Immunogenicity Characterization of the Recombinant gI Protein Fragment from Pseudorabies Virus and an Evaluation of Its Diagnostic Use in Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Strain, Plasmids, Bacterial Strains and Antibodies
2.2. Primer Design and Synthesis
2.3. Construction of Recombinant Expression Vector
2.4. Expression and Purification of Truncated gI Protein
2.5. SDS–PAGE and Western Blot
2.6. Animal Ethic Statements
2.7. Preparation of Anti-gI Protein Mouse Serum
2.8. Characterization of Murine gI Protein Polyclonal Antibody against PRV
2.9. Indirect ELISA
3. Results
3.1. Construction of the Expression Vector
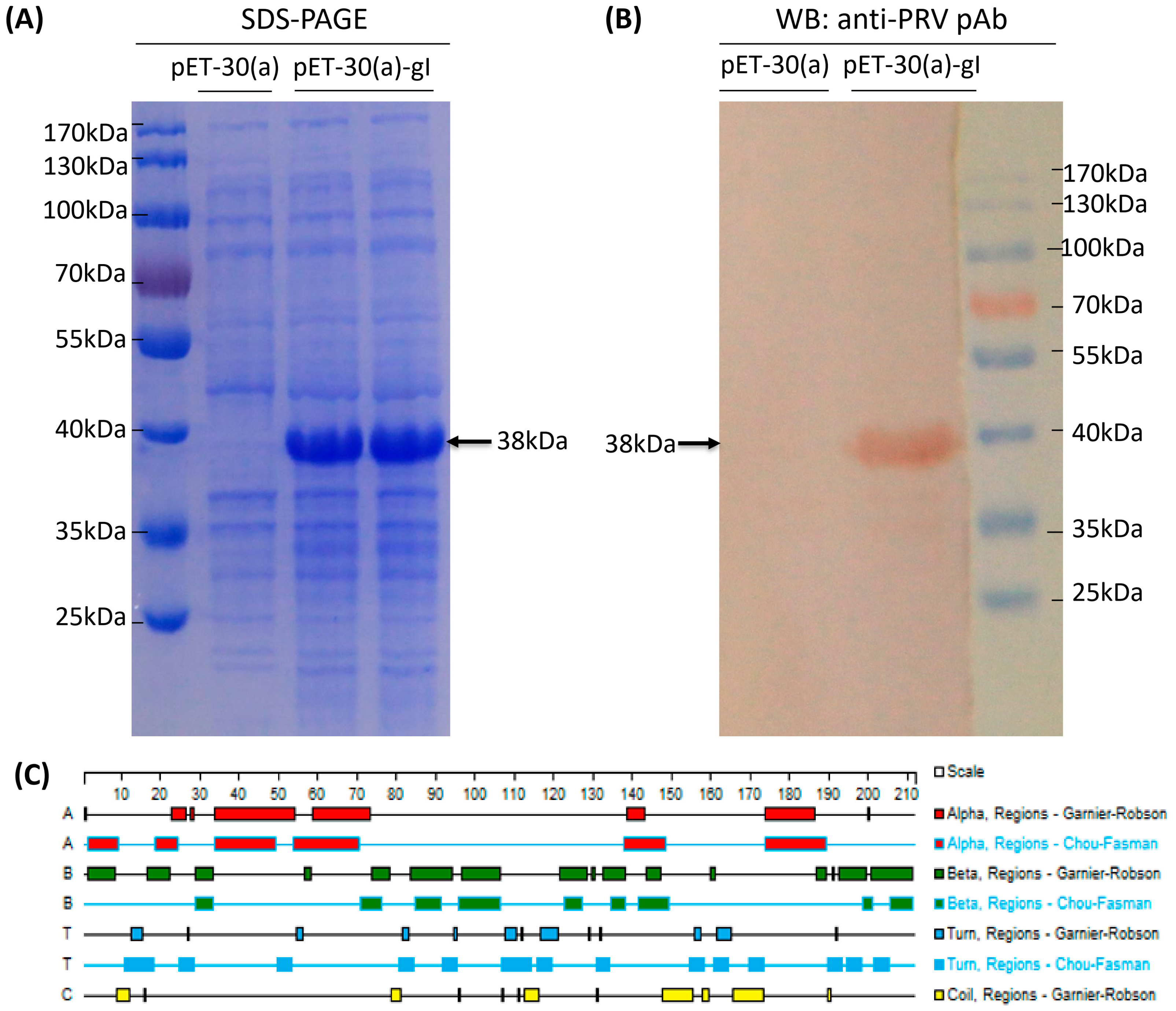
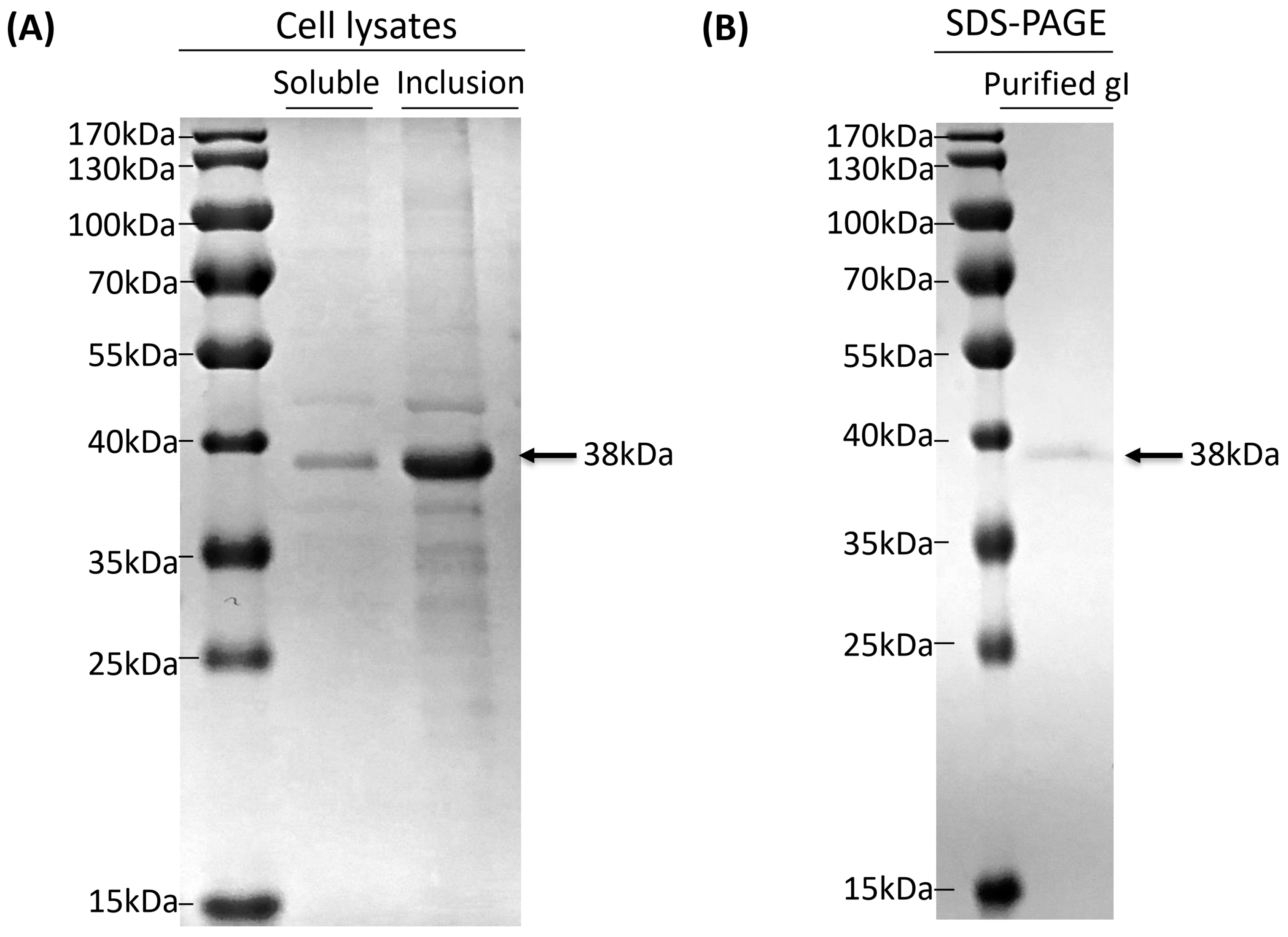
3.2. Expression and Identification of Truncated gI Protein
3.3. Purification of Truncated gI Protein
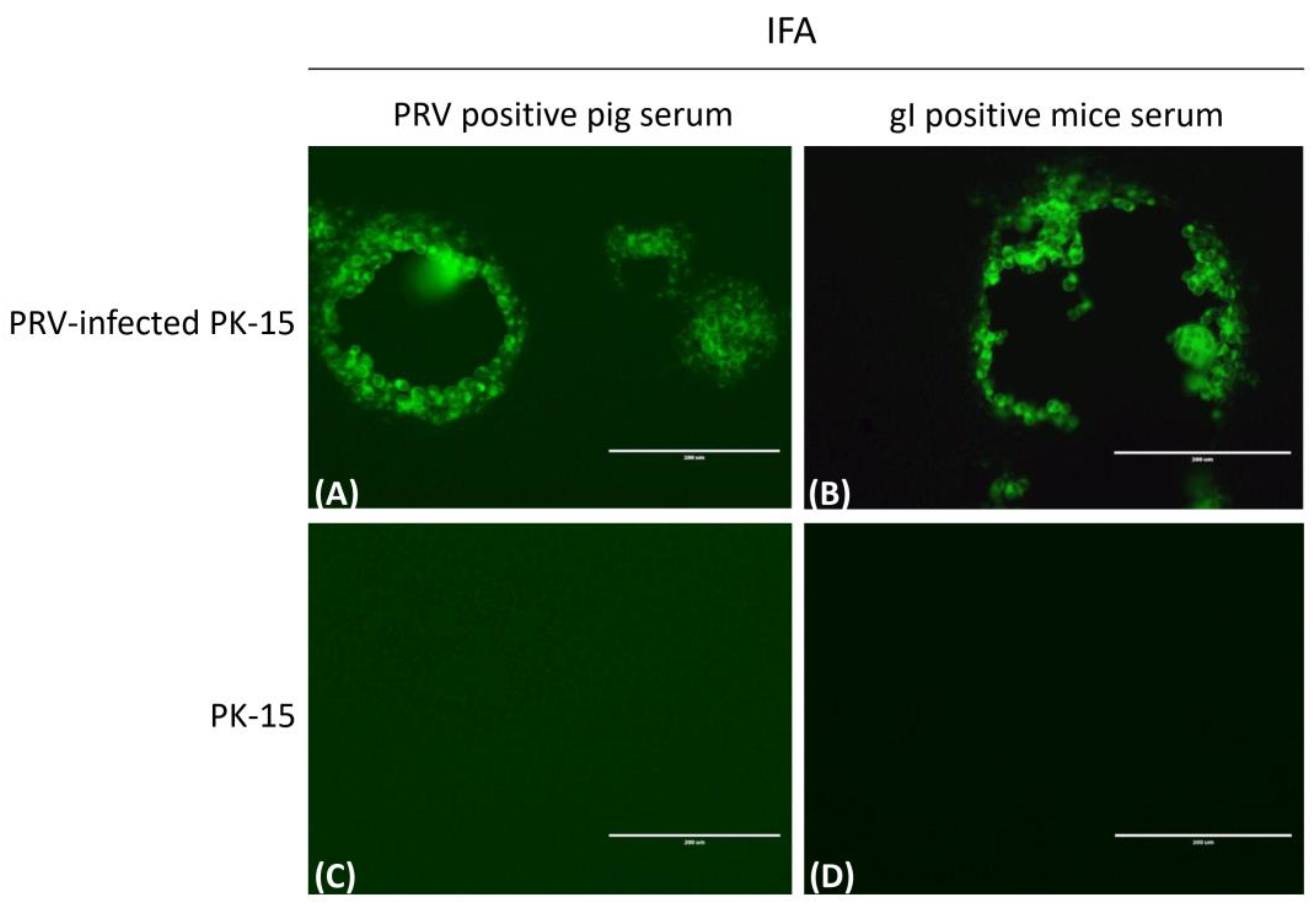
3.4. Immunogenicity Identification of Truncated gI Protein
3.5. Preliminary Clinical Application of Expressed gI Protein in ELISA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Klupp, B.G. Pseudorabies Virus Infections. Pathogens 2021, 10, 719. [Google Scholar] [CrossRef] [PubMed]
- Kondibaeva, Z.B.; Yespembetov, B.A.; Abeuov, K.B.; Mussayeva, A.K.; Siyabekov, S.T.; Nussupova, S.T.; Akmatova, E.K.; Pazylov, Y.K.; Maikhin, K.T.; Syrym, N.S. Inactivated vaccine against Aujeszky’s disease. Vet. World 2021, 14, 2957–2963. [Google Scholar] [CrossRef]
- Laval, K.; Enquist, L.W. The Neuropathic Itch Caused by Pseudorabies Virus. Pathogens 2020, 9, 254. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Luo, Y.; Wang, C.H.; Yuan, J.; Li, N.; Song, K.; Qiu, H.J. Control of swine pseudorabies in China: Opportunities and limitations. Vet. Microbiol. 2016, 183, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Minamiguchi, K.; Kojima, S.; Sakumoto, K.; Kirisawa, R. Isolation and molecular characterization of a variant of Chinese gC-genotype II pseudorabies virus from a hunting dog infected by biting a wild boar in Japan and its pathogenicity in a mouse model. Virus Genes 2019, 55, 322–331. [Google Scholar] [CrossRef]
- Kong, H.; Zhang, K.; Liu, Y.; Shang, Y.; Wu, B.; Liu, X. Attenuated live vaccine (Bartha-K16) caused pseudorabies (Aujeszky’s disease) in sheep. Vet. Res. Commun. 2013, 37, 329–332. [Google Scholar] [CrossRef]
- Cheng, Z.; Kong, Z.; Liu, P.; Fu, Z.; Zhang, J.; Liu, M.; Shang, Y. Natural infection of a variant pseudorabies virus leads to bovine death in China. Transbound. Emerg. Dis. 2020, 67, 518–522. [Google Scholar] [CrossRef]
- Jin, H.L.; Gao, S.M.; Liu, Y.; Zhang, S.F.; Hu, R.L. Pseudorabies in farmed foxes fed pig offal in Shandong province, China. Arch. Virol. 2016, 161, 445–448. [Google Scholar] [CrossRef]
- Zanin, E.; Capua, I.; Casaccia, C.; Zuin, A.; Moresco, A. Isolation and characterization of Aujeszky’s disease virus in captive brown bears from Italy. J. Wildl. Dis. 1997, 33, 632–634. [Google Scholar] [CrossRef]
- Ai, J.W.; Weng, S.S.; Cheng, Q.; Cui, P.; Li, Y.J.; Wu, H.L.; Zhu, Y.M.; Xu, B.; Zhang, W.H. Human Endophthalmitis Caused By Pseudorabies Virus Infection, China, 2017. Emerg. Infect. Dis. 2018, 24, 1087–1090. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Xie, C.; Ding, S.; Yang, H.; Guo, S.; Li, J.; Qin, L.; Ban, F.; Wang, D.; et al. A Novel Human Acute Encephalitis Caused by Pseudorabies Virus Variant Strain. Clin. Infect. Dis. An. Off. Publ. Infect. Dis. Soc. Am. 2021, 73, e3690–e3700. [Google Scholar] [CrossRef] [PubMed]
- Freuling, C.M.; Müller, T.F.; Mettenleiter, T.C. Vaccines against pseudorabies virus (PrV). Vet. Microbiol. 2017, 206, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Pomeranz, L.E.; Reynolds, A.E.; Hengartner, C.J. Molecular biology of pseudorabies virus: Impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. MMBR 2005, 69, 462–500. [Google Scholar] [CrossRef] [PubMed]
- Klupp, B.G.; Hengartner, C.J.; Mettenleiter, T.C.; Enquist, L.W. Complete, annotated sequence of the pseudorabies virus genome. J. Virol. 2004, 78, 424–440. [Google Scholar] [CrossRef]
- Wang, G.; Zha, Z.; Huang, P.; Sun, H.; Huang, Y.; He, M.; Chen, T.; Lin, L.; Chen, Z.; Kong, Z.; et al. Structures of pseudorabies virus capsids. Nat. Commun. 2022, 13, 1533. [Google Scholar] [CrossRef]
- Kratchmarov, R.; Kramer, T.; Greco, T.M.; Taylor, M.P.; Ch’ng, T.H.; Cristea, I.M.; Enquist, L.W. Glycoproteins gE and gI are required for efficient KIF1A-dependent anterograde axonal transport of alphaherpesvirus particles in neurons. J. Virol. 2013, 87, 9431–9440. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, L.Q.; Zheng, H.H.; Yang, Y.R.; Liu, F.; Zheng, L.L.; Jin, Y.; Chen, H.Y. Construction and immunogenicity of a gE/gI/TK-deleted PRV based on porcine pseudorabies virus variant. Mol. Cell. Probes 2020, 53, 101605. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, L.; Fu, Z.F. Effective cross-protection of a lyophilized live gE/gI/TK-deleted pseudorabies virus (PRV) vaccine against classical and variant PRV challenges. Vet. Microbiol. 2022, 267, 109387. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, W.; Liu, Q.; Zhao, T.; Zhu, H.; Hua, L.; Peng, Z.; Tang, X.; Stratton, C.W.; Zhou, D.; et al. Epidemiological and genetic characteristics of swine pseudorabies virus in mainland China between 2012 and 2017. PeerJ 2018, 6, e5785. [Google Scholar] [CrossRef]
- Wang, T.; Xiao, Y.; Yang, Q.; Wang, Y.; Sun, Z.; Zhang, C.; Yan, S.; Wang, J.; Guo, L.; Yan, H.; et al. Construction of a gE-Deleted Pseudorabies Virus and Its Efficacy to the New-Emerging Variant PRV Challenge in the Form of Killed Vaccine. BioMed Res. Int. 2015, 2015, 684945. [Google Scholar] [CrossRef][Green Version]
- Wu, Q.; Zhang, H.; Dong, H.; Mehmood, K.; Chang, Z.; Li, K.; Liu, S.; Rehman, M.U.; Nabi, F.; Javed, M.T.; et al. Seroprevalence and risk factors associated with Pseudorabies virus infection in Tibetan pigs in Tibet. BMC Vet. Res. 2018, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, S.; Xu, Q.; Wu, J.; Zhai, X.; Li, S.; Wang, J.; Ni, J.; Yuan, L.; Song, X.; et al. Investigation on pseudorabies prevalence in Chinese swine breeding farms in 2013–2016. Trop. Anim. Health Prod. 2018, 50, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Sun, Q.; Wang, J.; Chen, Q.; Liu, P.; Shen, C.; Sun, J.; Tu, Y.; Shen, S.; Zhu, J.; et al. Epidemiology of pseudorabies in intensive pig farms in Shanghai, China: Herd-level prevalence and risk factors. Prev. Vet. Med. 2018, 159, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Yao, J.; Yang, Y.; Luo, W.; Yuan, X.; Yang, L.; Wang, A. Current Status and Challenge of Pseudorabies Virus Infection in China. Virol. Sin. 2021, 36, 588–607. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fang, K.; Rong, Z.; Li, X.; Ren, X.; Ma, H.; Chen, H.; Li, X.; Qian, P. Comparison of gE/gI- and TK/gE/gI-Gene-Deleted Pseudorabies Virus Vaccines Mediated by CRISPR/Cas9 and Cre/Lox Systems. Viruses 2020, 12, 369. [Google Scholar] [CrossRef] [PubMed]
- Tu, F.; Zhang, Y.; Xu, S.; Yang, X.; Zhou, L.; Ge, X.; Han, J.; Guo, X.; Yang, H. Detection of pseudorabies virus with a real-time recombinase-aided amplification assay. Transbound. Emerg. Dis. 2022, 69, 2266–2274. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Wang, B.; Qi, H.; Jin, Z.; Qiu, H.J.; Sun, Y. A NanoLuc Luciferase Reporter Pseudorabies Virus for Live Imaging and Quantification of Viral Infection. Front. Vet. Sci. 2020, 7, 566446. [Google Scholar] [CrossRef]
- Fuchs, W.; Rziha, H.J.; Lukàcs, N.; Braunschweiger, I.; Visser, N.; Lütticken, D.; Schreurs, C.S.; Thiel, H.J.; Mettenleiter, T.C. Pseudorabies virus glycoprotein gI: In Vitro and In Vivo analysis of immunorelevant epitopes. J. Gen. Virol. 1990, 71 Pt 5, 1141–1151. [Google Scholar] [CrossRef]
- Tsumoto, K.; Ejima, D.; Kumagai, I.; Arakawa, T. Practical considerations in refolding proteins from inclusion bodies. Protein Expr. Purif. 2003, 28, 1–8. [Google Scholar] [CrossRef]
- Clark, E.D. Protein refolding for industrial processes. Curr. Opin. Biotechnol. 2001, 12, 202–207. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Qi, B.; Yang, Y.; Cui, X.; Chen, T.; Cai, X.; An, T.; Wang, S. Immunogenicity Characterization of the Recombinant gI Protein Fragment from Pseudorabies Virus and an Evaluation of Its Diagnostic Use in Pigs. Vet. Sci. 2023, 10, 506. https://doi.org/10.3390/vetsci10080506
He H, Qi B, Yang Y, Cui X, Chen T, Cai X, An T, Wang S. Immunogenicity Characterization of the Recombinant gI Protein Fragment from Pseudorabies Virus and an Evaluation of Its Diagnostic Use in Pigs. Veterinary Sciences. 2023; 10(8):506. https://doi.org/10.3390/vetsci10080506
Chicago/Turabian StyleHe, Haijuan, Baojie Qi, Yongbo Yang, Xiaowen Cui, Tianfeng Chen, Xuehui Cai, Tongqing An, and Shujie Wang. 2023. "Immunogenicity Characterization of the Recombinant gI Protein Fragment from Pseudorabies Virus and an Evaluation of Its Diagnostic Use in Pigs" Veterinary Sciences 10, no. 8: 506. https://doi.org/10.3390/vetsci10080506
APA StyleHe, H., Qi, B., Yang, Y., Cui, X., Chen, T., Cai, X., An, T., & Wang, S. (2023). Immunogenicity Characterization of the Recombinant gI Protein Fragment from Pseudorabies Virus and an Evaluation of Its Diagnostic Use in Pigs. Veterinary Sciences, 10(8), 506. https://doi.org/10.3390/vetsci10080506







