Regulation of IkappaB Protein Expression by Early Gestation in the Thymus of Ewes
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Tissue Collection
2.2. RNA Extraction and qRT-PCR Assay
2.3. Western Blot
2.4. Immunohistochemistry Analysis
2.5. Statistical Analysis
3. Results
3.1. Expression of IκB Genes in the Thymus
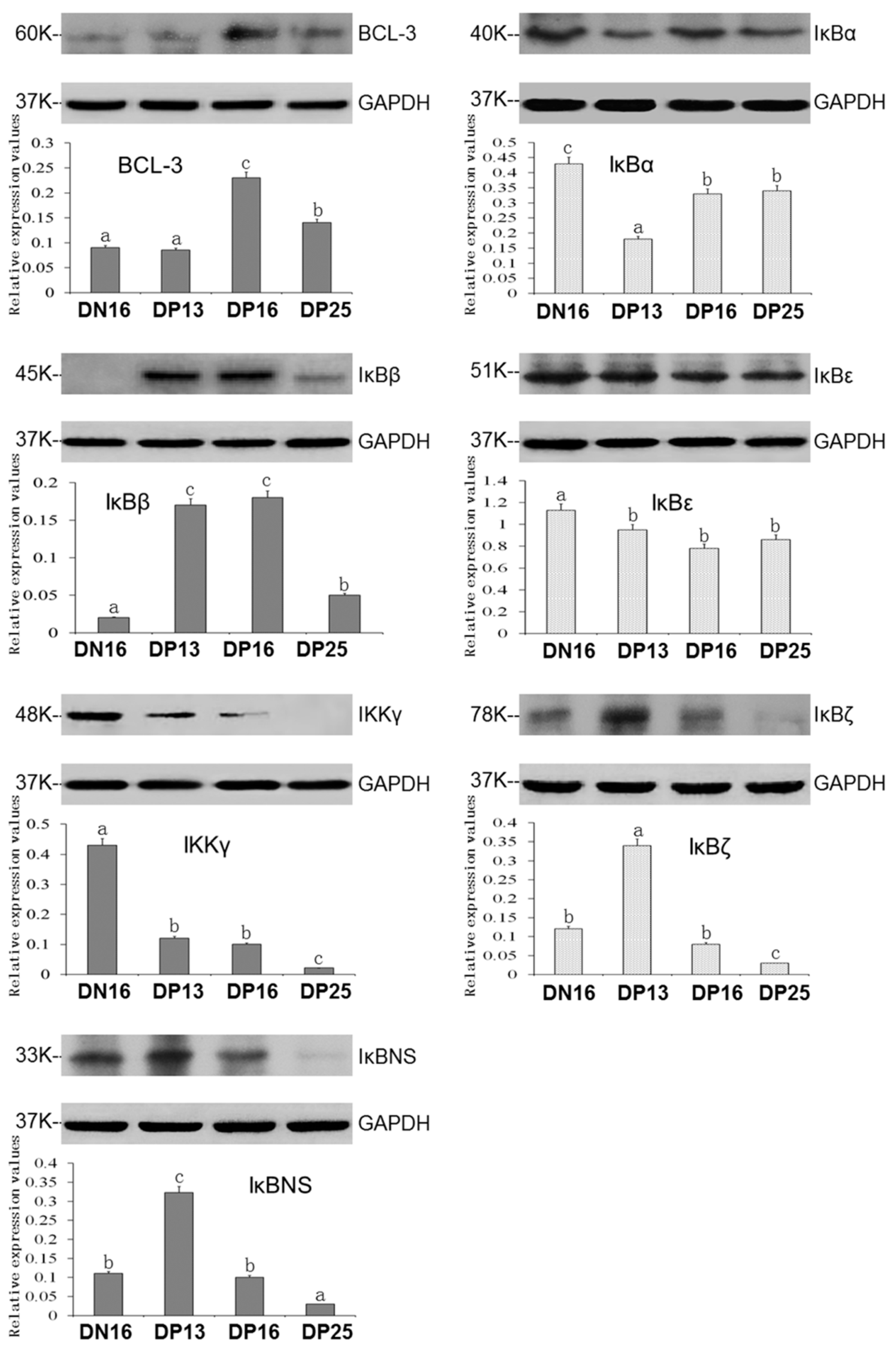
3.2. Expression of IκB Proteins in the Thymus
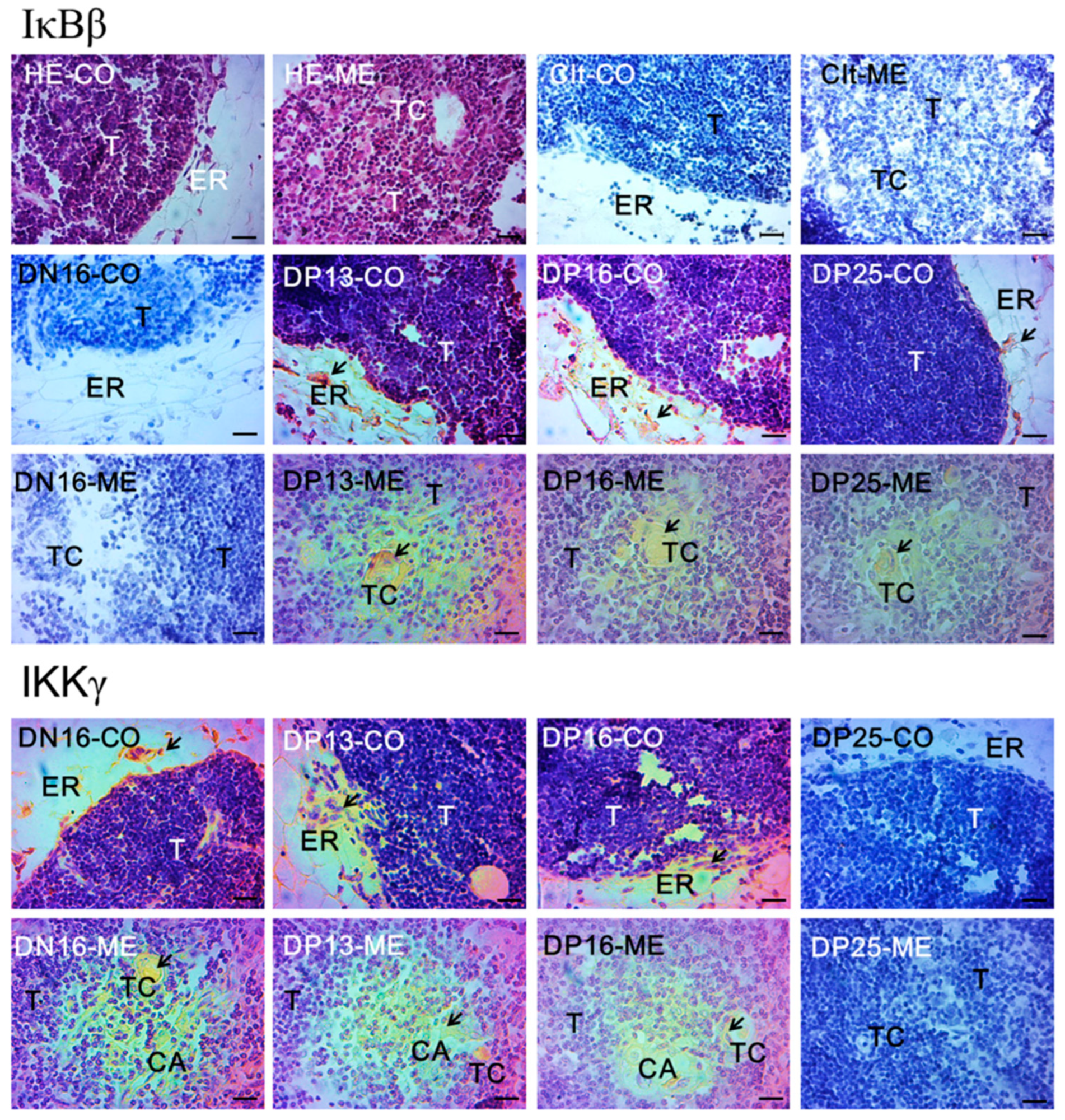
3.3. Immunohistochemistry for IκBβ and IKKγ Proteins
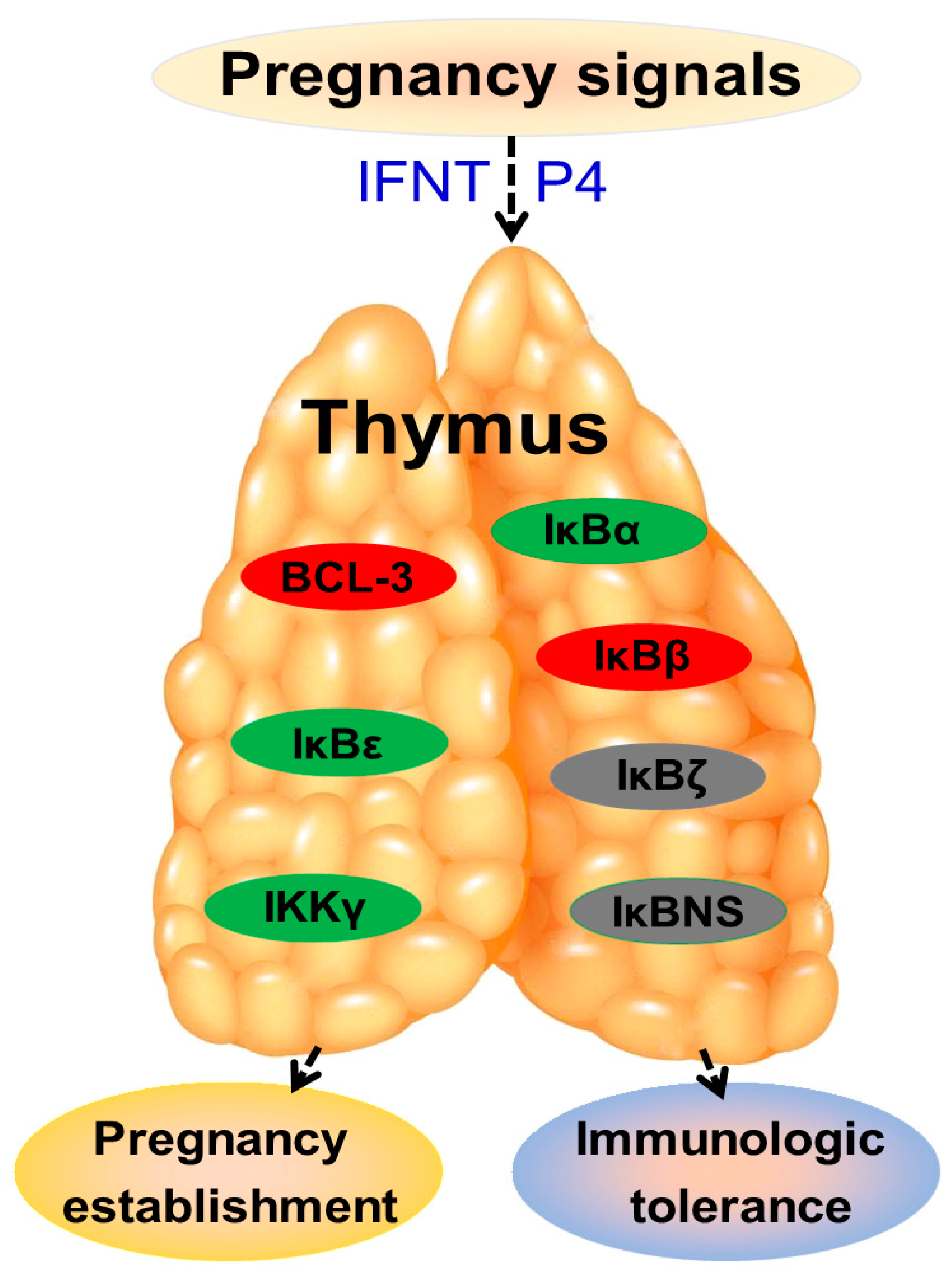
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moffett, A.; Shreeve, N. Local immune recognition of trophoblast in early human pregnancy: Controversies and questions. Nat. Rev. Immunol. 2022, 3, 222–235. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, G.; Brooks, J.F.; Tuomivaara, S.T.; McIntyre, T.I.; Ma, S.; Rideaux, D.; Zikherman, J.; Fisher, S.J.; Erlebacher, A. Establishment of fetomaternal tolerance through glycan-mediated B cell suppression. Nature 2022, 603, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Ott, T.L. Immunological detection of pregnancy: Evidence for systemic immune modulation during early pregnancy in ruminants. Theriogenology 2020, 150, 498–503. [Google Scholar] [CrossRef]
- Rocha, C.C.; da Silveira, J.C.; Forde, N.; Binelli, M.; Pugliesi, G. Conceptus-modulated innate immune function during early pregnancy in ruminants: A review. Anim. Reprod. 2021, 18, e20200048. [Google Scholar] [CrossRef]
- Cai, C.; Ren, Y.; Cao, J.; Fang, S.; Zhang, L.; Yang, L. Expression of IkappaB family in the ovine liver during early pregnancy. Animals 2023, 13, 1057. [Google Scholar] [CrossRef]
- Cheng, Q.J.; Ohta, S.; Sheu, K.M.; Spreafico, R.; Adelaja, A.; Taylor, B.; Hoffmann, A. NF-κB dynamics determine the stimulus specificity of epigenomic reprogramming in macrophages. Science 2021, 372, 1349–1353. [Google Scholar] [CrossRef] [PubMed]
- Ariyakumar, G.; Morris, J.M.; McKelvey, K.J.; Ashton, A.W.; McCracken, S.A. NF-κB regulation in maternal immunity during normal and IUGR pregnancies. Sci. Rep. 2021, 11, 20971. [Google Scholar] [CrossRef]
- Mulero, M.C.; Huxford, T.; Ghosh, G. NF-κB, IκB, and IKK: Integral components of immune system signaling. Adv. Exp. Med. Biol. 2019, 1172, 207–226. [Google Scholar] [CrossRef]
- McCracken, S.A.; Drury, C.L.; Lee, H.S.; Morris, J.M. Pregnancy is associated with suppression of the nuclear factor kappaB/IkappaB activation pathway in peripheral blood mononuclear cells. J. Reprod. Immunol. 2003, 58, 27–47. [Google Scholar] [CrossRef]
- Zozo, B.; Govender, N.; Moodley, J.; Naicker, T. Expression of plasma nuclear factor-kappa B cells (NF-κB) and Inhibitory subunit kappa B alpha (IκB-α) in HIV-associated pre-eclampsia. Hypertens Pregnancy 2021, 40, 15–20. [Google Scholar] [CrossRef]
- Fang, S.; Cai, C.; Bai, Y.; Zhang, L.; Yang, L. Early Pregnancy regulates expression of IkappaB family in ovine spleen and lymph nodes. Int. J. Mol. Sci. 2023, 24, 5156. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.F.A.P. The function of the thymus and its impact on modern medicine. Science 2020, 369, eaba2429. [Google Scholar] [CrossRef] [PubMed]
- Thapa, P.; Farber, D.L. The role of the thymus in the immune response. Thorac. Surg. Clin. 2019, 29, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Paolino, M.; Koglgruber, R.; Cronin, S.J.F.; Uribesalgo, I.; Rauscher, E.; Harreiter, J.; Schuster, M.; Bancher-Todesca, D.; Pranjic, B.; Novatchkova, M.; et al. RANK links thymic regulatory T cells to fetal loss and gestational diabetes in pregnancy. Nature 2021, 589, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Nguyen, S.L.; Petroff, M.G. Exploring the origin and antigenic specificity of maternal regulatory T cells in pregnancy. Front. Immunol. 2020, 11, 1302. [Google Scholar] [CrossRef]
- Feng, P.; Wu, J.; Ren, Y.; Zhang, L.; Cao, J.; Yang, L. Early pregnancy regulates the expression of prolactin and its receptor in the thymus, the liver, the spleen and lymph nodes in sheep. Domest. Anim. Endocrinol. 2022, 81, 106731. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Zhao, Z.; Cai, J.; Zhao, S.; Yang, L. Modulation of nod-like receptor expression in the thymus during early pregnancy in ewes. Vaccines 2022, 10, 2128. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Q.; Wang, H.; Feng, P.; Yang, G.; Yang, L. Effects of early pregnancy on the complement system in the ovine thymus. Vet. Res. Commun. 2022, 46, 137–145. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Liu, H.; Zeng, L.; Yang, Y.; Guo, C.; Wang, H. Bcl-3: A double-edged sword in immune cells and inflammation. Front. Immunol. 2022, 13, 847699. [Google Scholar] [CrossRef]
- Tang, W.; Saret, S.; Tian, R.; Wang, H.; Claudio, E.; Murphy, P.M.; Siebenlist, U. Bcl-3 suppresses differentiation of RORγt+ regulatory T cells. Immunol. Cell Biol. 2021, 99, 586–595. [Google Scholar] [CrossRef]
- Tassi, I.; Claudio, E.; Wang, H.; Tang, W.; Ha, H.L.; Saret, S.; Sher, A.; Jankovic, D.; Siebenlist, U. Adaptive immune-mediated host resistance to Toxoplasma gondii is governed by the NF-κB regulator Bcl-3 in dendritic cells. Eur. J. Immunol. 2015, 45, 1972–1979. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Mondragón, E.; Gómez-Chávez, F.; Murrieta-Coxca, M.; Vázquez-Sánchez, E.A.; Martínez-Torres, I.; Cancino-Díaz, M.E.; Rojas-Espinosa, O.; Cancino-Díaz, J.C.; Reyes-Sánchez, J.L.; Rodríguez-Muñóz, R.; et al. Low expression of IL-6 and TNF-α correlates with the presence of the nuclear regulators of NF-κB, IκBNS and BCL-3, in the uterus of mice. Mol. Immunol. 2015, 68, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Chávez, F.; Correa, D.; Navarrete-Meneses, P.; Cancino-Diaz, J.C.; Cancino-Diaz, M.E.; Rodríguez-Martínez, S. NF-κB and its regulators during pregnancy. Front. Immunol. 2021, 12, 679106. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Claudio, E.; Brown, K.; Siebenlist, U. A role for the IkappaB family member Bcl-3 in the control of central immunologic tolerance. Immunity 2007, 27, 438–452. [Google Scholar] [CrossRef]
- Caamaño, J.H.; Perez, P.; Lira, S.A.; Bravo, R. Constitutive expression of Bc1-3 in thymocytes increases the DNA binding of NF-kappaB1 (p50) homodimers in vivo. Mol. Cell. Biol. 1996, 16, 1342–1348. [Google Scholar] [CrossRef]
- Paxian, S.; Merkle, H.; Riemann, M.; Wilda, M.; Adler, G.; Hameister, H.; Liptay, S.; Pfeffer, K.; Schmid, R.M. Abnormal organogenesis of Peyer’s patches in mice deficient for NF-kappaB1, NF-kappaB2, and Bcl-3. Gastroenterology 2002, 122, 1853–1868. [Google Scholar] [CrossRef]
- Guo, D.; Tong, Y.; Jiang, X.; Meng, Y.; Jiang, H.; Du, L.; Wu, Q.; Li, S.; Luo, S.; Li, M.; et al. Aerobic glycolysis promotes tumor immune evasion by hexokinase2-mediated phosphorylation of IκBα. Cell Metab. 2022, 34, 1312–1324.e6. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, J.H.; Kim, J.; Choi, S.; Park, M.; Park, W.; Kim, S.; Lee, K.S.; Kim, T.; Jung, J.; et al. REDD-1 aggravates endotoxin-induced inflammation via atypical NF-κB activation. FASEB J. 2018, 32, 4585–4599. [Google Scholar] [CrossRef]
- Mooster, J.L.; Le Bras, S.; Massaad, M.J.; Jabara, H.; Yoon, J.; Galand, C.; Heesters, B.A.; Burton, O.T.; Mattoo, H.; Manis, J.; et al. Defective lymphoid organogenesis underlies the immune deficiency caused by a heterozygous S32I mutation in IκBα. J. Exp. Med. 2015, 212, 185–202. [Google Scholar] [CrossRef]
- Wen, Z.S.; Tang, Z.; Gu, L.X.; Xiang, X.W.; Qu, Y.L. Immunomodulatory effect of low molecular-weight seleno-aminopolysaccharide on immunosuppressive mice. Int. J. Biol. Macromol. 2019, 123, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Sakowicz, A.; Bralewska, M.; Pietrucha, T.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W.; Gach, A.; Rybak-Krzyszkowska, M.; Witas, P.J.; Huras, H.; Grzesiak, M.; et al. Canonical, Non-canonical and atypical pathways of nuclear factor кb activation in preeclampsia. Int. J. Mol. Sci. 2020, 21, 5574. [Google Scholar] [CrossRef] [PubMed]
- Tsui, R.; Kearns, J.D.; Lynch, C.; Vu, D.; Ngo, K.A.; Basak, S.; Ghosh, G.; Hoffmann, A. IκBβ enhances the generation of the low-affinity NFκB/RelA homodimer. Nat. Commun. 2015, 6, 7068. [Google Scholar] [CrossRef] [PubMed]
- McKenna, S.; Wright, C.J. Inhibiting IκBβ-NFκB signaling attenuates the expression of select pro-inflammatory genes. J. Cell Sci. 2015, 128, 2143–2155. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.J.; Agboke, F.; Muthu, M.; Michaelis, K.A.; Mundy, M.A.; La, P.; Yang, G.; Dennery, P.A. Nuclear factor-κB (NF-κB) inhibitory protein IκBβ determines apoptotic cell death following exposure to oxidative stress. J. Biol. Chem. 2012, 287, 6230–6239. [Google Scholar] [CrossRef]
- Cook, S.; Hung, V.; Duncan, K.A. Crosstalk between estrogen withdrawal and NFκB signaling following penetrating brain injury. Neuroimmunomodulation 2018, 25, 193–200. [Google Scholar] [CrossRef]
- Simeonidis, S.; Liang, S.; Chen, G.; Thanos, D. Cloning and functional characterization of mouse IkappaBepsilon. Proc. Natl. Acad. Sci. USA 1997, 94, 14372–14377. [Google Scholar] [CrossRef]
- Della-Valle, V.; Roos-Weil, D.; Scourzic, L.; Mouly, E.; Aid, Z.; Darwiche, W.; Lecluse, Y.; Damm, F.; Mémet, S.; Mercher, T.; et al. Nfkbie-deficiency leads to increased susceptibility to develop B-cell lymphoproliferative disorders in aged mice. Blood Cancer J. 2020, 10, 38. [Google Scholar] [CrossRef]
- Kearns, J.D.; Basak, S.; Werner, S.L.; Huang, C.S.; Hoffmann, A. IkappaBepsilon provides negative feedback to control NF-kappaB oscillations, signaling dynamics, and inflammatory gene expression. J. Cell. Biol. 2006, 173, 659–664. [Google Scholar] [CrossRef]
- Lee, S.H.; Hannink, M. Characterization of the nuclear import and export functions of Ikappa B(epsilon). J. Biol. Chem. 2002, 277, 23358–23366. [Google Scholar] [CrossRef]
- Tanaka, K.; Nakabayashi, K.; Kawai, T.; Tanigaki, S.; Matsumoto, K.; Hata, K.; Kobayashi, Y. Gene expression and DNA methylation changes in BeWo cells dependent on tumor necrosis factor-α and insulin-like growth factor-I. Hum. Cell. 2020, 33, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M.; Aleksiyadis, K.; Martin, A.; McNamee, K.; Tharmalingam, T.; Williams, R.O.; Mémet, S.; Cope, A.P. Inhibitor of kappa B epsilon (IκBε) is a non-redundant regulator of c-Rel-dependent gene expression in murine T and B cells. PLoS ONE 2011, 6, e24504. [Google Scholar] [CrossRef] [PubMed]
- Maubach, G.; Schmädicke, A.C.; Naumann, M. NEMO links nuclear factor-κB to human diseases. Trends Mol. Med. 2017, 23, 1138–1155. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Ea, C.K.; Fang, Y.; Chen, Z.J. Liquid phase separation of NEMO induced by polyubiquitin chains activates NF-κB. Mol. Cell. 2022, 82, 2415–2426.e5. [Google Scholar] [CrossRef]
- Wackernagel, L.M.; Abdi Sarabi, M.; Weinert, S.; Zuschratter, W.; Richter, K.; Fischer, K.D.; Braun-Dullaeus, R.C.; Medunjanin, S. IKKγ/NEMO localization into multivesicular bodies. Int. J. Mol. Sci. 2022, 23, 6778. [Google Scholar] [CrossRef] [PubMed]
- Surucu Yilmaz, N.; Bilgic Eltan, S.; Kayaoglu, B.; Geckin, B.; Heredia, R.J.; Sefer, A.P.; Kiykim, A.; Nain, E.; Kasap, N.; Dogru, O.; et al. Low density granulocytes and dysregulated neutrophils driving autoinflammatory manifestations in NEMO deficiency. J. Clin. Immunol. 2022, 42, 582–596. [Google Scholar] [CrossRef]
- Sakowicz, A.; Hejduk, P.; Pietrucha, T.; Nowakowska, M.; Płuciennik, E.; Pospiech, K.; Gach, A.; Rybak-Krzyszkowska, M.; Sakowicz, B.; Kaminski, M.; et al. Finding NEMO in preeclampsia. Am. J. Obstet. Gynecol. 2016, 214, 538.e1–538.e7. [Google Scholar] [CrossRef]
- Zeng, M.; Xu, M.; Li, X.; Li, J.; Liu, Y. PAD4 silencing inhibits inflammation whilst promoting trophoblast cell invasion and migration by inactivating the NEMO/NF-κB pathway. Exp. Ther. Med. 2022, 24, 568. [Google Scholar] [CrossRef]
- Tsagaratou, A.; Grammenoudi, S.; Mosialos, G. Differential requirement of IKK2 for CYLD-dependent representation of thymic and peripheral T-cell populations. Eur. J. Immunol. 2011, 41, 3054–3062. [Google Scholar] [CrossRef]
- Miyake, T.; Satoh, T.; Kato, H.; Matsushita, K.; Kumagai, Y.; Vandenbon, A.; Tani, T.; Muta, T.; Akira, S.; Takeuchi, O. IκBζ is essential for natural killer cell activation in response to IL-12 and IL-18. Proc. Natl. Acad. Sci. USA 2010, 107, 17680–17685. [Google Scholar] [CrossRef]
- Onitsuka, M.; Kinoshita, Y.; Nishizawa, A.; Tsutsui, T.; Omasa, T. Enhanced IgG1 production by overexpression of nuclear factor kappa B inhibitor zeta (NFKBIZ) in Chinese hamster ovary cells. Cytotechnology 2018, 70, 675–685. [Google Scholar] [CrossRef]
- Muta, T. IkappaB-zeta: An inducible regulator of nuclear factor-kappaB. Vitam. Horm. 2006, 74, 301–316. [Google Scholar] [CrossRef] [PubMed]
- MaruYama, T.; Kobayashi, S.; Ogasawara, K.; Yoshimura, A.; Chen, W.; Muta, T. Control of IFN-γ production and regulatory function by the inducible nuclear protein IκB-ζ in T cells. J. Leukoc. Biol. 2015, 98, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Cho, J.; Kwon, B.E.; Lee, G.S.; Yoon, S.I.; Kang, S.G.; Kim, P.H.; Kweon, M.N.; Yang, H.; Vallance, B.A.; et al. IκBζ facilitates protective immunity against Salmonella infection via Th1 differentiation and IgG production. Sci. Rep. 2019, 9, 8397. [Google Scholar] [CrossRef]
- Raghupathy, R.; Makhseed, M.; Azizieh, F.; Omu, A.; Gupta, M.; Farhat, R. Cytokine production by maternal lymphocytes during normal human pregnancy and in unexplained recurrent spontaneous abortion. Hum. Reprod. 2000, 15, 713–718. [Google Scholar] [CrossRef]
- Yang, L.; Wang, Y.; Li, S.; Zhu, M.; He, K.; Yao, X.; Zhang, L. Differential expression of interferon-gamma, IL-4 and IL-10 in peripheral blood mononuclear cells during early pregnancy of the bovine. Reprod. Biol. 2018, 18, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Haczku, A. Targeting IκBNS in allergic asthma: Where it resides, matters. Allergy 2017, 72, 1003–1005. [Google Scholar] [CrossRef]
- Erikson, E.; Ádori, M.; Khoenkhoen, S.; Zhang, J.; Rorbach, J.; Castro Dopico, X.; Karlsson Hedestam, G. Impaired plasma cell differentiation associates with increased oxidative metabolism in IκBNS-deficient B cells. Cell Immunol. 2022, 375, 104516. [Google Scholar] [CrossRef]
- Schuster, M.; Plaza-Sirvent, C.; Visekruna, A.; Huehn, J.; Schmitz, I. Generation of Foxp3+CD25- regulatory T-cell precursors requires c-Rel and IκBNS. Front. Immunol. 2019, 10, 1583. [Google Scholar] [CrossRef]
- Dwyer, J.R.; Racine, J.J.; Chapman, H.D.; Quinlan, A.; Presa, M.; Stafford, G.A.; Schmitz, I.; Serreze, D.V. Nfkbid overexpression in nonobese diabetic mice elicits complete type 1 diabetes resistance in part associated with enhanced thymic deletion of pathogenic CD8 T cells and increased numbers and activity of regulatory T cells. J. Immunol. 2022, 209, 227–237. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Cao, X. Regulation of type I interferon signaling in immunity and inflammation: A comprehensive review. J. Autoimmun. 2017, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xue, J.; Wang, Q.; Lv, W.; Mi, H.; Liu, Y.; Yang, L. Changes in expression of ISG15, progesterone receptor and progesterone-induced blocking factor in ovine thymus during early pregnancy. Theriogenology 2018, 121, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cai, C.; Fang, S.; Hao, S.; Zhang, T.; Zhang, L. Changes in expression of nuclear factor kappa B subunits in the ovine thymus during early pregnancy. Sci. Rep. 2022, 12, 17683. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Wang, X.; Jameson, S.C.; Hogquist, K.A. Late stages of T cell maturation in the thymus involve NF-κB and tonic type I interferon signaling. Nat. Immunol. 2016, 17, 565–573. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.; Yang, Z.; Quan, Y.; Zhao, S.; Zhang, L.; Yang, L. Regulation of IkappaB Protein Expression by Early Gestation in the Thymus of Ewes. Vet. Sci. 2023, 10, 462. https://doi.org/10.3390/vetsci10070462
Meng Y, Yang Z, Quan Y, Zhao S, Zhang L, Yang L. Regulation of IkappaB Protein Expression by Early Gestation in the Thymus of Ewes. Veterinary Sciences. 2023; 10(7):462. https://doi.org/10.3390/vetsci10070462
Chicago/Turabian StyleMeng, Yao, Zhen Yang, Yaodong Quan, Shuxin Zhao, Leying Zhang, and Ling Yang. 2023. "Regulation of IkappaB Protein Expression by Early Gestation in the Thymus of Ewes" Veterinary Sciences 10, no. 7: 462. https://doi.org/10.3390/vetsci10070462
APA StyleMeng, Y., Yang, Z., Quan, Y., Zhao, S., Zhang, L., & Yang, L. (2023). Regulation of IkappaB Protein Expression by Early Gestation in the Thymus of Ewes. Veterinary Sciences, 10(7), 462. https://doi.org/10.3390/vetsci10070462







