Analysis of Retrospective Laboratory Data on the Burden of Bacterial Pathogens Isolated at the National Veterinary Research Institute Nigeria, 2018–2021
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Data Collection
2.2. Key Pathogens
2.2.1. Escherichia coli
2.2.2. Staphylococcus spp.
2.2.3. Salmonella spp.
2.2.4. Klebsiella spp.
2.3. Laboratory Investigation
2.4. Data Analysis
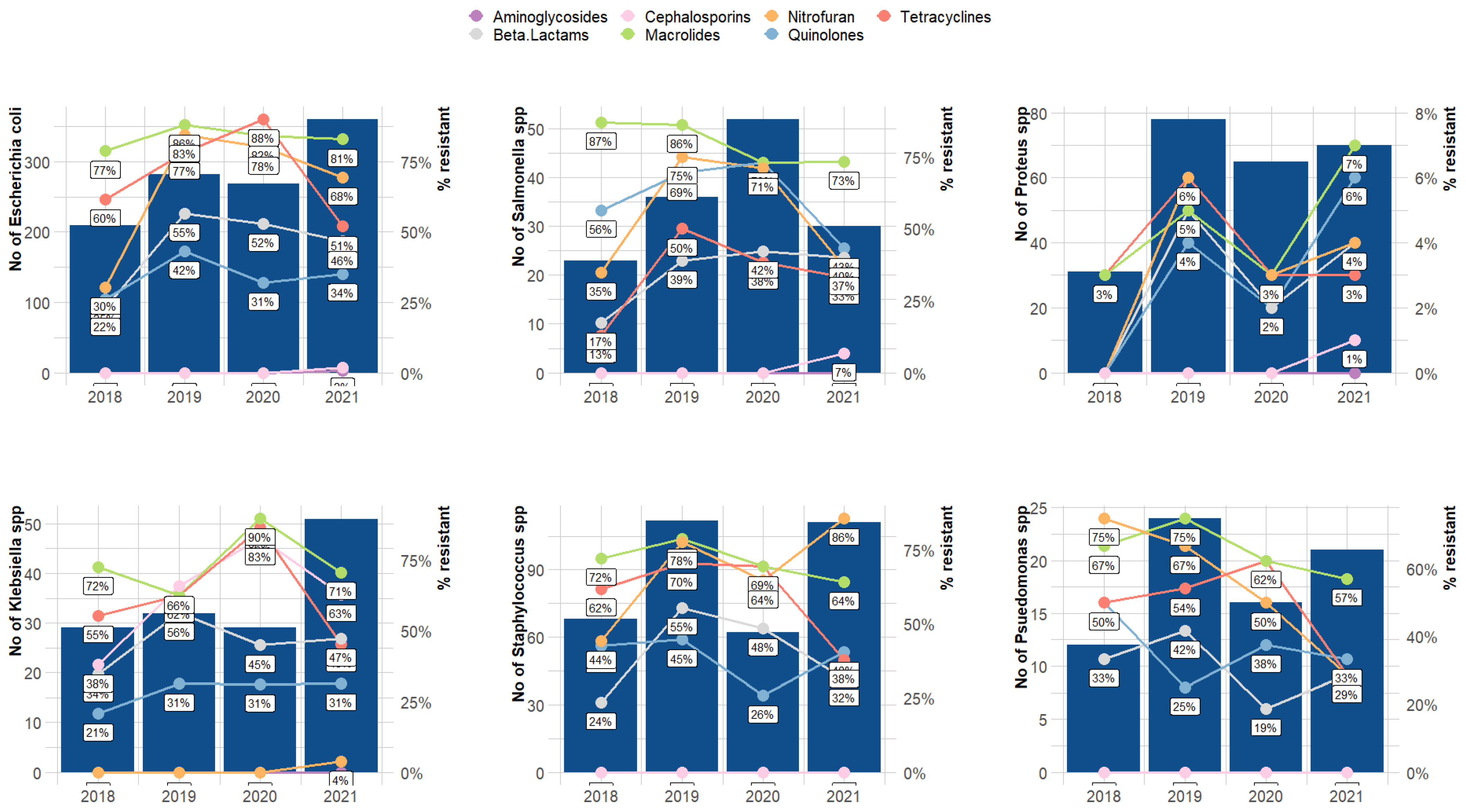
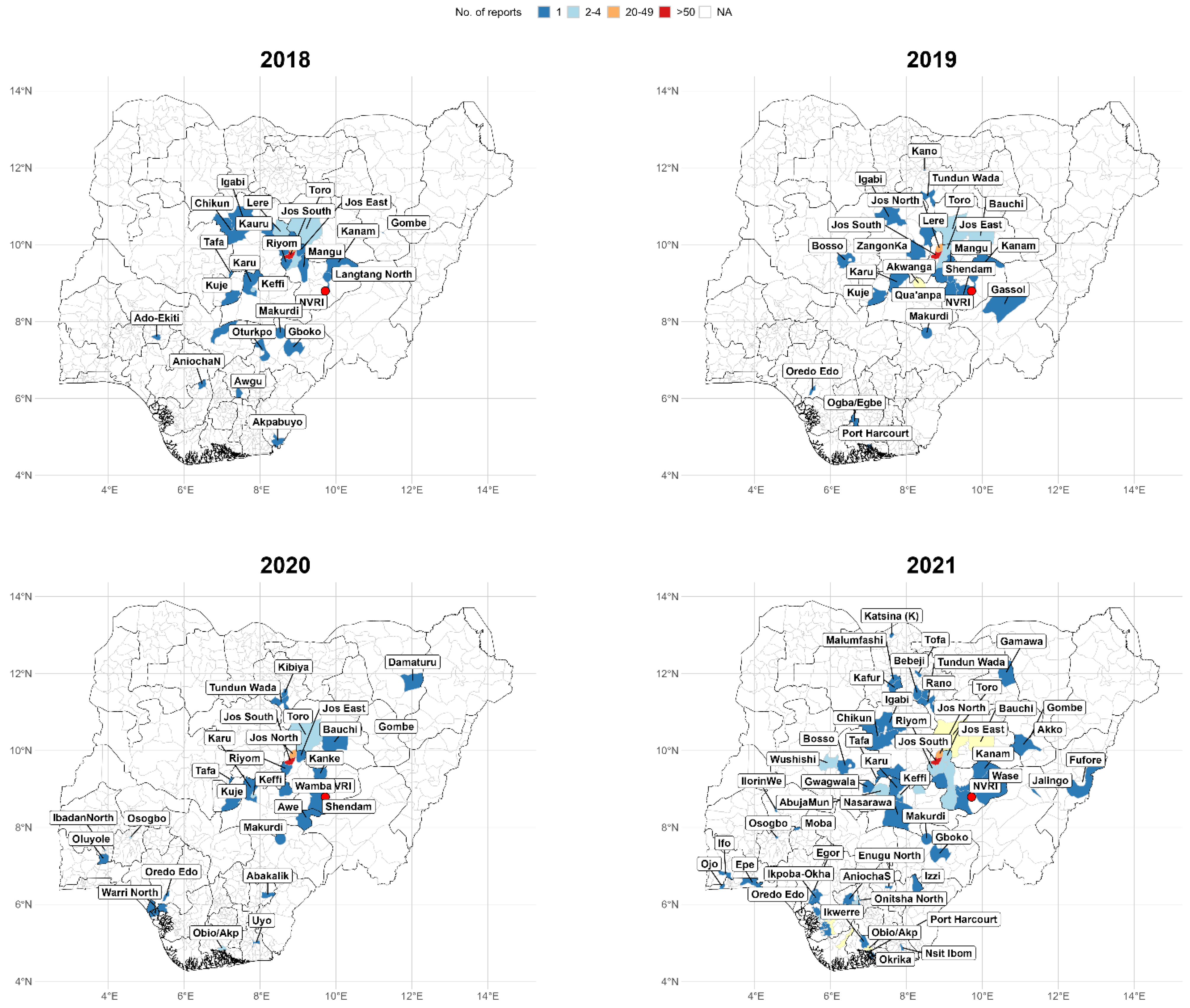
3. Results
4. Discussion
4.1. AMR Spilling from Animal to Humans
4.2. The Influence of the COVID-19 Pandemic on the Livestock Industry
4.3. Veterinary and Food Safety Implications of Bacterial Pathogens on Public Health
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abebe, E.; Gugsa, G.; Ahmed, M. Review on Major Food-Borne Zoonotic Bacterial Pathogens. J. Trop. Med. 2020, 2020, 4674235. [Google Scholar] [CrossRef]
- Prieto, M.; Colin, P.; Fernández-Escámez, P.; Alvarez-Ordóñez, A. Editorial Epidemiology, Detection, and Control of Foodborne Microbial Pathogens. BioMed Res. Int. 2015, 2015, 617417. [Google Scholar] [CrossRef] [PubMed]
- Argaw, S.; Addis, M. A Review on Staphylococcal Food Poisoning. Food Sci. Qual. Manag. 2015, 40, 59–72. [Google Scholar]
- Pillai, N.; Ramkumar, M.; Nanduri, B. Artificial Intelligence Models for Zoonotic Pathogens: A Survey. Microorgisms 2022, 10, 1911. [Google Scholar] [CrossRef] [PubMed]
- Caniça, M.; Igrejas, G. Escherichia coli as Commensal and Pathogenic Bacteria among Food-Producing Animals: Health Implications of Extended Spectrum β-Lactamase (ESBL) Production. Animals 2020, 10, 2239. [Google Scholar]
- Landers, T.F.; Cohen, B.; Wittum, T.E.; Larson, E.L. A Review of Antibiotic Use in Food Animals: Perspective, Policy, and Potential. Public Health Rep. 2012, 127, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Almshawt, N.F.; Hiblu, M.A.; Abid, A.S.; Abbassi, M.S.; Elkady, A.A.; Abouzeed, Y.M.; Ahmed, M.O. Antimicrobial resistance among commensal enteric bacteria isolated from healthy cattle in Libya. PAMJ-OH 2020, 13, 3. [Google Scholar] [CrossRef]
- De Jong, A.; Simjee, S.; Rose, M.; Moyaert, H.; El Garch, F.; Youala, M. Antimicrobial resistance monitoring in commensal enterococci from healthy cattle, pigs and chickens across Europe during 2004–14 (EASSA Study). J. Antimicrob. Chemother. 2019, 74, 921–930. [Google Scholar] [CrossRef]
- Aasmäe, B.; Häkkinen, L.; Kaart, T.; Kalmus, P. Antimicrobial resistance of Escherichia coli and Enterococcus spp. isolated from Estonian cattle and swine from 2010 to 2015. Acta Vet. Scand. 2019, 61, 5. [Google Scholar] [CrossRef]
- Bhunia, A.K. Foodborne Microbial Pathogens: Mechanisms and Pathogenesis; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar] [CrossRef]
- De Vries, S.P.W.; Vurayai, M.; Holmes, M.; Gupta, S.; Bateman, M.; Goldfarb, D.; Maskell, D.J.; Matsheka, M.I.; Grant, A.J. Phylogenetic analyses and antimicrobial resistance profiles of Campylobacter spp. from diarrhoeal patients and chickens in Botswana. PLoS ONE 2018, 13, e0194481. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing antimicrobial use in food animals. Science (80-) 2017, 357, 1350–1352. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, J.; Khan, S.; Su, J.Q.; Hesham, A.E.L.; Ditta, A.; Nawab, J.; Ali, A. Antibiotics in poultry manure and their associated health issues: A systematic review. J. Soils Sediments 2020, 20, 486–497. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef] [PubMed]
- Gemeda, B.A.; Amenu, K.; Magnusson, U.; Dohoo, I.; Hallenberg, G.S.; Alemayehu, G.; Desta, H.; Wieland, B. Antimicrobial Use in Extensive Smallholder Livestock Farming Systems in Ethiopia: Knowledge, Attitudes, and Practices of Livestock Keepers. Front. Vet. Sci. 2020, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Grace, D. Review of Evidence on Antimicrobial Resistance and Animal Agriculture in Developing Countries Delia Grace; CABI Publishing: Wallingford, UK, 2015. [Google Scholar]
- EFSA. The European Union Summary Report on antimicrobial resistance in zoonotic and indicator bacteria from humans, animals and food in 2010. EFSA J. 2012, 10, 7209. [Google Scholar] [CrossRef]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Ejo, M.; Garedew, L.; Alebachew, Z.; Worku, W. Prevalence and Antimicrobial Resistance of Salmonella Isolated from Animal-Origin Food Items in Gondar, Ethiopia. BioMed Res. Int. 2016, 2016, 4290506. [Google Scholar] [CrossRef]
- Estimating the Burden of Foodborne Diseases. Available online: https://www.who.int/activities/estimating-the-burden-of-foodborne-diseases (accessed on 24 December 2022).
- Hedman, H.D.; Vasco, K.A.; Zhang, L. A Review of Antimicrobial Resistance in Poultry Farming within Low-Resource Settings. Animals 2020, 10, 1264. [Google Scholar] [CrossRef]
- Caleb Ike, E. A Review of Small holder Farming in Nigeria: Need for Transformation. Agric. Eng. Res. J. 2015, 5, 19–26. [Google Scholar] [CrossRef]
- Odunze, E.; Boussini, H.; Mikecz, O.; Pica-Ciamarra, U. Livestock and livelihoods spotlight Nigeria: Cattle and poultry sectors. Food Agric. Organ. Afr. Sustain. Livest. 2018, 2050, 11–12. [Google Scholar]
- The Future of Livestock in Nigeria: Opportunities and Challenges in the Face of Uncertainty—Nigeria|ReliefWeb. Available online: https://reliefweb.int/report/nigeria/future-livestock-nigeria-opportunities-and-challenges-face-uncertainty (accessed on 24 December 2022).
- Contact—Contact. Available online: https://nvri.gov.ng/contact (accessed on 28 April 2023).
- Denamur, E.; Clermont, O.; Bonacorsi, S.; Gordon, D. The population genetics of pathogenic Escherichia coli. Nat. Rev. Microbiol. 2021, 19, 37–54. [Google Scholar] [CrossRef]
- Alkeskas, A.; Ogrodzki, P.; Saad, M.; Masood, N.; Rhoma, N.R.; Moore, K.; Farbos, A.; Paszkiewicz, K. The molecular characterisation of Escherichia coli K1 isolated from neonatal nasogastric feeding tubes. BMC Infect. Dis. 2015, 15, 449. [Google Scholar] [CrossRef] [PubMed]
- Croxen, M.A.; Finlay, B.B. Molecular mechanisms of Escherichia coli pathogenicity. Nat. Rev. Microbiol. 2010, 8, 26–38. [Google Scholar] [CrossRef]
- Rodriguez-Siek, K.E.; Giddings, C.W.; Doetkott, C.; Johnson, T.J.; Fakhr, M.K.; Nolan, L.K. Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology 2005, 151, 2097–2110. [Google Scholar] [CrossRef] [PubMed]
- Casella, T.; Nogueira, M.; Saras, E.; Haenni, M.; Madec, J.Y. High prevalence of ESBLs in retail chicken meat despite reduced use of antimicrobials in chicken production, France. Int. J. Food Microbiol. 2017, 257, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Caxito, M.; Benavides, J.A.; Adell, A.D.; Paes, A.C.; Moreno-Switt, A.I. Global prevalence and molecular characterization of extended-spectrum β-lactamase producing-Escherichia coli in dogs and cats—A scoping review and meta-analysis. One Health 2021, 12, 100236. [Google Scholar] [CrossRef]
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Kristiansson, E.; Larsson, D.G.J. Environmental factors influencing the development and spread of antibiotic resistance. FEMS Microbiol. Rev. 2018, 42, 68–80. [Google Scholar] [CrossRef]
- World Health Organization. Foodborne Disease Outbreaks: Guidelines for Investigation and Control; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Shrivastava, S. World health organization releases global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. J. Med. Soc. 2018, 32, 76–77. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020, 33, 10–1128. [Google Scholar] [CrossRef]
- Peton, V.; Le Loir, Y. Staphylococcus aureus in veterinary medicine. Infect. Genet. Evol. 2014, 21, 602–615. [Google Scholar] [CrossRef]
- Michalik, M.; Samet, A.; Podbielska-Kubera, A.; Savini, V.; Międzobrodzki, J.; Kosecka-Strojek, M. Coagulase-negative staphylococci (CoNS) as a significant etiological factor of laryngological infections: A review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 26. [Google Scholar] [CrossRef] [PubMed]
- Nawrotek, P.; Karakulska, J.; Fijałkowski, K. The Staphylococcal Panton-Valentine leukocidin (PVL). In Pet-to-Man Travelling Staphylococci; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Huebner, J.; Goldmann, D.A. Coagulase-Negative Staphylococci: Role as Pathogens. Annu. Rev. Med. 2003, 50, 223–236. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, C.; Ziebuhr, W.; Becker, K. Are coagulase-negative staphylococci virulent? Clin. Microbiol. Infect. 2019, 25, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Salmonella (Non-Typhoidal). Available online: https://www.who.int/news-room/fact-sheets/detail/salmonella-(non-typhoidal) (accessed on 9 January 2023).
- Ferrari, R.G.; Rosario, D.K.A.; Cunha-Neto, A.; Mano, S.B.; Figueiredo, E.E.S.; Conte-Juniora, C.A. Worldwide epidemiology of Salmonella serovars in animal-based foods: A meta-analysis. Appl. Environ. Microbiol. 2019, 85, e00591-19. [Google Scholar] [CrossRef] [PubMed]
- Mezal, E.H.; Sabol, A.; Khan, M.A.; Ali, N.; Stefanova, R.; Khan, A.A. Isolation and molecular characterization of Salmonella enterica serovar Enteritidis from poultry house and clinical samples during 2010. Food Microbiol. 2014, 38, 67–74. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Li, T.; Liu, F.; Cheng, Y.; Guo, X.; Zhang, X. Characterization of Salmonella spp. isolated from chickens in Central China. BMC Vet. Res. 2020, 16, 258. [Google Scholar] [CrossRef]
- Nair, D.V.T.; Kollanoor, J.A. Salmonella in Poultry Meat Production. In Food Safety in Poultry Meat Production; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–24. [Google Scholar] [CrossRef]
- Antunes, P.; Mourão, J.; Campos, J. Salmonellosis: The role of poultry meat. Clin. Microbiol. Infect. 2016, 22, 110–121. [Google Scholar] [CrossRef]
- Scallan, E.; Hoekstra, R.; Angulo, F. Foodborne illness acquired in the United States. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Gast, R.K.; Porter, R.E., Jr. Salmonella Infections. In Diseases of Poultry, 13th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 675–736. [Google Scholar] [CrossRef]
- Sylejmani, D.; Musliu, A. Associations between the Level of Biosecurity and Occurrence of Dermanyssus gallinae and Salmonella spp. in Layer Farms. Avian Dis. 2016, 60, 454–459. [Google Scholar] [CrossRef]
- Salmonella Homepage|CDC. Available online: https://www.cdc.gov/salmonella/index.html (accessed on 9 January 2023).
- Ribeiro, M.G.; de Morais, A.B.C.; Alves, A.C.; Bolaños, C.A.D.; de Paula, C.L.; Portilho, F.V.R.; de Nardi Júnior, G.; Lara, G.H.B.; de Souza Araújo Martins, L.; Moraes, L.S.; et al. Klebsiella-induced infections in domestic species: A case-series study in 697 animals (1997–2019). Braz. J. Microbiol. 2022, 53, 455–464. [Google Scholar] [CrossRef]
- Wyres, K.; Lam, M. Population genomics of Klebsiella pneumoniae. Nat. Rev. Microbiol. 2020, 18, 344–359. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Yang, X.; Chan, E.W.C.; Zhang, R.; Chen, S. Klebsiella species: Taxonomy, hypervirulence and multidrug resistance. EBioMedicine 2022, 79, 103998. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J.; Markey, B.K.; Carter, M.E.; Donnelly, W.J.C.; Leonard, F.C. Veterinary microbiology and microbial disease. Vet. Microbiol. Microb. Dis. 2002, 44, 986. [Google Scholar]
- Washington, J. Laboratory Procedures in Clinical Microbiology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Bauer, A.W.; Perry, D.M.; Kirby, W.M.M. Single-Disk Antibiotic-Sensitivity Testing of Staphylococci: An Analysis of Technique and Results. AMA Arch. Intern. Med. 1959, 104, 208–216. [Google Scholar] [CrossRef]
- Vineetha, N.; Sridhar, D.; Vignesh, R.A. Preparation, Standardization of Antibiotic Discs and Study of Resistance Pattern for First-Line Antibiotics in Isolates from Clinical Samples. Int. J. Appl. Res. 2015, 1, 624–631. [Google Scholar]
- Benklaouz, M.B.; Aggad, H.; Benameur, Q. Resistance to multiple first-line antibiotics among Escherichia coli from poultry in Western Algeria. Vet. World 2020, 13, 290–295. [Google Scholar] [CrossRef]
- Samreen Ahmad, I.; Malak, H.A.; Abulreesh, H.H. Environmental antimicrobial resistance and its drivers: A potential threat to public health. J. Glob. Antimicrob. Resist. 2021, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Wee, B.A.; Muloi, D.M.; van Bunnik, B.A.D. Quantifying the transmission of antimicrobial resistance at the human and livestock interface with genomics. Clin. Microbiol. Infect. 2020, 26, 1612–1616. [Google Scholar] [CrossRef]
- Magouras, I.; Carmo, L.P.; Stärk, K.D.C.; Schüpbach-Regula, G. Antimicrobial usage and -resistance in livestock: Where should we focus? Front. Vet. Sci. 2017, 4, 148. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science (80-) 2019, 365, eaaw1944. [Google Scholar] [CrossRef]
- Innes, G.K.; Randad, P.R.; Korinek, A.; Davis, M.F.; Price, L.B.; So, A.D.; Heaney, C.D. External societal costs of antimicrobial resistance in humans attributable to antimicrobial use in livestock. Annu. Rev. Public Health 2019, 41, 141–157. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 521–547. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; He, X.; Feng, Y.; Wang, W.; Chen, H.; Gong, M.; Liu, D.; Clarke, L.J.; van Eerde, A. Pollution by Antibiotics and Antimicrobial Resistance in LiveStock and Poultry Manure in China, and Countermeasures. Antibiotics 2021, 10, 539. [Google Scholar] [CrossRef]
- Heuer, H.; Schmitt, H.; Smalla, K. Antibiotic resistance gene spread due to manure application on agricultural fields. Curr. Opin. Microbiol. 2011, 14, 236–243. [Google Scholar] [CrossRef]
- Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2021. Available online: https://www.who.int/publications/i/item/9789240027336 (accessed on 5 July 2023).
- Magnusson, U.; Moodley, A.; Osbjer, K. Antimicrobial resistance at the livestock–human interface: Implications for Veterinary Services. OIE Rev. Sci. Tech. 2021, 40, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Ono, H.K.; Shimojima, Y.; Kubota, H.; Kato, R.; Kakuda, T.; Hirose, S.; Hu, D.-L.; Nakane, A.; Takai, S.; et al. A novel staphylococcal enterotoxin SE02 involved in a staphylococcal food poisoning outbreak that occurred in Tokyo in 2004. Food Microbiol. 2020, 92, 103588. [Google Scholar] [CrossRef]
- Vidovic, N.; Vidovic, S. Antimicrobial resistance and food animals: Influence of livestock environment on the emergence and dissemination of antimicrobial resistance. Antibiotics 2020, 9, 52. [Google Scholar] [CrossRef]
- Bacanlı, M.; Başaran, N. Importance of antibiotic residues in animal food. Food Chem. Toxicol. 2019, 125, 462–466. [Google Scholar] [CrossRef]
- Awoyomi, O.J.; Olasoju, M.I.; Kehinde, O.O.; Adebowale, O.O.; Awoyomi, F.S.O.; Agbajelola, V.I.; Ogugua, A.J. Impact of COVID-19 on livestock production and marketing in Nigeria. Niger. J. Anim. Prod. 2022, 49, 195–212. [Google Scholar] [CrossRef]
- Rahimi, P.; Islam, M.S.; Duarte, P.M.; Tazerji, S.S.; Sobur, M.A.; El Zowalaty, M.E.; Ashour, H.M.; Rahman, T. Impact of the COVID-19 pandemic on food production and animal health. Trends Food. Sci. Technol. 2022, 121, 105–113. [Google Scholar] [CrossRef]
- Galgallo, D.A.; Roka, Z.G.; Boru, W.G.; Abill, K.; Ransom, J. Investigation of a typhoid fever epidemic in Moyale Sub-County, Kenya, 2014–2015. J. Health Popul. Nutr. 2018, 37, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Bamidele, O.; Amole, T.A. Impact of COVID-19 on Smallholder Poultry Farmers in Nigeria. Sustainability 2021, 13, 11475. [Google Scholar] [CrossRef]
- Morens, D.M.; Folkers, G.K.; Fauci, A.S. The challenge of emerging and re-emerging infectious diseases. Nature 2004, 430, 242–249. [Google Scholar] [CrossRef]
- Okolie, C.C.; Ogundeji, A.A. Effect of COVID-19 on agricultural production and food security: A scientometric analysis. Hum. Soc. Sci. Commun. 2022, 9, 64. [Google Scholar] [CrossRef]
- Sykes, J.E.; Rankin, S.C.; Papich, M.G.; Weese, J.S.; Little, S.E. Greene’s Infectious Diseases of the Dog and Cat; Springer: Berlin/Heidelberg, Germany, 1799. [Google Scholar]
- Schukken, Y.; Chuff, M.; Moroni, P.; Gurjar, A.; Santisteban, C.; Welcome, F.; Zadoks, R. The “Other” Gram-Negative Bacteria in Mastitis. Klebsiella, Serratia, and More. Vet. Clin. N. Am. Food Anim. Pract. 2012, 28, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Shon, A.S.; Bajwa, R.P.S.; Russo, T.A. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: A new and dangerous breed. Virulence 2013, 4, 107–118. [Google Scholar] [CrossRef]
- Gu, D.; Dong, N.; Zheng, Z.; Lin, D.; Huang, M.; Wang, L.; Chan, E.W.-C.; Shu, L.; Yu, J.; Zhang, R.; et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: A molecular epidemiological study. Lancet Infect. Dis. 2018, 18, 37–46. [Google Scholar] [CrossRef]
- Marr, C.M.; Russo, T.A. Hypervirulent Klebsiella pneumoniae: A new public health threat. Expert Rev. Anti-Infect. Ther. 2019, 17, 71–73. [Google Scholar] [CrossRef]
- Nolan, L.K.; John, B.H.; Vaillancourt, J.P.; Abdul-Aziz, T.; Logue, C.M. Colibacillosis. In Diseases of Poultry, 13th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 751–805. [Google Scholar] [CrossRef]
- Smith, T.C.; Male, M.J.; Harper, A.L.; Kroeger, J.S.; Tinkler, G.P.; Moritz, E.D.; Capuano, A.W.; Herwaldt, L.A.; Diekema, D.J. Methicillin-resistant Staphylococcus aureus (MRSA) strain ST398 is present in midwestern U.S. swine and swine workers. PLoS ONE 2009, 4, e4258. [Google Scholar] [CrossRef]
- Nemati, M.; Hermans, K.; Lipinska, U.; Denis, O.; Deplano, A.; Struelens, M.; Devriese, L.A.; Pasmans, F.; Haesebrouck, F. Antimicrobial resistance of old and recent Staphylococcus aureus isolates from poultry: First detection of livestock-associated methicillin-resistant strain ST398. Antimicrob. Agents Chemother. 2008, 52, 3817–3819. [Google Scholar] [CrossRef]
- Uro, T.W. International Journal of Advanced Research in Biological Sciences Salmonellosis: A Review. Int. J. Adv. Res. Biol. Sci. 2019, 6, 79–88. [Google Scholar] [CrossRef]
- Ngogo, F.A.; Joachim, A.; Abade, A.M.; Rumisha, S.F.; Mizinduko, M.M.; Majigo, M.V. Factors associated with Salmonella infection in patients with gastrointestinal complaints seeking health care at Regional Hospital in Southern Highland of Tanzania. BMC Infect. Dis. 2020, 20, 135. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Ge, Y.; Guo, F. Salmonella bacteremia and its disseminated infection should not be neglected. Zhonghua Nei Ke Za Zhi 2021, 60, 102–105. [Google Scholar] [PubMed]
- Haeusler, G.M.; Curtis, N. Non-typhoidal salmonella in children: Microbiology, epidemiology and treatment. Adv. Exp. Med. Biol. 2013, 764, 13–26. [Google Scholar] [CrossRef]
- Barua, H.; Biswas, P.K.; Olsen, K.E.P.; Christensen, J.P. Prevalence and characterization of motile Salmonella in commercial layer poultry farms in Bangladesh. PLoS ONE 2012, 7, e35914. [Google Scholar] [CrossRef] [PubMed][Green Version]


| Sample | 2018 | 2019 | 2020 | 2021 |
|---|---|---|---|---|
| Avian | 310 | 383 | 323 | 468 |
| Bovine | 29 | 33 | 24 | 29 |
| Canine | 19 | 24 | 27 | 27 |
| Caprine | 8 | 11 | 11 | 15 |
| Equine | 1 | 1 | 0 | 7 |
| Feed | 1 | 4 | 1 | 0 |
| Feline | 1 | 0 | 0 | 3 |
| Laprine | 22 | 23 | 21 | 27 |
| Ovine | 10 | 10 | 13 | 16 |
| Pisces | 2 | 0 | 2 | 4 |
| Porcine | 6 | 9 | 32 | 58 |
| Grand Total | 409 | 498 | 464 | 654 |
| Microorganisms | % Bacterial Isolates for Each Animal | Number of Reports 2018–2021 | % Annual Increase (95% CI) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Av | Bov | Can | Cap | Equ | Fel | Lap | Ov | Pcs | Por | Total | Mean | SD | ||
| Escherichia coli | 54 | 31 | 30 | 36 | 33 | 0 | 40 | 49 | 25 | 41 | 1120 | 280 | 62.09 | 15.83 (0.54, 21.13) |
| Salmonella spp. | 9.5 | 0.9 | 0 | 4.4 | 22 | 0 | 0 | 0 | 25 | 1 | 142 | 35.5 | 12.23 | 11.31 (−3.44, 26.19) |
| Klebsiella spp. | 4.5 | 8.7 | 5 | 2.2 | 0 | 25 | 8 | 14 | 0 | 8 | 141 | 35.3 | 10.59 | 18.04 (3.16, 33.14) |
| Proteus spp. | 10 | 10 | 16 | 2.2 | 0 | 0 | 11 | 12 | 0 | 17 | 244 | 61 | 20.7 | 17.19 (5.88, 28.63) |
| Staphylococcus spp. | 13 | 18 | 24 | 22 | 22 | 2 | 15 | 12 | 25 | 22 | 361 | 90.3 | 29.49 | 7.88 (−1.36, 17.16) |
| Streptococcus spp. | 0.6 | 5.2 | 1 | 2.2 | 0 | 0 | 0 | 0 | 0 | 1 | 29 | 7.25 | 4.27 | 20.95 (−11.83, 55.01) |
| Psuedomonas spp. | 2.4 | 5.2 | 3 | 4.4 | 0 | 0 | 3.2 | 4.1 | 0 | 4 | 73 | 18.3 | 5.32 | 10.44 (−10.11, 31.24) |
| Shighella spp. | 0.5 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 10 | 2.5 | 2.65 | 0.00 (−56.64, 56.64) |
| Enterobacter spp. | 0.9 | 0.9 | 0 | 2.2 | 0 | 0 | 1.1 | 2 | 0 | 0 | 23 | 5.75 | 2.36 | 30.33 (−6.84, 69.89) |
| Other organisms | 562 | 141 | 54.07 | 30.33 (−6.84, 69.89) | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabantiyok, D.; Gyang, M.D.; Agada, G.O.; Ogundeji, A.; Nyam, D.; Uhiara, U.G.; Abiayi, E.; Dashe, Y.; Ngulukun, S.; Muhammad, M.; et al. Analysis of Retrospective Laboratory Data on the Burden of Bacterial Pathogens Isolated at the National Veterinary Research Institute Nigeria, 2018–2021. Vet. Sci. 2023, 10, 505. https://doi.org/10.3390/vetsci10080505
Kabantiyok D, Gyang MD, Agada GO, Ogundeji A, Nyam D, Uhiara UG, Abiayi E, Dashe Y, Ngulukun S, Muhammad M, et al. Analysis of Retrospective Laboratory Data on the Burden of Bacterial Pathogens Isolated at the National Veterinary Research Institute Nigeria, 2018–2021. Veterinary Sciences. 2023; 10(8):505. https://doi.org/10.3390/vetsci10080505
Chicago/Turabian StyleKabantiyok, Dennis, Moses D. Gyang, Godwin O. Agada, Alice Ogundeji, Daniel Nyam, Uchechi G. Uhiara, Elmina Abiayi, Yakubu Dashe, Sati Ngulukun, Maryam Muhammad, and et al. 2023. "Analysis of Retrospective Laboratory Data on the Burden of Bacterial Pathogens Isolated at the National Veterinary Research Institute Nigeria, 2018–2021" Veterinary Sciences 10, no. 8: 505. https://doi.org/10.3390/vetsci10080505
APA StyleKabantiyok, D., Gyang, M. D., Agada, G. O., Ogundeji, A., Nyam, D., Uhiara, U. G., Abiayi, E., Dashe, Y., Ngulukun, S., Muhammad, M., Adegboye, O. A., & Emeto, T. I. (2023). Analysis of Retrospective Laboratory Data on the Burden of Bacterial Pathogens Isolated at the National Veterinary Research Institute Nigeria, 2018–2021. Veterinary Sciences, 10(8), 505. https://doi.org/10.3390/vetsci10080505








