Age at Tumor Diagnosis in 14,636 Canine Cases from the Pathology-Based UNIPI Animal Cancer Registry, Italy: One Size Doesn’t Fit All
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source
2.2. Data Subsetting
2.3. Statistical Analysis
2.3.1. Univariate Analysis
2.3.2. Event History Analysis
3. Results
3.1. Demographic Features of the Study Population
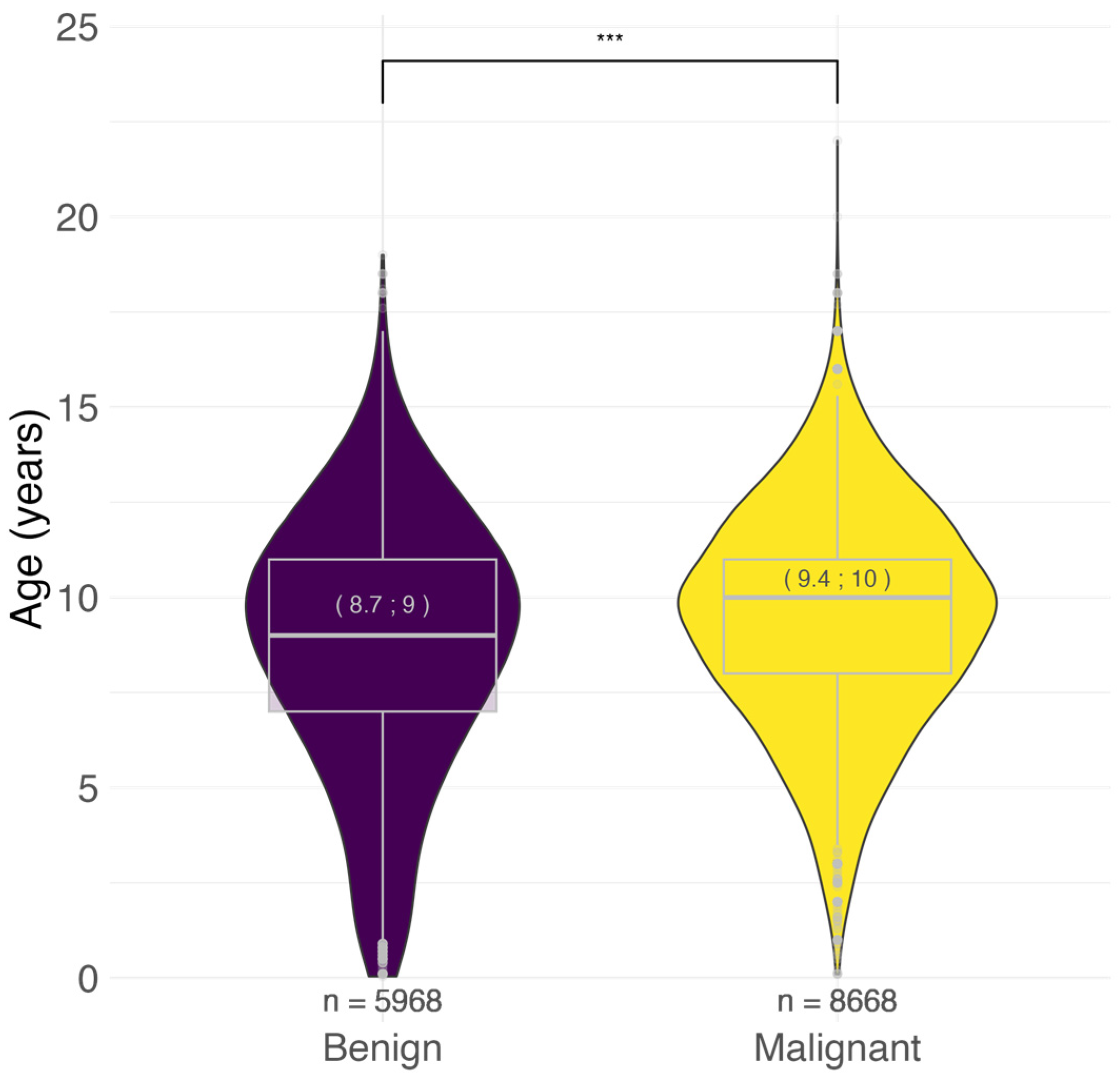
3.2. Age at Tumor Diagnosis
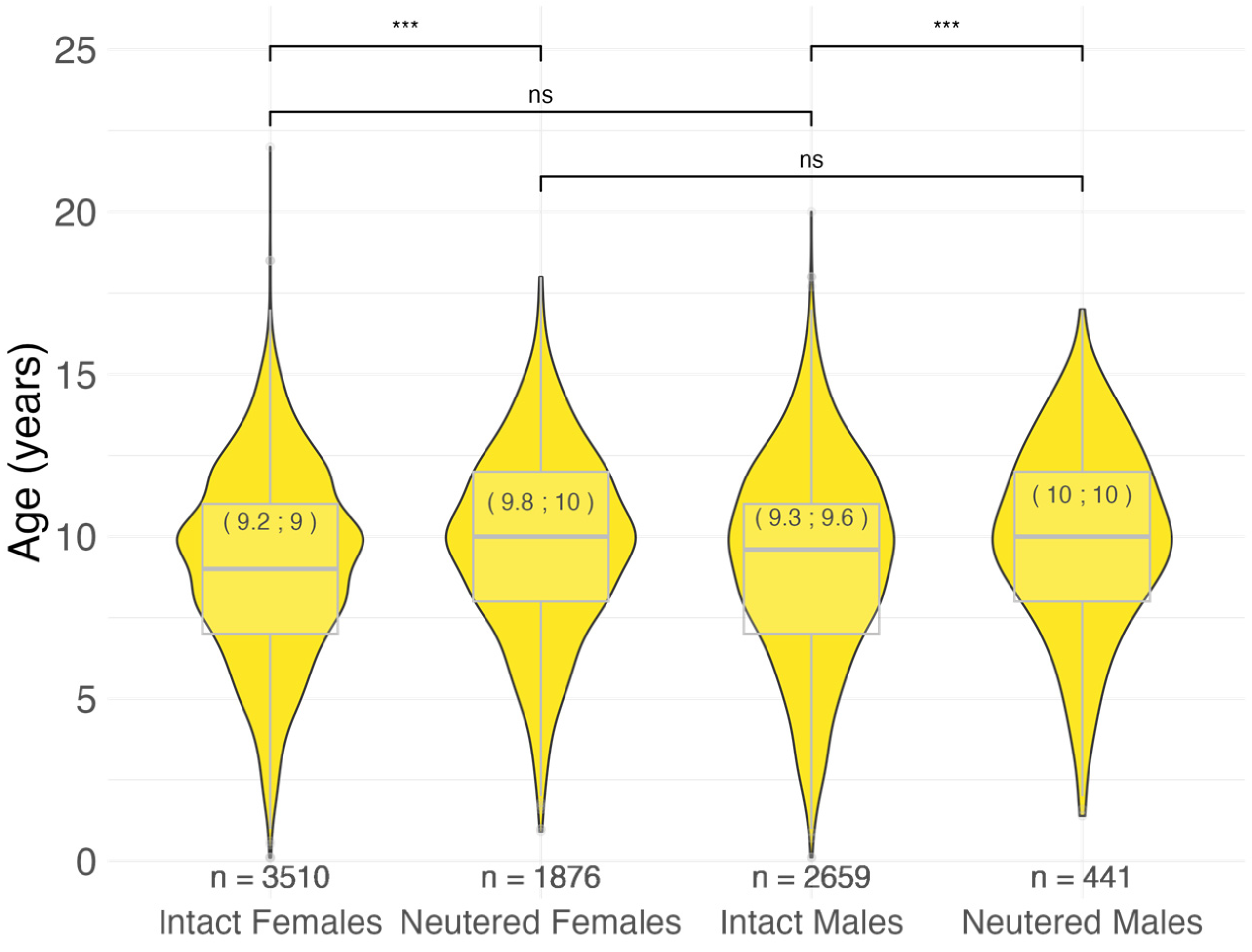
3.2.1. Age at Malignant Tumor Diagnosis by Sex and Neutering Status
3.2.2. Age at Malignant Tumor Diagnosis by Size and Cephalic Index
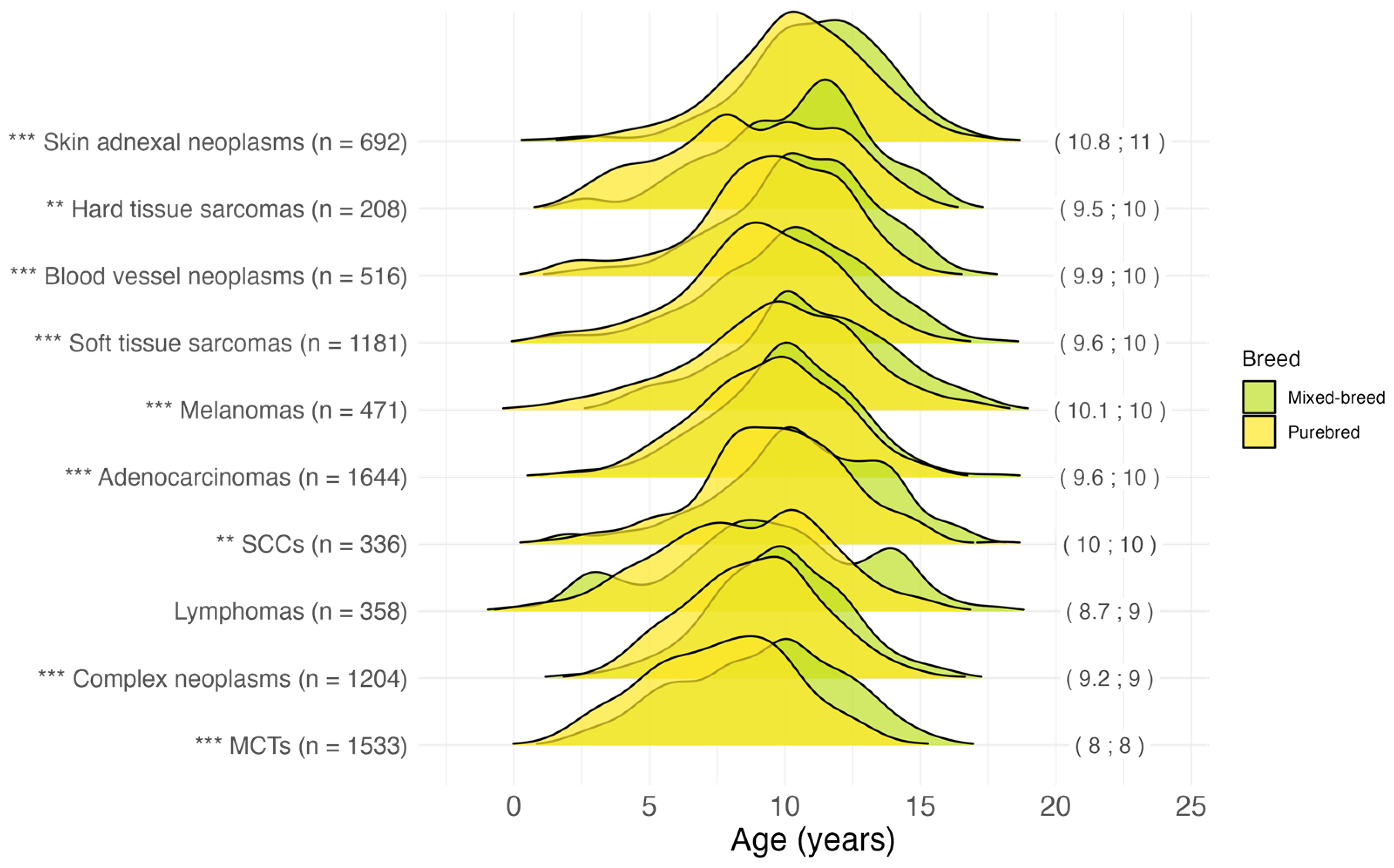
3.2.3. Age at Malignant Tumor Diagnosis by Breed
3.2.4. Age at Malignant Tumor Diagnosis by Histotype
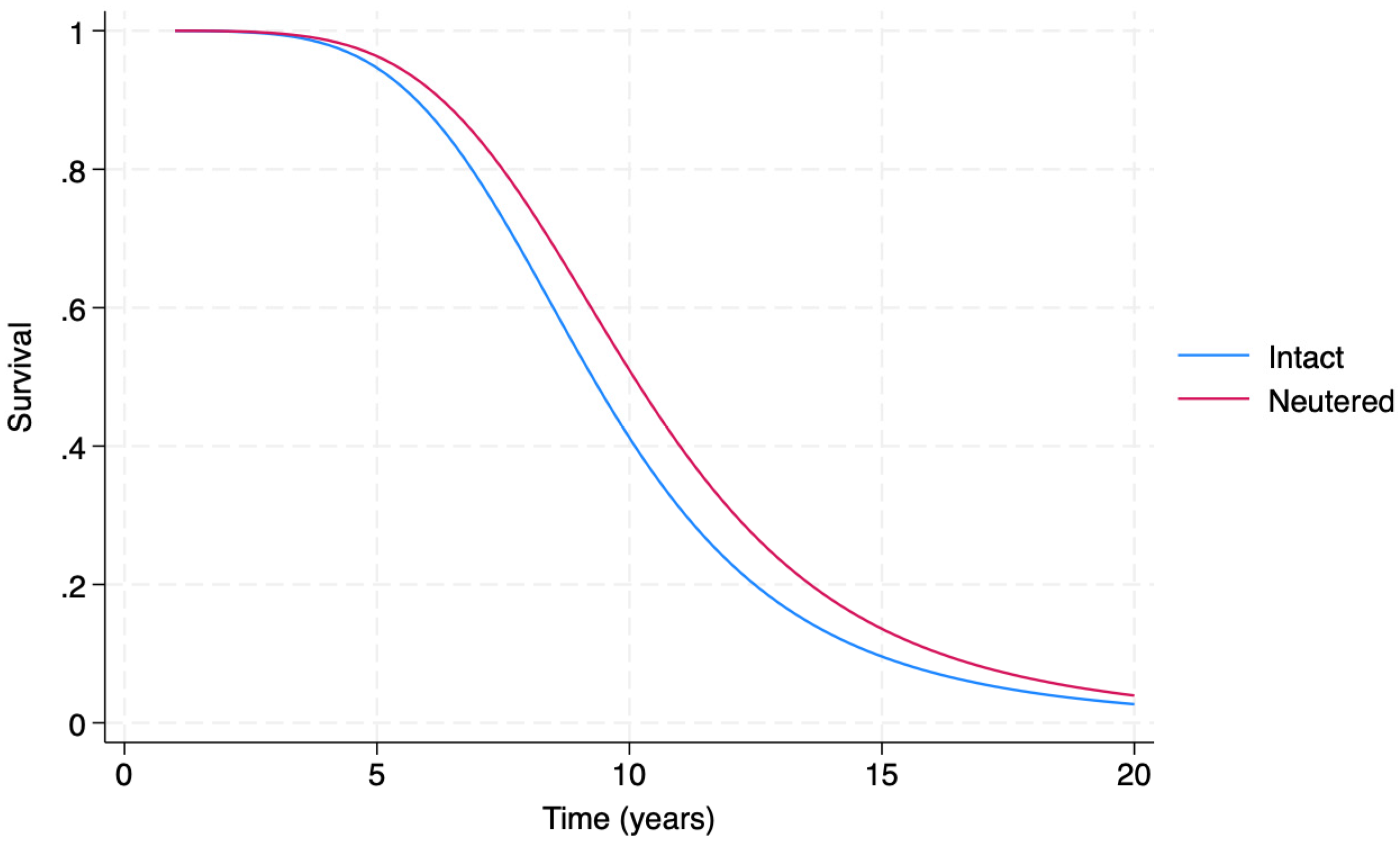
3.3. Event History Analysis
4. Discussion
4.1. Tumor Behavior
4.2. Sex and Neutering Status
4.3. Size and Cephalic Index
4.4. Breed
4.5. The EHA Model
4.6. Research Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Schiffman, J.D.; Breen, M. Comparative oncology: What dogs and other species can teach us about humans with cancer. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140231. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, B.; Ballarin, C.; Mantovani, R.; Rota, A. Aging and Veterinary Care of Cats, Dogs, and Horses through the Records of Three University Veterinary Hospitals. Front. Vet. Sci. 2017, 4, 14. [Google Scholar] [CrossRef]
- Oh, J.H.; Cho, J.Y. Comparative Oncology: Overcoming Human Cancer through Companion Animal Studies. Exp. Mol. Med. 2023, 55, 725–734. [Google Scholar] [CrossRef]
- Sarver, A.L.; Makielski, K.M.; DePauw, T.A.; Schulte, A.J.; Modiano, J.F. Increased risk of cancer in dogs and humans: A consequence of recent extension of lifespan beyond evolutionarily determined limitations? Aging Cancer 2022, 3, 3–19. [Google Scholar] [CrossRef]
- Powell, L.; Chia, D.; McGreevy, P.; Podberscek, A.L.; Edwards, K.M.; Neilly, B.; Guastella, A.J.; Lee, V.; Stamatakis, E. Expectations for dog ownership: Perceived physical, mental and psychosocial health consequences among prospective adopters. PLoS ONE 2018, 13, e0200276. [Google Scholar] [CrossRef] [PubMed]
- Gardner, H.L.; Fenger, J.M.; London, C.A. Dogs as a Model for Cancer. Annu. Rev. Anim. Biosci. 2016, 4, 199–222. [Google Scholar] [CrossRef]
- Hegedus, C.; Andronie, L.; Uiuiu, P.; Jurco, E.; Lazar, E.A.; Popescu, S. Pets, Genuine Tools of Environmental Pollutant Detection. Animals 2023, 13, 2923. [Google Scholar] [CrossRef]
- Khanna, C.; Lindblad-Toh, K.; Vail, D.; London, C.; Bergman, P.; Barber, L.; Breen, M.; Kitchell, B.; McNeil, E.; Modiano, J.F.; et al. The dog as a cancer model. Nat. Biotechnol. 2006, 24, 1065–1066. [Google Scholar] [CrossRef]
- Marsden, C.D.; Ortega-Del Vecchyo, D.; O’Brien, D.P.; Taylor, J.F.; Ramirez, O.; Vila, C.; Marques-Bonet, T.; Schnabel, R.D.; Wayne, R.K.; Lohmueller, K.E. Bottlenecks and selective sweeps during domestication have increased deleterious genetic variation in dogs. Proc. Natl. Acad. Sci. USA 2016, 113, 152–157. [Google Scholar] [CrossRef]
- Turcsán, B.; Kubinyi, E. Differential behavioral aging trajectories according to body size, expected lifespan, and head shape in dogs. GeroScience 2024, 46, 1731–1754. [Google Scholar] [CrossRef]
- McMillan, K.M.; Bielby, J.; Williams, C.L.; Upjohn, M.M.; Casey, R.A.; Christley, R.M. Longevity of Companion Dog Breeds: Those at Risk from Early Death. Sci. Rep. 2024, 14, 531. [Google Scholar] [CrossRef]
- Adams, V.J.; Evans, K.M.; Sampson, J.; Wood, J.L.N. Methods and mortality results of a health survey of purebred dogs in the UK. J. Small Anim. Pract. 2010, 51, 512–524. [Google Scholar] [CrossRef]
- Fleming, J.M.; Creevy, K.E.; Promislow, D.E. Mortality in north american dogs from 1984 to 2004: An investigation into age-, size-, and breed-related causes of death. J. Vet. Intern. Med. 2011, 25, 187–198. [Google Scholar] [CrossRef]
- Dobson, J.M. Breed-predispositions to cancer in pedigree dogs. ISRN Vet. Sci. 2013, 2013, 941275. [Google Scholar] [CrossRef]
- Flory, A.; Wilson-Robles, H. Noninvasive Blood-Based Cancer Detection in Veterinary Medicine. Vet. Clin. N. Am. Small Anim. Pract. 2024, 54, 541–558. [Google Scholar] [CrossRef]
- Colombe, P.; Béguin, J.; Benchekroun, G.; Le Roux, D. Blood biomarkers for canine cancer, from human to veterinary oncology. Vet. Comp. Oncol. 2022, 20, 767–777. [Google Scholar] [CrossRef]
- Ferreira, T.; da Costa, R.M.G.; Dias, F.; Gama, A.; Gaspar, V.M.; Mano, J.F.; Oliveira, P.A.; Medeiros, R. Exploring the role of microRNAs as diagnostic and prognostic biomarkers in canine mammary tumors. GeroScience 2024, 2, 1–17. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Approaches to canine health surveillance. Canine Genet. Epidemiol. 2014, 1, 2. [Google Scholar] [CrossRef]
- Pinello, K.C.; Queiroga, F.; de Matos, A.; Santos, A.; Ribeiro, J.N.; Guscetti, F.; Palmieri, C.; Soberano, M.; Momanyi, K.; Torres, J.R.; et al. The Global Initiative for Veterinary Cancer Surveillance (GIVCS): Report of the first meeting and future perspectives. Vet. Comp. Oncol. 2020, 18, 141–142. [Google Scholar] [CrossRef]
- Meuten, D.J.; Moore, F.M.; Donovan, T.A.; Bertram, C.A.; Klopfleisch, R.; Foster, R.A.; Smedley, R.C.; Dark, M.J.; Milovancev, M.; Stromberg, P.; et al. International Guidelines for Veterinary Tumor Pathology: A Call to Action. Vet. Pathol. 2021, 58, 766–794. [Google Scholar] [CrossRef] [PubMed]
- Pinello, K.; Baldassarre, V.; Steiger, K.; Paciello, O.; Pires, I.; Laufer-Amorim, R.; Oevermann, A.; Niza-Ribeiro, J.; Aresu, L.; Rous, B.; et al. Vet-ICD-O-Canine-1, a System for Coding Canine Neoplasms Based on the Human ICD-O-3.2. Cancers 2022, 14, 1529. [Google Scholar] [CrossRef] [PubMed]
- Merlo, D.F.; Rossi, L.; Pellegrino, C.; Ceppi, M.; Cardellino, U.; Capurro, C.; Ratto, A.; Sambucco, P.L.; Sestito, V.; Tanara, G.; et al. Cancer incidence in pet dogs: Findings of the animal tumor registry of Genoa, Italy. J. Vet. Intern. Med. 2008, 22, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Vascellari, M.; Baioni, E.; Ru, G.; Carminato, A.; Mutinelli, F. Animal tumour registry of two provinces in northern Italy: Incidence of spontaneous tumours in dogs and cats. BMC Vet. Res. 2009, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Baioni, E.; Scanziani, E.; Vincenti, M.C.; Leschiera, M.; Bozzetta, E.; Pezzolato, M.; Desiato, R.; Bertolini, S.; Maurella, C.; Ru, G. Estimating canine cancer incidence: Findings from a population-based tumour registry in northwestern Italy. BMC Vet. Res. 2017, 13, 203. [Google Scholar] [CrossRef]
- Manuali, E.; Morgante, R.A.; Maresca, C.; Leonardi, L.; Purificato, I.; Giaimo, M.D.; Giovannini, G. A web-based tumor registration system for a regional Canine Cancer Registry in Umbria, central Italy. Ann. Ist. Super. Sanita 2019, 55, 357–362. [Google Scholar] [CrossRef]
- De Biase, D.; Baldassarre, V.; Piegari, G.; Rosato, G.; Caputo, V.; Pompameo, M.; Sarnelli, P.; Russo, V.; D’Angelo, D.; Papparella, S.; et al. Animal sentinels and cancer registries: State of the art and new perspectives. Ann. Res. Oncol. 2023, 3, 14–23. [Google Scholar] [CrossRef]
- Crescio, M.I.; Ru, G.; Aresu, L.; Bozzetta, E.; Cancedda, M.G.; Capello, K.; Castagnaro, M.; Carnio, A.; Cocumelli, C.; Degli Uberti, B.; et al. The Italian network of laboratories for veterinary oncology (NILOV) 2.0: Improving knowledge on canine tumours. Vet. Sci. 2022, 9, 394. [Google Scholar] [CrossRef]
- Dorn, C.R.; Taylor, D.O.; Frye, F.L.; Hibbard, H.H. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. I. Methodology and description of cases. J. Natl. Cancer Inst. 1968, 40, 295–305. [Google Scholar]
- Dorn, C.R.; Taylor, D.O.; Schneider, R.; Hibbard, H.H.; Klauber, M.R. Survey of animal neoplasms in Alameda and Contra Costa Counties, California. II. Cancer morbidity in dogs and cats from Alameda County. J. Natl. Cancer Inst. 1968, 40, 307–318. [Google Scholar]
- MacVean, D.W.; Monlux, A.W.; Anderson, P.S., Jr.; Silberg, S.L.; Roszel, J.F. Frequency of Canine and Feline Tumors in a Defined Population. Vet. Pathol. 1978, 15, 700–715. [Google Scholar] [CrossRef]
- Dobson, J.M.; Samuel, S.; Milstein, H.; Rogers, K.; Wood, J.L. Canine neoplasia in the UK: Estimates of inci- dence rates from a population of insured dogs. J. Small Anim. Pract. 2002, 43, 240–246. [Google Scholar] [CrossRef]
- Gruntzig, K.; Graf, R.; Hassig, M.; Welle, M.; Meier, D.; Lott, G.; Erni, D.; Schenker, N.S.; Guscetti, F.; Boo, G.; et al. The Swiss canine cancer registry: A retrospective study on the occurrence of tumours in dogs in Switzerland from 1955 to 2008. J. Comp. Pathol. 2015, 152, 161–171. [Google Scholar] [CrossRef]
- Komazawa, S.; Sakai, H.; Itoh, Y.; Kawabe, M.; Murakami, M.; Mori, T.; Maruo, K. Canine tumor development and crude incidence of tumors by breed based on domestic dogs in Gifu prefecture. J. Vet. Med. Sci. 2016, 78, 1269–1275. [Google Scholar] [CrossRef]
- Dhein, E.S.; Heikkilä, U.; Oevermann, A.; Blatter, S.; Meier, D.; Hartnack, S.; Guscetti, F. Incidence rates of the most common canine tumors based on data from the Swiss Canine Cancer Registry (2008 to 2020). PLoS ONE 2024, 19, e0302231. [Google Scholar] [CrossRef]
- Richards, H.G.; McNeil, P.E.; Thompson, H.; Reid, S.W. An epidemiological analysis of a canine-biopsies database compiled by a diagnostic histopathology service. Prev. Vet. Med. 2001, 51, 125–136. [Google Scholar] [CrossRef]
- Gruntzig, K.; Graf, R.; Boo, G.; Guscetti, F.; Hassig, M.; Axhausen, K.W.; Fabrikant, S.; Welle, M.; Meier, D.; Folkers, G.; et al. Swiss Canine Cancer Registry 1955–2008: Occurrence of the Most Common Tumour Diagnoses and Influence of Age, Breed, Body Size, Sex and Neutering Status on Tumour Development. J. Comp. Pathol. 2016, 155, 156–170. [Google Scholar] [CrossRef]
- Pinello, K.; Pires, I.; Castro, A.F.; Carvalho, P.T.; Santos, A.; de Matos, A.; Queiroga, F.; Canadas-Sousa, A.; Dias-Pereira, P.; Catarino, J.; et al. Cross Species Analysis and Comparison of Tumors in Dogs and Cats, by Age, Sex, Topography and Main Morphologies. Data from Vet-OncoNet. Vet. Sci. 2022, 9, 167. [Google Scholar] [CrossRef]
- Pinello, K.; Amorim, I.; Pires, I.; Canadas-Sousa, A.; Catarino, J.; Faísca, P.; Branco, S.; Peleteiro, M.C.; Silva, D.; Severo, M.; et al. Vet-OncoNet: Malignancy Analysis of Neoplasms in Dogs and Cats. Vet. Sci. 2022, 9, 535. [Google Scholar] [CrossRef]
- Aupperle-Lellbach, H.; Grassinger, J.M.; Floren, A.; Torner, K.; Beitzinger, C.; Loesenbeck, G.; Muller, T. Tumour Incidence in Dogs in Germany: A Retrospective Analysis of 109,616 Histopathological Diagnoses (2014–2019). J. Comp. Pathol. 2022, 198, 33–55. [Google Scholar] [CrossRef]
- Brønden, L.B.; Nielsen, S.S.; Toft, N.; Kristensen, A.T.; Dvm, L.B.B.; Msc, N.T.; Dvm, A.T.K. Data from the Danish Veterinary Cancer Registry on the occurrence and distribution of neoplasms in dogs in Denmark. Vet. Rec. 2010, 166, 586–590. [Google Scholar] [CrossRef]
- Rafalko, J.M.; Kruglyak, K.M.; McCleary-Wheeler, A.L.; Goyal, V.; Phelps-Dunn, A.; Wong, L.K.; Warren, C.D.; Rafalko, J.M.; Kruglyak, K.M.; McCleary-Wheeler, A.L.; et al. Age at Cancer Diagnosis by Breed, Weight, Sex, and Cancer Type in a Cohort of More than 3,000 Dogs: Determining the Optimal Age to Initiate Cancer Screening in Canine Patients. PLoS ONE 2023, 18, e0280795. [Google Scholar] [CrossRef]
- Biller, B.; Berg, J.; Garrett, L.; Ruslander, D.; Wearing, R.; Abbott, B.; Patel, M.; Smith, D.; Bryan, C. AAHA oncology guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 2016, 52, 181–204. [Google Scholar] [CrossRef]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early Detection of Cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Diseases for Oncology (ICD-O)—3rd Edition, 1st Revision. Available online: https://apps.who.int/iris/bitstream/handle/10665/96612/9789241548496_eng.pdf (accessed on 2 September 2024).
- Kiupel, M. Mast Cell Tumors. In Tumors in Domestic Animals; Wiley Blackwell: Hoboken, NJ, USA, 2016; pp. 176–202. [Google Scholar]
- Bellamy, E.; Berlato, D. Canine cutaneous and subcutaneous mast cell tumours: A review. J. Small Anim. Pract. 2021, 63, 497–511. [Google Scholar] [CrossRef]
- Burrai, G.P.; Baldassarre, V.; Brunetti, B.; Iussich, S.; Maniscalco, L.; Mariotti, F.; Sfacteria, A.; Cocumelli, C.; Grieco, V.; Millanta, F.; et al. Canine and feline in situ mammary carcinoma: A comparative review. Vet. Pathol. 2022, 59, 894–902. [Google Scholar] [CrossRef]
- Fédération Cynologique Internationale. Available online: https://www.fci.be/fr/ (accessed on 2 September 2024).
- Edmunds, G.; Beck, S.; Kale, U.K.; Spasic, I.; O’Neill, D.; Brodbelt, B.; Smalley, M.J. Associations between dog breed and clinical features of mammary epithelial neoplasia in bitches: An Epidemiological Study of Submissions to a Single Diagnostic Pathology Centre Between 2008–2021. J. Mammary Gland. Biol. Neoplasia 2023, 28, 6. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Pegram, C.; Crocker, P.; Brodbelt, D.C.; Church, D.B.; Packer, R.M.A. Unravelling the health status of brachycephalic dogs in the UK using multivariable analysis. Sci. Rep. 2020, 10, 17251. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Yamaguchi, K. Event History Analysis; Sage Publications, Inc.: Newbury Park, CA, USA, 1991. [Google Scholar]
- Aleksić-Kovačević, S.; Gligorić, S.; Vučićević, I.; Kukolj, V. Seven-Year Follow-Up of Tumors in Young Dogs in the Republic of Serbia. Acta Vet. 2024, 74, 302–312. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Bozic, I.; Wu, C.J. Delineating the evolutionary dynamics of cancer from theory to reality. Nat. Cancer 2020, 1, 580–588. [Google Scholar] [CrossRef]
- Slater, M.R.; di Nardo, A.; Pediconi, O.; Villa, P.D.; Candeloro, L.; Alessandrini, B.; del Papa, S. Cat and Dog Ownership and Management Patterns in Central Italy. Prev. Vet. Med. 2008, 85, 267–294. [Google Scholar] [CrossRef] [PubMed]
- Carvelli, A.; Scaramozzino, P.; Iacoponi, F.; Condoleo, R.; Marta, U.D. Size, demography, ownership profiles, and identification rate of the owned dog population in Central Italy. PLoS ONE 2020, 15, e0240551. [Google Scholar] [CrossRef]
- Kraus, C.; Snyder-Mackler, N.; Promislow, D.E. How size and genetic diversity shape lifespan across breeds of purebred dogs. GeroScience 2023, 45, 627–643. [Google Scholar] [CrossRef]
- Nunney, L. The Effect of Body Size and Inbreeding on Cancer Mortality in Breeds of the Domestic Dog: A Test of the Multi-Stage Model of Carcinogenesis. R. Soc. Open Sci. 2024, 11, 231356. [Google Scholar] [CrossRef]
- Peto, R.; Roe, F.J.C.; Lee, P.N.; Levy, L.; Clack, J. Cancer and ageing in mice and men. Br. J. Cancer 1975, 32, 411–426. [Google Scholar] [CrossRef]
- Vincze, O.; Colchero, F.; Lemaître, J.F.; Conde, D.A.; Pavard, S.; Bieuville, M.; Urrutia, A.O.; Ujvari, B.; Boddy, A.M.; Maley, C.C.; et al. Cancer risk across mammals. Nature 2022, 601, 263–267. [Google Scholar] [CrossRef]
- Roberts, T.; McGreevy, P.; Valenzuela, M. Human induced rotation and reorganization of the brain of domestic dogs. PLoS ONE 2010, 5, e11946. [Google Scholar] [CrossRef]
- Bannasch, D.; Famula, T.; Donner, J.; Anderson, H.; Honkanen, L.; Batcher, K.; Safra, N.; Thomasy, S.; Rebhun, R. The Effect of Inbreeding, Body Size and Morphology on Health in Dog Breeds. Canine Med. Genet. 2021, 8, 12. [Google Scholar] [CrossRef]
- Yordy, J.; Kraus, C.; Hayward, J.J.; White, M.E.; Shannon, L.M.; Creevy, K.E.; Promislow, D.E.L.; Boyko, A.R. Body size, inbreeding, and lifespan in domestic dogs. Conserv. Genet. 2020, 21, 137–148. [Google Scholar] [CrossRef]
- Schwartz, S.M.; Urfer, S.R.; White, M.; Megquier, K.; Shrager, S.; The Dog Aging Project Consortium; Ruple, A. Lifetime Prevalence of Malignant and Benign Tumours in Companion Dogs: Cross-sectional Analysis of Dog Aging Project Baseline Survey. Vet. Comp. Oncol. 2022, 20, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Kaldrymidou, H.; Leontides, L.; Koutinas, A.F.; Saridomichelakis, M.N.; Karayannopoulou, M. Prevalence, distribution and factors associated with the presence and the potential for malignancy of cutaneous neoplasms in 174 dogs admitted to a clinic in northern Greece. J. Vet. Med. Ser. 2002, 49, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Nationwide. Diversity of Risk: Purebred Dogs and Cancer. Available online: https://assets.ctfassets.net/440y9b545yd9/4inMq7Attnq5K1kInWrqcR/01160a4e7cfcbbda1c0248d2bafc39be/Nationwide_Diversity_Of_Risk_Purebred_Cancer_White_Paper.pdf (accessed on 2 September 2024).
- Bray, J.P. Soft tissue sarcoma in the dog—Part 1: A current review. J. Small Anim. Pract. 2016, 57, 510–519. [Google Scholar] [CrossRef]
- Rasotto, R.; Berlato, D.; Goldschmidt, M.H.; Zappulli, V. Prognostic significance of canine mammary tumor histologic subtypes: An observational cohort study of 229 cases. Vet. Pathol. 2017, 54, 571–578. [Google Scholar] [CrossRef]
- Carnio, A.; Eleni, C.; Cocumelli, C.; Bartolomé Del Pino, L.E.; Simeoni, S.; Spallucci, V.; Scaramozzino, P. Evaluation of Intrinsic and Extrinsic Risk Factors for Dog Visceral Hemangiosarcoma: A Retrospective Case-Control Study Register-Based in Lazio Region, Italy. Prev. Vet. Med. 2020, 181, 105074. [Google Scholar] [CrossRef] [PubMed]
- Celant, E.; Marconato, L.; Stefanello, D.; Moretti, P.; Aresu, L.; Comazzi, S.; Martini, V. Clinical and Clinical Pathological Presentation of 310 Dogs Affected by Lymphoma with Aberrant Antigen Expression Identified via Flow Cytometry. Vet. Sci. 2022, 9, 184. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Edmunds, G.L.; Urquhart-Gilmore, J.; Church, D.B.; Rutherford, L.; Smalley, M.J.; Brodbelt, D.C. Dog breeds and conformations predisposed to osteosarcoma in the UK: A VetCompass study. Canine Med. Genet. 2023, 10, 8. [Google Scholar] [CrossRef]
- Polton, G.; Borrego, J.F.; Clemente-Vicario, F.; Clifford, C.A.; Jagielski, D.; Kessler, M.; Kobayashi, T.; Lanore, D.; Queiroga, F.L.; Rowe, A.T.; et al. Melanoma of the Dog and Cat: Consensus and Guidelines. Front. Vet. Sci. 2024, 11, 1359426. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, M.; Salini, R.; Pietra, M.; Sgorbini, M.; Gori, E.; Dondi, M.; Crisi, P.E.; Conte, A.; Dalla Villa, P.; Podaliri, M.; et al. Factors related to longevity and mortality of dogs in Italy. Prev. Vet. Med. 2024, 225, 106155. [Google Scholar] [CrossRef]
- Kleinbaum, D.G.; Klein, M. Survival Analysis a Self-Learning Text, 3rd ed.; Springer: New York, NY, USA, 2012; pp. 1–54. [Google Scholar]
- Aivaliotis, G.; Palczewski, J.; Atkinson, R.; Cade, J.E.; Morris, M.A. A comparison of time to event analysis methods, using weight status and breast cancer as a case study. Sci. Rep. 2021, 11, 14058. [Google Scholar] [CrossRef]
- Kraus, C.; Pavard, S.; Promislow, D.E.L. The Size–Life Span Trade-Off Decomposed: Why Large Dogs Die Young. Am. Nat. 2013, 181, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Montoya, M.; Morrison, J.A.; Arrignon, F.; Spofford, N.; Charles, H.; Hours, M.-A.; Biourge, V. Life expectancy tables for dogs and cats derived from clinical data. Front. Vet. Sci. 2023, 10, 1082102. [Google Scholar] [CrossRef] [PubMed]
- Flory, A.; Gray, S.; McLennan, L.M.; Rafalko, J.M.; Marshall, M.A.; Wotrang, K.; Kroll, M.; Flesner, B.K.; O’Kell, A.L.; Cohen, T.A.; et al. Study Design and Interim Analysis of the Cancer Lifetime Assessment Screening Study in Canines (CLASSiC): The First Prospective Cancer Screening Study in Dogs Using Next-Generation Sequencing-Based Liquid Biopsy. bioRxiv 2024. [Google Scholar] [CrossRef]
- Barnes, M.; Duray, P.; DeLuca, A.; Anderson, W.; Sindelar, W.; Kinsella, T. Tumor induction following intraoperative radiotherapy: Late results of the National Cancer Institute canine trials. Int. J. Radiat. Oncol. Biol. Phys. 1990, 19, 651–660. [Google Scholar] [CrossRef]
- Hahn, F.F.; Muggenburg, B.A.; Boecker, B.B. Hepatic neoplasms from internally deposited 144CeCl3. Toxicol. Pathol. 1996, 24, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Del Monte, U. Does the cell number 109 still really fit one gram of tumor tissue? Cell Cycle 2009, 8, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Saganuwan, S.A. Mathematical determination of some oncological parameters and their therapeutic implications in dogs. Comp. Clin. Pathol. 2019, 28, 1025–1030. [Google Scholar] [CrossRef]
- Vaghi, C.; Rodallec, A.; Fanciullino, R.; Ciccolini, J.; Mochel, J.P.; Mastri, M. Population modeling of tumor growth curves and the reduced Gompertz model improve prediction of the age of experimental tumors. PLoS Comput. Biol. 2020, 16, e1007178. [Google Scholar] [CrossRef]
- Papparella, S.; Crescio, M.I.; Baldassarre, V.; Brunetti, B.; Burrai, G.P.; Cocumelli, C.; Grieco, V.; Iussich, S.; Maniscalco, L.; Mariotti, F.; et al. Reproducibility and Feasibility of Classification and National Guidelines for Histological Diagnosis of Canine Mammary Gland Tumours: A Multi-Institutional Ring Study. Vet. Sci. 2022, 9, 357. [Google Scholar] [CrossRef]


| Variables | Dogs | Total Tumors | Malignant Tumors | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Sex | Male | Intact | 4984 | 37.8 | 5486 | 37.4 | 2659 | 30.7 |
| Neutered | 644 | 4.9 | 689 | 4.7 | 441 | 5.1 | ||
| N.d. | 4 | <0.1 | 4 | <0.1 | 4 | <0.1 | ||
| Female | Intact | 4718 | 35.8 | 5318 | 36.2 | 3510 | 40.5 | |
| Neutered | 2561 | 19.4 | 2847 | 19.5 | 1876 | 21.6 | ||
| N.d. | 1 | <0.1 | 1 | <0.1 | 1 | <0.1 | ||
| N.d. | 277 | 2.1 | 291 | 2.0 | 177 | 2.0 | ||
| Breed | Mixed-breed | 4822 | 36.6 | 5303 | 36.2 | 3146 | 36.3 | |
| Purebred | 8367 | 63.4 | 9333 | 63.8 | 5522 | 63.7 | ||
| Size 1 | Small | 2122 | 25.4 | 2351 | 25.2 | 1323 | 24.0 | |
| Medium | 1740 | 20.8 | 1943 | 20.8 | 1152 | 20.9 | ||
| Large | 4209 | 50.3 | 4700 | 50.4 | 2862 | 51.8 | ||
| Cephalic index 1 | Brachycephalic | 1415 | 16.9 | 1586 | 17.0 | 988 | 17.9 | |
| Mesocephalic | 4886 | 58.4 | 5458 | 58.5 | 3150 | 57.0 | ||
| Dolichocephalic | 2051 | 24.5 | 2274 | 24.4 | 1373 | 24.9 | ||
| Covariates | Variables | CE (p Value) 1 |
|---|---|---|
| Sex | Male Female | Ref |
| −0.01 (0.667) | ||
| Neutering status | Neutered | Ref |
| Intact | 0.02 (0.426) | |
| Sex # Neutering status | Female # Intact | −0.10 (<0.001) |
| Size | Small | Ref |
| Medium | −0.06 (<0.001) | |
| Large | −0.12 (<0.001) | |
| Cephalic index | Mesocephalic | Ref |
| Brachycephalic | −0.15 (<0.001) | |
| Dolichocephalic | −0.02 (0.034) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonti, N.; Parisi, F.; Lachi, A.; Dhein, E.S.; Guscetti, F.; Poli, A.; Millanta, F. Age at Tumor Diagnosis in 14,636 Canine Cases from the Pathology-Based UNIPI Animal Cancer Registry, Italy: One Size Doesn’t Fit All. Vet. Sci. 2024, 11, 485. https://doi.org/10.3390/vetsci11100485
Fonti N, Parisi F, Lachi A, Dhein ES, Guscetti F, Poli A, Millanta F. Age at Tumor Diagnosis in 14,636 Canine Cases from the Pathology-Based UNIPI Animal Cancer Registry, Italy: One Size Doesn’t Fit All. Veterinary Sciences. 2024; 11(10):485. https://doi.org/10.3390/vetsci11100485
Chicago/Turabian StyleFonti, Niccolò, Francesca Parisi, Alessio Lachi, Elena Sophie Dhein, Franco Guscetti, Alessandro Poli, and Francesca Millanta. 2024. "Age at Tumor Diagnosis in 14,636 Canine Cases from the Pathology-Based UNIPI Animal Cancer Registry, Italy: One Size Doesn’t Fit All" Veterinary Sciences 11, no. 10: 485. https://doi.org/10.3390/vetsci11100485






