Effect of Solid-State Fermentation Products of Lactobacillus plantarum, Candida utilis, and Bacillus coagulans on Growth Performance of Broilers and Prevention of Avian Colibacillosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
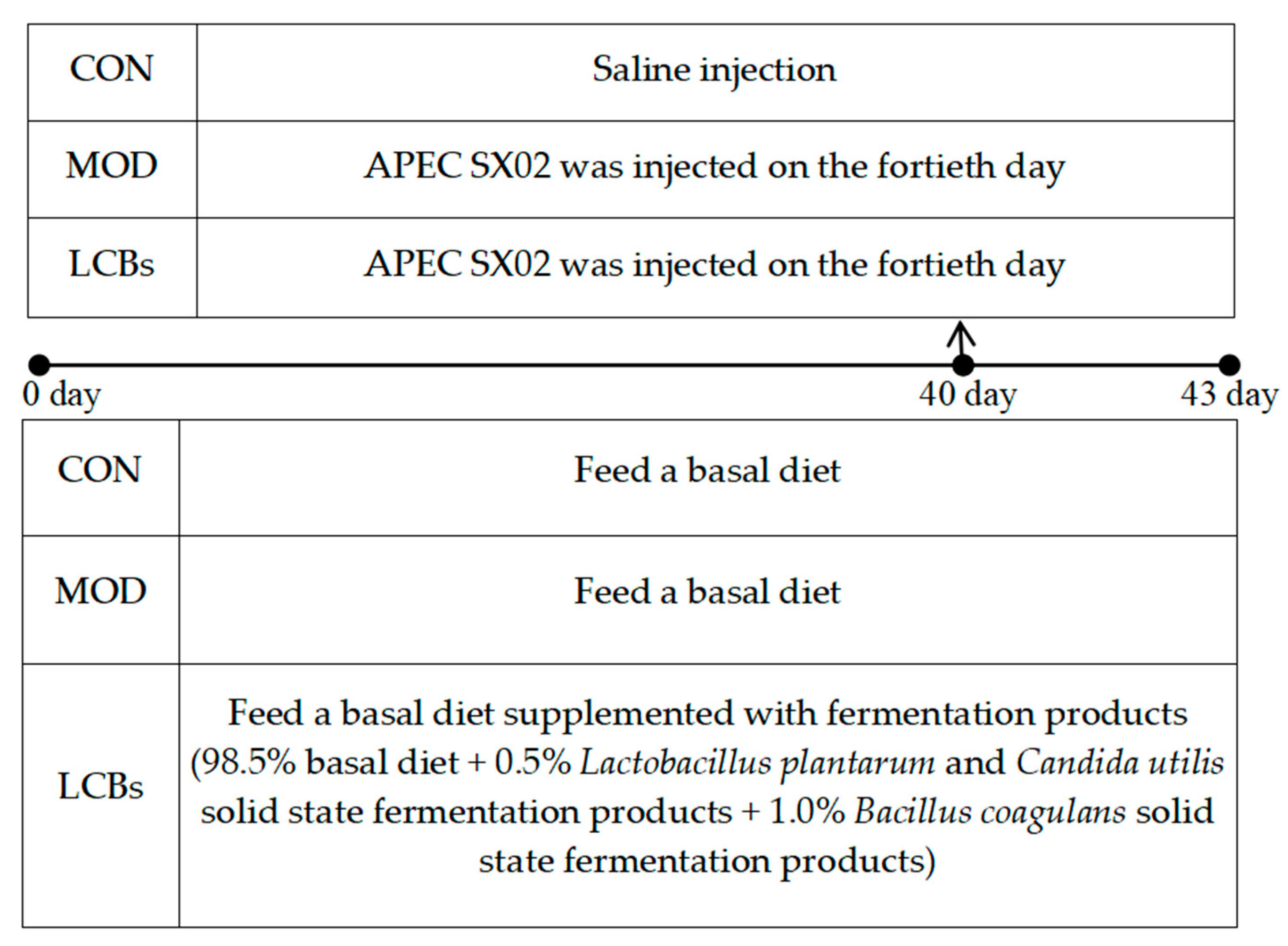
2.1. Bacterial Strains, Broilers, and Diets
2.2. Sample Collection
2.3. Determination of Heart, Liver, Spleen, and Bursa of Fabricius Organ Indices
2.4. Determination of Macroscopic Lesions and Histomorphology of the Heart, Liver, and Jejunum
2.5. Collection of Tissue Samples for Histology Assessment
2.6. Determination of Heart, Liver, and Lung Bacterial Load
2.7. Molecular Analysis of Microbial Communities
2.8. Statistical Analysis
2.9. Use of AI or AI-Assisted Technologies
3. Results
3.1. Growth Performance
3.2. Organ Index
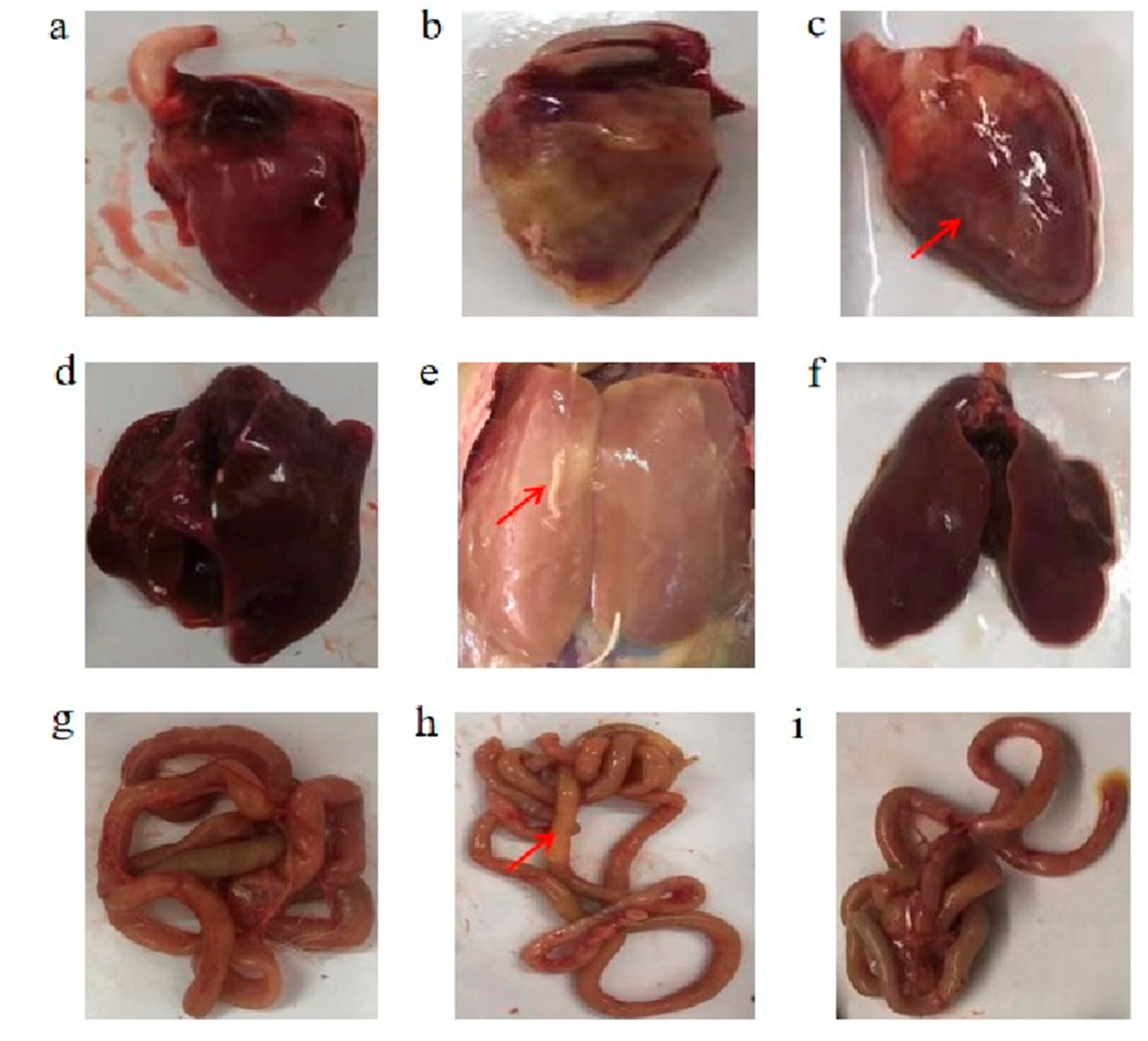
3.3. Macroscopic Lesions of the Heart, Liver, and Jejunum
3.4. Histomorphology of the Heart, Liver, and Jejunum
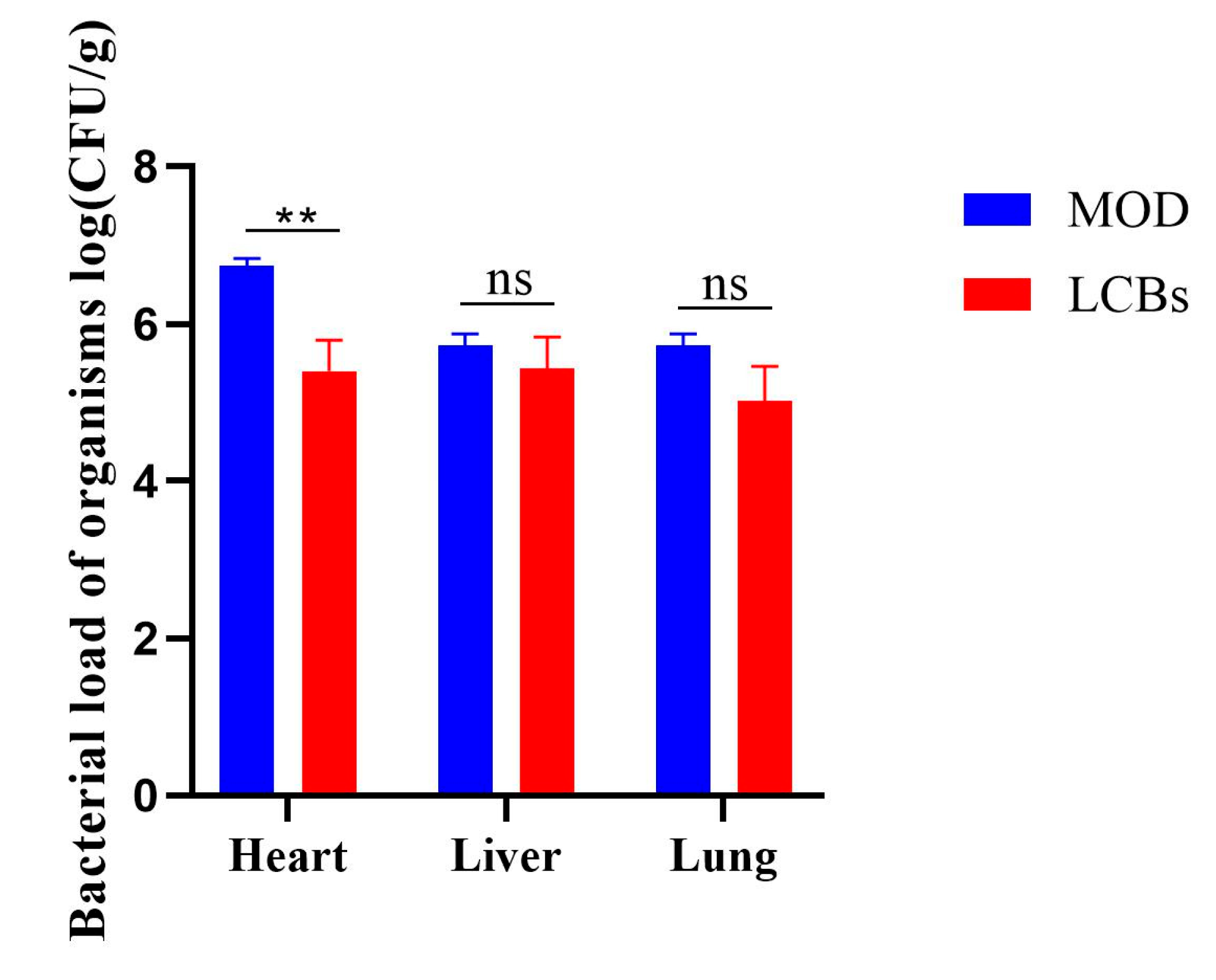
3.5. Bacterial Load of Organisms
3.6. Jejunal Villus Length and Crypt Depth
3.7. Cecal Microbiota Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lone, A.; Mottawea, W.; Mehdi, Y.; Hammami, R. Bacteriocinogenic probiotics as an integrated alternative to antibiotics in chicken production—Why and how? Crit. Rev. Food Sci. Nutr. 2022, 62, 8744–8752. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C.; Shim, W.Y.; Patel, S.K.S.; Gong, C.; Lee, J.-K. Recent developments in antimicrobial growth promoters in chicken health: Opportunities and challenges. Sci. Total Environ. 2022, 8, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Duan, X.; Li, X.; Cao, H.; Wang, Y.; Zheng, S.J. Emerging of a highly pathogenic and multi-drug resistant strain of Escherichia coli causing an outbreak of colibacillosis in chickens. Infect. Genet. Evol. 2018, 65, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalaifah, H.S. Benefits of probiotics and/or prebiotics for antibiotic-reduced poultry. Poult. Sci. 2018, 97, 3807–3815. [Google Scholar] [CrossRef]
- Dai, P.; Wu, H.; Ding, G.; Fan, J.; Li, Y.; Li, S.; Bao, E.; Li, Y.; Gao, X.; Li, H.; et al. Recombinant Salmonella gallinarum (S. gallinarum) Vaccine Candidate Expressing Avian Pathogenic Escherichia coli Type I Fimbriae Provides Protections against APEC O78 and O161 Serogroups and S. gallinarum Infection. Vaccines 2023, 11, 1778. [Google Scholar] [CrossRef]
- Azam, M.; Mohsin, M.; Johnson, T.J.; Smith, E.A.; Johnson, A.; Umair, M.; Saleemi, M.K.; Sajjad-Ur-Rahman. Genomic landscape of multi-drug resistant avian pathogenic Escherichia coli recovered from broilers. Vet. Microbiol. 2020, 247, 108766–108770. [Google Scholar] [CrossRef]
- Ahmadzadeh, A.; Nobakht, A.; Mehmannavaz, Y. Supplementary Prebiotics, Probiotics, and Thyme (Thymus vulgaris) Essential Oil for Broilers: Performance, Intestinal Morphology, and Fecal Nutrient Composition. Probiot. Antimicrob. Proteins 2022, 2, 903–911. [Google Scholar] [CrossRef]
- Yang, J.; Yang, H. Recent development in Se-enriched yeast, lactic acid bacteria and bifidobacteria. Crit. Rev. Food Sci. Nutr. 2023, 63, 411–420. [Google Scholar] [CrossRef]
- Ibarra-Cantún, D.; Ramos-Cassellis, M.E.; Marín-Castro, M.A.; Castelán-Vega, R.D.C. Secondary Metabolites and Antioxidant Activity of the Solid-State Fermentation in Apple (Pirus malus L.) and Agave Mezcalero (Agave angustifolia H.) Bagasse. J. Fungi 2020, 6, 137. [Google Scholar] [CrossRef]
- Yang, L.; Zeng, X.; Qiao, S. Advances in research on solid-state fermented feed and its utilization: The pioneer of private customization for intestinal microorganisms. Anim. Nutr. 2021, 7, 905–916. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jeon, H.-J.; Kim, J.-Y.; Shim, J.-J.; Lee, J.-H. Lactiplantibacillus plantarum HY7718 Improves Intestinal Integrity in a DSS-Induced Ulcerative Colitis Mouse Model by Suppressing Inflammation through Modulation of the Gut Microbiota. Int. J. Mol. Sci. 2024, 25, 575. [Google Scholar] [CrossRef] [PubMed]
- Vimon, S.; Angkanaporn, K.; Nuengjamnong, C. Microencapsulation of Lactobacillus plantarum MB001 and its probiotic effect on growth performance, cecal microbiome and gut integrity of broiler chickens in a tropical climate. Anim. Biosci. 2023, 36, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, Y.; He, T.; Bumbie, G.Z.; Wu, L.; Sun, Z.; Sun, W.; Tang, Z. Effects of Dietary Yucca schidigera Extract and Oral Candida utilis on Growth Performance and Intestinal Health of Weaned Piglets. Front. Nutr. 2021, 8, 685540. [Google Scholar] [CrossRef] [PubMed]
- Hyrslova, I.; Kana, A.; Nesporova, V.; Mrvikova, I.; Doulgeraki, A.I.; Lampova, B.; Doskocil, I.; Musilova, S.; Kieliszek, M.; Krausova, G. In vitro digestion and characterization of selenized Saccharomyces cerevisiae, Pichia fermentans and probiotic Saccharomyces boulardii. J. Trace Elem. Med. Biol. 2024, 83, 127402. [Google Scholar] [CrossRef]
- Hirai, S.; Kawasumi, T. Enhanced lactic acid bacteria viability with yeast coincubation under acidic conditions. Biosci. Biotechnol. Biochem. 2020, 84, 1706–1713. [Google Scholar] [CrossRef]
- Buerth, C.; Tielker, D.; Ernst, J.F. Candida utilis and Cyberlindnera (Pichia) jadinii: Yeast relatives with expanding applications. Appl. Microbiol. Biotechnol. 2016, 100, 6981–6986. [Google Scholar] [CrossRef]
- Xu, L.; Zhou, Y.; Zhan, Z.; Zhang, W.; Fu, D.; Zhao, R.; Chen, X. Research Note: Effects of Bacillus coagulans X26 on the production performance, intestinal structure, short-chain fatty acids and flora composition of laying hens during the peak laying period. Poult. Sci. 2022, 101, 101835. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, W.; Lu, Q.; Zhang, G. Effects of Bacillus coagulans and Lactobacillus plantarum on the Fermentation Characteristics, Microbial Community, and Functional Shifts during Alfalfa Silage Fermentation. Animals 2023, 13, 932. [Google Scholar] [CrossRef]
- Sun, T.; Miao, H.; Zhang, C.; Wang, Y.; Liu, S.; Jiao, P.; Li, W.; Li, Y.; Huang, Z. Effect of dietary Bacillus coagulans on the performance and intestinal microbiota of weaned piglets. Animal 2022, 16, 100561. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, T.; Ai, F.; Ji, C.; Wu, Y.; Huang, X.; Zheng, X.; Yan, F. Bacillus coagulans XY2 ameliorates copper-induced toxicity by bioadsorption, gut microbiota and lipid metabolism regulation. J. Hazard. Mater. 2023, 445, 130585. [Google Scholar] [CrossRef]
- Dolati, M.; Tafvizi, F.; Salehipour, M.; Movahed, T.K.; Jafari, P. Biogenic copper oxide nanoparticles from Bacillus coagulans induced reactive oxygen species generation and apoptotic and anti-metastatic activities in breast cancer cells. Sci. Rep. 2023, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zuo, J.; Chen, Z.; Fu, L.; Lv, X.; Hu, S.; Shi, X.; Jing, Y.; Wang, Y.; Wang, Z.; et al. Use of a modified bacterial ghost lysis system for the construction of an inactivated avian pathogenic Escherichia coli vaccine candidate. Vet. Microbiol. 2019, 229, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-K.; Kim, W.-S.; Paik, H.-D. Bacillus strains as human probiotics: Characterization, safety, microbiome, and probiotic carrier. Food Sci. Biotechnol. 2019, 28, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Fan, H.; Song, B.; Li, G.; Liu, D.; Guo, Y. Supplementing Genistein for Breeder Hens Alters the Fatty Acid Metabolism and Growth Performance of Offsprings by Epigenetic Modification. Oxid. Med. Cell. Longev 2019, 2019, 9214209. [Google Scholar] [CrossRef] [PubMed]
- Elbing, K.L.; Brent, R. Growth of E. coli on Solid Media. Curr. Protoc. Mol. Biol. 2019, 125, e82. [Google Scholar] [CrossRef]
- Zuo, J.; Yin, H.; Hu, J.; Miao, J.; Chen, Z.; Qi, K.; Wang, Z.; Gong, J.; Phouthapane, V.; Jiang, W.; et al. Lsr operon is associated with AI-2 transfer and pathogenicity in avian pathogenic Escherichia coli. Vet. Res. 2019, 50, 109–115. [Google Scholar] [CrossRef]
- Joseph, J.; Zhang, L.; Adhikari, P.; Evans, J.D.; Ramachandran, R. Avian Pathogenic Escherichia coli (APEC) in Broiler Breeders: An Overview. Pathogens 2023, 12, 1280. [Google Scholar] [CrossRef]
- Tarabees, R.; Gafar, K.M.; El-Sayed, M.S.; Shehata, A.A.; Ahmed, M. Effects of Dietary Supplementation of Probiotic Mix and Prebiotic on Growth Performance, Cecal Microbiota Composition, and Protection against Escherichia coli O78 in Broiler Chickens. Probiot. Antimicrob. Proteins 2019, 11, 981–989. [Google Scholar] [CrossRef]
- Zhang, M.; Kou, J.; Wu, Y.; Wang, M.; Zhou, X.; Yang, Y.; Wu, Z. Dietary genistein supplementation improves intestinal mucosal barrier function in Escherichia coli O78-challenged broilers. J. Nutr. Biochem. 2020, 77, 108267. [Google Scholar] [CrossRef]
- Dho-Moulin, M.; Fairbrother, J.M. Avian pathogenic Escherichia coli (APEC). Vet. Res. 1999, 30, 299–316. [Google Scholar]
- Song, K.; Li, J.; Tan, Y.; Yu, J.; Li, M.; Shen, S.; Peng, L.; Yi, P.; Fu, B. Xiaochaihu Decoction Treatment of Chicken Colibacillosis by Improving Pulmonary Inflammation and Systemic Inflammation. Pathogens 2022, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.; Duan, Y.; Wang, F.; Guo, F.; Yan, F.; Yang, X.; Yang, X. Intestinal toxicity of deoxynivalenol is limited by supplementation with Lactobacillus plantarum JM113 and consequentially altered gut microbiota in broiler chickens. J. Anim. Sci. Biotechnol. 2018, 9, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Xie, Y.; Zhang, H.; Jin, J.; Xiong, L.; Liu, H. Effects of a probiotic-fermented herbal blend on the growth performance, intestinal flora and immune function of chicks infected with Salmonella pullorum. Poult. Sci. 2021, 100, 101196. [Google Scholar] [CrossRef] [PubMed]
- Papouskova, A.; Rychlik, I.; Harustiakova, D.; Cizek, A. Research Note: A mixture of Bacteroides spp. and other probiotic intestinal anaerobes reduces colonization by pathogenic E. coli strain O78:H4-ST117 in newly hatched chickens. Poult. Sci. 2023, 102, 102529. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xiang, Y.; Zhou, W.; Chen, J.; Li, K.; Yang, H. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2017, 96, 1387–1393. [Google Scholar] [CrossRef]
- Liao, X.; Shao, Y.; Sun, G.; Yang, Y.; Zhang, L.; Guo, Y.; Luo, X.; Lu, L. The relationship among gut microbiota, short-chain fatty acids, and intestinal morphology of growing and healthy broilers. Poult. Sci. 2020, 99, 5883–5895. [Google Scholar] [CrossRef]
- Zhang, H.; Pertiwi, H.; Hou, Y.; Majdeddin, M.; Michiels, J. Protective effects of Lactobacillus on heat stress-induced intestinal injury in finisher broilers by regulating gut microbiota and stimulating epithelial development. Sci. Total Environ. 2024, 25, 170410. [Google Scholar] [CrossRef]
- Li, C.; Niu, Z.; Zou, M.; Liu, S.; Wang, M.; Gu, X.; Lu, H.; Tian, H.; Jha, R. Probiotics, prebiotics, and synbiotics regulate the intestinal microbiota differentially and restore the relative abundance of specific gut microorganisms. J. Dairy Sci. 2020, 103, 5816–5820. [Google Scholar] [CrossRef]



| Types | Bacillus coagulans Preparation | Lactobacillus plantarum and Candida utilis Preparation |
|---|---|---|
| Dry wheat bran (g) | 100 | 100 |
| Tap water (mL) | 80 | 80 |
| Liquid fermentation medium (mL) | 8 | 8 |
| Fermentation conditions | Under natural conditions (temperature was 25–30 °C, humidity was 35–40%), fermentation for 1–3 days | Under natural conditions (temperature was 25–30 °C, humidity was 35–40%), fermentation for 2–6 days |
| Spore, CFU/g | >108 | >109, >107 |
| Group | Liver Index | Bursal Index | Heart Index | Spleen Index |
|---|---|---|---|---|
| CON | 28.02 ± 2.62 c | 1.43 ± 0.21 b | 8.75 ± 1.29 c | 3.37 ± 0.74 a |
| MOD | 55.39 ± 5.76 a | 3.89 ± 0.78 a | 14.83 ± 3.99 a | 1.83 ± 0.91 c |
| LCBs | 45.07 ± 5.23 b | 4.43 ± 0.77 a | 13.05 ± 3.62 ab | 2.19 ± 0.72 ab |
| Group | Villi Height (μm) | Crypt Depth (μm) | Villi Height/Crypt Depth |
|---|---|---|---|
| CON | 376.39 ± 16.28 a | 48.45 ± 3.79 ab | 7.81 ± 0.81 b |
| MOD | 332.30 ± 21.29 b | 44.77 ± 3.14 b | 7.43 ± 0.47 b |
| LCBs | 374.58 ± 21.22 a | 37.24 ± 2.94 c | 10.09 ± 0.71 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Lv, B.; Zuo, J.; Nawaz, S.; Wang, Z.; Lian, L.; Yin, H.; Chen, S.; Han, X.; Wang, H. Effect of Solid-State Fermentation Products of Lactobacillus plantarum, Candida utilis, and Bacillus coagulans on Growth Performance of Broilers and Prevention of Avian Colibacillosis. Vet. Sci. 2024, 11, 468. https://doi.org/10.3390/vetsci11100468
Li F, Lv B, Zuo J, Nawaz S, Wang Z, Lian L, Yin H, Chen S, Han X, Wang H. Effect of Solid-State Fermentation Products of Lactobacillus plantarum, Candida utilis, and Bacillus coagulans on Growth Performance of Broilers and Prevention of Avian Colibacillosis. Veterinary Sciences. 2024; 11(10):468. https://doi.org/10.3390/vetsci11100468
Chicago/Turabian StyleLi, Fangfang, Bing Lv, Jiakun Zuo, Saqib Nawaz, Zhihao Wang, Liyan Lian, Huifang Yin, Shuming Chen, Xiangan Han, and Haidong Wang. 2024. "Effect of Solid-State Fermentation Products of Lactobacillus plantarum, Candida utilis, and Bacillus coagulans on Growth Performance of Broilers and Prevention of Avian Colibacillosis" Veterinary Sciences 11, no. 10: 468. https://doi.org/10.3390/vetsci11100468
APA StyleLi, F., Lv, B., Zuo, J., Nawaz, S., Wang, Z., Lian, L., Yin, H., Chen, S., Han, X., & Wang, H. (2024). Effect of Solid-State Fermentation Products of Lactobacillus plantarum, Candida utilis, and Bacillus coagulans on Growth Performance of Broilers and Prevention of Avian Colibacillosis. Veterinary Sciences, 11(10), 468. https://doi.org/10.3390/vetsci11100468







