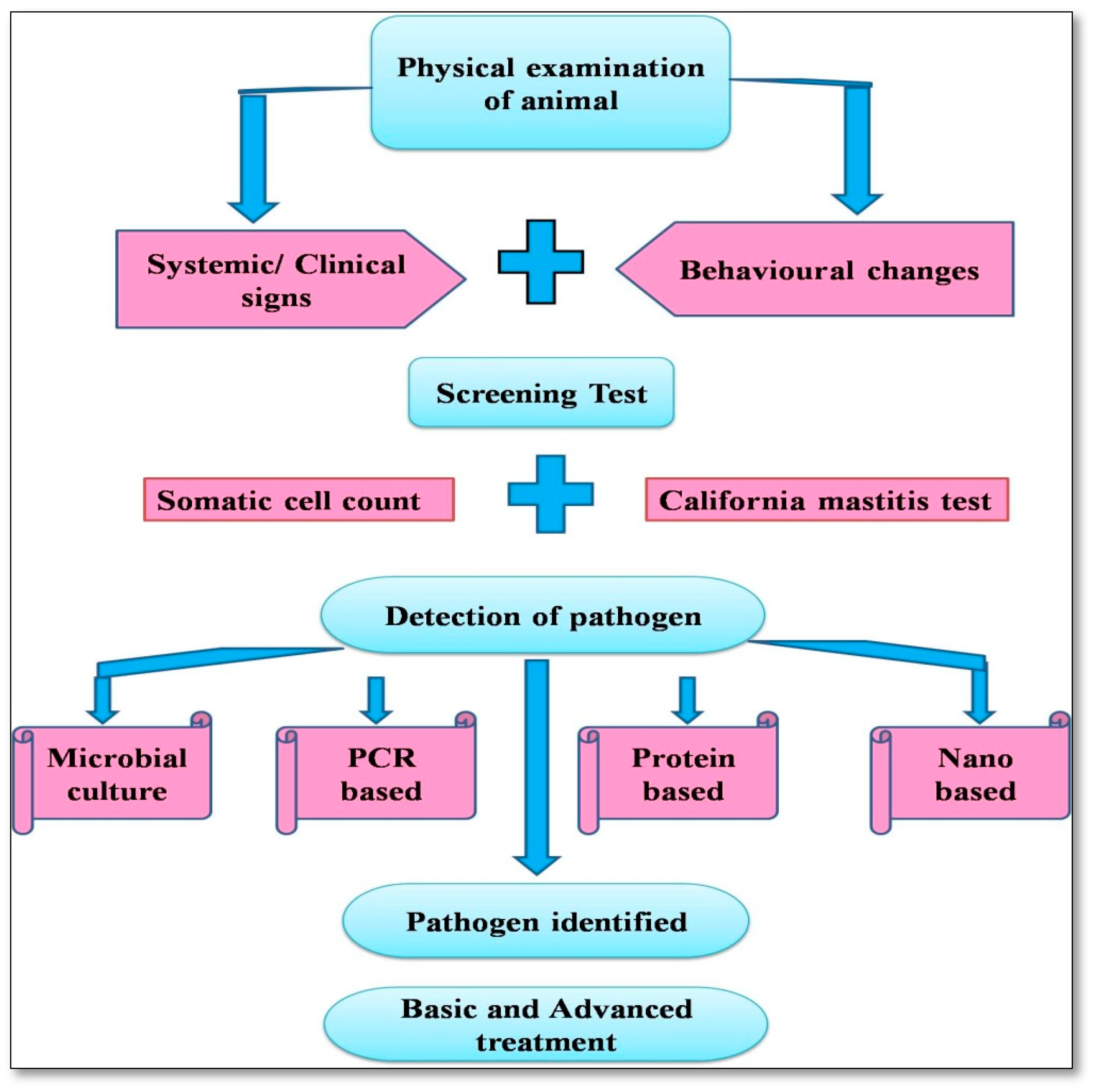


| S No. | Causative Agent of Mastitis | References |
|---|---|---|
| 1. | Staphylococcus spp. | |
| Staphylococcus spp. | [45,58,59,60,61,62] | |
| S. aureus | [63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82] | |
| S.mastitidis | [58,83,84] | |
| Coagulase-negative Staphylococcus (CNS) | [65,85,86,87,88,89,90,91,92,93] | |
| 2. | Streptococcus spp. | |
| Streptococcus spp. | [6,59,94,95,96] | |
| Strep. agalacticae | [11,97,98,99,100,101,102,103,104,105,106] | |
| Strep. viridians | [107] | |
| Strep. lactis | [108] | |
| Strep. mastitidis, Strep. acidominimus | [109,110,111] | |
| Strep.uberis | [97,100,112,113,114,115,116,117,118,119] | |
| 3. | E. coli | [6,63,66,67,74,96,118,120,121,122,123,124,125,126,127] |
| 4. | Bacillus spp. | [67,69,126,128,129,130,131] |
| 5. | Tuberculous type bacilli | [58,119,132] |
| 6. | Mycoplasma spp. | [69,119] |
| 7. | Aerobacter aerogens | [84] |
| 8. | Pseudomonas aeruginosa | [69,97,119,133,134] |
| 9. | Corynebacterium spp. | [135,136,137,138,139,140,141] |
| Key Features of Coliform Vaccine Trails | |||
|---|---|---|---|
| Vaccine Antigen | Efficacy | Knowledge Gap | References |
| Escherichia coliJ5 bacterins | Decreased coliform mastitis severity in field experiments | Variable effect on incidence of cases, unknown mechanism | [310] |
| Salmonella Re-17 bacterin toxoid | Decreased severity in field experiment | Unknown mechanism | [311] |
| E. coli J5 bacterin with killed Staphylococcus aureus (StartVac, Hipra) | Less severity in field cases | Unknown mechanism | [27] |
| Whole E. coli (P4), intramammary booster with bacterial extract | Reduction in severity, likely independent of antibodies, related to Th17 response | Test for heterologous protection not performed | [191] |
| Klebsiella recombinant YidR | Reduced incidence of Kleibsiella mastitis | Unknown mechanism and little antibody response to whole bacteria | [312] |
| Key Features of Staphylococcus Aureus Vaccine Trials | |||
| Vaccine Antigen | Efficacy | Knowledge Gap | References |
| Protein A (SpA) | Spontaneous cure of Staph. Aureus infection with experimental challenge | Mechanism not identified, no field trial | [313] |
| Killed vaccine, “in vivo” antigen and dextran sulfate | Severity of infection low | Mechanism not identified | [314] |
| Bacterial lysate (5 strains) Lysigin (Boehringer Ingelheim Vetmedica, Lyon, France) | Reduction in intramammary infection | Variable results | [315] |
| Recombinant IsdB and IsdH | IgG2 antibodies and antigen-specific lymphoproliferation | Protection study in cows not documented | [316] |
| Slime on killed bacteria, StartVac (Hipra) | Reduction in bacterial shedding in milk | Mechanism not identified | [317] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kour, S.; Sharma, N.; N., B.; Kumar, P.; Soodan, J.S.; Santos, M.V.d.; Son, Y.-O. Advances in Diagnostic Approaches and Therapeutic Management in Bovine Mastitis. Vet. Sci. 2023, 10, 449. https://doi.org/10.3390/vetsci10070449
Kour S, Sharma N, N. B, Kumar P, Soodan JS, Santos MVd, Son Y-O. Advances in Diagnostic Approaches and Therapeutic Management in Bovine Mastitis. Veterinary Sciences. 2023; 10(7):449. https://doi.org/10.3390/vetsci10070449
Chicago/Turabian StyleKour, Savleen, Neelesh Sharma, Balaji N., Pavan Kumar, Jasvinder Singh Soodan, Marcos Veiga dos Santos, and Young-Ok Son. 2023. "Advances in Diagnostic Approaches and Therapeutic Management in Bovine Mastitis" Veterinary Sciences 10, no. 7: 449. https://doi.org/10.3390/vetsci10070449
APA StyleKour, S., Sharma, N., N., B., Kumar, P., Soodan, J. S., Santos, M. V. d., & Son, Y. -O. (2023). Advances in Diagnostic Approaches and Therapeutic Management in Bovine Mastitis. Veterinary Sciences, 10(7), 449. https://doi.org/10.3390/vetsci10070449










