Exploring the Differential Expression of a Set of Key Genes Involved in the Regulation and Functioning of the Stomach in the Post-Weaned Pig
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
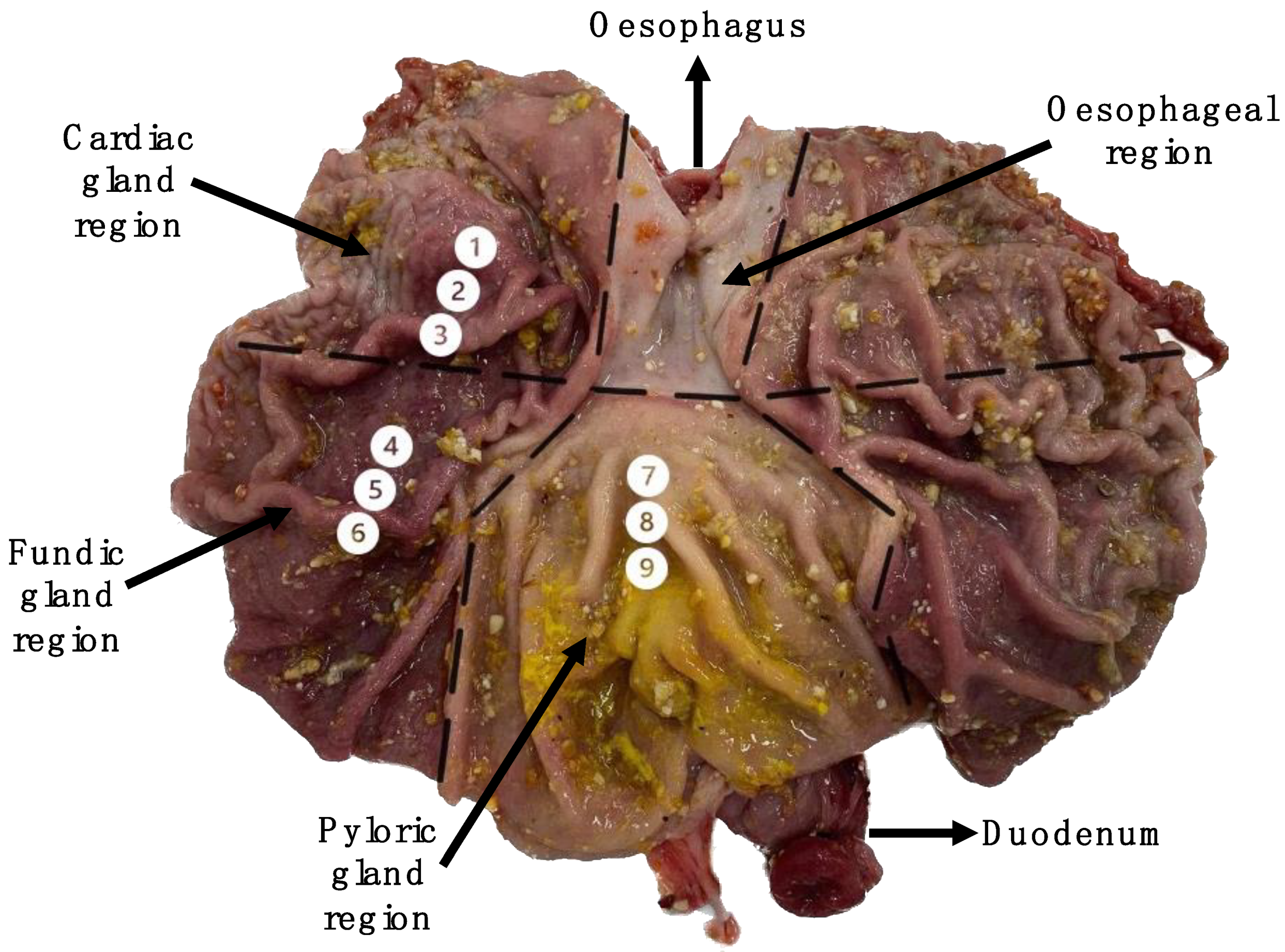
2.2. Sample Collection
2.3. Gene Expression
2.3.1. RNA Extraction and cDNA Synthesis
2.3.2. Target Selection
2.3.3. Quantitative PCR
2.3.4. Statistical Analysis
3. Results
3.1. Stomach Weights
3.2. Stomach pH
3.3. Comparing Gene Expression between the Cardiac, Fundic and Pyloric Regions
3.3.1. The Cardiac vs. Fundic vs. Pyloric Gland Regions
The Cardiac Gland Region
The Fundic Gland Region
The Pyloric Gland Region
3.4. Comparing Gene Expression within the Cardiac, Fundic and Pyloric Regions
3.4.1. The Cardiac Gland Region (Location 1 vs. Location 2 vs. Location 3)
3.4.2. The Fundic Gland Region (Location 4 vs. Location 5 vs. Location 6)
3.4.3. The Pyloric Gland Region (Location 7 vs. Location 8 vs. Location 9)
3.5. Gene Expression Dynamics per Tissue
3.5.1. The Cardiac Gland Region
3.5.2. The Fundic Gland Region
3.5.3. The Pyloric Gland Region
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Function | Gene | Tissue | SEM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cardiac (C) | Fundic (F) | Pyloric (P) | C | F | P | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |||||
| Acid secretion | ATP4A | 0.0100 | 0.0700 | 0.1663 | 0.4763 | 0.4013 | 0.4838 | 0.0000 | 0.0000 | 0.0000 | 0.0462 | 0.1142 | 0.0000 |
| HRH2 | 0.0300 | 0.0950 | 0.1638 | 0.3850 | 0.3850 | 0.4363 | 0.0313 | 0.0288 | 0.0375 | 0.0426 | 0.1052 | 0.0036 | |
| CLIC6 | 0.0075 | 0.0600 | 0.1813 | 0.4425 | 0.3125 | 0.3275 | 0.0000 | 0.0000 | 0.0000 | 0.0564 | 0.1049 | 0.0000 | |
| KCNQ1 | 0.1338 | 0.1400 | 0.1800 | 0.4037 | 0.3650 | 0.4175 | 0.0588 | 0.0775 | 0.0788 | 0.0224 | 0.0912 | 0.0130 | |
| Digestive enzyme production | CHIA | 0.0225 | 0.0763 | 0.1388 | 0.4188 | 0.3813 | 0.4087 | 0.0075 | 0.0063 | 0.0088 | 0.0339 | 0.1036 | 0.0025 |
| PGA5 | 0.0038 | 0.0075 | 0.0138 | 0.1688 | 0.1712 | 0.1162 | 0.0138 | 0.0125 | 0.0138 | 0.0040 | 0.0979 | 0.0021 | |
| Gastrin production | GAST | 0.0000 | 0.0000 | 0.0000 | 0.0025 | 0.0000 | 0.0025 | 0.1713 | 0.2375 | 0.2263 | 0.0000 | 0.0002 | 0.0604 |
| Gastrin receptor | CCKBR | 0.0075 | 0.0400 | 0.1100 | 0.3912 | 0.2675 | 0.2913 | 0.0000 | 0.0000 | 0.0000 | 0.0334 | 0.0944 | 0.0000 |
| Ghrelin production | GHRL | 0.1875 | 0.2500 | 0.3238 | 0.4288 | 0.4100 | 0.4875 | 0.1263 | 0.1425 | 0.1250 | 0.0582 | 0.8652 | 0.0345 |
| MBOAT4 | 0.3163 | 0.2575 | 0.2838 | 0.3900 | 0.3625 | 0.3362 | 0.1363 | 0.1513 | 0.1500 | 0.0766 | 0.0417 | 0.0202 | |
| Intrinsic factor production | CBLIF | 0.0038 | 0.0413 | 0.1325 | 0.2300 | 0.1913 | 0.2588 | 0.3563 | 0.5100 | 0.5575 | 0.0368 | 0.0635 | 0.0803 |
| Histamine production | HDC | 0.0637 | 0.1338 | 0.1638 | 0.4200 | 0.2950 | 0.2975 | 0.0350 | 0.0412 | 0.0437 | 0.0391 | 0.0788 | 0.0093 |
| Inflammation | CXCL8 | 0.3600 | 0.3975 | 0.2687 | 0.1450 | 0.1725 | 0.1288 | 0.1588 | 0.1225 | 0.1600 | 0.0613 | 0.0554 | 0.0385 |
| Mucosal defence | PIGR | 0.6550 | 0.5338 | 0.3787 | 0.1750 | 0.1650 | 0.1538 | 0.2400 | 0.2550 | 0.2925 | 0.0895 | 0.0555 | 0.0494 |
| OLFM4 | 0.4850 | 0.4062 | 0.2125 | 0.1275 | 0.1638 | 0.1263 | 0.2338 | 0.2463 | 0.3475 | 0.0856 | 0.0728 | 0.0590 | |
| Mucus production | MUC1 | 0.3075 | 0.2875 | 0.2975 | 0.3513 | 0.3913 | 0.4213 | 0.3400 | 0.3462 | 0.3437 | 0.0477 | 0.0644 | 0.0455 |
| MUC2 | 0.3363 | 0.2150 | 0.1837 | 0.0100 | 0.0025 | 0.0025 | 0.0100 | 0.0125 | 0.0063 | 0.0597 | 0.0004 | 0.0004 | |
| MUC5AC | 0.1463 | 0.1388 | 0.1500 | 0.3500 | 0.3612 | 0.3687 | 0.4262 | 0.4350 | 0.4425 | 0.0112 | 0.0660 | 0.0837 | |
| MUC6 | 0.2038 | 0.1937 | 0.2150 | 0.3425 | 0.2962 | 0.3388 | 0.3100 | 0.3225 | 0.3700 | 0.0422 | 0.0452 | 0.0946 | |
| Parietal cell water channel | AQP4 | 0.0050 | 0.0688 | 0.1538 | 0.4362 | 0.3888 | 0.4562 | 0.0000 | 0.0000 | 0.0000 | 0.0452 | 0.1219 | 0.0000 |
| Prohormone processing | PCSK1 | 0.1388 | 0.2150 | 0.2663 | 0.5500 | 0.4750 | 0.5125 | 0.3325 | 0.4475 | 0.4487 | 0.0519 | 0.0679 | 0.0828 |
| Somatostatin production | SST | 0.1525 | 0.1675 | 0.1788 | 0.2725 | 0.2050 | 0.2425 | 0.4600 | 0.5762 | 0.6500 | 0.0259 | 0.0292 | 0.0937 |
| Toll-like receptors | TLR2 | 0.2669 | 0.3214 | 0.2219 | 0.1890 | 0.2089 | 0.1757 | 0.2563 | 0.2276 | 0.2043 | 0.0521 | 0.0246 | 0.0172 |
| TLR3 | 0.2825 | 0.3037 | 0.3788 | 0.5640 | 0.5237 | 0.5592 | 0.6329 | 0.5789 | 0.6527 | 0.0531 | 0.0473 | 0.0472 | |
| TLR4 | 0.6543 | 0.6567 | 0.5785 | 0.5805 | 0.5962 | 0.5644 | 0.6199 | 0.5314 | 0.5436 | 0.0668 | 0.0556 | 0.0685 | |
| TLR5 | 0.0971 | 0.1024 | 0.1199 | 0.5138 | 0.4571 | 0.4678 | 0.3870 | 0.4447 | 0.5029 | 0.0149 | 0.0417 | 0.0550 | |
References
- Cranwell, P.D. The development of acid and pepsin (EC 3. 4. 23. 1) secretory capacity in the pig; the effects of age and weaning: 1. Studies in anaesthetized pigs. Br. J. Nutr. 1985, 54, 305–320. [Google Scholar] [CrossRef]
- Du, G.M.; Shi, Z.M.; Wei, X.H.; Liu, M.J.; Zhang, L.; Zhao, R.Q. Expression of gastric ghrelin and H+–K+-ATPase MRNA in weanling piglets and effect of ghrelin on H+–K+-ATPase expression and activity in gastric mucosal cells in vitro. Res. Vet. Sci. 2007, 82, 99–104. [Google Scholar] [CrossRef]
- Trevisi, P.; Luise, D.; Correa, F.; Messori, S.; Mazzoni, M.; Lallès, J.P.; Bosi, P. Maternal antibiotic treatment affects offspring gastric sensing for umami taste and ghrelin regulation in the pig. J. Anim. Sci. Biotechnol. 2021, 12, 31. [Google Scholar] [CrossRef]
- Barrow, P.; Fuller, R.; Newport, M. Changes in the microflora and physiology of the anterior intestinal tract of pigs weaned at 2 days, with special reference to the pathogenesis of diarrhea. Infect. Immun. 1977, 18, 586–595. [Google Scholar] [CrossRef]
- Cranwell, P.; Noakes, D.; Hill, K. Gastric secretion and fermentation in the suckling pig. Br. J. Nutr. 1976, 36, 71–86. [Google Scholar] [CrossRef]
- Suiryanrayna, M.V.A.N.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef]
- Lawlor, P.G.; Lynch, P.B.; Caffrey, P.J.; O’Reilly, J.J.; O’Connell, M.K. Measurements of the acid-binding capacity of ingredients used in pig diets. Ir. Vet. J. 2005, 58, 447. [Google Scholar] [CrossRef]
- Lawlor, P.G.; Lynch, P.B.; Caffrey, P.J. Effect of Fumaric Acid, Calcium Formate and Mineral Levels in Diets on the Intake and Growth Performance of Newly Weaned Pigs. Ir. J. Agric. Food Res. 2006, 45, 61–71. [Google Scholar]
- Hedemann, M.S.; Jensen, B.B. Variations in enzyme activity in stomach and pancreatic tissue and digesta in piglets around weaning. Arch. Anim. Nutr. 2004, 58, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Luise, D.; Motta, V.; Salvarani, C.; Chiappelli, M.; Fusco, L.; Bertocchi, M.; Mazzoni, M.; Maiorano, G.; Costa, L.N.; Van Milgen, J.; et al. Long-term administration of formic acid to weaners: Influence on intestinal microbiota, immunity parameters and growth performance. Anim. Feed. Sci. Technol. 2017, 232, 160–168. [Google Scholar] [CrossRef]
- Colombo, M.; Priori, D.; Gandolfi, G.; Boatto, G.; Nieddu, M.; Bosi, P.; Trevisi, P. Effect of free thymol on differential gene expression in gastric mucosa of the young pig. Animal 2014, 8, 786–791. [Google Scholar] [CrossRef]
- Mazzoni, M.; Le Gall, M.; De Filippi, S.; Minieri, L.; Trevisi, P.; Wolinski, J.; Lalatta-Costerbosa, G.; Lallès, J.-P.; Guilloteau, P.; Bosi, P. Supplemental Sodium Butyrate Stimulates Different Gastric Cells in Weaned Pigs. J. Nutr. 2008, 138, 1426–1431. [Google Scholar] [CrossRef]
- Bosi, P.; Mazzoni, M.; De Filippi, S.; Trevisi, P.; Casini, L.; Petrosino, G.; Lalatta-Costerbosa, G. A continuous dietary supply of free calcium formate negatively affects the parietal cell population and gastric RNA expression for H+/K+-ATPase in weaning pigs. J. Nutr. 2006, 136, 1229–1235. [Google Scholar] [CrossRef]
- Yin, J.; Li, X.; Li, D.; Yue, T.; Fang, Q.; Ni, J.; Zhou, X.; Wu, G. Dietary supplementation with zinc oxide stimulates ghrelin secretion from the stomach of young pigs. J. Nutr. Biochem. 2009, 20, 783–790. [Google Scholar] [CrossRef]
- Fothergill, L.J.; Galiazzo, G.; Hunne, B.; Stebbing, M.J.; Fakhry, J.; Weissenborn, F.; Fazio Coles, T.E.; Furness, J.B. Distribution and co-expression patterns of specific cell markers of enteroendocrine cells in pig gastric epithelium. Cell Tissue Res. 2019, 378, 457–469. [Google Scholar] [CrossRef]
- Colombo, M.; Priori, D.; Trevisi, P.; Bosi, P. Differential Gene Expression in the Oxyntic and Pyloric Mucosa of the Young Pig. PLoS ONE 2014, 9, e111447. [Google Scholar] [CrossRef]
- Mazzoni, M.; Bosi, P.; De Sordi, N.; Lalatta-Costerbosa, G. Distribution, organization and innervation of gastric MALT in conventional piglet. J. Anat. 2011, 219, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, G.C.; Devine, W.A.; Redel, B.K.; Whitworth, K.M.; Samuel, M.; Spate, L.D.; Cecil, R.F.; Prather, R.S.; Wu, Y.L.; Wells, K.D.; et al. Profiling development of abdominal organs in the pig. Sci. Rep. 2022, 12, 16245. [Google Scholar] [CrossRef] [PubMed]
- McCracken, K.W.; Wells, J.M. Mechanisms of embryonic stomach development. Semin. Cell Dev. Biol. 2017, 66, 36–42. [Google Scholar] [CrossRef]
- Xian, Y.; Zhao, X.; Wang, C.; Kang, C.; Ding, L.; Zhu, W.; Hang, S. Phenylalanine and tryptophan stimulate gastrin and somatostatin secretion and H+-K+-ATPase activity in pigs through calcium-sensing receptor. Gen. Comp. Endocrinol. 2018, 267, 1–8. [Google Scholar] [CrossRef]
- Trevisi, P.; Gandolfi, G.; Priori, D.; Messori, S.; Colombo, M.; Mazzoni, M.; Lallès, J.-P.; Bosi, P. Age-Related Expression of the Polymeric Immunoglobulin Receptor (pIgR) in the Gastric Mucosa of Young Pigs. PLoS ONE 2013, 8, e81473. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package ‘corrplot’. Statistician 2017, 56, e24. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 1 June 2023).
- Yao, X.; Forte, J.G. Cell biology of acid secretion by the parietal cell. Annu. Rev. Physiol. 2003, 65, 103–131. [Google Scholar] [CrossRef]
- Shin, J.M.; Munson, K.; Vagin, O.; Sachs, G. The gastric HK-ATPase: Structure, function, and inhibition. Pflügers Arch. -Eur. J. Physiol. 2009, 457, 609–622. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, N.W.G.; Yakubov, I.; Scott, D.; Sachs, G. Identification of the K efflux channel coupled to the gastric H-K-ATPase during acid secretion. Physiol. Genom. 2005, 21, 81–91. [Google Scholar] [CrossRef]
- Grahammer, F.; Wittekindt, O.H.; Nitschke, R.; Herling, A.W.; Lang, H.J.; Bleich, M.; Schmitt-Gräff, A.; Barhanin, J.; Warth, R. The cardiac K+ channel KCNQ1 is essential for gastric acid secretion. Gastroenterology 2001, 120, 1363–1371. [Google Scholar] [CrossRef]
- Fujita, A.; Horio, Y.; Nielsen, S.; Nagelhus, E.A.; Hata, F.; Ottersen, O.P.; Kurachi, Y. High-resolution immunogold cytochemistry indicates that AQP4 is concentrated along the basal membrane of parietal cell in rat stomach. FEBS Lett. 1999, 459, 305–309. [Google Scholar] [CrossRef]
- Wang, K.S.; Komar, A.R.; Ma, T.; Filiz, F.; McLeroy, J.; Hoda, K.; Verkman, A.S.; Bastidas, J.A. Gastric acid secretion in aquaporin-4 knockout mice. Am. J. Physiol. -Gastrointest. Liver Physiol. 2000, 279, G448–G453. [Google Scholar] [CrossRef]
- Arciszewski, M.B.; Matysek, M.; Sienkiewicz, W. Immunohistochemical localization of aquaporin 4 (AQP4) in the porcine gastrointestinal tract. Acta Vet. Brno 2015, 84, 321–326. [Google Scholar] [CrossRef]
- Sawada, M.; Finniss, S.; Dickinson, C.J. Diminished prohormone convertase 3 expression (PC1/PC3) inhibits progastrin post-translational processing. Regul. Pept. 2000, 89, 19–28. [Google Scholar] [CrossRef]
- Rehfeld, J.F.; Zhu, X.; Norrbom, C.; Bundgaard, J.R.; Johnsen, A.H.; Nielsen, J.E.; Vikesaa, J.; Stein, J.; Dey, A.; Steiner, D.F. Prohormone convertases 1/3 and 2 together orchestrate the site-specific cleavages of progastrin to release gastrin-34 and gastrin-17. Biochem. J. 2008, 415, 35–43. [Google Scholar] [CrossRef]
- Cleverdon, E.R.; McGovern-Gooch, K.R.; Hougland, J.L. The octanoylated energy regulating hormone ghrelin: An expanded view of ghrelin’s biological interactions and avenues for controlling ghrelin signaling. Mol. Membr. Biol. 2017, 33, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Kopin, A.S.; Lee, Y.-M.; McBride, E.W.; Miller, L.J.; Lu, M.; Lin, H.Y.; Kolakowski, L.F., Jr.; Beinborn, M. Expression cloning and characterization of the canine parietal cell gastrin receptor. Proc. Natl. Acad. Sci. USA 1992, 89, 3605–3609. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Matsui, T.; Ito, M.; Taniguchi, T.; Naribayashi, Y.; Arima, N.; Nakamura, A.; Kinoshita, Y.; Chihara, K.; Hosoda, S. Cloning and characterization of gastrin receptor from ECL carcinoid tumor of Mastomys natalensis. Biochem. Biophys. Res. Commun. 1992, 187, 1151–1157. [Google Scholar] [CrossRef]
- Kulaksiz, H.; Arnold, R.; Göke, B.; Maronde, E.; Meyer, M.; Fahrenholz, F.; Forssmann, W.-G.; Eissele, R. Expression and cell-specific localization of the cholecystokinin B/gastrin receptor in the human stomach. Cell Tissue Res. 2000, 299, 289–298. [Google Scholar] [CrossRef]
- Samuelson, L.C.; Hinkle, K.L. Insights into the regulation of gastric acid secretion through analysis of genetically engineered mice. Annu. Rev. Physiol. 2003, 65, 383. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Aguilar, M.D.; López-Arjona, M.; Martínez-Miró, S.; Escribano, D.; Hernández-Ruipérez, F.; Cerón, J.J.; Tecles, F. Changes in saliva analytes during pregnancy, farrowing and lactation in sows: A sialochemistry approach. Vet. J. 2021, 273, 105679. [Google Scholar] [CrossRef]
- Soll, A.; Walsh, J.H. Regulation of gastric acid secretion. Annu. Rev. Physiol. 1979, 41, 35–53. [Google Scholar] [CrossRef]
- Kamoshida, S.; Saito, E.; Fukuda, S.; Kato, K.; Iwasaki, A.; Arakawa, Y. Anatomical location of enterochromaffin-like (ECL) cells, parietal cells, and chief cells in the stomach demonstrated by immunocytochemistry and electron microscopy. J. Gastroenterol. 1999, 34, 315–320. [Google Scholar] [CrossRef]
- Hunne, B.; Stebbing, M.J.; McQuade, R.M.; Furness, J.B. Distributions and relationships of chemically defined enteroendocrine cells in the rat gastric mucosa. Cell Tissue Res. 2019, 378, 33–48. [Google Scholar] [CrossRef]
- Fakhry, J.; Stebbing, M.J.; Hunne, B.; Bayguinov, Y.; Ward, S.M.; Sasse, K.C.; Callaghan, B.; McQuade, R.M.; Furness, J.B. Relationships of endocrine cells to each other and to other cell types in the human gastric fundus and corpus. Cell Tissue Res. 2019, 376, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Raptis, S.; Dollinger, H.; Von Berger, L.; Schlegel, W.; Schröder, K.; Pfeiffer, E. Effects of somatostatin on gastric secretion and gastrin release in man. Digestion 1975, 13, 15–26. [Google Scholar] [CrossRef]
- Prinz, C.; Kajimura, M.; Scott, D.R.; Mercier, F.; Helander, H.F.; Sachs, G. Histamine secretion from rat enterochromaffinlike cells. Gastroenterology 1993, 105, 449–461. [Google Scholar] [CrossRef]
- D’sa, A.B.; Bloom, S.; Baron, J. Inhibition by somatostatin (growth-hormone release-inhibiting hormone, GH-RIH) of gastric acid and pepsin and G-cell release of gastrin. Gut 1978, 19, 315–320. [Google Scholar]
- Choi, E.; Roland, J.T.; Barlow, B.J.; O’Neal, R.; Rich, A.E.; Nam, K.T.; Shi, C.; Goldenring, J.R. Cell lineage distribution atlas of the human stomach reveals heterogeneous gland populations in the gastric antrum. Gut 2014, 63, 1711–1720. [Google Scholar] [CrossRef]
- Vizcarra, J.A.; Kirby, J.D.; Kim, S.K.; Galyean, M.L. Active immunization against ghrelin decreases weight gain and alters plasma concentrations of growth hormone in growing pigs. Domest. Anim. Endocrinol. 2007, 33, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Barretero-Hernandez, R.; Galyean, M.L.; Vizcarra, J.A. The Effect of Feed Restriction on Plasma Ghrelin, Growth Hormone, Insulin, and Glucose Tolerance in Pigs. Prof. Anim. Sci. 2010, 26, 26–34. [Google Scholar] [CrossRef]
- Zhang, H.; Yin, J.; Li, D.; Zhou, X.; Li, X. Tryptophan enhances ghrelin expression and secretion associated with increased food intake and weight gain in weanling pigs. Domest. Anim. Endocrinol. 2007, 33, 47–61. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Xu, J.; Tang, S.-Q.; Li, H.-Y.; Jiang, Q.-Y.; Zou, X.-T. Ghrelin and its biological effects on pigs. Peptides 2009, 30, 1203–1211. [Google Scholar] [CrossRef]
- Scrimgeour, K.; Gresham, M.J.; Giles, L.R.; Thomson, P.C.; Wynn, P.C.; Newman, R.E. Ghrelin secretion is more closely aligned to energy balance than with feeding behaviour in the grower pig. J. Endocrinol. 2008, 198, 135. [Google Scholar] [CrossRef]
- Govoni, N.; De Iasio, R.; Cocco, C.; Parmeggiani, A.; Galeati, G.; Pagotto, U.; Brancia, C.; Spinaci, M.; Tamanini, C.; Pasquali, R. Gastric immunolocalization and plasma profiles of acyl-ghrelin in fasted and fasted-refed prepuberal gilts. J. Endocrinol. 2005, 186, 505–513. [Google Scholar] [CrossRef]
- Priori, D.; Trevisi, P.; Mazzoni, M.; Messori, S.; Bosi, P. Effect of fasting and refeeding on expression of genes involved in the gastric nutrient sensing and orexigenic control of pigs. J. Anim. Physiol. Anim. Nutr. 2015, 99, 692–700. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.V.; Ren, P.G.; Avsian-Kretchmer, O.; Luo, C.W.; Rauch, R.; Klein, C.; Hsueh, A.J. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 2005, 310, 996–999. [Google Scholar] [CrossRef]
- Steinert, R.E.; Feinle-Bisset, C.; Geary, N.; Beglinger, C. Digestive physiology of the pig symposium: Secretion of gastrointestinal hormones and eating control. J. Anim. Sci. 2013, 91, 1963–1973. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Goebel, M.; Wang, L.; Taché, Y.; Sachs, G.; Lambrecht, N.W.G. Differential distribution of ghrelin-O-acyltransferase (GOAT) immunoreactive cells in the mouse and rat gastric oxyntic mucosa. Biochem. Biophys. Res. Commun. 2010, 392, 67–71. [Google Scholar] [CrossRef]
- Sakata, I.; Yang, J.; Lee, C.E.; Osborne-Lawrence, S.; Rovinsky, S.A.; Elmquist, J.K.; Zigman, J.M. Colocalization of ghrelin O-acyltransferase and ghrelin in gastric mucosal cells. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E134–E141. [Google Scholar] [CrossRef] [PubMed]
- Moreau, H.; Bernadac, A.; Gargouri, Y.; Benkouka, F.; Laugier, R.; Verger, R. Immunocytolocalization of human gastric lipase in chief cells of the fundic mucosa. Histochemistry 1989, 91, 419–423. [Google Scholar] [CrossRef]
- Ohno, M.; Kimura, M.; Miyazaki, H.; Okawa, K.; Onuki, R.; Nemoto, C.; Tabata, E.; Wakita, S.; Kashimura, A.; Sakaguchi, M.; et al. Acidic mammalian chitinase is a proteases-resistant glycosidase in mouse digestive system. Sci. Rep. 2016, 6, 37756. [Google Scholar] [CrossRef]
- Goto, M.; Fujimoto, W.; Nio, J.; Iwanaga, T.; Kawasaki, T. Immunohistochemical demonstration of acidic mammalian chitinase in the mouse salivary gland and gastric mucosa. Arch. Oral Biol. 2003, 48, 701–707. [Google Scholar] [CrossRef]
- Suzuki, M.; Fujimoto, W.; Goto, M.; Morimatsu, M.; Syuto, B.; Iwanaga, T. Cellular expression of gut chitinase mRNA in the gastrointestinal tract of mice and chickens. J. Histochem. Cytochem. 2002, 50, 1081–1089. [Google Scholar] [CrossRef]
- Peniche, C.; Argüelles-Monal, W.; Goycoolea, F.M. Chapter 25-Chitin and Chitosan: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 517–542. [Google Scholar] [CrossRef]
- Hong, J.; Kim, Y.Y. Insect as feed ingredients for pigs. Anim. Biosci. 2022, 35, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Osafune, T.; Tamehira, S.; Yano, K. Piglets can secrete acidic mammalian chitinase from the pre weaning stage. Sci. Rep. 2021, 11, 1297. [Google Scholar] [CrossRef] [PubMed]
- Tabata, E.; Kashimura, A.; Wakita, S.; Ohno, M.; Sakaguchi, M.; Sugahara, Y.; Imamura, Y.; Seki, S.; Ueda, H.; Matoska, V. Protease resistance of porcine acidic mammalian chitinase under gastrointestinal conditions implies that chitin-containing organisms can be sustainable dietary resources. Sci. Rep. 2017, 7, 12963. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, N.G.; Bal, H.S. Comparative morphology of the stomach of some laboratory mammals. Lab. Anim. 1989, 23, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Dieckgraefe, B.K.; Seetharam, B.; Alpers, D.H. Developmental regulation of rat intrinsic factor mRNA. Am. J. Physiol. Gastrointest. Liver Physiol. 1988, 254, G913–G919. [Google Scholar] [CrossRef]
- O’Neil, A.; Petersen, C.P.; Choi, E.; Engevik, A.C.; Goldenring, J.R. Unique Cellular Lineage Composition of the First Gland of the Mouse Gastric Corpus. J. Histochem. Cytochem. 2017, 65, 47–58. [Google Scholar] [CrossRef]
- Padra, M.; Adamczyk, B.; Benktander, J.; Flahou, B.; Skoog, E.C.; Padra, J.T.; Smet, A.; Jin, C.; Ducatelle, R.; Samuelsson, T.; et al. Helicobacter suis binding to carbohydrates on human and porcine gastric mucins and glycolipids occurs via two modes. Virulence 2018, 9, 898–918. [Google Scholar] [CrossRef]
- Duc, M.; Johansen, F.-E.; Corthésy, B. Antigen binding to secretory immunoglobulin A results in decreased sensitivity to intestinal proteases and increased binding to cellular Fc receptors. J. Biol. Chem. 2010, 285, 953–960. [Google Scholar] [CrossRef]
- Kim, A.; Park, W.Y.; Shin, N.; Lee, H.J.; Kim, Y.K.; Lee, S.J.; Hwang, C.S.; Park, D.Y.; Kim, G.H.; Lee, B.E.; et al. Cardiac mucosa at the gastroesophageal junction: An Eastern perspective. World J. Gastroenterol. 2015, 21, 9126–9133. [Google Scholar] [CrossRef]




| Ingredient | Quantity (g/kg) |
|---|---|
| Wheat | 328.0 |
| Barley | 150.0 |
| Full-fat soya bean | 170.0 |
| Soya bean meal | 105.0 |
| Whey powder (90%) | 50.0 |
| Soya oil | 30.0 |
| Soya concentrate | 65.0 |
| Maize | 77.4 |
| Lysine-HCl | 4.0 |
| Dl-methionine | 2.0 |
| L-threonine | 1.8 |
| Tryptophan | 0.3 |
| Sodium bicarbonate | 2.0 |
| Monocalcium phosphate | 4.0 |
| Mineral and vitamins | 2.5 |
| Calcium carbonate (limestone) | 6.0 |
| Salt | 2.0 |
| Composition | |
| Protein | 207.0 |
| Ether extract | 83.0 |
| Crude fibre | 30.0 |
| DE (MJ/kg) | 15.0 |
| NE (MJ/kg) | 11.0 |
| Lysine | 14.7 |
| SID lysine | 13.3 |
| Methionine + cysteine | 9.0 |
| Threonine | 10.5 |
| Tryptophan | 3.0 |
| Calcium | 7.9 |
| Total phosphorous | 5.9 |
| Target Gene | Gene Name | Accession No. | Forward Primer (5‣-3‣) Reverse Primer (5‣-3‣) | Amplicon Length (bp) |
|---|---|---|---|---|
| Acid secretion | ||||
| ATP4A | ATPase H+/K+ Transporting Subunit Alpha | XM_021093570.1 | F:GGACATGGCAGCCAAGATG R:TGTTCTCCAGCTTCTCCTTCCT | 74 |
| CLIC6 | Chloride Intracellular Channel 6 | XM_003358948.4 | F:CGGAACCAGTCAGAAGAACGA R:TCCTACCGCCCAAGAAGCT | 87 |
| HRH2 | Histamine Receptor 2 | XM_003354192.4 | F:CCAGCCTGGATGTCATGCCT R:CCGGTCGAGGCTGATCAT | 65 |
| KCNQ1 | Potassium Voltage-Gated Channel Subfamily Q Member 1 | XM_021082620.1 | F:CGCGTCTACAACTTCCTCGAA R:CGATAAGGAAGACAGCAAAGTGGTA | 73 |
| Cobalamin binding intrinsic factor | ||||
| CBLIF | Cobalamin Binding Intrinsic Factor | XM_003122682.3 | F:CGGAATCATTGGAAACATCTATAGC R:GGTCGCTCAGGTGTCACAGA | 69 |
| Digestive enzymes | ||||
| CHIA | Chitinase Acidic | NM_001258377.1 | F:GCCTTTTGTACCCACCTGGTCTA R:TCAGTGGTGGTGATCTCGTTGT | 65 |
| PGA5 | Pepsinogen A5 | NM_213872.2 | F:CGGCAGCGTGGTGGTGTTGT R:GGAAACAGGCACCCAGTTCA | 73 |
| Gastrin | ||||
| GAST | Gastrin | NM_001004036.2 | F:TCCCAGCTCTGCAGTCAAGA R:CCAGAGCCAGCACATGGA | 65 |
| Gastrin receptor | ||||
| CCKBR | Cholecystokinin B Receptor | XM_021062350 | F:CATGGGCACGTTTATCTTTGG R:TCACAGACACCCCCATGAAGT | 68 |
| Ghrelin production | ||||
| GHRL | Ghrelin | XM_013981924.2 | F:AAGCTGGAAATCCGGTTCAA R:CGGACTGAGCCCCTGACA | 64 |
| MBOAT4 | Membrane Bound O-Acyltransferase Domain Containing 4 | NM_001190423.1 | F:GCTCCCACCAAACCCAGA R:CCCACTGGATCCTGGATGAG | 65 |
| Histamine production | ||||
| HDC | Histidine Decarboxylase | XM_001925342.5 | F:ATCTGCCAGTACCTGAGCACTGT R:GCAGGTAGCCAGGTCTCACATC | 67 |
| Inflammation | ||||
| CXCL8 | C-X-C Motif Chemokine Ligand 8 | NM_213867.1 | F:TGCACTTACTCTTGCCAGAACTG R:CAAACTGGCTGTTGCCTTCTT | 82 |
| Mucins | ||||
| MUC1 | Mucin 1 | XM_001926883.1 | F:ACACCCATGGGCGCTATGT R:GCCTGCAGAAACCTGCTCAT | 68 |
| MUC2 | Mucin 2 | AK231524 | F:CAACGGCCTCTCCTTCTCTGT R:GCCACACTGGCCCTTTGT | 70 |
| MUC5AC | Mucin 5AC | XM_021092583.1 | F:GGATGTCGCCAGAGACTGAGTA R:CCCCCTCGTCTCCTTTTACC | 71 |
| MUC6 | Mucin 6 | XM_021082474.1 | F:AAAACGTGGGCAGGATGTGT R:GCCATCCTCGCTCAGAAACT | 77 |
| Mucosal defence | ||||
| OLFM4 | Olfactomedin 4 | XM_003482903.4 | F:GGCGCCAGGGAGCTGTA R:TGAGTTGAACAATAGCCGGTTTG | 65 |
| PIGR | Polymeric Immunoglobulin Receptor | XM_021102216.1 | F:GGGCTCGGTGACATTTGACT R:TTTAGCTGGCACAGAAATTTGG | 72 |
| Aquaporin water channel protein | ||||
| AQP4 | Aquaporin 4 | XM_021093195.1 | F:GCAAAGCTAGCCAACAAACAAA R:CCTCGGTCTCAACCTGACTTCT | 72 |
| Prohormone processing | ||||
| PCSK1 | Proprotein Convertase Subtilisin/Kexin Type 1 | HE599222.1 | F:GCAATTCTTTCTGGCTTTTCTACCT R:CACACTCGCCCGCATACA | 72 |
| Somatostatin | ||||
| SST | Somatostatin | NM_001009583.1 | F:CCCTGGAGCCTGAAGATTTG R:GCCGGGTTTGAGTTAGCTGAT | 85 |
| Toll-like receptors | ||||
| TLR2 | Toll-Like Receptor 2 | NM_213761.1 | F:CATCTTCGTGCTTTCCGAGAAC R:AAAGAGACGGAAGTGGGAGAAGT | 79 |
| TLR3 | Toll-Like Receptor 3 | NM_001097444.1 | F:CATTGAGAATCTATCCCTGAGCAA R: TGCTGAGGTTTGTCTGCTTTAGTC | 86 |
| TLR4 | Toll-Like Receptor 4 | NM_001293317.1 | F:TGCATGGAGCTGAATTTCTACAA R: GATAAATCCAGCACCTGCAGTTC | 140 |
| TLR5 | Toll-Like Receptor 5 | NM_001348771.1 | F:CAGCCAGGCCGTCAATG R:AAGCCAAACCCAGAACCCATA | 75 |
| Reference genes | ||||
| ACTB | Beta Actin | XM_001927228.1 | F:GGACATCGGATACCCAAGGA R:AAGTTGGAAGGCCGGTTAATTT | 71 |
| B2M | Beta-2-Microglobulin | NM_213978.1 | F:CGGAAAGCCAAATTACCTGAAC R:TCTCCCCGTTTTTCAGCAAAT | 83 |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase | AF017079.1 | F:CAGCAATGCCTCCTGTACCA R:ACGATGCCGAAGTTGTCATG | 72 |
| PPIA | Peptidylprolyl Isomerase A | NM_214353.1 | F:CGGGTCCTGGCATCTTGT R:TGGCAGTGCAAATGAAAAACT | 75 |
| OAZ1 | Ornithine Decarboxylase Antizyme 1 | NM_125342.1 | F:CATCCCCTTGTCCCCAA R:ACCAGAGGACTCTCTCTCAAACGT | 69 |
| RPS29 | Ribosomal Protein S29 | NM_001001633.2 | F:CGCATGCGTGCGCTAAG R:TGGTGACCCATCTTGCTCTCT | 64 |
| RPL27 | Ribosomal Protein L27 | NM_001097479.1 | F:GTCCTGGCTGGTCGCTACTC R:GGTCTGAGGTGCCATCATCA | 70 |
| RPL29 | Ribosomal Protein L29 | XM_0023442034.1 | F:GCCAATGTGAGGACAGAAGGA R:CAGGACACCAGCCCCGTATA | 65 |
| Function | Gene | Cardiac (C) | Fundic (F) | Pyloric (P) | SEM | p-Values | ||
|---|---|---|---|---|---|---|---|---|
| CF | CP | FP | ||||||
| Acid secretion | ATP4A | 0.082 | 0.454 | 0.000 | 0.040 | <0.0001 | 0.1540 | <0.0001 |
| CLIC6 | 0.083 | 0.361 | 0.000 | 0.040 | <0.0001 | 0.1428 | <0.0001 | |
| HRH2 | 0.096 | 0.402 | 0.033 | 0.037 | <0.0001 | 0.2260 | <0.0001 | |
| KCNQ1 | 0.151 | 0.395 | 0.072 | 0.030 | <0.0001 | 0.0689 | <0.0001 | |
| Digestive enzyme production | CHIA | 0.079 | 0.403 | 0.007 | 0.035 | <0.0001 | 0.1552 | <0.0001 |
| PGA5 | 0.008 | 0.152 | 0.013 | 0.031 | 0.0018 | 0.9106 | 0.0026 | |
| Gastrin production | GAST | 0.000 | 0.002 | 0.212 | 0.020 | 0.9521 | <0.0001 | <0.0001 |
| Gastrin receptor | CCKBR | 0.053 | 0.317 | 0.000 | 0.034 | <0.0001 | 0.2637 | <0.0001 |
| Ghrelin production | GHRL | 0.254 | 0.442 | 0.131 | 0.036 | 0.0004 | 0.0184 | <0.0001 |
| MBOAT4 | 0.286 | 0.363 | 0.146 | 0.029 | 0.0626 | 0.0010 | <0.0001 | |
| Histamine production | HDC | 0.120 | 0.338 | 0.040 | 0.029 | <0.0001 | 0.0579 | <0.0001 |
| Inflammation | CXCL8 | 0.342 | 0.149 | 0.144 | 0.030 | <0.0001 | <0.0001 | 0.9065 |
| Intrinsic factor production | CBLIF | 0.059 | 0.227 | 0.475 | 0.037 | 0.0020 | <0.0001 | <0.0001 |
| Mucosal defence | PIGR | 0.523 | 0.165 | 0.263 | 0.040 | <0.0001 | <0.0001 | 0.0848 |
| OLFM4 | 0.368 | 0.139 | 0.276 | 0.043 | 0.0004 | 0.1364 | 0.0286 | |
| Mucus production | MUC1 | 0.298 | 0.388 | 0.343 | 0.030 | 0.0341 | 0.2771 | 0.2903 |
| MUC2 | 0.245 | 0.005 | 0.010 | 0.021 | <0.0001 | <0.0001 | 0.8760 | |
| MUC5AC | 0.145 | 0.360 | 0.435 | 0.034 | <0.0001 | <0.0001 | 0.1272 | |
| MUC6 | 0.204 | 0.326 | 0.338 | 0.036 | 0.0204 | 0.0113 | 0.8206 | |
| Water channel protein | AQP4 | 0.076 | 0.427 | 0.000 | 0.042 | <0.0001 | 0.2081 | <0.0001 |
| Prohormone processing | PCSK1 | 0.207 | 0.513 | 0.410 | 0.039 | <0.0001 | 0.0005 | 0.0672 |
| Somatostatin production | SST | 0.166 | 0.240 | 0.562 | 0.034 | 0.1282 | <0.0001 | <0.0001 |
| Toll-like receptor | TLR2 | 0.267 | 0.178 | 0.243 | 0.020 | 0.0022 | 0.3959 | 0.0218 |
| TLR3 | 0.323 | 0.550 | 0.621 | 0.028 | <0.0001 | <0.0001 | 0.0816 | |
| TLR4 | 0.628 | 0.607 | 0.538 | 0.036 | 0.6854 | 0.0809 | 0.1722 | |
| TLR5 | 0.107 | 0.481 | 0.442 | 0.024 | <0.0001 | <0.0001 | 0.2526 | |
| Function | Genes | Cardiac (C) | Fundic (F) | Pyloric (P) | SEM | p-Values | ||
|---|---|---|---|---|---|---|---|---|
| CF | CP | FP | ||||||
| Acid secretion | ATP4A, KCNQ1, CLIC6, HRH2 | 0.103 | 0.403 | 0.026 | 0.034 | <0.0001 | 0.1159 | <0.0001 |
| Digestive enzymes | CHIA, PGA5 | 0.044 | 0.278 | 0.010 | 0.031 | <0.0001 | 0.4531 | <0.0001 |
| Ghrelin production | GHRL, MBOAT4 | 0.270 | 0.403 | 0.139 | 0.028 | 0.0015 | 0.0017 | <0.0001 |
| Mucus production | MUC1, MUC2, MUC5AC, MUC6 | 0.223 | 0.270 | 0.281 | 0.018 | 0.0645 | 0.0220 | 0.6437 |
| Toll-like receptors | TLR2, TLR3, TLR4, TLR5 | 0.331 | 0.454 | 0.461 | 0.0137 | <0.0001 | <0.0001 | 0.7216 |
| Function | Gene | Location * | SEM | p-Values | ||||
|---|---|---|---|---|---|---|---|---|
| 1(a) | 2(b) | 3(c) | ab | ac | bc | |||
| Acid secretion | ATP4A | 0.010 | 0.070 | 0.166 | 0.046 | 0.3693 | 0.0263 | 0.1559 |
| CLIC6 | 0.008 | 0.060 | 0.181 | 0.056 | 0.5178 | 0.0410 | 0.1436 | |
| HRH2 | 0.030 | 0.095 | 0.164 | 0.043 | 0.2936 | 0.0378 | 0.2673 | |
| Digestive enzyme | CHIA | 0.023 | 0.076 | 0.139 | 0.034 | 0.2756 | 0.0246 | 0.2071 |
| PGA5 | 0.004 | 0.008 | 0.014 | 0.004 | 0.5174 | 0.0937 | 0.2849 | |
| Gastrin receptor | CCKBR | 0.008 | 0.040 | 0.110 | 0.033 | 0.4991 | 0.0417 | 0.1533 |
| Histamine production | HDC | 0.064 | 0.134 | 0.164 | 0.039 | 0.2199 | 0.0852 | 0.5935 |
| Intrinsic factor | CBLIF | 0.004 | 0.041 | 0.133 | 0.037 | 0.4800 | 0.0222 | 0.0947 |
| Mucosal defence | OLFM4 | 0.485 | 0.406 | 0.213 | 0.086 | 0.5226 | 0.0353 | 0.1246 |
| PIGR | 0.655 | 0.534 | 0.379 | 0.090 | 0.3492 | 0.0407 | 0.2345 | |
| Mucus production | MUC2 | 0.336 | 0.215 | 0.183 | 0.060 | 0.1657 | 0.0852 | 0.7150 |
| Water channel protein | AQP4 | 0.001 | 0.069 | 0.154 | 0.045 | 0.3300 | 0.0301 | 0.1979 |
| Prohormone processing | PCSK1 | 0.139 | 0.215 | 0.266 | 0.052 | 0.3103 | 0.0968 | 0.4924 |
| Role | Genes | Location * | SEM | p-Values | ||||
|---|---|---|---|---|---|---|---|---|
| 1(a) | 2(b) | 3(c) | ab | ac | bc | |||
| Acid secretion | ATP4A, CLIC6, HRH2, KCNQ1 | 0.045 | 0.091 | 0.173 | 0.038 | 0.4127 | 0.0305 | 0.1527 |
| Digestive enzyme | CHIA, PGA5 | 0.013 | 0.042 | 0.076 | 0.019 | 0.2924 | 0.0274 | 0.2107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiernan, D.P.; O’Doherty, J.V.; Connolly, K.R.; Ryan, M.; Sweeney, T. Exploring the Differential Expression of a Set of Key Genes Involved in the Regulation and Functioning of the Stomach in the Post-Weaned Pig. Vet. Sci. 2023, 10, 473. https://doi.org/10.3390/vetsci10070473
Kiernan DP, O’Doherty JV, Connolly KR, Ryan M, Sweeney T. Exploring the Differential Expression of a Set of Key Genes Involved in the Regulation and Functioning of the Stomach in the Post-Weaned Pig. Veterinary Sciences. 2023; 10(7):473. https://doi.org/10.3390/vetsci10070473
Chicago/Turabian StyleKiernan, Dillon P., John V. O’Doherty, Kathryn Ruth Connolly, Marion Ryan, and Torres Sweeney. 2023. "Exploring the Differential Expression of a Set of Key Genes Involved in the Regulation and Functioning of the Stomach in the Post-Weaned Pig" Veterinary Sciences 10, no. 7: 473. https://doi.org/10.3390/vetsci10070473
APA StyleKiernan, D. P., O’Doherty, J. V., Connolly, K. R., Ryan, M., & Sweeney, T. (2023). Exploring the Differential Expression of a Set of Key Genes Involved in the Regulation and Functioning of the Stomach in the Post-Weaned Pig. Veterinary Sciences, 10(7), 473. https://doi.org/10.3390/vetsci10070473






